Abstract
Perturbations in methyl group metabolism and homocysteine balance have emerged over the past few decades as having defining roles in a number of pathological conditions. Numerous nutritional, hormonal, and genetic factors that are characterized by elevations in circulating homocysteine concentrations are also associated with specific pathological conditions, including cancer development, autoimmune diseases, vascular dysfunction, and neurodegenerative disease. Although much remains to be explored, our understanding of the relationship between disease, methyl balance, and epigenetic control of gene expression has steadily progressed. However, homocysteine balance and its role in health and disease are not as clearly understood. This review presents our current understanding of homocysteine metabolism and its link to specific pathologies.
Methyl group and homocysteine metabolism have emerged as a metabolic process that has profound effects on health and disease, particularly when it is disrupted (1–5). Although the link between methyl groups and epigenetic control of gene expression is relatively clear from a mechanistic point of view (6, 7), the relationship between aberrant homocysteine metabolism and numerous pathological conditions is not well understood. Homocysteine is a product of all S-adenosylmethionine (SAM)2-dependent transmethylation reactions, including those involved in epigenetic regulation of DNA silencing and posttranslational modification of histones. It is clear that elevated concentrations of homocysteine in the circulation are characteristic of a number of disease states; thus, homocysteine management represents an important goal for optimizing health.
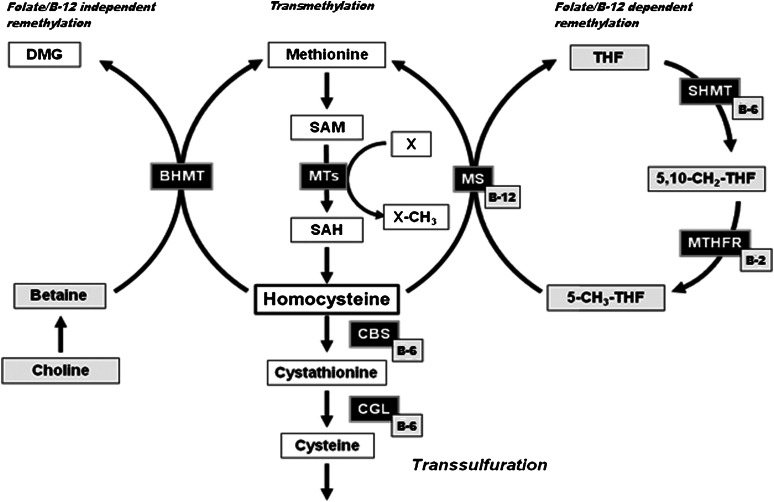
Homocysteine is not a classic amino acid found in dietary protein or used for the endogenous synthesis of proteins, but rather a sulfur-containing amino acid derived from the metabolism of methionine via methyl group metabolism (Fig. 1). Methionine is activated via the action of MAT to generate SAM, the ubiquitous methyl donor in a vast array of intracellular transmethylation reactions. Methyl groups derived from SAM are used in the synthesis of many compounds, including creatine, phosphatidylcholine, and neurotransmitters. SAM-derived methylation also exerts a regulatory role in the control of gene expression. Posttranslational modification of histones via methylation can function to either condense or relax chromatin, whereas the methylation of CpG regions present in DNA typically results in gene silencing. S-adenosylhomocysteine (SAH) is the product of all SAM-dependent transmethylation reactions, and because SAH is a potent allosteric inhibitor of most methyltransferases (8), the intracellular ratio of SAM to SAH is considered an index of transmethylation potential (9, 10).
Figure 1.
Hepatic folate, methyl group, and homocysteine metabolism. For this review, important SAM-dependent methyltransferases include glycine N-methyltransferase (GNMT), guanidinoacetate N-methyltransferase (GAMT), and phosphatidylethanolamine N-methyltransferase (PEMT). These 3 methyltransferases respectively catalyze the conversion of glycine to sarcosine, guanidinoacetate to creatine, and phosphatidylethanolamine to phosphatidylcholine. In addition to folate, these reactions are dependent on a number of other B vitamins, including riboflavin (vitamin B-2), vitamin B-6, and vitamin B-12. Metabolites and enzymes: BHMT, betaine-homocysteine S-methyltransferase; CBS, cystathionine β-synthase; CGL, cystathionine γ-lyase; DMG, dimethylglycine; MS, methionine synthase; MTs, methyltransferases; MTHFR, 5,10-methylene-THF reductase; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SHMT, serine hydroxymethyltransferase; THF, tetrahydrofolate; X, methyl acceptor. Adapted from reference (5) with permission.
Although SAM-dependent transmethylation exists in most tissues, the full complement of enzymes involved in methyl group and homocysteine metabolism (Fig. 1) is tissue specific, with the liver possessing the most activity and thus has a major influence on methyl group supply for other tissues as well as circulating homocysteine concentrations. The kidney also contains many of the key enzymes involved in homocysteine balance, including betaine-homocysteine S-methyltransferase (BHMT), cystathionine β-synthase (CBS), and glycine N-methyltransferase (GNMT). Different cell types may also express isoforms of a given enzyme that possess different characteristics. For example, methionine adenosyltransferase (MAT)I and MATIII in the liver exhibit different enzyme kinetics, namely, a higher Km, than MATII found in extrahepatic tissues; thus, the liver has a much greater capacity to convert methionine to SAM. MATII is also subject to product inhibition, a regulatory control mechanism that prevents excessive accumulation of SAM in extrahepatic tissues. Therefore, although plasma homocysteine concentrations are highly dependent on adequate intracellular homocysteine metabolism in the liver and kidney, it may also reflect 1-carbon metabolism in a number of other cell types. As discussed later in this review, kidney dysfunction can have a significant impact on homocysteine balance in humans.
After SAM-dependent transmethylation, SAH is rapidly metabolized to adenosine and homocysteine by SAH hydrolase. Therefore, production of homocysteine is highly dependent on the action of phosphatidylethanolamine N-methyltransferase (PEMT) in phosphatidylcholine synthesis, and guanidinoacetate N-methyltransferase (GAMT) for creatine synthesis, as well as other SAM-dependent methyltransferases. The actions of PEMT and GAMT are major contributors to the production of homocysteine in the cell, comprising ∼85% of all SAM-dependent transmethylation, a finding that is supported by the classic work of Mudd et al. (11, 12). However, a more recent review by Stead et al. (13) suggests that PEMT is the largest consumer of SAM-derived methyl groups and thus plays a major role in homocysteine balance.
