Abstract
Many epidemiologic studies have considered whether markers of B-vitamin status are associated with cognitive function and cognitive decline. This avenue of research was sparked by the homocysteine (Hcy) theory of cardiovascular disease, which was extended to Alzheimer’s disease when a link between vascular dementia and Alzheimer’s disease was discovered. Hcy could cause cognitive impairment via direct neurotoxicity. However, decreased remethylation of Hcy to methionine might also compromise cognitive function by means other than mere Hcy lowering. Folate and vitamin B-12 participate in Hcy remethylation and largely determine Hcy status. Consequently, much of the relevant research has focused on these 2 B vitamins. The many subtly different hypotheses that investigators have addressed by attempting to link several B-vitamin status indicators to diverse cognition-related outcomes have created a confusing body of conflicting studies that seems to defy summarization. Nevertheless, themes are discernible that aid interpretation, foster hypothesis generation, and inform future study design. For example, despite a shared metabolic pathway, Hcy, vitamin B-12, and folate are differently related to specific cognitive outcomes. Although consistency of findings across studies is often touted as essential to distinguishing causal from coincidental relationships, discrepancies among study findings can be even more informative.
Introduction
The potential for low B-vitamin status to affect cognitive function is actually well-known (1). What has changed in recent years is emerging evidence that B-vitamin status plays a role in age-related cognitive impairment and decline. Revolutionary ideas about the etiology of cardiovascular disease (CVD)4 and its likely connection to Alzheimer’s disease (AD), along with revelations about the prevalence, causes, characteristics, and susceptibility to exacerbation by high folic acid intakes of vitamin B-12 deficiency converged in the late 1990s to spark an explosion of research.
Biochemical rationale
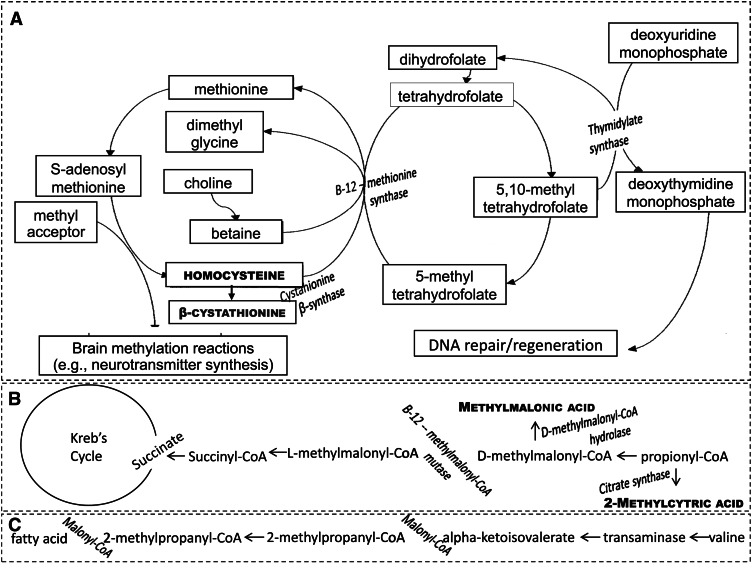
In part due to growing acceptance of the homocysteine (Hcy) theory of CVD (2) and the roles that folate and vitamin B-12 play in Hcy remethylation (3), most of the research has focused on these 2 vitamins. Hcy remethylation is catalyzed by the enzyme methionine synthase (Fig. 1), which uses vitamin B-12 as a cofactor and transfers a methyl group from 5-methyltetrahydrofolate, or food folate, to Hcy. This reaction has several consequences with the potential to affect cognition. First, it results in the regeneration of the active form of folate, tetrahydrofolate, which is needed for thymidine synthesis and thus DNA replication. This could benefit cognition by fostering adult neurogenesis in the hippocampus (4), a brain area critical for learning and memory that is especially vulnerable to the adverse effects of aging (5). Next, it leads to less Hcy, which may be neurotoxic (6), and has been widely believed to be a direct cause of atherosclerosis, although that hypothesis has become more controversial lately (7). Finally, a downstream product is S-adenosyl methionine, the methyl donor for the central nervous system (8). Methylation reactions are vital to brain function, one example being their role in neurotransmitter synthesis (9).
Figure 1.
Diagram illustrating the biochemical rationale for associations among vitamin B-12, folate, and cognitive function. A, The roles of the 2 vitamins in homocysteine remethylation, which results in the regeneration of the active form of folate, tetrahydrofolate, and the production of S-adenosyl methionine, the methyl donor for the central nervous system. Accumulation of homocysteine and β-cystathionine indicates the loss of the cofactor and substrate functions of vitamin B-12 and folate in homocysteine remethylation. B, The role of vitamin B-12 as a cofactor to the enzyme methylmalonyl-CoA mutase. Buildup of methylmalonic acid and 2-methylcitric acid indicates the loss of this vitamin B-12 function. C, The role of malonyl-CoA in fatty acid synthesis. When vitamin B-12 is lacking, methylmalonyl-CoA builds up, perhaps substituting for malonyl-CoA, which might result in the accumulation of abnormal fatty acids in membranes of neural tissue.
A low vitamin B-12 concentration could also affect cognitive function by virtue of the vitamin’s only other known function—acting as a cofactor for the enzyme methylmalonyl-CoA mutase. If that enzyme were inactivated, as it would be in the absence of vitamin B-12, methylmalonyl-CoA would build up and possibly substitute for malonyl-CoA in fatty acid synthesis. Cognitive dysfunction might then ensue due to the resultant accumulation of abnormal fatty acids in the membranes of neural tissue (10).
Historical underpinnings
The vitamin B-12 deficiency disease pernicious anemia (PA), which is now known to be caused by autoimmune destruction of the parietal cells that produce intrinsic factor (11), was first described in 1849. In that era, Addison noted delirium and other mental disturbances in his patients (12). Before the role of vitamin B-12 status in PA was known, the diagnostic hallmark was a potentially fatal megaloblastic anemia. However, by the late 1970s, it was recognized that PA could be expressed entirely neurologically and without vitamin B-12 deficiency, as traditionally defined (13). A related development was the recognition of poor vitamin B-12 function not attributable to intrinsic factor deficiency and detectable by the markers Hcy, methylmalonic acid (MMA), 2-methylcitrtic acid, and β-cystathionine (14). These markers indicate poor vitamin B-12 function in the body because they build up in the circulation when vitamin B-12 is not performing its cofactor roles (Fig. 1). Use of these markers has revealed the insensitivity of a low vitamin B-12 concentration as traditionally defined (i.e., <148 pmol/L) to poor vitamin B-12 function. Thus, the possible prevalence of cognitively significant low vitamin B-12 status increased from the 0.1–0.2% prevalence of classically presenting PA (15) to the 3% prevalence of low vitamin B-12 status, as traditionally defined (16), to the 11–50% prevalence of elevated MMA (17–20). Most of this so-called biochemical vitamin B-12 deficiency is believed to be caused by age-related food-cobalamin malabsorption (21, 22). Carmel (23) uses the term subclinical deficiency to describe the syndrome because it does not present with the classic PA features of megaloblastic anemia and spinal cord degeneration. However, food-cobalamin malabsorption has been linked to confusion and dementia (22).
