Abstract
Objective:
To assess autonomic function by heart rate variability (HRV) during sleep in patients with sleep related alveolar hypoventilation (SRAH) and to compare it with that of patients with obstructive sleep apnea (OSA) and control patients.
Design:
Cross-sectional study.
Setting:
Sleep Unit, University Hospital of University of Navarra.
Patients:
Fifteen idiopathic and obesity related-SRAH patients were studied. For each patient with SRAH, a patient with OSA, matched in age, sex, body mass index (BMI), minimal oxygen saturation (SatO2), and mean SatO2 was selected. Control patients were also matched in age, sex, and BMI with patients with OSA and those with SRAH, and in apnea/hypopnea index (AHI) with patients with SRAH.
Interventions:
N/A.
Measurements and Results:
Time- and frequency-domain HRV measures (R-R, standard deviation of normal-to-normal RR interval [SDNN], very low frequency [VLF], low frequency [LF], high frequency [HF], LF/HF ratio) were calculated across all sleep stages as well as during wakefulness just before and after sleep during a 1-night polysomnography. In patients with SRAH and OSA, LF was increased during rapid eye movement (REM) when compared with control patients, whereas HF was decreased during REM and N1-N2 sleep stages. The LF/HF ratio was equally increased in patients with SRAH and OSA during REM and N1-N2. Correlation analysis showed that LF and HF values during REM sleep were correlated with minimal SatO2 and mean SatO2.
Conclusions:
Patients with SRAH exhibited an abnormal cardiac tone during sleep. This fact appears to be related to the severity of nocturnal oxygen desaturation. Moreover, there were no differences between OSA and SRAH, supporting the hypothesis that autonomic changes in OSA are primarily related to a reduced nocturnal oxygen saturation, rather than a consequence of other factors such as nocturnal respiratory events.
Citation:
Palma JA; Urrestarazu E; Lopez-Azcarate J; Alegre M; Fernandez S; Artieda J; Iriarte J. Increased sympathetic and decreased parasym-pathetic cardiac tone in patients with sleep related alveolar hypoventilation. SLEEP 2013;36(6):933-940.
Keywords: Autonomic nervous system, heart rate variability, hypoxia, obesity hypoventilation syndrome, sleep apnea, sleep disordered breathing
INTRODUCTION
Sleep related alveolar hypoventilation (SRAH) is characterized by decreased alveolar ventilation resulting in sleep related oxygen arterial desaturation in patients with normal mechanical properties of the lung. SRAH can be distinguished from central and obstructive sleep apnea (OSA) by the fact that OSA is defined by periodic alterations in the respiratory flow (i.e., apnea, hypopnea, or respiratory effort-related arousals [RERAs]) that are not typically present in SRAH, even though both conditions (sleep apnea and SRAH) may coexist.1
In the absence of pulmonary parenchymal or vascular pathology, lower airway obstruction, neuromuscular disorders, or kyphoscoliosis, the disorder is referred to as idiopathic SRAH. When severe obesity is present, it predisposes individuals to developing SRAH by increasing the burden on disadvantaged respiratory muscles, and then the disorder can be referred to as obesity-related sleep hypoventilation syndrome, and is usually included in the spectrum of obesity-related hypoventilation (pickwickian syndrome).2,3 Not all patients with SRAH have an increased body mass index (BMI), nor will all patients with obesity develop SRAH. The magnitude of obesity and SRAH is not sufficiently correlated to predict what will occur during sleep in individual patients.1
The chief feature of SRAH is reduced ventilation secondary to decreased tidal volume, resulting in hypercapnia, hypoxemia, and leading to a disruption of sleep architecture with increased fragmentation and awakenings. Hypoxemia may also give rise to arrhythmia during sleep.1
One of the simplest noninvasive methods to study changes in cardiovascular autonomic control is to measure heart rate variability (HRV), which can be defined as a physiologic phenomenon where the time interval between heartbeats varies. It is measured by the variation in the beat-to-beat interval. HRV reflects the relationship between the parasympathetic nervous system (PNS) and the sympathetic nervous system (SNS), and it has been reported to be a good predictor for future cardiovascular disease.4 HRV can be analyzed by using a time- or frequency-domain analysis in several intervals (see Methods section).
There is evidence supporting the fact that the autonomic nervous system is dysregulated during sleep and wakefulness in patients with OSA even without evidence of cardiovascular disease. When HRV is analyzed, patients with OSA exhibit increased sympathetic activity and decreased parasympathetic activity.5,6 The HRV improves after initiation of continuous positive airway pressure, even in the first night of treatment.7 However, it remains unclear whether the changes in HRV seen in patients with OSA are a consequence of the hypoxemia during sleep or, conversely, are a result of the dramatic fluctuations in intrathoracic pressure that occur during the repetitive nocturnal respiratory events (i.e. apnea, hypopnea, or RERAs).
In this regard, patients with SRAH represent a uniquely valuable group of individuals to explore this question, as they have significant oxygen desaturations during sleep but respiratory events are almost absent. We hypothesized that, if HRV changes are a consequence of hypoxemia (and not an effect of the changes in intrathoracic pressure during respiratory events), spectral components of HRV should not differ between patients with SRAH and those with OSA.