Another important enzyme that uses SAM-derived methyl groups and subsequently results in homocysteine production is GNMT. GNMT is an abundant cytosolic protein that catalyzes the SAM-dependent methylation of glycine to generate sarcosine, a reaction of questionable importance. GNMT also has a regulatory role, namely, to control the ratio of SAM to SAH as a means to optimize intracellular transmethylation reactions (14). A metabolic network between SAM, 5,10-methylenetetrahydrofolate reductase (MTHFR) 5-methyltetrahydrofolate (5-methyl-THF), and GNMT exists to conserve methyl groups under conditions of low supply and dispose of them as sarcosine when the groups are abundant. This balance is achieved by the allosteric inhibition of MTHFR by SAM (15, 16) and the allosteric inhibition of GNMT by 5-methyl-THF (17). Thus, under conditions of excess methionine, the subsequent increase in SAM inhibits MTHFR, thereby reducing 5-methyl-THF production and alleviating any potential inhibition on GNMT, allowing it to divert methyl groups to sarcosine production. Conversely, the lack of MTHFR inhibition by SAM when methyl group supply is reduced allows sufficient 5-methyl-THF synthesis to inhibit GNMT activity, thereby conserving methyl groups for SAM-dependent transmethylation reactions. The relationship between PEMT, GAMT, and homocysteine balance, as well as its importance, is discussed later in this review.
In addition to production, homocysteine balance is also dependent on its remethylation back to methionine or its irreversible catabolism to other compounds of biological significance. For the latter, CBS catalyzes the condensation of homocysteine and serine to form cystathionine, a vitamin B-6–dependent reaction. The transsulfuration pathway is completed by the formation of cysteine and α-ketobutyrate, another vitamin B-6–dependent reaction catalyzed by cystathionine γ-lyase. Some of the initial linkage between elevations in homocysteine and cardiovascular disease resided in the inborn error of metabolism termed homocystinuria, wherein genetic defects of CBS and/or cystathionine γ-lyase resulted in elevated concentrations of homocysteine in the plasma and urine. Because it can be synthesized from methionine through the transmethylation and transsulfuration pathway, cysteine is considered a conditionally essential amino acid, provided adequate methionine is available. In turn, cysteine can serve as a substrate for gluconeogenesis via its conversion to pyruvate, the synthesis of the tripeptide antioxidant glutathione, or further catabolism to taurine.
Two distinct routes exist for the remethylation of homocysteine back to methionine to complete the methyl cycle. The first reaction is dependent on the B vitamins folate and B-12. The folate coenzyme 5-methyl-THF can donate a methyl group to homocysteine in a reaction catalyzed by the vitamin B-12–dependent enzyme methionine synthase (MS). Thus, both folate and/or vitamin B-12 status play an important role in homocysteine balance within the cell and subsequently the circulation. It should also be noted that sufficient supply of 5-methyl-THF for folate-dependent remethylation of homocysteine is dependent on the enzymatic reduction of 5,10-methylene-THF and the catalytic activity of MTHFR, a physiologically irreversible reaction. A single nucleotide polymorphism of the MTHFR gene (C677T) can result in inadequate folate-dependent remethylation and subsequent elevations in homocysteine concentrations. The second route for homocysteine remethylation is independent of folate and the 1-carbon pool, using betaine, derived from the oxidation of choline, as a methyl group source in a reaction catalyzed by BHMT. BHMT-dependent remethylation of homocysteine is primarily hepatic and renal, whereas the folate–vitamin B-12–dependent route is found universally in all tissues. The relationship between MS, MTHFR, BHMT, homocysteine balance, and disease is further discussed later in this review.
Current status of knowledge
Regulation of homocysteine balance
Homocysteine production.
Because homocysteine has many metabolic routes for its production and utilization, a number of key proteins involved in these processes factor heavily in the regulation of homocysteine balance. As mentioned, PEMT and GAMT collectively represent the most predominant SAM-dependent methyltransferases that contribute to homocysteine production, and GNMT exerts regulatory control over methyl group metabolism and concomitantly the generation of homocysteine. GAMT is a hepatic enzyme that catalyzes the synthesis of creatine by the SAM-dependent transmethylation of guanidinoacetate, a compound synthesized in the kidney from arginine and glycine. The biosynthesis of creatine for muscle creatine phosphate is a major consumer of SAM-derived methyl groups and thus produces a significant amount of intracellular homocysteine in the liver. Stead et al. (18) showed that dietary provision of creatine alleviates the requirement of hepatic creatine synthesis, and subsequently the production of homocysteine was diminished. Moreover, homocysteine production was elevated when guanidinoacetate was provided in the diet, likely owing to the metabolic need to methylate it to creatine.
In addition to the direct synthesis of phosphatidylcholine from choline, hepatic PEMT serves as an additional route to generate phosphatidylcholine from phosphatidylethanolamine. Because this biosynthetic pathway requires 3 methyl groups from SAM, it has been proposed that PEMT represents the largest consumption of SAM-derived methyl groups and subsequent production of homocysteine (13). This potential regulatory relationship between hepatic PEMT and homocysteine has been explored by Vance et al. (19–21). Pemt-deficient mice exhibited significantly reduced concentrations of homocysteine in the circulation, and overexpression of PEMT resulted in hyperhomocysteinemia. Whether this later finding can be linked to hyperhomocysteinemia-associated pathologies remains to be determined.
Knockout mouse models have also been useful in further understanding the role of GNMT as a potential regulator of homocysteine balance. However, unlike PEMT, GNMT-knockout mice had normal circulating concentrations of homocysteine, whereas plasma methionine and SAM concentrations were markedly elevated (22). Thus, a direct relationship between GNMT regulation and homocysteine concentrations does not appear to exist, in contrast to PEMT and other related enzymes, as discussed in the following. It has also been shown that hepatic PEMT expression is estrogen regulated (23), thus, sex and age may be additional factors in PEMT function and its impact on homocysteine balance.
Homocysteine catabolism.
The irreversible catabolism of homocysteine by the sequential action of CBS and γ-cystathionase to generate cysteine exists largely in the liver and kidney, but is also present in a few other tissues. Inborn defects in either vitamin B-6–dependent enzyme results in homocystinuria and was some of the initial evidence linking elevations in homocysteine to vascular disease. The link between CBS, hyperhomocysteinemia, and cardiovascular disease continues to be explored and is further discussed in this review. It is clear that inadequate CBS expression, combined with specific diets, results in well-defined adverse vascular outcomes (24, 25). The use of a low-folate, high-methionine diet fed to Cbs+/− mice has proved to be a very successful approach to produce varying degrees of hyperhomocysteinemia and linked to specific measure of endothelial dysfunction as well as elevated SAH concentrations.