Occurring concurrently with these advances in our understanding of low vitamin B-12 status were increasing acceptance of McCully’s Hcy theory of arteriosclerosis (2) and the realization that vascular dementia and AD were related (24). In 1968, Kilmer proposed that the underlying cause of arteriosclerosis (and thus vascular dementia) in the general population was not fat or cholesterol, but B-vitamin deficiency and consequent hyperhomocysteinemia (25). Bell et al. (26) were the first investigators to attempt to apply the Hcy theory of CVD to dementia. In a small study, they found that patients with dementia had higher Hcy concentrations than those without dementia. However, the studies by Riggs et al. (27) and Clarke et al. (28) in the late 1990s set in motion the race to prove the Hcy theory of dementia. The relevant studies include those that attempted to link Hcy, folate status, and vitamin B-12 status to dementia/AD, poor or worsening performance on the Mini-Mental State Examination (MMSE) and more sensitive neuropsychological tests, as well as studies that attempted to prevent or slow cognitive decline with B-vitamin supplements. Ellinson et al. (29) reviewed the extant body of literature in 2004 and concluded that results of the 6 studies that fulfilled their search criteria did not support a correlation between serum vitamin B-12 or folate and cognitive impairment in people aged older than 60 y of age. In 2007, Raman et al. (30) lamented the low quality of some of the 24 studies that they reviewed as well as the lack of standardization among them, both in cognition-assessment methodology and low B-vitamin status definitions. More recently, Smith (31) noted that, by 2008, 77 cross-sectional studies and 33 prospective studies had shown associations between Hcy and/or B vitamins and cognitive deficit/dementia and called for large-scale randomized, controlled trials (RCT) of Hcy-lowering vitamins. Such trials have now been published, but no reviewer has found the evidence compelling enough to recommend B-vitamin supplementation for the prevention of age-related cognitive impairment and decline (32–36). Once RCTs have been published, results of observational studies tend to be forgotten. However, ignoring a vast body of observational research in favor of a few RCTs of uneven quality seems imprudent (37). The purpose of this review is to guide future research by reconciling conflicting results of observational studies and trials and refining testable working hypotheses.
Current status of knowledge
Indicators of B-vitamin status in relation to dementia/AD
The initial studies that hinted at associations between B-vitamin status indicators and dementia/AD or cognitive impairment were small and had retrospective designs (26–28). Eventually, however, the various hypotheses were tested in large, international, population-based cohort studies and surveys. Among 12 studies relating Hcy or hyperhomocysteinemia to dementia/AD (28, 38–48), 8 of which were prospective (38, 39, 41, 43, 45–48), all but 2 found an association. The 2 null studies were a South Korean prospective study with a very short follow-up period and few incident AD cases (38) and an analysis of data from the WHICAP (Washington Heights-Inwood Columbia Aging Project) (39). Diabetes prevalence in the WHICAP cohort was ∼30%, and the inverse relationship between diabetes and Hcy (49, 50) likely explained that study’s null findings.
In some of the same studies that investigated the association between Hcy and dementia/AD, attempts were made to link circulating levels of folate, vitamin B-12, or the biologically active fraction of the circulating vitamin B-12, holotranscobalamin (holoTC) (51), to this outcome, but with very different results. In fact, the initial observation of Clarke et al. (28) regarding vitamin B-12 status and dementia/AD stands in stark contrast to all of the null findings that have been reported since (38, 41, 42, 45, 48, 52, 53). Some of the authors claimed that their findings were supportive of an association, but the evidence was actually equivocal. For example, with data from the Kungsholmen Project, Wang et al. (53) found an association with combined low vitamin B-12 and folate status, but not with low status for either vitamin alone. Furthermore, Kivipelto et al. (41) found significant protection against AD in association with the third, but not the fourth, holoTC quartile category compared with the first, and the small South Korean study by Kim et al. (38) found an increased risk of dementia in association with 2.4-y decline in circulating vitamin B-12 levels, but not with baseline vitamin B-12 status.
Evidence of an association between low folate status and dementia/AD is more consistent, with only 3 of 10 (28, 38, 42, 44, 45, 48, 52–55) studies failing to find a significant association. The most likely explanation for the null findings of the CAIDE (Cardiovascular Risk Factors, Aging, and Dementia) study is the low folate status of the population (mean = 6.3 nmol/L), which would have yielded few values in the acceptable range (i.e., ≥13.6 nmol/L) (56) for comparison. In addition to the Kungsholmen study by Wang et al. (53) previously mentioned , the only other null finding came from the CHAP (Chicago Health and Aging Project) (55). These seemingly aberrant results are not easily dismissed, however, because the CHAP was a well-designed, long-term follow-up study.