According to the hypothesis previously mentioned, our aim in this study was to evaluate nocturnal autonomic cardiovascular function in patients with SRAH using frequency- and time-domain HRV analysis during 1-night polysomnography (PSG) and compare it with that of patients with OSA and control patients. Further, we analyzed whether HRV changes differed depending on the different sleep stages (rapid eye movement [REM], N1-N2, N3, and wakefulness just before and after sleep), and whether HRV changes were correlated with any of the patients' characteristics such as body mass index (BMI), minimal oxygen saturation (SatO2), mean SatO2, or wake time after sleep onset (WASO).
Previous studies analyzing HRV have been reported in congenital central hypoventilation syndrome (Ondine's course)8,9 and in hypoventilation associated with chest wall deformities and neuromuscular disease10; however, no studies have investigated the spectral HRV components across sleep stages in patients with idiopathic or obesity-related SRAH in comparison with patients with OSA or healthy control patients. An understanding of the changes in HRV during sleep in patients with SRAH may offer insights into the physiopathology of the autonomic nervous system disruption seen in patients with sleep related breathing disorders.
METHODS
Patients
This was a single-center cross-sectional study involving patients with idiopathic SRAH and obesity-related SRAH. For each patient with SRAH, a patient with OSA matched by age, sex, BMI, minimal SatO2, and mean SatO2 was selected. Control patients with a normal PSG study were also matched in age, sex, and BMI to the remaining two groups and matched in apnea-hypopnea index (AHI) with patients with SRAH.
Patients were retrospectively selected from the sleep database of the Sleep Unit of the University Clinic of Navarra. Patients were either referred to the Sleep Unit from other departments within the University Clinic of Navarra (neurology, pulmonology, endocrinology, ear, nose, and throat, etc.) or from other hospitals of Spain. Most patients were referred because of primary complaints of poor nocturnal sleep, daytime sleepiness, morning headache, or other symptoms suggesting a sleep related breathing disorder. Before PSG studies, all patients completed a questionnaire to document clinically relevant data including current medications, current medical problems, alcohol, tobacco, and recreational drugs consumption, Epworth Sleepiness Scale score, height, and weight. During each hospitalization, sitting systolic and diastolic blood pressures (SBP and DBP) were measured using a mercury sphygmomanometer just before the PSG study. Physical and neurologic examinations were performed to exclude other pathologies.
Inclusion criteria for patients with SRAH were (1) PSG showing more than 30% of total sleep time at an SatO2 of less than 90%; (2) five or fewer scoreable respiratory events (i.e. apnea or hypopnea) per hour of sleep; (3) absence of periodic alterations of air flow; (4) absence of kyphoscoliosis and pulmonary, neurologic, muscular, or cardiac cause.
Inclusion criteria for patients with OSA were (1) five or more scoreable respiratory events (i.e. apnea or hypopnea) per hour of sleep; (2) evidence of respiratory effort during all or a portion of each of the respiratory events; (3) matching with patients with SRAH in terms of BMI, age, sex, minimal SatO2, and mean SatO2.
Inclusion criteria for control patients were (1) five or fewer scoreable respiratory events (i.e. apnea or hypopnea) per hour of sleep; (2) PSG showing more than 30% of total sleep time at an SatO2 of more than 90%; (3) matching with patients with SRAH in terms of BMI, age, sex, and AHI.
Exclusion criteria for the three groups were (1) atrial fibrillation and other cardiac arrhythmias; (2) myocardial ischemia, cardiomyopathy or myocardial infarction; (3) cardiac pacemaker; (4) history of neurologic or psychiatric disorders; (5) thyroid diseases; (6) other sleep disorders such as periodic limb movement disorder, restless limb syndrome, or narcolepsy; (7) treatment with calcium channels blocker, beta-blockers, or any other drugs known to affect the autonomic nervous system; and (8) a previous diagnosis of congenital central hypoventilation syndrome.
Data Acquisition and Handling
OSA and SRAH were diagnosed based on an overnight PSG study. PSG studies were performed using Lamont amplifiers, 20 bit, 32 channels, and dedicated inputs for electroencephalogram, tibial and chin electromyogram, oronasal flow, respiratory effort, oxymetry, heart rate, and body position. Sleep stage classification was performed following the current American Academy of Sleep Medicine (AASM) criteria,11 and hypopneas, apneas, and arousals were scored using the standard recommended AASM scoring criteria.1 We recorded data on the following features: total sleep time, percentage of sleep spent in REM, N1-N2, and N3; REM latency; WASO; number of periodic leg movements per hour; number of awakenings per hour; SatO2; minimal SatO2; number of oxygen desaturations per hour of sleep; and AHI.
HRV Data Handling and Analysis
For each patient, we carefully selected several consecutive apnea-free, arousal-free, 10-min electrocardiogram (ECG) samples, from each sleep stage (REM, N1-N2, N3 and wakefulness before sleep [W-pre] and after sleep [W-post]). ECG was recorded with two derivations (V3 and V5), amplified, band-pass filtered (0.3-30 Hz), and digitized at 250 Hz. The beat series derived from the ECG segments was interpolated and resampled, using cubic splines, to obtain equally spaced RR intervals with an interpolation rate of 4 Hz.
Sleep data in the form of digital files were collected using the Stellate Reviewer software program (Harmonie 6.0). The sleep segments were saved as text files from their digital recording in Stellate Reviewer Version 6 (Stellate Inc., Montreal, Canada). The text files containing the sleep data were converted into a Spike2 data file (S2R) using Spike2 (version 6.02, Cambridge Electronic Design Limited, Cambridge, UK). Then, two of the authors (JAP, EU) assessed HRV of each patient blinded to the PSG results using the S2R files in a HRV analysis program created with MatLab (Mathworks Inc., Nattick, MA, USA) by one of the authors (JLA). Each ECG recording was manually inspected to avoid abnormal QRS wave morphology, ectopic cardiac beats, and movement artifacts, and to ensure that R-waves were correctly marked by the HRV analysis program to allow an accurate detection of R-R intervals.