Homocysteine remethylation.
Hepatic folate-dependent remethylation of homocysteine depends on 2 enzymes, MTHFR and MS. The MTHFR C677T polymorphism has been shown to result in hyperhomocysteinemia and indices of vascular disease (26–28). Similar to CBS, animal knockout models of MTHFR and MS have proved useful tools in examining the regulation of homocysteine with respect to vascular dysfunction (29). For folate-independent remethylation, the regulation and/or expression of BHMT has been shown to affect homocysteine concentrations. The use of a highly specific inhibitor of BHMT activity, S-(α-carboxybutyl)-dl-homocysteine, inhibited BHMT activity by 90% and resulted in a 7-fold increase in circulating homocysteine concentrations (30). Because this compound did not have an effect on other homocysteine-metabolizing enzymes, this study demonstrated that inhibition of BHMT alone is sufficient to result in homocysteine imbalance. Recently, a Bhmt-deficient mouse model was developed that confirms these earlier findings (31). Bhmt+/− mice were characterized by a 50% reduction in BHMT activity and normal circulating homocysteine concentrations, whereas Bhmt−/− mice have a complete absence of activity and approximately an 8-fold increase in plasma homocysteine concentrations. Although the Bhmt-deficient mouse exhibits hepatic steatosis and increased hepatocellular carcinoma, it has not yet been determined whether the characteristic homocysteine imbalance has an impact on the pathogenesis of the diseases described in the following.
Homocysteine and disease
Cardiovascular disease.
There is little doubt that hyperhomocysteinemia plays a role in the development of cardiovascular disease (CVD) (32–34). This is not only supported by human population studies identifying it as an independent risk factor, but strong evidence resides in animal models with diet- and/ or genetic-based elevations in homocysteine concentrations (29, 35–38). Homocysteine and the incidence of myocardial infarction are positively correlated, even after adjustment for other CVD risk factors (39). Meta-analysis of >80 studies on folate metabolism and CVD has shown an association with hyperhomocysteinemia and a variety of cardiovascular events, including atherosclerotic vessel damage and thrombosis (40). In contrast, plasma homocysteine is not related to total CVD risk burden and may or may not be related to CVD-related mortality (41). However, it should be noted that clinical trials targeting homocysteine management by the use of vitamin B supplementation as a means to lower circulating homocysteine concentrations have not been as effective as anticipated (42–47). Numerous reviews have debated the various explanations for these findings and the associative versus causal role of homocysteine in vascular disease (25, 48, 49). Nonetheless, it is clear from animal studies that genetic and dietary-induced increases in plasma homocysteine result in well-defined indices of vascular disease (29, 35–38). Heterozygous mice deficient in CBS, MS, and MTHFR are all characterized by elevated circulation homocysteine concentrations and vascular dysfunction, as well as neurological disorders. This is supported in humans possessing the MTHFR C677T polymorphism, which results in reduced activity of the enzyme, resulting in hyperhomocysteinemia and increased incidence of vascular disease (26–28).
Recently, homocysteine has been shown to negatively affect endothelial cell function, revealing novel mechanisms involved in hyperhomocysteinemia and cardiovascular health. Homocysteine can hypomethylate CpG islands in the promoter region of P66shc, an adaptor protein, leading to increased P66shc expression and causing increased oxidative stress (50). In endothelial cells of the coronary arteries, hyperhomocysteinemia appeared to result in diminished tetrahydrobiopterin function, an important cofactor for vasodilation (51). However, the impact of homocysteine on vascular oxidation was shown to be unimportant compared with the impact of 5-methyl-THF, which appears to regulate nitric oxide balance in blood vessels (52). This indicates that elevated homocysteine may not only be a cause of endothelial dysfunction, but also a biomarker for other complications. Attempts to improve endothelial cell function by lowering homocysteine have had moderate success. Homocysteine-lowering therapy did not lower markers of endothelial cell dysfunction in stroke patients, perhaps because the vascular damage was too advanced (53). In a mouse model, homocysteine-lowering gene therapy corrected endothelial cell dysfunction, thereby delaying development of thrombosis, but had no impact on atherogenesis or vessel narrowing (54).
The impact of homocysteine on endothelial cell health may contribute to the development of hypertension. In an epidemiological study of 500 patients, those in the highest tertile with respect to plasma homocysteine concentrations, had an odds risk of 1.66 for the development of hypertension within 10 y compared with patients in the lowest tertile (55). This finding is in agreement with a recent study comparing plasma homocysteine with total antioxidant status, C-reactive protein (an index of inflammation), endothelial progenitor cells, and intima-media thickness of the carotid arteries. In hypertensive patients, homocysteine, intima-media thickness and C-reactive protein were significantly higher, whereas total antioxidant status and endothelial progenitor cells were significantly lower (56). Researchers speculate that the reduced total antioxidant status, possibly affected by homocysteine, may reduce the number of endothelial progenitor cells. Elevated plasma homocysteine may also be a valuable predictive factor for hypertension because circulating homocysteine is related to increased arterial stiffness in prehypertensive patients (50).
Autoimmune disease.
A number of autoimmune diseases are characterized by aberrant methyl group metabolism and homocysteine imbalance. For humans, both types 1 and 2 diabetes are characterized by hypohomocysteinemia, which progresses to hyperhomocysteinemia as renal function becomes compromised (57–59). From animal studies using chemically induced type 1 diabetes and genetic-based type 2 diabetes, the initial phase of hypohomocysteinemia appears to be the result of increased remethylation and catabolism of homocysteine by the induction of BHMT and CBS, respectively (60–65). This is not surprising because increased expression of hepatic and renal CBS in transgenic mice has been reported to effectively lower circulating homocysteine concentrations (66). These alterations in homocysteine balance in type 1 diabetes are specific for the pathology because insulin administration abrogates all of the metabolic anomalies (60, 65, 67, 68). Diabetes is also characterized by induction of specific methyltransferases that should result in increased homocysteine production, namely, GNMT and PEMT (62, 63, 67). However, the concomitant induction of CBS and BHMT appears to promote remethylation and transsulfuration as a means to prevent homocysteine accumulation in the cell and subsequently the circulation. An increased flux through the transsulfuration pathway has also been reported in humans with type 2 diabetic nephropathy (69). One clear species-specific difference is that in Zucker diabetic fatty rats with clear indices of renal dysfunction, the circulating concentration remains lower than normal and does not progress to hyperhomocysteinemia as it does in humans (70). The basis for this discrepancy remains an area for future investigation, but may be attributed to the fact the rodents possess very little renal BHMT activity compared with humans; hence, they may be less susceptible to the hyperhomocysteinemia owing to renal dysfunction and the associated loss of BHMT-dependent remethylation of homocysteine.