Hcy in relation to cognitive function and decline
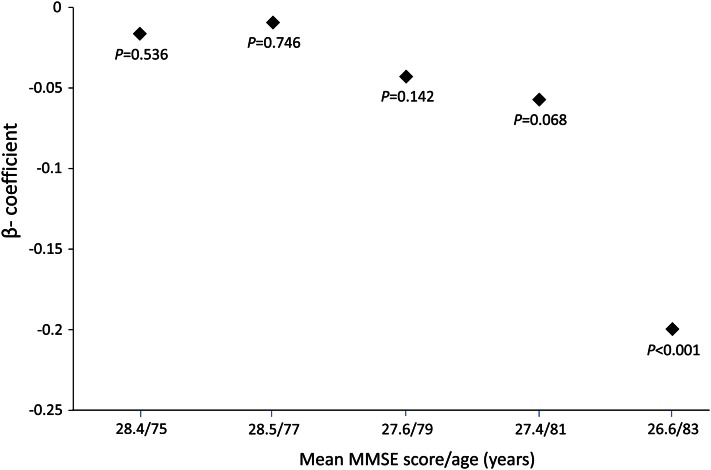
Many population-based studies have used the 30-point MMSE to assess cognitive function and decline. The disadvantages of the MMSE include insensitivity to mild or isolated cognitive deficits (57). Furthermore, although the MMSE can be used successfully to test the hypothesis that an exposure is associated with severe global cognitive deficits suggestive of dementia, its ability to do so depends on the prevalence of low scores in the population (57). Consequently, the null findings of studies that attempted to relate Hcy concentrations to MMSE performance in samples with MMSE scores mainly between 28 and 30 (58, 59), including young subsets of subjects studied by Duthie et al. (60) and Robbins et al. (61), are not surprising. Similarly predictable are the mixed findings of studies of samples with MMSE scores mainly between 27 and 28 (62, 63). In samples in which lower MMSE scores were more common (64–67), however, including older subjects studied by Duthie et al. (60) and Robbins et al. (61), higher Hcy concentrations were associated with lower MMSE scores. Data collected on members of the Framingham Offspring Cohort illustrate the principle. Figure 2 shows β coefficients for the cross-sectional association between the plasma total Hcy concentration and MMSE performance at 5 consecutive examination cycles. The plot illustrates the inability to demonstrate a significant inverse relationship until the cohort members had aged to a point when poor MMSE performance was common.
Figure 2.
Cross-sectional association between plasma homocysteine and Mini-Mental State Examination (MMSE) performance at 5 successive examinations of the Framingham Original Cohort (N = 541). Examinations occurred 2 y apart. Plotted β coefficients give the decrease in the MMSE score per micromole per liter increase in homocysteine. Results are adjusted for age, sex, education, smoking status, serum creatinine, and body mass index.
Results of studies examining the association between baseline Hcy concentration and MMSE score decrease illustrate another principle—that to connect an exposure to decline, decline must occur over the follow-up period. Observation of cognitive decline using the MMSE score requires either a long follow-up period or a cohort whose MMSE scores are decreasing relatively rapidly. Thus, too-short follow-up times likely explain the null findings of 2 studies that followed cognitively intact subjects as young as 50 y of age for ≤3 y, on average (58, 68). Seshadri et al. (43) reported that they could find an association between Hcy and MMSE score decrease only after ≥4 y of follow-up from the baseline examination for their analyses. However, data presented in Figure 2 suggest that the time needed would depend on which examination was considered to be the baseline. Clarke et al. (69) successfully linked Hcy to MMSE score decrease after following subjects with a mean baseline age of 75.4 y for 10 y, when MMSE scores decreased by 1.3 points, on average. More recently, Hooshmand et al. (67) demonstrated an association between Hcy and 7.4-y MMSE score decrease despite almost identical mean MMSE scores at baseline and follow-up. However, that relationship was found to depend on the inclusion in analyses of 20 study participants who deteriorated to dementia during the follow-up period. Dufouil et al. (62) found a significant association between hyperhomocysteinemia (i.e., Hcy ≥15 μmol/L) and MMSE score decrease of at least 3 points over the 2-y follow-up period, a definition that suggests a high frequency of rapid cognitive decline in the cohort.
The null finding of the Leiden 85-Plus study (70) is likely explained by the advanced age of the subjects. Among Framingham original cohort members, the ability to find a significant interaction between Hcy and time in a mixed-model, repeated-measures analysis depended on the age of the subjects at baseline. Specifically, significant acceleration of 8-y MMSE score decrease was associated with increasing baseline Hcy concentration among subjects who were 73–78 y of age at baseline (β = −0.02, P < 0.001), but not among subjects who were younger (β = −0.007, P = 0.114), or older (β = −0.002, P = 0.854) (M.S. Morris, unpublished results).
Advantages of neuropsychological test batteries over the MMSE include sensitivity to mild cognitive deficits that reflect early-stage decline, the opportunity to detect isolated impairments, and the ability to determine the specific cognitive domains affected by an exposure or disease (71). There can be disadvantages in terms of specificity, however, in that there may be multiple causes of the cognitive deficits detectable by the sensitive tests. Another problem is the lack of a generally accepted standard test battery. Even if there were one, the included tests might not be the best ones for detecting the cognitive deficits caused by B-vitamin deficiency or combining results of multiple tests into a global score might obscure the most relevant findings.
Many studies have used tests of delayed verbal recall. The likely reason for this choice is the reputed relevance to AD (72, 73). Of 13 studies that assessed cross-sectional associations between Hcy and recall or memory performance, 9 found at least marginally significant associations (59, 67, 68, 74–79). Two American studies (80, 81) were among the 4 exceptions (63, 80–82). Results obtained by our group with data collected in the NHANES III may provide a clue to some of the discrepancies (74). In that study, we found an inverse association between Hcy and delayed story recall that was restricted to survey participants with serum folate <17 nmol/L (74). This seems to suggest that Hcy affects memory only when it is due to low folate status. In other words, the apparent Hcy effect is really the effect of low folate, for which Hcy is a marker. If there is no low folate status in the population, Hcy reflects other phenomena (83). The prevalence of serum folate <17 nmol/L was 50% in the NHANES III, and it was even higher in the other American and European studies that successfully linked Hcy to poor memory function (67, 76, 79). Elias et al. (76), who found the association with data from the Framingham Offspring Cohort, did not replicate their findings with data from the Maine-Syracuse Study (80). However, the latter study and analysis by Miller et al. (81) of data collected in the Sacramento Health and Aging Study were conducted in the United States in the era of food folic acid fortification, when very little of the hyperhomocysteinemia could have been due to low folate (83).
Variation in the tests used makes summarizing results for Hcy and performance in nonmemory functions challenging. Nevertheless, it is worth pointing out that all 7 studies that evaluated the association between Hcy and performance on the Digit-Symbol Substitution (DSST) or similar tests of information-processing speed (84) found associations (60, 62, 63, 75, 78, 80, 85). Although fewer in number, all studies that used the Stroop test (60, 75, 78), block design test (60, 63, 80), and finger tapping test (62, 78) also found associations. These tests assess executive function/processing speed (86), executive function/visuospatial ability (87), and simple motor speed (88), respectively. Impairments in these cognitive functions characterize vascular dementia (89, 90) and have been linked to white matter hyperintensity volume, a possible sign of asymptomatic CVD (91). Consequently, the many positive findings likely reflect the link between Hcy and CVD.
Most prospective studies that used neuropsychological test batteries found associations between baseline Hcy and decline in global functioning (92), memory performance (68, 72, 93), and/or other cognitive functions (68, 72, 93, 94). However, no association was observed between Hcy and decline in memory function in a study conducted in postfortification Canada (94). Furthermore, Tangney et al. (95) failed to link Hcy to decrease in a global composite score that included results of tests of both immediate and delayed recall over a follow-up period that spanned the pre- and postfortification eras in Chicago.