The following HRV measures were computed in the time and frequency domain based on the measurement standards12:
Time Domain Measures
Mean R-R: the mean normal-to-normal RR interval, in ms.
The standard deviation of normal-to-normal RR interval (SDNN), reflecting the overall HRV, in ms.
Frequency Domain Measures
For HRV analysis in the frequency domain, RR time series were interpolated at 250 ms to obtain equidistant values. Then, fast Fourier transform was applied. We quantified HRV power in four frequency bands that have been associated with different physiologic rhythms4:
Ultra low frequency (ULF) band (ULF power in the range of 0.0001 to 0.003 Hz), in ms2, which is associated with circadian variation, and variations in the activity of neuroendocrine system.
Very low frequency (VLF) band (VLF power in the range of 0.003 to 0.04 Hz), in ms2, which reflects vagal and renin-angiotensin system effects on heart rate.
Low frequency (LF) band (LF power in the range of 0.04 to 0.15 Hz), in ms2, which reflects a combination of sympathetic and parasympathetic influences and baroreflex function.13
High frequency (HF) band (HF power in the range of 0.15 to 0.40 Hz), in ms2, which primarily reflects parasympathetic activity.
We also considered the LF/HF ratio, a unitless measure thought to reflect sympathovagal balance or sympathetic modulations.12
Statistical Analyses
Comparisons of patients' characteristics and sleep characteristics in the three samples were made. The effect of sleep on the HRV variables across sleep stages and within the same sleep stage among the three groups was calculated by using analysis of variance (ANOVA), with Bonferroni correction for multiple comparisons, and the interaction where appropriate. The correlation between the clinical parameters of SRAH patients and the various measures of HRV was analyzed with Spearman correlation coefficient. In all cases, statistical significance was defined as P < 0.05. All statistical tests were performed using SPSS version 15.0.1 (SPSS Inc., Chicago, IL, USA).
Standard Protocol Approval
This study was approved by the Institutional Review Board (IRB) of the University of Navarra. The IRB specifically waived the need for consent of participants, as this was a retrospective study. Data were made anonymous by removal of direct identifiers from the data file (a variable was removed when it was highly identifying such as name, surname, or place of birth; other variables irrelevant for analyses were also removed).
RESULTS
Sample Characteristics
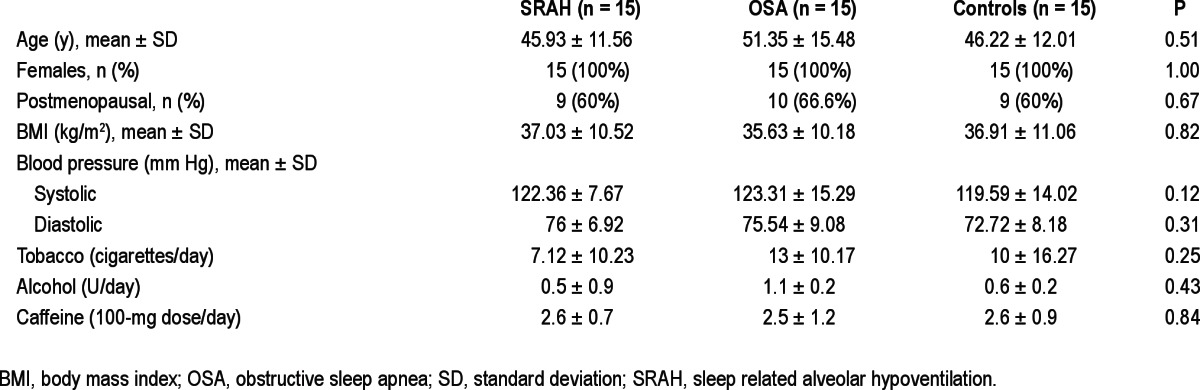
Fifteen female patients with SRAH who fulfilled the inclusion criteria were selected. Fifteen female patients with OSA and 15 female control patients were also selected. All groups had similar age, BMI, and tobacco, alcohol, and caffeine intake levels. Eleven patients with SRAH had severe obesity (BMI ≥ 30 kg/m2), fulfilling criteria for obesity hypoventilation syndrome; the remaining four patients had a BMI ≤ 25 kg/m2, fulfilling criteria for idiopathic SRAH. Characteristics of patients with SRAH, patients with OSA, and control patients are summarized in Table 1.
Table 1.