Gastrointestinal disorders.
Hyperhomocysteinemia contributes to inflammatory remodeling of the gastrointestinal tract, resulting in elevated levels of matrix metalloproteinase-9, reactive oxygen species, and superoxide (71). Additionally, hyperhomocysteinemia caused by the C677T MTHFR polymorphism was associated with mesenteric venous thrombosis and bowel infarction (72). Increased plasma homocysteine concentration has been implicated in a variety of gastrointestinal diseases, including constipation, inflammatory bowel disease, Crohn’s disease, and colorectal cancer (73–76). Constipation, lowered fecal output, and increased superoxide species quickly develop in Cbs+/− mice, indicating that hyperhomocysteinemia itself may be causing gastrointestinal distress (73). Specific medications that interfere with methyl group metabolism have the negative side effects of stomach pain and indigestion. In particular, isotretinoin (i.e., 13-cis-retinoic acid), a therapeutic retinoid derivative used for the treatment of severe acne, has been associated with severe bowel problems, possibly due to the marked increase in homocysteine concentrations observed in isotretinoin patients (77, 78). However, the increased homocysteine concentrations in patients with inflammatory bowel disease may also be a consequence of the disease itself because the gastrointestinal tract is responsible for much of the metabolism of sulfur amino acids (75).
Skeletal maintenance.
Homocysteine can impair bone health by interfering with proper osteoclast activity. In vitro incubation of homocysteine and bone marrow cells showed that homocysteine up-regulates osteoclast formation and suppresses osteoclast apoptosis by increasing reactive oxygen species concentrations in bone marrow cells. This increase in oxidative stress induces the activity of RANK-L (receptor activator for nuclear factor κB ligand), an osteoclast differentiation factor. Increased osteoclast activity will result in increased bone resorption, explaining the increased risk of fractures and decreased bone mineral density seen in patients with high circulating homocysteine concentrations (79, 80). Furthermore, homocysteine induces caspase-dependent apoptosis of human bone marrow stromal cells, thereby impairing bone repair (81). A rat model of hyperhomocysteinemia led to increased accumulation of homocysteine in bone tissue, 65% of which was localized in the collagen extracellular matrix. This accumulation of homocysteine was associated with a dramatic reduction of trabecular or “spongy” bone and a corresponding decrease in bone strength (82). Furthermore, homocysteine can epigenetically modify expression of Lox, thereby down-regulating expression of lysyl oxidase, essential for collagen cross-linking and stability (83). This indicates a novel mechanism for bone damage resulting from elevated homocysteine concentrations in the circulation. Recently, studies in a rat model showed that homocysteine may lower blood flow through bone tissue, an effect that can be partially corrected by folic acid supplementation (84). However, homocysteine-lowering therapy has not been conclusively shown to improve bone health (85).
Neurodegenerative disorders.
An area that has received a lot of attention recently is the relationship between homocysteine and neurological problems, such as depression and Parkinson’s disease. Major depressive disorder is commonly attributed to low levels or impaired transmission of the neurotransmitters serotonin, dopamine, and norepinephrine, some of which are dependent on methyl group donation from SAM for synthesis or metabolism. In a recent longitudinal study of >11,000 patients, elevated homocysteine concentrations were associated with a 26% increase in the likelihood of depressive symptoms (86). Dietary supplementation with folate, vitamin B-12, and SAM has been shown to effectively lower plasma homocysteine concentrations and reduce depressive symptoms (87).
Hyperhomocysteinemia occurs in 10% to 30% of patients with Parkinson’s disease (88). Parkinson’s disease is a neurodegenerative disorder characterized by loss of motor control, often resulting in tremors caused by damage to dopamine-producing brain cells, which can be managed with the neurotransmitter precursor levodopa (L-DOPA). Metabolism of L-DOPA by catechol-O-methyltransferase requires SAM, rapidly depleting the methyl group supply and leading to increased concentrations of homocysteine (89). Elevated plasma homocysteine concentrations have been associated with dementia, depression, and dyskinesia in Parkinson’s patients; however, determination of homocysteine concentrations alone was not sufficient for prognosis (90). As previously mentioned, hyperhomocysteinemia has been linked to low bone mineral density, a serious concern for Parkinson’s disease patients, who have an increased fracture risk due to loss of motor control and comorbid dementia (91). Patients are often encouraged to minimize protein intake and take vitamin B supplements to prevent or control perturbations in methyl group metabolism (92). Recently, Parkinson’s disease patients who exercised regularly were shown to completely avoid an increase in plasma homocysteine concentrations after l-DOPA treatment, in contrast to sedentary Parkinson’s disease patients (93). Reduction of plasma homocysteine concentration remains a primary goal in controlling the symptoms of Parkinson’s disease.
Homocysteine can also complicate the progression of Alzheimer’s disease. In a recent population study of >1200 Swedish women, a high plasma homocysteine concentration in middle age was an independent risk factor for later dementia and Alzheimer’s disease (94). In patients who already have a diagnosis of Alzheimer’s disease, the rate of cognitive decline positively correlated with the concentration of plasma homocysteine (95). Furthermore, patients with moderate Alzheimer’s disease and elevated homocysteine concentrations experienced greater behavioral disturbances associated with major depressive disorder (96). A recent study involving a transgenic mouse model of Alzheimer’s disease showed that a methionine-rich, homocysteine-lowering diet may effectively lower brain amyloidosis and improve cognitive defects (97). However, these benefits were not confirmed in a randomized, placebo-controlled trial with human Alzheimer’s patients (98).
Conclusions
Homocysteine balance, and hyperhomocysteinemia in particular, appears to be a consistent characteristic of a number of pathologies. It remains unclear whether excessive homocysteine concentrations directly contribute to the pathogenesis of disease or represent a biomarker of metabolic aberrations, such as aberrant methyl group metabolism. It is clear from dietary and genetic animal models of hyperhomocysteinemia that well-defined adverse outcomes, such as vascular dysfunction, can be demonstrated. Intervention strategies to reduce plasma homocysteine concentrations have met with mixed results, not just in the case of vascular disease, but also with respect to neurodegenerative disorders, diabetes, and bone health. Therefore, a more precise understanding of the relationship between homocysteine balance and disease remains an important area of investigation, particularly for those populations that may be at the greatest risk of hyperhomocysteinemia.