Folate and vitamin B-12 in relation to cognitive function and decline
With the exception of studies with little chance of finding low MMSE scores due to the exclusion of people with low scores (63, 96) or that focused on relatively young people [e.g., the 1936 cohort of Duthie et al. (60)], cross-sectional studies generally found associations between B-vitamin status and MMSE scores (44, 60, 66, 67, 69, 97, 98). Lindeman et al. (98) speculated that their failure to link low vitamin B-12 status to poor MMSE performance might have resulted from their use of serum vitamin B-12 rather than a more sensitive indicator of vitamin B-12 status. However, 3 published studies found cross-sectional associations between low serum vitamin B-12 and poor MMSE performance (69, 97, 99).
Only a few studies have considered serum vitamin B-12 or folate levels in relation to decrease in MMSE score. These included 1 of the short-term studies (68) and the Leiden 85-Plus (70), both with null results. However, 3 well-designed studies [1 from the United Kingdom (69), the CHAP (94), and our group’s analysis of Framingham data (99)] successfully linked low vitamin B-12 status to accelerated decrease in MMSE scores (69, 99) or a composite score that included MMSE results (95) over 6–10 y of follow-up. Although some favor MMA over plasma vitamin B-12 for assessing vitamin B-12 status, the CHAP and Framingham studies revealed associations with both markers. In the Framingham study, being in either of the 2 quintile categories indicative of the worst vitamin B-12 status predicted accelerated decrease in MMSE score. On the other hand, considering both markers together revealed that only the 16% of cohort members with both plasma vitamin B-12 below the second quintile (i.e., <258 pmol/L) and MMA above the third quintile (i.e., ≥228 nmol/L) actually experienced accelerated decline (M.S. Morris, unpublished).
Almost all of 15 studies that attempted to demonstrate cross-sectional associations between serum folate and neuropsychological test performance found an association with at least 1 test (44, 60, 63, 68, 74, 80, 82, 85, 92, 98, 100). However, of 13 published studies that included memory tests in the battery (44, 60, 63, 67, 68, 74, 80, 82, 92, 98, 100–102), 6 failed to find an association with serum folate. The null findings of these studies may have been due to insufficient variation in folate status (67) or overreliance on immediate versus delayed verbal recall (68, 82, 100–102).
A majority of studies of relationships between vitamin B-12 status and sensitive neuropsychological test performance found associations (67, 68, 76, 80, 85, 100, 103, 104), although the evidence is not as strong as that for folate, with 6 of 14 studies finding no association with any test (63, 74, 92, 98, 101, 102). Four of the positive studies used sensitive indicators of vitamin B-12 status (67, 85, 103, 104), but associations have also been reported between circulating vitamin B-12 and performance on some tests (68, 76, 80, 100). The positive studies betray little consistency in terms of the tests and domains for which associations were observed, and it seems that all of the associations between circulating vitamin B-12 and test performance could have been confounded by folate status. Nevertheless, there is some evidence to suggest that vitamin B-12 status is independently related to memory function. When we conducted our 2001 study of Hcy and folate in relation to story recall in senior participants in the NHANES III, we found no significant association between serum vitamin B-12 and that outcome (85). Since the publication of that study, however, we have measured MMA in approximately half of the serum samples. Somewhat poorer delayed verbal recall ability was associated with being in the bottom 2 vitamin B-12 quintile categories (i.e., serum vitamin B-12 <283 pmol/L) and the top 3 MMA quintile categories (i.e., MMA ≥211 nmol/L) after controlling for age, sex, race/ethnicity, education, creatinine, and serum folate. Furthermore, after multivariate adjustment, the 34% of seniors with serum vitamin B-12 <283 pmol/L and MMA ≥211 nmol/L could recall, on average, <5 of 6 main story ideas after a delay compared with 5.3 for the 22% with the combination serum vitamin B-12 ≥283 pmol/L and MMA <211 nmol/L (P = 0.03) (M.S. Morris, unpublished). Other supporting evidence comes from the CHAP. In that study, MMA, β-cystathionine, and 2-methylcitric acid, but not serum vitamin B-12, were associated with episodic memory function, and the latter 2 indicators were also associated with semantic memory function (103). Somewhat consistent with the CHAP results, 2-methylcitric acid, but not MMA or serum vitamin B-12, was associated with recall and semantic learning in a Canadian study (104).
In our group’s study using NHANES (1999–2002) data, we defined low vitamin B-12 status using both serum vitamin B-12 and plasma MMA and found an association with performance on the DSST (85), the only cognitive function test administered to senior participants in that survey. Furthermore, plasma MMA, by itself, was associated with this outcome, but serum vitamin B-12 was not. Consistent with the latter result, neither Feng et al. (63) nor Durga et al. (100) found serum vitamin B-12 to be related to DSST performance.
One of the first studies to be published on the subject of B-vitamin status and cognition was a prospective analysis of CHAP data (105). Only intake data were available, and results were contrary to expectation and inconsistent with results of the many mainly cross-sectional studies supporting the hypothesis that high folate status was beneficial to cognition. In fact, the results of the CHAP suggested that high folate intakes were associated with accelerated cognitive decline over time. Among other studies of B-vitamin status in relation to decline in performance on sensitive neuropsychological tests, Tucker et al. (68) found that higher folate status (i.e., ≥20 nmol/L) protected against a 3-y decline in figure copying ability, but that the serum vitamin B-12 level was not independently associated with a decline in performance on any test. Furthermore, Kado et al. (92) linked low folate status (i.e., <7.14 nmol/L), but not low serum vitamin B-12 (i.e., <217 pmol/L), to an accelerated 7-y decline in a composite score on several tests, including tests of figure copying and memory. Kang et al. (101) found no associations between either folate status or vitamin B-12 status and the decrease in a verbal memory composite score that occurred over 4 y of follow-up.
Folate/B-12 interaction in relation to cognitive function and decline
The unique findings of the CHAP may be explained by a combination of high folic acid intake by CHAP participants and a high prevalence of low vitamin B-12 status in the cohort.