Sample characteristics
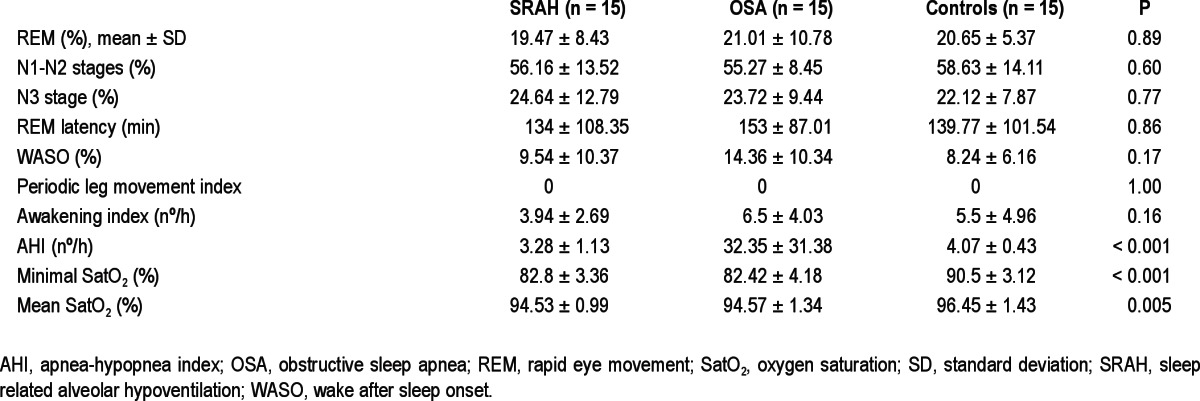
Sleep structure was similar in all groups, including REM latency, percentage of REM, N1-N2 and N3 sleep, and periodic limb movement index. As expected, patients with OSA had a higher AHI when compared with patients with SRAH and control patients, whose AHI was similar. Patients with SRAH and those with OSA had a similar mean and minimal SatO2, but it was lower in comparison with control patients. WASO and the awakening index were higher in OSA patients, although the differences were not statistically significant. Sleep characteristics of all groups are summarized in Table 2.
Table 2.
Sleep characteristic in patients with sleep related alveolar hypoventilation, patients with obstructive sleep apnea, and control patients
HRV Parameters
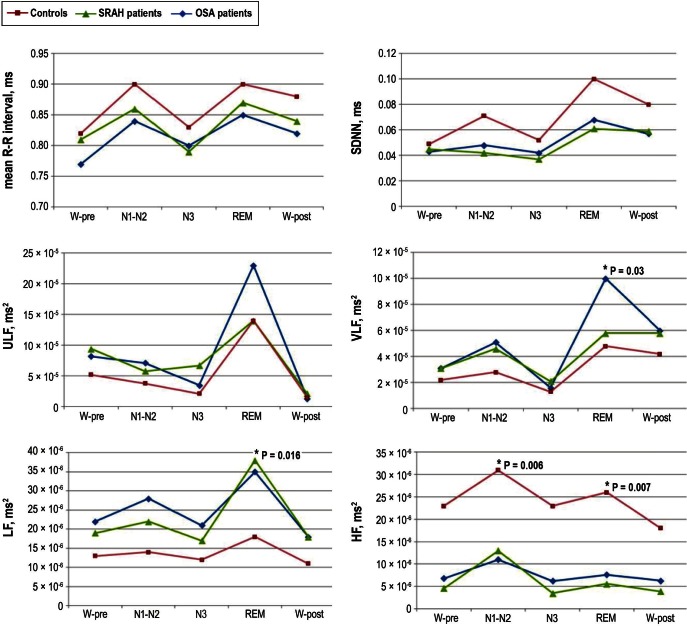
The ANOVA between-group analysis revealed that mean R-R intervals and SDNN were lower in patients with OSA and SRAH when compared with control patients in all sleep stages, although the differences did not reach statistical significance. Across sleep stages, no significant differences were observed in W-pre, W-post, and N3 stages. However, during N1-N2 stage, HF was lower in patients with OSA and SRAH (P = 0.006), and the LF/HF was higher in patients with SRAH when compared with control patients (P = 0.014). During REM, LF was higher in patients with OSA and SRAH than in control patients (P = 0.016), as well as VLF (P = 0.030). Conversely, HF was lower in patients with OSA and SRAH (P = 0.007). The LF/HF ratio was increased in patients with SRAH when compared with control patients (P = 0.011) but not with patients with OSA. No differences were seen when we compared the spectral values of patients with OSA against those with SRAH (Figure 1). For further details, see Table S1 in the supplemental material.
Figure 1.
Mean R-R interval, standard deviation of the NN interval (SDNN), ultra low-frequency components (ULF), very low-frequency components (VLF), low-frequency components (LF), and high-frequency components (HF) during different sleep stages (N1-N2, N3, REM) as well as during wakefulness before (W-pre) and after (W-post) sleep in patients with SRAH, patients with OSA, and control patients. Asterisks indicate statistical significance, and the analysis of variance P value is given. OSA, obstructive sleep apnea; REM, rapid eye movement; SRAH, sleep related alveolar hypoventilation.
When studying the effects of sleep stage on HRV in patients with SRAH, the ANOVA within-group analysis only showed changes during REM, comprising a significant increase of ULF (P = 0.003), and VLF (P = 0.003) components. In patients with OSA, LF was higher during REM (P = 0.004) and HF was higher during N1-N2 (P = 0.03). In control patients, only significant increases in ULF (P = 0.002) during REM and in HF during N1-N2 (P = 0.04) were observed.