Acknowledgments
The manuscript was written and edited by both authors; K.L.S. had responsibility for the final copy. Both authors have read and approved the final manuscript.
Footnotes
Abbreviations used: BHMT, betaine-homocysteine S-methyltransferase; CBS, cystathionine β-synthase; CVD, cardiovascular disease; GAMT, guanidinoacetate N-methyltransferase; GNMT, glycine N-methyltransferase; L-DOPA, levodopa; MAT, methionine adenosyltransferase; 5-methyl-THF, 5-methyltetrahydrofolate; MTHFR, 5,10-methylenetetrahydrofolate reductase; MS, methionine synthase; PEMT, phosphatidylethanolamine N-methyltransferase; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
Literature Cited
- 1.Kang S-S, Wong PW, Malinow MR. Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu Rev Nutr. 1992;12:279–98 [DOI] [PubMed] [Google Scholar]
- 2.Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–46 [DOI] [PubMed] [Google Scholar]
- 3.Newberne PM, Rogers AE. Labile methyl groups and the promotion of cancer. Annu Rev Nutr. 1986;6:407–32 [DOI] [PubMed] [Google Scholar]
- 4.Scott JM, Kirke PN, Weir DG. The role of nutrition in neural tube defects. Annu Rev Nutr. 1990;10:277–95 [DOI] [PubMed] [Google Scholar]
- 5.Williams KT, Schalinske KL. Homocysteine metabolism and its relation to health and disease. Biofactors. 2010;36:19–24 [DOI] [PubMed] [Google Scholar]
- 6.Shenker N, Flanagan JM. Intragenic DNA methylation: implications of this epigenetic mechanism for cancer research. Br J Cancer. 2012;106:248–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van den Veyver IB. Genetic effects of methylation diets. Annu Rev Nutr. 2002;22:255–82 [DOI] [PubMed] [Google Scholar]
- 8.Kerr SJ. Competing methyltransferase systems. J Biol Chem. 1972;247:4248–52 [PubMed] [Google Scholar]
- 9.Cantoni GL. S-adenosylamino acids thirty years later: 1951–1981. In: Usdin E, Borchardt RT, Creveling CR, edtors. The biochemistry of S-adenosylmethionine and related compounds. New York: Macmillan Publishers; 1982. p. 3–10.
- 10.Cantoni GL, Chiang PK. The role of S-adenosylhomocysteine and S-adenosylhomocysteine hydrolase in the control of biological methylations. In: Cavallini D, Gaull GE, Zappia V, editors. Natural sulfur compounds, New York: Plenum Press; 1980. p. 67–80.
- 11.Mudd SH, Poole JR. Labile methyl balances for normal humans on various dietary regimens. Metabolism. 1975;24:721–35 [DOI] [PubMed] [Google Scholar]
- 12.Mudd SH, Ebert MH, Scriver CR. Labile methyl group balances in the human: the role of sarcosine. Metabolism. 1980;29:707–20 [DOI] [PubMed] [Google Scholar]
- 13.Stead LM, Brosnan JT, Brosnan ME, Vance DE, Jacobs RL. Is it time to reevaluate methyl balance in humans? Am J Clin Nutr. 2006;83:5–10 [DOI] [PubMed] [Google Scholar]
- 14.Wagner C. Biochemical role of folate in cellular metabolism. In: Bailey LB, editors. Folate in health and disease. New York: Marcel Dekker; 1995. p. 23–42.
- 15.Jencks DA, Matthews RG. Allosteric inhibition of methylenetetrahydrofolate reductase by adenosylmethionine. J Biol Chem. 1987;262:2485–93 [PubMed] [Google Scholar]
- 16.Kutzbach C, Stokstad ELR. Feedback inhibition of methylenetetrahydrofolate reductase. Partial purification, properties, and inhibition by S-adenosylmethionine. Biochim Biophys Acta. 1967;139:217–20 [DOI] [PubMed] [Google Scholar]
- 17.Wagner C, Briggs WT, Cook RJ. Inhibition of glycine N-methyltransferase activity by folate derivatives: implications for regulation of methyl group metabolism. Biochem Biophys Res Commun. 1985;127:746–52 [DOI] [PubMed] [Google Scholar]
- 18.Stead LM, Au KP, Jacobs RL, Brosnan ME, Brosnan JT. Methylation demand and homocysteine metabolism: effects of dietary provision of creatine and guanidinoacetate. Am J Physiol Endocrinol Metab. 2001;281:E1095–100 [DOI] [PubMed] [Google Scholar]
- 19.Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988;263:2998–3004 [PubMed] [Google Scholar]
- 20.Jacobs RL, Stead LM, Devlin C, Tabas I, Brosnan ME, Brosnan JT, Vance DE. Physiological regulation of phospholipid methylation alters plasma homocysteine in mice. J Biol Chem. 2005;280:28299–305 [DOI] [PubMed] [Google Scholar]
- 21.Noga AA, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J Biol Chem. 2002;277:42358–65 [DOI] [PubMed] [Google Scholar]
- 22.Luka Z, Capdevila A, Mato JM, Wagner CA. Glycine N-methyltransferase knockout mouse model for humans with deficiency of this enzyme. Transgenic Res. 2006;15:393–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Resseguie M, Song J, Nuculescu MD, da Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007;21:2622–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dayal S, Lentz SR. Murine models of hyperhomocysteinemia and their vascular phenotypes. Arterioscler Thromb Vasc Biol. 2008;28:1596–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodionov RN, Lentz SR. The homocysteine paradox. Arterioscler Thromb Vasc Biol. 2008;28:1031–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJH, den Heijer M, Kluijtmans LAJ, van den Heuvel LP, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3 [DOI] [PubMed] [Google Scholar]
- 27.Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, Selhub J, Rozen R. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7–9 [DOI] [PubMed] [Google Scholar]