A general adverse effect of folic acid on cognition would be unexpected, but concern for the elderly was expressed during debates over folic acid fortification policy due to the possibility of rapid neuropsychiatric deterioration of the many older people with undiagnosed low vitamin B-12 status (106, 107). This fear was based on results of folic acid intervention studies conducted on PA patients in the 1940s and 1950s (108). In our group’s 2007 study using NHANES (1999–2002) data, we found especially poor DSST performance in association with very high folate status combined with low vitamin B-12 status (85). The vitamin B-12 status of the CHAP cohort was unknown at the time that the landmark study of Morris et al. (105) was published, but a later report revealed a nearly 40% prevalence of elevated MMA (i.e., >271 nmol/L) and a 14.2% prevalence of probable vitamin B-12 deficiency (95). Circulating folate levels were not assessed, but 17% of the subjects received ≥400 μg/d of folic acid from supplements alone at baseline. Furthermore, the median total folate intake in the top quintile category was 742 μg/d, and mandatory food folic acid fortification was instituted in the United States during the study’s 6-y follow-up period. Considering the high prevalence of low vitamin B-12 status in the CHAP and the inclusion of the Symbol Digit Modalities Test of information-processing speed in the CHAP cognitive test battery, the accelerated decline in cognitive function associated with high folate intake in that study could be consistent with our group’s finding of especially slow information processing among NHANES participants with a combination of low vitamin B-12 status and either very high folate status (85) or circulating unmetabolized folic acid (109). Thus, results of both studies could reflect precipitation or exacerbation by folic acid of cognitive consequences of low vitamin B-12 status.
Clarke et al. (110) did not replicate our group’s 2007 findings with data collected in the Banbury B12 Study and the Oxford Healthy Aging Project. In the combined sample of Clarke et al., the odds of poor MMSE performance (i.e., MMSE <25) associated with plasma holoTC <45 pmol/L was increased to approximately the same extent in subgroups with serum folate >30 nmol/L (OR = 1.5; 95% CI = 0.91–2.46) and ≤30 nmol/L (OR = 1.45; 95% CI = 1.19–1.76). However, the median serum folate level in the 2 compared groups was 53 nmol/L and 14 nmol/L, respectively, as opposed to 80 nmol/L and 30 nmol/L, respectively, for the folate categories compared in our group’s NHANES (1999–2002) analysis. Consequently, Clarke et al. and we addressed different hypotheses. Whereas our study was designed to test the hypothesis that the supraphysiologic status achievable in postfortification America was associated with precipitation or exacerbation of cognitive problems associated with low vitamin B-12 status, the Clark et al. study was only capable of demonstrating the benefits of replete folate status over relative folate deficiency in people stratified by vitamin B-12 status.
The prevalence of supplement use among Framingham Offspring Cohort members is high. At examination 7 (1998–2001), in the postfortification era, 25% of cohort members received ≥400 μg/d of folic acid from supplements alone, and the median total folate intake in the top quintile category was 917 μg/d (M.S. Morris, unpublished results). Among cohort members 65 y of age and older, higher folate status at examination 7 was associated with an accelerated 5-y decline in visuospatial ability and executive functioning (M.S. Morris, unpublished results). Marginally significantly poorer episodic memory performance was associated with plasma folate above the top quintile (i.e., 66 nmol/L) as well as with plasma vitamin B-12 <258 pmol/L. There was also a significant interaction, however. Specifically, among the cohort members with plasma vitamin B-12 ≥258 pmol/L, supraphysiologic folate status was not associated with performance on a test of delayed story recall. Among the 35% of the cohort with plasma vitamin B-12 <258 pmol/L, on the other hand, the subgroup with plasma folate >66 nmol/L had significantly worse episodic memory performance than the subgroup with lower (i.e., replete but not supraphysiologic) plasma folate. These findings also appear consistent with exacerbation of an adverse neuropsychiatric effect of low vitamin B-12 status by supraphysiologic folate status reflecting high folic acid intake.
Randomized, controlled trials
A number of RCTs have addressed the hypothesis that B-vitamin supplementation or Hcy lowering protects against cognitive decline, and several systematic reviews have been published (32–34, 36). The earliest published trial included by most reviewers is the 1997 folic acid trial by Fioravanti et al. (111) However, Balk et al. (33) included 2 studies from the 1970s among 14 using treatment with folic acid, vitamin B-12, or both. All reviewers reached the same conclusion; there was no evidence that B-vitamin treatment is effective at slowing cognitive decline. However, the following statement by Balk et al. bears consideration: “Fourteen trials met our criteria; most were of low quality and limited applicability (33).” Balk et al. and the other reviewers remarked on the inconsistency across studies in outcome assessment and on the short duration of most trials. Rather than attempting to draw conclusions from a collection of studies of low quality and limited applicability, I focus on high-quality trials, the discrepant findings of which appear to be reconcilable by differences in design. These particular trials were selected because they were of relatively large size and long duration, did not specifically select cognitively impaired subjects or those with dementia, and provided data on the subjects’ baseline B-vitamin status.
The salient details of 3 trials are summarized in Table 1. The trials conducted by McMahon et al. (112) and Durga et al. (113) were Hcy-lowering trials, whereas the recently published Walker et al. (114) trial was not. This is reflected in the mean baseline Hcy concentrations. Also worth noting is the exclusion of vitamin B-12–deficient subjects from the Durga et al. trial only. These differences, plus the variation across studies in the treatment, help to explain the trials’ disparate findings. Specifically, the treatment in the McMahon et al. trial was at a level that might have resulted in circulating unmetabolized folic acid (115) because folate status among the subjects was high, and total folic acid intake likely often exceeded the upper tolerable limit of 1000 μg/d (114). Although the McMahon et al. study was interpreted as null, the general pattern of treatment effects was one of nonsignificantly greater cognitive decline among the treated subjects compared with the controls. Furthermore, processing speed actually slowed more over time among the treated subjects compared with the placebo group. This finding could be consistent with our group’s results of a cross-sectional analysis of NHANES (1999–2002) data. We showed that, among subjects with low vitamin B-12 status, circulating unmetabolized folic acid was associated with poor DSST performance (109). The treatment in the McMahon et al. trial included a dose of vitamin B-12 that far exceeded recommendations (i.e., 500 vs. 2.4 μg/d) (116), but that might not have been enough to correct vitamin B-12 deficiency (116, 117). Even though Durga et al. also treated subjects with a rather high dose of folic acid and no vitamin B-12 at all, their treatment effected benefits to both memory and processing speed. These benefits might be explained by the combination of a safe folic acid dose, exclusion of vitamin B-12–deficient subjects, and low baseline folate status. The results generate the hypothesis that Hcy lowering benefited processing speed and that correction of folate deficiency benefited memory.
Table 1.