Correlation Analysis
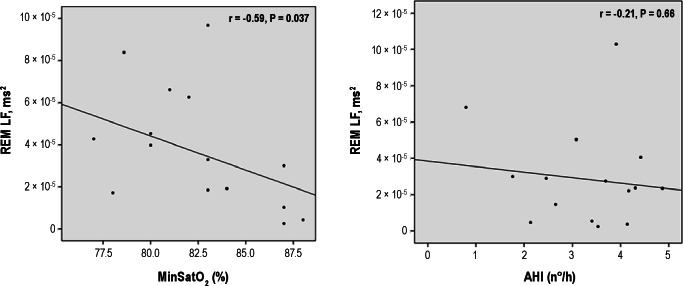
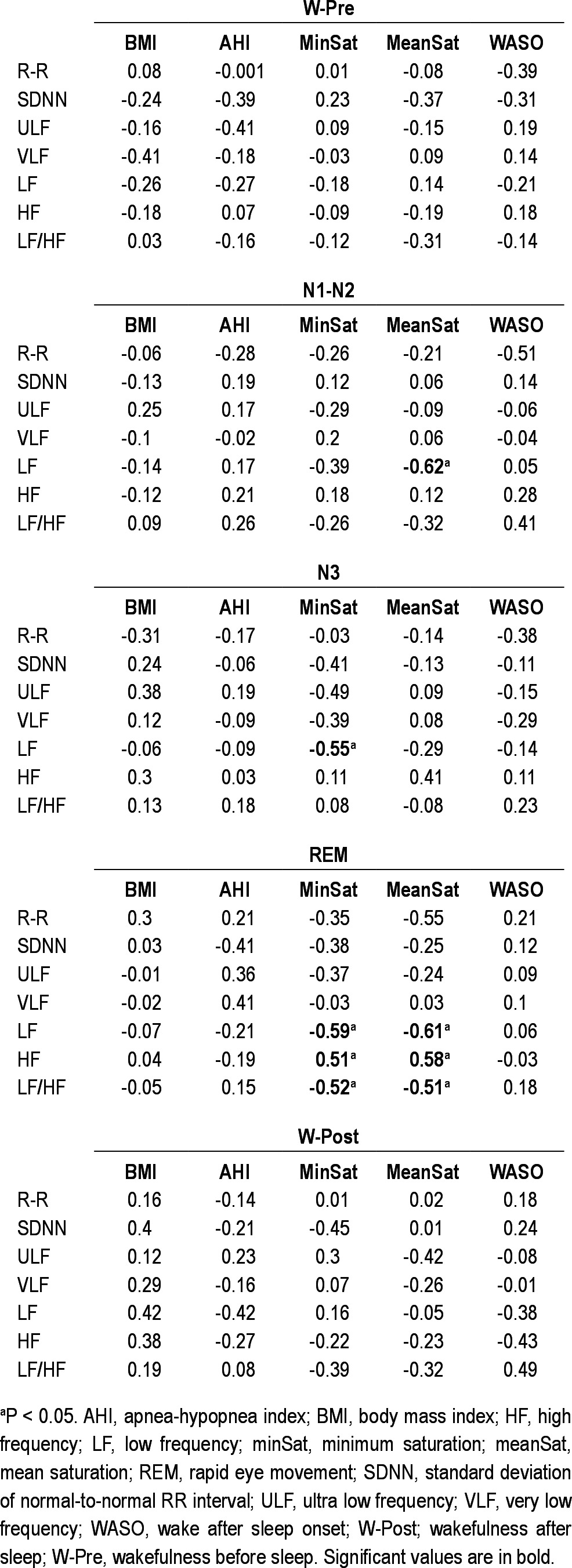
In patients with SRAH, mean SatO2 was inversely correlated with LF during N1-N2, and LF and LF/HF during REM; it was positively correlated with HF during REM. Minimal SatO2 showed a similar pattern, as it was inversely correlated with LF during N3, and LF and LF/HF during REM; and positively correlated with HF during REM. Figure 2 shows an example of this correlation analysis. The remaining features (BMI, AHI, and WASO) did not correlate in patients with SRAH with any of the HRV measures (Table 3).
Figure 2.
Examples of correlation analysis. Low-frequency (LF) heart rate variability during rapid eye movement (REM) sleep in relation to minimal oxygen saturation (SatO2) and apnea-hypopnea index (AHI). The y-axis is given in ms2. Significant increases in LF variability occurred with decreasing SatO2 (r = -0.59; P = 0.037) but not with increasing AHI (r = -0.21; P = 0.66).
Table 3.
Spearman correlation coefficient (rho) between different measures and heart rate variability parameters in patients with sleep related alveolar hypoventilation
DISCUSSION
In this work, we aimed to study HRV across sleep stages in a homogenous SRAH patient sample (free from medications, comorbidities and any other concurrent sleep related breathing disorders) and to compare it with a well-matched sample of patients with OSA and control patients. Another of our aims was to investigate whether the HRV differences were correlated with BMI, AHI, WASO, or oxygen saturation.
Our results revealed that patients with SRAH exhibit an increased LF heart rate (which is thought to be dependent on negative feedback of the baroreflex arch mediated by both SNS and PNS, and traditionally has been considered a measure of sympathetic activity) and decreased HF heart rate (which is modulated by PNS inputs only), suggesting an impaired cardiovascular tone during sleep, particularly during REM sleep, when compared with control patients with similar sleep structure, BMI, and AHI. Furthermore, when patients with SRAH were compared with a group of patients with OSA with similar sleep structure, BMI, mean SatO2 and minimal SatO2, no differences were observed.
Moreover, in patients with SRAH, the LF band was inversely correlated with the mean and minimal SatO2 values, but not with BMI or AHI. The HF band was directly correlated with the mean and minimal SatO2 values, but not with the BMI or the AHI. The influence of BMI on HRV has remained controversial, with some studies finding that obese patients have changes in HRV,14,15 whereas other investigations found no association.7 This discrepancy might be a consequence of the fact that those studies finding changes in HRV in obese patients did not measure oxygen saturation nor were PSGs performed, and therefore the possibility that patients had sleep disordered breathing could not be excluded.