- 28.Nelen WLDM, Blom HJ, Thomas CMG, Steegers EAP, Boers GHJ, Eskes TKAB. Methylenetetrahydrofolate reductase polymorphism affects the change in homocysteine and folate concentrations resulting from low dose folic acid supplementation in women with unexplained recurrent miscarriages. J Nutr. 1998;128:1336–41 [DOI] [PubMed] [Google Scholar]
- 29.Devlin AM, Arning E, Bottiglieri T, Faraci FM, Rozen R, Lentz SR. Effect of Mthfr genotype on diet-induced hyperhomocysteinemia and vascular function in mice. Blood. 2004;103:2624–9 [DOI] [PubMed] [Google Scholar]
- 30.Strakova J, Williams KT, Gupta S, Schalinske KL, Kruger WD, Rozen R, Jiracek J, Lucas L, Garrow TA. Dietary intake of S-(α-carboxybutyl)-dl-homocysteine induces hyperhomocysteinemia in rats. Nutr Res. 2010;30:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teng YW, Mehedint MG, Garrow TA, Zeisel SH. Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J Biol Chem. 2011;286:36258–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro R, Rivera I, Blom HJ, Jakobs C, Travares de Almeida I. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: an overview. J Inherit Metab Dis. 2006;29:3–20 [DOI] [PubMed] [Google Scholar]
- 33.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–55 [DOI] [PubMed] [Google Scholar]
- 34.Zaina S, Lindholm MW, Lund G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J Nutr. 2005;135:5–8 [DOI] [PubMed] [Google Scholar]
- 35.Dayal S, Arning E, Bottiglieri T, Böger RH, Sigmund CD, Faraci FM, Lentz SR. Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke. 2004;35:1957–62 [DOI] [PubMed] [Google Scholar]
- 36.Dayal S, Bottiglieri T, Arning E, Maeda N, Malinow MR, Sigmund CD, Heistad DD, Faraci FM, Lentz SR. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine β-synthase-deficient mice. Circ Res. 2001;88:1203–9 [DOI] [PubMed] [Google Scholar]
- 37.Dayal S, Wilson KM, Leo L, Arning E, Bottiglieri T, Lentz SR. Enhanced susceptibility to arterial thrombosis in a murine model of hyperhomocysteinemia. Blood. 2006;108:2237–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson KM, Lynch CM, Faraci FM, Lentz SR. Effect of mechanical ventilation on carotid artery thrombosis induced by photochemical injury in mice. J Thromb Haemost. 2003;1:2669–74 [DOI] [PubMed] [Google Scholar]
- 39.Woodward M, Rumley A, Rumley C, Lewington S, Morrison CE, Lowe GD. The association between homocysteine and myocardial infarction is independent of age, sex, blood pressure, cholesterol, smoking and markers of inflammation: the Glasgow Myocardial Infarction Study. Blood Coagul Fibrinolysis. 2006;17:1–5 [DOI] [PubMed] [Google Scholar]
- 40.Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62 [DOI] [PubMed] [Google Scholar]
- 41.De Chiara B, Sedda V, Parolini M, Campolo J, De Maria R, Caruso R, Pizzi G, Disoteo O, Dellanoce C, Corno AR, et al. Plasma total cysteine and cardiovascular risk burden: action and interaction. ScientificWorld Journal. 2012;2012:303654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blacher J, Czernichow S, Paillard F. Cardiovascular effects of B-vitamins and/or N-3 fatty acids: the Su.Fol.Om3 trial. Int J Cardiol. 2012 Feb 22 [DOI] [PubMed] [Google Scholar]
- 43.Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K; NORVIT Trial Investigators Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–88 [DOI] [PubMed] [Google Scholar]
- 44.Clarke R, Halsey J, Lewington S, Manson JE, Bønaa KH, Spence JD, Nygård O, Jamison R, Gariano JM, Guarino P, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170:1622–31 [DOI] [PubMed] [Google Scholar]
- 45.den Heijer M, Willems HP, Blom HJ, Gerrits WB, Cattaneo M, Eichinger S, Rosendaal FR, Bos GM. Homocysteine lowering by B vitamins and the secondary prevention of deep vein thrombosis and pulmonary embolism: a randomized, placebo-controlled, double-blind trial. Blood. 2007;109:139–44 [DOI] [PubMed] [Google Scholar]
- 46.Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM; Veterans Affairs Site Investigators Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007;298:1163–70 [DOI] [PubMed] [Google Scholar]
- 47.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, et al. Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77 [DOI] [PubMed] [Google Scholar]
- 48.McCully KS. Homocysteine, vitamins, and vascular disease prevention. Am J Clin Nutr. 2007;86:1563S–8S [DOI] [PubMed] [Google Scholar]
- 49.McNulty H, Pentieva K, Hoey L, Ward M. Homocysteine, B-vitamins and CVD. Proc Nutr Soc. 2008;67:232–7 [DOI] [PubMed] [Google Scholar]
- 50.Kim BJ, Seo M, Huh J, Kwon C, Kim J, Sung K, Kim B, Kang J. Associations of plasma homocysteine levels with arterial stiffness in prehypertensive individuals. Clin Exp Hypertens. 2011;33:411–7 [DOI] [PubMed] [Google Scholar]
- 51.He L, Zeng H, Fuwang L, Feng J, Liu S, Liu J, Yu J, Mao J, Hong T, Chen A, et al. Homocysteine impairs coronary artery endothelial function by inhibiting tetrabiopterin in patients with hyperhomocysteinemia. Am J Physiol Endocrinol Metab. 2010;299:E1061–5 [DOI] [PubMed] [Google Scholar]
- 52.Antoniades C, Shirodaria C, Leeson P, Baarholm O, Van-Assche T, Cunnington C, Pillai R, Ratnatunga C, Tousoulis D, Stefanadis C, et al. MTHFR 677 C>T polymorphism reveals functional importance for 5-methyltetrahydrofolate, not homocysteine, in regulation of vascular redox state and endothelial function in human atherosclerosis. Circulation. 2009;119:2507–15 [DOI] [PubMed] [Google Scholar]