Comparison among 3 randomized, controlled trials assessing effects of B-vitamin treatment on cognitive change1
| Trials |
|||
| McMahon et al. (112) | Durga et al. (113) | Walker et al. (114) | |
| No. | 276 | 818 | 900 |
| Country | New Zealand | The Netherlands | Australia |
| Age, y | ≥65 | 50–70 | 60–74 |
| Baseline plasma Hcy, μmol/L | 16.82 | 132 | 9.7 |
| Baseline plasma/RBC folate, nmol/L | 22.63 | 123 | 5734 |
| Follow-up plasma/RBC folate, nmol/L | 753 | 763 | 9514 |
| Baseline plasma vitamin B-12, pmol/L | 284 | 2905 | 305 |
| Length, y | 2 | 3 | 2 |
| MMSE | 29 | 29 | ? |
| Folic acid intake, μg/d | 1000 | 800 | 400 |
| Vitamin B-12 intake, μg/d | 500 | ─ | 100 |
| Vitamin B-6 intake, mg/d | 10 | ─ | ─ |
| Memory effect | None | More improvement | More improvement |
| Processing-speed effect | More worsening | Less worsening | None |
Hcy, homocysteine; MMSE, Mini-Mental State Examination.
Potential subjects with plasma homocysteine <13μmol/L were excluded.
Plasma folate was reported.
RBC folate was reported.
Potential subjects with serum vitamin B-12 <200 pmol/L were excluded.
Results of the Walker et al. (114) RCT help to reinforce this idea, in that the lack of hyperhomocysteinemia among their subjects might have precluded treatment benefits to processing speed, and the correction of folate deficiency could account for the observed benefits to memory. Mean baseline RBC folate was well above the cutoff point for deficiency (e.g., 317 nmol/L) (119), but it was comparable to that of senior NHANES III participants (i.e., 532 nmol/L), 21% of whom had RBC folate in the deficient range (M.S. Morris, unpublished). Also in the NHANES III, seniors with RBC folate ≥317 nmol/L performed significantly better on a test of delayed story recall than those with RBC folate <317 nmol/L (P < 0.001), after controlling for demographic factors and vitamin B-12 deficiency (M.S. Morris, unpublished).
In a recent large (N = 2009) American trial, mean folate intake at baseline exceeded the dose delivered by most multivitamins, and no overall benefit was found with a B-vitamin supplement containing 2.5 mg of folic acid, 50 mg of vitamin B-6, and 1 mg of vitamin B-12 (120). However, subgroup analyses suggested that, among the 15% of trial participants who had folate intakes <279 μg/d at baseline, the treated subjects experienced significantly less decline than the placebo group in performance on the Telephone Interview of Cognitive Status. Interestingly, despite a treatment that contained more than twice as much folic acid as that used by McMahon et al., a treatment benefit was also observed among those trial participants with baseline vitamin B-12 intakes <2.4 μg/d. In other words, despite long-term treatment with a megadose of folic acid, the vitamin B-12–deficient subjects in the treatment group experienced less cognitive decline than the vitamin B-12–deficient subjects in the placebo group. Trial participants with low vitamin B-12 intake were few, and plasma vitamin B-12 concentrations were not available, but the larger dose of vitamin B-12, compared with that used by McMahon et al., might have accounted for the different findings of the 2 trials.
Conclusions
The body of literature on B-vitamin status in relation to cognitive function and decline has grown dramatically since 2004, and even published reviews on the subject are now numerous. Reviewers point to null studies and studies suggesting adverse effects of high folic acid intake or status and conclude that the evidence does not support a recommendation to combat cognitive impairment and decline with B-vitamin supplements. However, unrealistic expectations and subtle differences among the hypotheses addressed have contributed to the overall impression of inconsistency. For example, it is surely naïve to think that everyone will benefit from more folic acid and vitamin B-12, regardless of their status. Equally naïve are the assumptions that all cognitive functions will be similarly affected by low B-vitamin status, and that all markers will be able to detect true associations. Thus, we should not expect studies with no replete or deficient subjects to find associations, nor should we expect that all single-outcome studies will produce the same results, or that circulating vitamin B-12 and functional vitamin B-12 status indicators will generate equivalent findings. Furthermore, based on historical PA case studies, megadoses of folic acid unaccompanied by treatment for vitamin B-12 deficiency should not be expected to benefit cognition in vitamin B-12–deficient individuals. Considered in this regard, the seemingly aberrant results of studies of folate status in relation to cognitive function conducted in populations with fortified food supplies and many supplement users should not be thought of as inconsistent with the majority. They simply test a different hypothesis, i.e., whether raising the folate status of already replete people is beneficial. What is the proper definition of replete, however? Many studies have found that folate status well above traditionally used cutoff points confers benefits over low-normal status, and results of some studies have suggested that even so-called low-normal vitamin B-12 status is cognitively significant. This argues for exploration of dose–response relationships rather than dichotomizing exposure or reporting a β coefficient for a continuous association whose shape is unknown.
To detect a true association epidemiologically, not only must the exposure vary sufficiently, but so must the outcome. Thus, the null results of studies conducted in cognitively intact or nondeclining samples do not really conflict with the positive findings of studies that merely had a better chance of success.
If the extant body of literature does not support firm conclusions, it at least generates some promising working hypotheses. Two that come to mind are that hyperhomocysteinemia slows processing speed, but that only low folate status impairs memory. Unfortunately, however, there may be a subgroup within the population for whom supraphysiologic folate status is actually harmful to memory function. It is also important to point out that, in our ongoing study of the Framingham Offspring Cohort, we identified no cognitive benefits of supraphysiologic over merely replete folate status (M.S. Morris, unpublished). Although intakes of folic acid in excess of the current upper tolerable limit are reportedly rare in the general US population, effort should be invested in determining how high might be too high when it comes to preserving good cognitive function during aging, and who, precisely, is at risk.
Low vitamin B-12 status has been linked to both slow information processing and poor memory function, but sensitive indicators of vitamin B-12 function appear to be needed to detect these effects in studies. On the other hand, associations have been detected between low circulating vitamin B-12 levels and poor global cognitive functioning and accelerated global cognitive decline. Such a relationship should not be surprising considering the literature on PA. However, the apparent equivalence of both a functional marker and plasma B-12 at identifying people at risk may suggest that the phenomenon applies specifically to a subgroup that both indicators tend to misclassify (e.g., those with PA or severe malabsorption).