The individual effects of intermittent hypoxia and intrathoracic pressure swings on the sympathetic nervous system activity during sleep disordered breathing are difficult to disentangle.16 We added further insight into the pathophysiology of abnormal autonomic tone during sleep observed in OSA patients. Our results suggest that the increased sympathetic and decreased parasympathetic tone observed in patients with OSA and SRAH might be a direct consequence of the hypoxemia during sleep; acute changes in intrathoracic pressure during nocturnal respiratory events (i.e., apnea and hypopneas that give rise to intrathoracic swings) probably have a minor relevance in the dysregulation of cardiac autonomic control during sleep in patients with sleep disordered breathing.
Some authors have underlined the importance of increased intrathoracic pressure swings, which are an important feature of OSA, in the abnormal cardiac function.17,18 Increased negative inspiratory intrathoracic pressures generated against the occluded upper airway increase left ventricular (LV) pressure and amplify LV afterload. These changes increase venous return, augmenting right ventricular (RV) preload, whereas OSA-induced hypoxemia will result in pulmonary vasoconstriction, thus giving rise to an increased RV afterload. These phenomena (i.e., RV distension and impairment of LV filling) can synergistically increase myocardial oxygen demand in the context of reduced tissue oxygen delivery during apnea-related hypoxemia.19
However, our results support the importance of hypoxia per se, independently of other phenomena, and nicely extends the findings by Sforza et al.20 who found that, during REM sleep, hypoxic responsiveness does not account for the observed changes in intrathoracic pressure during apnea. In our study, autonomic disturbances were observed primarily during REM sleep. Additional experimental reports have emphasized the key role of hypoxemia in the autonomic changes seen in OSA.21,22
The arterial chemoreceptors, particularly the carotid bodies that operate as primary sensors for hypoxemia, and the ensuing carotid chemoreflex trigger a reactive oxygen species-mediated signaling that increases sympathetic activity, elevates blood pressure, and stimulates breathing, giving rise to arousal from sleep.23 Upon arousal (which is a direct consequence of hypoxemia) autonomic activity, blood pressure, cardiac output, and heart rate rapidly increase, resulting in more increased cardiac oxygen demand at a time when arterial SatO2 is at its lowest level.24 In patients with OSA, repetitive apnea episodes aggravate the hypoxemia and CO2 retention, both of which augment sympathetic activity and reduce parasympathetic activity.25,26
Our findings indicating that patients with OSA exhibit changes of spectral components of the HRV are mainly in accordance with previous reports showing that patients with OSA have a an increased sympathetic predominance, primarily during REM sleep (where apneas and hypopneas, and also hypoxemia, predominate).27–30 Although a similar mechanism might take place in patients with SRAH, the pathways by which nocturnal hypoxemia causes autonomic dysfunction in this population remain unclear.
Previous experimental studies clearly support the fact that hypoxemia elevates sympathetic basal activity.21,25,31 Patients with congenital alveolar hypoventilation exhibit a decreased cortical thickness and extensive axonal injury as a consequence of sustained hypoxia.32 It is possible that autonomic neurons may be able to survive prolonged periods of mild hypoxia, but that damage occurs following more severe hypoxemia, even if this lasts only for a few min. This could explain why HRV was correlated both with the minimal oxygen saturation and the mean nocturnal oxygen saturation. Alhough bilevel pressure ventilation has been shown to improve oxygenation and sleep quality in obesity hypoventilation syndrome,33 it is unknown whether the autonomic damage is reversible, and further studies to investigate the effects of treatment with noninvasive ventilation on autonomic function in patients with SRAH are required.
The similar HRV findings in patients with SRAH and those with OSA raises the question whether both conditions represent two stages of a clinical continuum of sleep disordered breathing, ranging from normality to severe OSA.34,35 This vision is supported by the fact that upper airway collapsibility during sleep is variable (which would help to explain why not all patients with severe obesity develop OSA)36,37; and by the fact that nonapneic snorers as well as patients with upper airway resistance syndrome (two of the conditions also included in the continuum of sleep disordered breathing) exhibit alterations in HRV during sleep.38,39 However, the initiating feature of OSA would be the obstructive episodes leading to hypoxia (and, in the long-term, diminished chemoreceptor responsiveness24), whereas the initiating feature of SRAH (and also in central sleep apnea40) would be the impaired chemoreceptor responsiveness per se, resulting in diminished drive to breathe, hypoventilation, and hypoxemia.
This is the first study to analyze the HRV both in idiopathic and obesity-related SRAH, as previous studies have only investigated patients with congenital central hypoventilation syndrome8,9 and hypoventilation associated with chest wall deformities and neuromuscular disease.10 These previous studies, as with the current study, also found that autonomic dysfunction appears to be related to the severity of oxygen desaturation, although patients with less severe nocturnal oxygen desaturation had significant abnormalities in comparison with those with no oxygen desaturation.10
The observed differences of HRV in patients with SRAH when compared with control patients cannot be attributed to differences in BMI, AHI, or sleep structure, as both groups had similar characteristics. Remarkably, we were able to select control patients with severe obesity (BMI ≥ 30 kg/m2) without respiratory abnormalities, which confirms that the magnitude of obesity and SRAH are not sufficiently correlated to predict whether an obese individual will develop sleep disordered breathing.