- 53.Hankey G, Eikelbloom P, Dusitanond P, Thom J, Loh K, van Bockxmeer F, Jamrozik K. Homocysteine-lowering treatment with folic acid, cobalamin, and pyroidoxine does not suppress blood markers of inflammation, endothelial dysfunction, or hypercoagulability in patients with prior TIA or stroke. Stroke. 2005;36:474. [DOI] [PubMed] [Google Scholar]
- 54.Jacobs F, Van Craeyveld E, Muthuramu I, Gordts S, Emmerechts J, Hoylaerts M, Herljgers P, De Geest B. Correction of endothelial dysfunction after selective homocysteine lowering gene therapy reduces arterial thrombogenicity but has no effect on atherogenesis. J Mol Med. 2011;89:1051–8 [DOI] [PubMed] [Google Scholar]
- 55.Fukami A, Adachi H, Hirai Y, Enomoto M, Otsuka M, Nanjo Y, Yoshikawa K, Esaki E, Kumagai E, Ogata K, et al. High levels of plasma homocysteine predicts development of hypertension in a general population: the Tanushimaru study. J Epidemiol Community Health. 2011;65:A245–7 [Google Scholar]
- 56.Bogdanski P, Miller-Kasprzak E, Pupek-Musialik D, Jablecka A, Lacinski M, Jagodzinski P, Jakubowski H. Plasma total homocysteine is a determinant of carotid intima-media thickness and circulating endothelial progenitor cells in patients with newly diagnosed hypertension. Clin Chem Lab Med. 2012;50:1107–13 [DOI] [PubMed] [Google Scholar]
- 57.Hofmann MA, Kohl K, Zumbach MS, Borcea V, Bierhaus A, Henkels A, Amira J, Fiehn W, Ziegler R, Wahl P, et al. Hyperhomocyst(e)inemia and endothelial dysfunction in IDDM. Diabetes Care. 1997;20:1880–6 [DOI] [PubMed] [Google Scholar]
- 58.Poirier LA, Brown AT, Fink LM, Wise CK, Randolph CJ, Delongchamp RR, Fonseca VA. Blood S-adenosylmethionine concentrations and lymphocyte methylenetetrahydrofolate reductase activity in diabetes mellitus and diabetic nephropathy. Metabolism. 2001;50:1014–8 [DOI] [PubMed] [Google Scholar]
- 59.Robillon JF, Canivet B, Candito M, Sadoul JL, Jullien D, Morand P, Chambon P, Freychet P. Type 1 diabetes mellitus and homocyst(e)ine. Diabete Metab. 1994;20:494–6 [PubMed] [Google Scholar]
- 60.Jacobs RL, House JD, Brosnan ME, Brosnan JT. Effects of streptozotocin-induced diabetes and of insulin treatment on homocysteine metabolism in the rat. Diabetes. 1998;47:1967–70 [DOI] [PubMed] [Google Scholar]
- 61.Jacobs RL, Steads LM, Brosnan ME, Brosnan JT. Hyperglucagonemia in rats results in decreased plasma homocysteine and increased flux through the transsulfuration pathway in liver. J Biol Chem. 2001;276:43740–7 [DOI] [PubMed] [Google Scholar]
- 62.Nieman KM, Hartz CS, Szegedi SS, Garrow TA, Sparks JD, Schalinske KL. Folate status modulates the induction of hepatic glycine N-methyltransferase and homocysteine metabolism in diabetic rats. Am J Physiol Endocrinol Metab. 2006;291:E1235–42 [DOI] [PubMed] [Google Scholar]
- 63.Nieman KM, Rowling MJ, Garrow TA, Schalinske KL. Modulation of methyl group metabolism by streptozotocin-induced diabetes and all-trans-retinoic acid. J Biol Chem. 2004;279:45708–12 [DOI] [PubMed] [Google Scholar]
- 64.Ratnam S, Maclean KN, Jacobs RL, Brosnan ME, Kraus JP, Brosnan JT. Hormonal regulation of cystathionine β-synthase expression in liver. J Biol Chem. 2002;277:42912–8 [DOI] [PubMed] [Google Scholar]
- 65.Ratnam S, Wijekoon EP, Hall B, Garrow TA, Brosnan ME, Brosnan JT. Effects of diabetes and insulin on betaine-homocysteine S-methyltransferase expression in rat liver. Am J Physiol Endocrinol Metab. 2006;290:E933–39 [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Jhee K-H, Hua X, DiBello PM, Jacobsen DW, Kruger WD. Modulation of cystathionine β-synthase level regulates total serum homocysteine in mice. Circ Res. 2004;94:1318–24 [DOI] [PubMed] [Google Scholar]
- 67.Hartz CS, Nieman KM, Jacobs RL, Vance DE, Schalinske KL. Hepatic phosphatidyl-ethanolamine N-methyltransferase expression is increased in diabetic rats. J Nutr. 2006;136:3005–9 [DOI] [PubMed] [Google Scholar]
- 68.Nieman KM, Schalinske KL. Insulin abrogates perturbation of methyl group and homocysteine metabolism in diabetic rats. Am J Physiol Endocrinol Metab. 2011;301:E560–5 [DOI] [PubMed] [Google Scholar]
- 69.Tessari P, Coracina A, Kiwanuka E, Vedovato M, Vettore M, Valerio A, Zaramella M, Garibotto G. Effects of insulin on methionine and homocysteine kinetics in type 2 diabetes with nephropathy. Diabetes. 2005;54:2968–76 [DOI] [PubMed] [Google Scholar]
- 70.Williams KT, Schalinske KL. Tissue-specific alterations of methyl group metabolism and DNA hypermethylation in the Zucker (type 2) diabetic fatty (ZDF) rat. Diabetes Metab Res Rev. 2012;28:123–31 [DOI] [PubMed] [Google Scholar]
- 71.Danese S, Sgambato A, Papa A, Scaldaferri F, Pola R, Sans M, Lovecchio M, Gasbarrini G, Cittadini A, Gasbarrini A. Homocysteine triggers mucosal microvascular activation in inflammatory bowel disease. Am J Gastroenterol. 2005;100:886–95 [DOI] [PubMed] [Google Scholar]
- 72.Munjal C, Givvimani S, Qipshidze N, Tyagi N, Falcone JC, Tyagi SC. Mesenteric vascular remodeling in hyperhomocysteinemia. Mol Cell Biochem. 2011;348:99–108 [DOI] [PubMed] [Google Scholar]
- 73.Givvimani S, Munjal C, Narayanan N, Aqil F, Tyagi G, Metreveli N, Tyagi SC. Hyperhomocysteinemia decreases intestinal motility leading to constipation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G281–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wiernicki I, Millo B, Safranow K, Gorecka-Szyld B, Gutowski P. MMP-9, homocysteine and CRP circulating levels are associated with intraluminal thrombus thickness of abdominal aortic aneurysms: new implication of the old biomarkers. Dis Markers. 2011;31:67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chowers Y, Sela BA, Holland R, Fidder H, Simoni FB, Bar-Meir S. Increased levels of homocysteine in patients with Crohn's disease are related to folate levels. Am J Gastroenterol. 2000;95:3498–502 [DOI] [PubMed] [Google Scholar]