When design issues that may have prevented a few studies from finding a true association are taken into account, results of studies attempting to link hyperhomocysteinemia and low folate status to dementia/AD appear extremely consistent. However, cautious interpretation of the associations is warranted in light of the likely involvement of atherosclerosis in both vascular dementia and AD and the already established links among low folate status, hyperhomocysteinemia, and vascular disease. If the associations do reflect causal relationships, then the incidence of dementia/AD should decrease over time in countries with fortified food supplies and a high prevalence of supplement use. Also worth emphasizing is the overall lack of evidence for a link between low vitamin B-12 deficiency and dementia/AD, despite the suggestion from a few studies that low vitamin B-12 status predicts rapid cognitive decline. This may suggest that the elderly are susceptible to a preventable form of cognitive deterioration.
Future studies should attempt to clarify and expand upon the following observations: Hcy and low folate status are both related to dementia/AD, but low vitamin B-12 status is not. Hcy is related to slow information processing; low folate status is related to poor memory function; indicators of poor vitamin B-12 function are related to both outcomes, and low circulating vitamin B-12 levels predict accelerated global cognitive decline. RCTs have effected benefits to memory function and processing speed by correcting low folate status and hyperhomocysteinemia, respectively. However, supraphysiologic folate status, circulating unmetabolized folic acid, and high folic acid intake have been linked to slow information processing and accelerated decline in memory and global cognition. Whether these findings apply only to vitamin B-12–deficient individuals or even a subset of them requires clarification.
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Abbreviation used: AD, Alzheimer’s disease; CHAP, Chicago Health and Aging Project; CVD, cardiovascular disease; DSST, Digit-Symbol Substitution Test; holoTC, holotranscobalamin; Hcy, homocysteine; MMA, methylmalonic acid; MMSE, Mini-Mental State Examination; PA, pernicious anemia; RCT, randomized controlled trial.
Literature Cited
- 1.Hutto BR. Folate and cobalamin in psychiatric illness. Compr Psychiatry. 1997;38:305–14 [DOI] [PubMed] [Google Scholar]
- 2.McCully KS, Wilson RB. Homocysteine theory of arteriosclerosis. Atherosclerosis. 1975;22:215–27 [DOI] [PubMed] [Google Scholar]
- 3.Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993;270:2693–8 [DOI] [PubMed] [Google Scholar]
- 4.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7 [DOI] [PubMed] [Google Scholar]
- 5.Driscoll I, Sutherland RJ. The aging hippocampus: navigating between rat and human experiments. Rev Neurosci. 2005;16:87–121 [DOI] [PubMed] [Google Scholar]
- 6.Obeid R, Herrmann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006;580:2994–3005 [DOI] [PubMed] [Google Scholar]
- 7.Smulders YM, Blom HJ. The homocysteine controversy. J Inherit Metab Dis. 2011;34:93–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottiglieri T. S-Adenosyl-L-methionine (SAMe): from the bench to the bedside–molecular basis of a pleiotrophic molecule. Am J Clin Nutr. 2002;76:1151S–7S [DOI] [PubMed] [Google Scholar]
- 9.Selhub J, Troen A, Rosenberg IH. B vitamins and the aging brain. Nutr Rev. 2010;68: Suppl 2:S112–8 [DOI] [PubMed] [Google Scholar]
- 10.Metz J. Cobalamin deficiency and the pathogenesis of nervous system disease. Annu Rev Nutr. 1992;12:59–79 [DOI] [PubMed] [Google Scholar]
- 11.Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337:1441–8 [DOI] [PubMed] [Google Scholar]
- 12.De Gennes L, Bensoussan P, Klein F. [Addison's disease; acute mental disorders and chronic delirium]. Ann Med Psychol (Paris). 1954;112:522–7 [PubMed] [Google Scholar]
- 13.Carmel R. Pernicious anemia. The expected findings of very low serum cobalamin levels, anemia, and macrocytosis are often lacking. Arch Intern Med. 1988;148:1712–4 [DOI] [PubMed] [Google Scholar]
- 14.Allen RH, Stabler SP, Lindenbaum J. Relevance of vitamins, homocysteine and other metabolites in neuropsychiatric disorders. Eur J Pediatr. 1998;157: Suppl 2:S122–6 [DOI] [PubMed] [Google Scholar]
- 15.Scott E. Prevalence of pernicious anaemia in Great Britain. J Coll Gen Pract. 1960;3:80–4 [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82:442–50 [DOI] [PubMed] [Google Scholar]
- 17.Carmel R, Green R, Jacobsen DW, Rasmussen K, Florea M, Azen C. Serum cobalamin, homocysteine, and methylmalonic acid concentrations in a multiethnic elderly population: ethnic and sex differences in cobalamin and metabolite abnormalities. Am J Clin Nutr. 1999;70:904–10 [DOI] [PubMed] [Google Scholar]
- 18.Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr. 1994;60:2–11 [DOI] [PubMed] [Google Scholar]
- 19.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Elevated serum methylmalonic acid concentrations are common among elderly Americans. J Nutr. 2002;132:2799–803 [DOI] [PubMed] [Google Scholar]
- 20.Björkegren K, Svardsudd K. Serum cobalamin, folate, methylmalonic acid and total homocysteine as vitamin B12 and folate tissue deficiency markers amongst elderly Swedes–a population-based study. J Intern Med. 2001;249:423–32 [DOI] [PubMed] [Google Scholar]
- 21.Carmel R. Mean corpuscular volume and other concerns in the study of vitamin B-12 deficiency: epidemiology with pathophysiology. Am J Clin Nutr 2008;87:1962–3; author reply 3–4 [DOI] [PubMed] [Google Scholar]
- 22.Andrès E, Perrin AE, Demangeat C, Kurtz JE, Vinzio S, Grunenberger F, Goichot B, Schlienger JL. The syndrome of food-cobalamin malabsorption revisited in a department of internal medicine. A monocentric cohort study of 80 patients. Eur J Intern Med. 2003;14:221–6 [DOI] [PubMed] [Google Scholar]
- 23.Carmel R. Subclinical cobalamin deficiency. Curr Opin Gastroenterol. 2012;28:151–8 [DOI] [PubMed] [Google Scholar]
- 24.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–7 [PubMed] [Google Scholar]