Why all patients with SRAH in our sample were found to be women is unclear. The main reason is probably the sample selection: after patients with cardiovascular and other comorbid diseases, patients taking drugs, and patients with artifacted ECG recording were excluded, only a sample composed of women remained. However, epidemiology of idiopathic SRAH is poorly characterized, so it is not well known whether, in this particular condition, a female preponderance exists. Among patients with OHS, male patients have been reported to have a slightly increased frequency than women,41 although some studies have analyzed samples with more women than men.2
This fact is related to one of the limitations of this research: because all patients were female, we were unable to analyze the differences in HRV according to sex. This would be an interesting analysis in patients with SRAH, as it has been shown that healthy women have a higher parasympathetic tone than men42 and that non-REM to REM excitatory cardiac responses are more marked in women that in men, regardless of their hormonal status.43 Hormonal mechanisms are also important, as women exhibit sleep disordered breathing syndrome less frequently than men before but not after menopause, suggesting that progesterone and estrogens may exert a protective effect44,45 although the exact mode of action through which these hormones affect breathing is not completely understood. In our sample, the frequency of postmenopause was similar among the three groups, so differences could not be attributed to hormonal status.
Because this was a cross-sectional study, the prognostic value of our findings is unclear, although in other conditions, autonomic dysfunction as measured by HRV has been reported to be a marker of morbidity and mortality.46–48 It is therefore likely that HRV analysis may provide a tool for prognostic evaluation in patients with SRAH, but this warrants additional prospective studies.
In conclusion, HRV is abnormal in patients with SRAH, and appears to be related to the severity of nocturnal oxygen desaturation. Moreover, there were no differences between patients with OSA and patients with SRAH, supporting the hypothesis that HRV changes seen in patients with OSA may be primarily related to lower nocturnal oxygen saturation rather than a consequence of intrathoracic changes during nocturnal respiratory events. Further studies are needed to elucidate whether the abnormal autonomic function has prognostic significance, and whether it is affected by the treatment of SRAH.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
SUPPLEMENTAL MATERIAL
Heart rate variability in patients with sleep related alveolar hypoventilation, patients with obstructive sleep apnea, and control patients
REFERENCES
- 1.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. The International Classification of Sleep Disorders 2nd ed: Diagnostic and Coding Manual. [Google Scholar]
- 2.Berger KI, Ayappa I, Chatr-Amontri B, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest. 2001;120:1231–8. doi: 10.1378/chest.120.4.1231. [DOI] [PubMed] [Google Scholar]
- 3.Piper AJ, Grunstein RR. Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med. 2011;183:292–8. doi: 10.1164/rccm.201008-1280CI. [DOI] [PubMed] [Google Scholar]
- 4.Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput. 2006;44:1031–51. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 5.Aydin M, Altin R, Ozeren A, Kart L, Bilge M, Unalacak M. Cardiac autonomic activity in obstructive sleep apnea: time-dependent and spectral analysis of heart rate variability using 24-hour Holter electrocardiograms. Tex Heart Inst J. 2004;31:132–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Jo JA, Blasi A, Valladares E, Juarez R, Baydur A, Khoo MC. Determinants of heart rate variability in obstructive sleep apnea syndrome during wakefulness and sleep. Am J Physiol Heart Circ Physiol. 2005;288:H1103–12. doi: 10.1152/ajpheart.01065.2003. [DOI] [PubMed] [Google Scholar]
- 7.Kufoy E, Palma JA, Lopez J, et al. Changes in the heart rate variability in patients with obstructive sleep apnea and its response to acute CPAP treatment. PLoS One. 2012;7:e33769. doi: 10.1371/journal.pone.0033769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo MS, Woo MA, Gozal D, Jansen MT, Keens TG, Harper RM. Heart rate variability in congenital central hypoventilation syndrome. Pediatr Res. 1992;31:291–6. doi: 10.1203/00006450-199203000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Trang H, Girard A, Laude D, Elghozi JL. Short-term blood pressure and heart rate variability in congenital central hypoventilation syndrome (On-dine's curse) Clin Sci (Lond) 2005;108:225–30. doi: 10.1042/CS20040282. [DOI] [PubMed] [Google Scholar]
- 10.Watson JP, Nolan J, Elliott MW. Autonomic dysfunction in patients with nocturnal hypoventilation in extrapulmonary restrictive disease. Eur Respir J. 1999;13:1097–102. doi: 10.1034/j.1399-3003.1999.13e26.x. [DOI] [PubMed] [Google Scholar]
- 11.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–31. [PubMed] [Google Scholar]
- 12.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 13.Goldstein DS, Bentho O, Park MY, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. 2011;96:1255–61. doi: 10.1113/expphysiol.2010.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karason K, Molgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83:1242–7. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 15.Poliakova N, Despres JP, Bergeron J, Almeras N, Tremblay A, Poirier P. Influence of obesity indices, metabolic parameters and age on cardiac autonomic function in abdominally obese men. Metabolism. 2012;61:1270–9. doi: 10.1016/j.metabol.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Hakim F, Gozal D, Kheirandish-Gozal L. Sympathetic and catecholaminergic alterations in sleep apnea with particular emphasis on children. Front Neurol. 2012;3:7. doi: 10.3389/fneur.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Floras JS, Bradley TD. Treating obstructive sleep apnea: is there more to the story than 2 millimeters of mercury? Hypertension. 2007;50:289–91. doi: 10.1161/HYPERTENSIONAHA.107.092106. [DOI] [PubMed] [Google Scholar]