- 76.Oussalah A, Guéant JL, Peyrin-Biroulet L. Meta-analysis: hyperhomocysteinaemia in inflammatory bowel diseases. Aliment Pharmacol Ther. 2011;34:1173–84 [DOI] [PubMed] [Google Scholar]
- 77.Schulpis KH, Karikas GA, Georgala S, Michas T, Tsakiris S. Elevated plasma homocysteine levels in patients on isotretinoin therapy for cystic acne. Int J Dermatol. 2001;40:33–6 [DOI] [PubMed] [Google Scholar]
- 78.Thakrar BT, Robinson NJ. Isotretinoin use and the risk of inflammatory bowel disease. Am J Gastroenterol. 2011;106:1000–2, author reply 1002–3 [DOI] [PubMed] [Google Scholar]
- 79.Elshorbagy AK, Gjesdal CG, Nurk E, Tell GS, Ueland PM, Nygård O, Tverdal A, Vollset SE, Smith AD, Refsum H. Cysteine, homocysteine and bone mineral density: a role for body composition? Bone. 2009;44:954–8 [DOI] [PubMed] [Google Scholar]
- 80.van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, van der Klift M, de Jonge R, Lindemans J, de Groot LC, Hofman A, Witteman JC, van Leeuwen JP, et al. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. 2004;350:2033–41 [DOI] [PubMed] [Google Scholar]
- 81.Kim DJ, Koh JM, Lee O, Kim NJ, Lee YS, Kim YS, Park JY, Lee KU, Kim GS. Homocysteine enhances apoptosis in human bone marrow stromal cells. Bone. 2006;39:582–90 [DOI] [PubMed] [Google Scholar]
- 82.Herrmann M, Tami A, Wildemann B, Wolny M, Wagner A, Schorr H, Taban-Shomal O, Umanskaya N, Ross S, Garcia P, et al. Hyperhomocysteinemia induces a tissue specific accumulation of homocysteine in bone by collagen binding and adversely affects bone. Bone. 2009;44:467–75 [DOI] [PubMed] [Google Scholar]
- 83.Thaler R, Spitzer S, Rumpler M, Fratzl-Zelman N, Klaushofer K, Paschalis E, Varga F. Differential effects of homocysteine and beta aminopropionitrile on preosteoblastic MC3T3–E1 cells. Bone. 2010;46:703–9 [DOI] [PubMed] [Google Scholar]
- 84.Tyagi N, Vacek T, Fleming J, Vacek J, Tyagi S. Hyperhomocysteinemia reduces bone blood flow. Vasc Health Risk Manag. 2011;7:31–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herrmann M, Umanskaya N, Traber L, Schmidt-Gayk H, Menke W, Lanzer G, Lenhart M, Peter Schmidt J, Herrmann W. The effect of B-vitamins on biochemical bone turnover markers and bone mineral density in osteoporotic patients: a 1-year double blind placebo controlled trial. Clin Chem Lab Med. 2007;45:1785–92 [DOI] [PubMed] [Google Scholar]
- 86.Gu P, Defina LF, Leonard D, John S, Weiner MF, Brown ES. Relationship between serum homocysteine levels and depressive symptoms: the Cooper Center Longitudinal Study. J Clin Psychiatry. 2012;73:691–5 [DOI] [PubMed] [Google Scholar]
- 87.Gariballa S. Testing homocysteine-induced neurotransmitter deficiency, and depression of mood hypothesis in clinical practice. Age Ageing. 2011;40:702–5 [DOI] [PubMed] [Google Scholar]
- 88.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76 [DOI] [PubMed] [Google Scholar]
- 89.O'Suilleabhain PE, Sung V, Hernandez C, Lacritz L, Dewey RB, Jr, Bottiglieri T, Diaz-Arrastia R. Elevated plasma homocysteine level in patients with Parkinson disease: motor, affective, and cognitive associations. Arch Neurol. 2004;61:865–8 [DOI] [PubMed] [Google Scholar]
- 90.O'Suilleabhain PE, Oberle R, Bartis C, Dewey RB, Bottiglieri T, Diaz-Arrastia R. Clinical course in Parkinson's disease with elevated homocysteine. Parkinsonism Relat Disord. 2006;12:103–7 [DOI] [PubMed] [Google Scholar]
- 91.Lee SH, Kim MJ, Kim BJ, Kim SR, Chun S, Ryu JS, Kim GS, Lee MC, Koh JM, Chung SJ. Homocysteine-lowering therapy or antioxidant therapy for bone loss in Parkinson's disease. Mov Disord. 2010;25:332–40 [DOI] [PubMed] [Google Scholar]
- 92.Evatt ML. Nutritional therapies in Parkinson's disease. Curr Treat Options Neurol. 2007;9:198–204 [DOI] [PubMed] [Google Scholar]
- 93.Nascimento CM, Stella F, Garlipp CR, Santos RF, Gobbi S, Gobbi LT. Serum homocysteine and physical exercise in patients with Parkinson's disease. Psychogeriatrics. 2011;11:105–12 [DOI] [PubMed] [Google Scholar]
- 94.Zylberstein DE, Lissner L, Bjorkelund C, Mehlig K, Thelle D, Gustafson D, Ostling S, Waeren M, Guo X, Skoog I. Midlife homocysteine and late-life dementia in women: a prospective population study. Neurobiol Aging. 2011;32:380–6 [DOI] [PubMed] [Google Scholar]
- 95.Oulhaj A, Refsum H, Beaumont H, Williams J, King E, Jacoby R, Smith A. Homocysteine as a predictor of cognitive decline in Alzheimer’s disease. Int J Geriatr Psychiatry. 2010;25:82–90 [DOI] [PubMed] [Google Scholar]
- 96.Chen CS, Chou M, Yeh Y, Yang Y, Lai C, Uen C, Liu C, Liao Y. Plasma homocysteine levels and major depressive disorders in Alzheimer’s disease. Am J Geriatr Psychiatry. 2010;18:1045–8 [DOI] [PubMed] [Google Scholar]
- 97.Zhuo JM, Pratico D. Normalization of hyperhomocysteinemia improves cognitive deficits and ameliorates brain amyloidosis of a transgenic mouse model of Alzheimer’s disease. FASEB J. 2010;24:3895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kwok T, Lee J, Law C, Pan P, Yung C, Choi K, Lam L. A randomized placebo controlled trial of homocysteine lowering to reduce cognitive decline in older demented people. Clin Nutr. 2011;30:297–302 [DOI] [PubMed] [Google Scholar]