- 18.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7:677–85. doi: 10.1038/nrcardio.2010.145. [DOI] [PubMed] [Google Scholar]
- 19.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57:119–27. doi: 10.1016/j.jacc.2010.08.627. [DOI] [PubMed] [Google Scholar]
- 20.Sforza E, Boudewijns A, Schnedecker B, Zamagni M, Krieger J. Role of chemosensitivity in intrathoracic pressure changes during obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154:1741–7. doi: 10.1164/ajrccm.154.6.8970364. [DOI] [PubMed] [Google Scholar]
- 21.Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol. 1995;79:581–8. doi: 10.1152/jappl.1995.79.2.581. [DOI] [PubMed] [Google Scholar]
- 22.Smith ML, Niedermaier ON, Hardy SM, Decker MJ, Strohl KP. Role of hypoxemia in sleep apnea-induced sympathoexcitation. J Auton Nerv Syst. 1996;56:184–90. doi: 10.1016/0165-1838(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 23.Prabhakar NR, Kumar GK, Peng YJ. Sympatho-adrenal activation by chronic intermittent hypoxia. J Appl Physiol. 2012;113:1304–10. doi: 10.1152/japplphysiol.00444.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis. 2009;51:313–23. doi: 10.1016/j.pcad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol. 1989;67:2101–6. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- 26.Horner RL, Brooks D, Kozar LF, Tse S, Phillipson EA. Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. J Appl Physiol. 1995;79:151–62. doi: 10.1152/jappl.1995.79.1.151. [DOI] [PubMed] [Google Scholar]
- 27.Dingli K, Assimakopoulos T, Wraith PK, Fietze I, Witt C, Douglas NJ. Spectral oscillations of RR intervals in sleep apnoea/hypopnoea syndrome patients. Eur Respir J. 2003;22:943–50. doi: 10.1183/09031936.03.00098002. [DOI] [PubMed] [Google Scholar]
- 28.Phillips CL, Yang Q, Williams A, et al. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. J Sleep Res. 2007;16:217–25. doi: 10.1111/j.1365-2869.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 29.Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98:1071–7. doi: 10.1161/01.cir.98.11.1071. [DOI] [PubMed] [Google Scholar]
- 30.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster GE, Brugniaux JV, Pialoux V, et al. Cardiovascular and cerebrovascular responses to acute hypoxia following exposure to intermittent hypoxia in healthy humans. J Physiol. 2009;587:3287–99. doi: 10.1113/jphysiol.2009.171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macey PM, Moiyadi AS, Kumar R, Woo MA, Harper RM. Decreased cortical thickness in central hypoventilation syndrome. Cereb Cortex. 2012;22:1728–37. doi: 10.1093/cercor/bhr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest. 2006;130:815–21. doi: 10.1378/chest.130.3.815. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5:185–92. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535–42. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 37.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143:1300–3. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 38.Gates GJ, Mateika SE, Mateika JH. Heart rate variability in non-apneic snorers and controls before and after continuous positive airway pressure. BMC Pulm Med. 2005;5:9. doi: 10.1186/1471-2466-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guilleminault C, Poyares D, Rosa A, Huang YS. Heart rate variability, sympathetic and vagal balance and EEG arousals in upper airway resistance and mild obstructive sleep apnea syndromes. Sleep Med. 2005;6:451–7. doi: 10.1016/j.sleep.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: patho-physiology and treatment. Chest. 2007;131:595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mokhlesi B, Tulaimat A. Recent advances in obesity hypoventilation syndrome. Chest. 2007;132:1322–36. doi: 10.1378/chest.07-0027. [DOI] [PubMed] [Google Scholar]
- 42.Sztajzel J, Jung M, Bayes de Luna A. Reproducibility and gender-related differences of heart rate variability during all-day activity in young men and women. Ann Noninvasive Electrocardiol. 2008;13:270–7. doi: 10.1111/j.1542-474X.2008.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richard M, LeBlanc AR, Pennestri MH, et al. The effect of gender on autonomic and respiratory responses during sleep among both young and middle-aged subjects. Sleep Med. 2007;8:760–7. doi: 10.1016/j.sleep.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 44.McGinty D, Littner M, Beahm E, Ruiz-Primo E, Young E, Sowers J. Sleep related breathing disorders in older men: a search for underlying mechanisms. Neurobiol Aging. 1982;3:337–50. doi: 10.1016/0197-4580(82)90022-7. [DOI] [PubMed] [Google Scholar]
- 45.Pickett CK, Regensteiner JG, Woodard WD, Hagerman DD, Weil JV, Moore LG. Progestin and estrogen reduce sleep-disordered breathing in postmenopausal women. J Appl Physiol. 1989;66:1656–61. doi: 10.1152/jappl.1989.66.4.1656. [DOI] [PubMed] [Google Scholar]
- 46.Dewey FE, Freeman JV, Engel G, et al. Novel predictor of prognosis from exercise stress testing: heart rate variability response to the exercise tread-mill test. Am Heart J. 2007;153:281–8. doi: 10.1016/j.ahj.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Tateishi O, Shouda T, Honda Y, Sakai T, Mochizuki S, Machida K. Apnea-related heart rate variability and its clinical utility in congestive heart failure outpatients. Ann Noninvasive Electrocardiol. 2002;7:127–32. doi: 10.1111/j.1542-474X.2002.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilman MP, Floras JS, Usui K, Kaneko Y, Leung RS, Bradley TD. Continuous positive airway pressure increases heart rate variability in heart failure patients with obstructive sleep apnoea. Clin Sci (Lond) 2008;114:243–9. doi: 10.1042/CS20070172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heart rate variability in patients with sleep related alveolar hypoventilation, patients with obstructive sleep apnea, and control patients