- 25.Larkin M. Kilmer McCully: pioneer of the homocysteine theory. Lancet. 1998;352:1364. [DOI] [PubMed] [Google Scholar]
- 26.Bell IR, Edman JS, Selhub J, Morrow FD, Marby DW, Kayne HL, Cole JO. Plasma homocysteine in vascular disease and in nonvascular dementia of depressed elderly people. Acta Psychiatr Scand. 1992;86:386–90 [DOI] [PubMed] [Google Scholar]
- 27.Riggs KM, Spiro A, 3rd, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr. 1996;63:306–14 [DOI] [PubMed] [Google Scholar]
- 28.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–55 [DOI] [PubMed] [Google Scholar]
- 29.Ellinson M, Thomas J, Patterson A. A critical evaluation of the relationship between serum vitamin B, folate and total homocysteine with cognitive impairment in the elderly. J Hum Nutr Diet. 2004;17:371–83, quiz 85–7 [DOI] [PubMed] [Google Scholar]
- 30.Raman G, Tatsioni A, Chung M, Rosenberg IH, Lau J, Lichtenstein AH, Balk EM. Heterogeneity and lack of good quality studies limit association between folate, vitamins B-6 and B-12, and cognitive function. J Nutr. 2007;137:1789–94 [DOI] [PubMed] [Google Scholar]
- 31.Smith AD. The worldwide challenge of the dementias: a role for B vitamins and homocysteine? Food Nutr Bull. 2008; 29(Suppl 2)S143–72 [DOI] [PubMed] [Google Scholar]
- 32.Ford AH, Almeida OP. Effect of homocysteine lowering treatment on cognitive function: a systematic review and meta-analysis of randomized controlled trials. J Alzheimers Dis. 2012;29:133–49 [DOI] [PubMed] [Google Scholar]
- 33.Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21–30 [DOI] [PubMed] [Google Scholar]
- 34.Wald DS, Kasturiratne A, Simmonds M. doi: 10.1016/j.amjmed.2010.01.017. Effect of folic acid, with or without other B vitamins, on cognitive decline: meta-analysis of randomized trials. Am J Med 2010;123:522–7.e2. [DOI] [PubMed] [Google Scholar]
- 35.Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008; (4):CD004514. [DOI] [PubMed] [Google Scholar]
- 36.Vogel T, Dali-Youcef N, Kaltenbach G, Andres E. Homocysteine, vitamin B12, folate and cognitive functions: a systematic and critical review of the literature. Int J Clin Pract. 2009;63:1061–7 [DOI] [PubMed] [Google Scholar]
- 37.de Jager CA, Kovatcheva A. Summary and discussion: Methodologies to assess long-term effects of nutrition on brain function. Nutr Rev. 2010;68: Suppl 1:S53–8 [DOI] [PubMed] [Google Scholar]
- 38.Kim JM, Stewart R, Kim SW, Shin IS, Yang SJ, Shin HY, Yoon JS. Changes in folate, vitamin B12 and homocysteine associated with incident dementia. J Neurol Neurosurg Psychiatry. 2008;79:864–8 [DOI] [PubMed] [Google Scholar]
- 39.Luchsinger JA, Tang MX, Shea S, Miller J, Green R, Mayeux R. Plasma homocysteine levels and risk of Alzheimer disease. Neurology. 2004;62:1972–6 [DOI] [PubMed] [Google Scholar]
- 40.McIlroy SP, Dynan KB, Lawson JT, Patterson CC, Passmore AP. Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke. 2002;33:2351–6 [DOI] [PubMed] [Google Scholar]
- 41.Kivipelto M, Annerbo S, Hultdin J, Backman L, Viitanen M, Fratiglioni L, Lokk J. Homocysteine and holo-transcobalamin and the risk of dementia and Alzheimers disease: a prospective study. Eur J Neurol. 2009;16:808–13 [DOI] [PubMed] [Google Scholar]
- 42.Quadri P, Fragiacomo C, Pezzati R, Zanda E, Forloni G, Tettamanti M, Lucca U. Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr. 2004;80:114–22 [DOI] [PubMed] [Google Scholar]
- 43.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–83 [DOI] [PubMed] [Google Scholar]
- 44.Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN, Green R, Miller JW. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2005;82:1346–52 [DOI] [PubMed] [Google Scholar]
- 45.Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Porcellini E, Licastro F. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82:636–43 [DOI] [PubMed] [Google Scholar]
- 46.Redéen S, Ryberg A, Petersson F, Eriksson O, Nagga K, Borch K. Homocysteine levels in chronic gastritis and other conditions: relations to incident cardiovascular disease and dementia. Dig Dis Sci. 2010;55:351–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zylberstein DE, Lissner L, Bjorkelund C, Mehlig K, Thelle DS, Gustafson D, Ostling S, Waern M, Guo X, Skoog I. Midlife homocysteine and late-life dementia in women. A prospective population study. Neurobiol Aging. 2011;32:380–6 [DOI] [PubMed] [Google Scholar]
- 48.Hooshmand B, Solomon A, Kareholt I, Leiviska J, Rusanen M, Ahtiluoto S, Winblad B, Laatikainen T, Soininen H, Kivipelto M. Homocysteine and holotranscobalamin and the risk of Alzheimer disease: a longitudinal study. Neurology. 2010;75:1408–14 [DOI] [PubMed] [Google Scholar]
- 49.Mazza A, Bossone E, Mazza F, Distante A. Reduced serum homocysteine levels in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2005;15:118–24 [DOI] [PubMed] [Google Scholar]
- 50.Mazza A, Giugliano D, Motti C, Cortese C, Andreotti F, Marra G, Nulli A. Glycemia, MTHFR genotype and low homocysteine in uncomplicated type 2 diabetic patients. Atherosclerosis. 2000;149:223–4 [DOI] [PubMed] [Google Scholar]
- 51.Herrmann W, Obeid R, Schorr H, Geisel J. Functional vitamin B12 deficiency and determination of holotranscobalamin in populations at risk. Clin Chem Lab Med. 2003;41:1478–88 [DOI] [PubMed] [Google Scholar]
- 52.Luchsinger JA, Tang MX, Miller J, Green R, Mayeux R. Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Arch Neurol. 2007;64:86–92 [DOI] [PubMed] [Google Scholar]
- 53.Wang HX, Wahlin A, Basun H, Fastbom J, Winblad B, Fratiglioni L. Vitamin B(12) and folate in relation to the development of Alzheimer's disease. Neurology. 2001;56:1188–94 [DOI] [PubMed] [Google Scholar]
- 54.Corrada MM, Kawas CH, Hallfrisch J, Muller D, Brookmeyer R. Reduced risk of Alzheimer’s disease with high folate intake: The Baltimore Longitudinal Study of Aging. Alzheimer Dement. 2005;1:11–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morris MC, Evans DA, Schneider JA, Tangney CC, Bienias JL, Aggarwal NT. Dietary folate and vitamins B-12 and B-6 not associated with incident Alzheimer's disease. J Alzheimers Dis. 2006;9:435–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shane B. Folate status assessment history: implications for measurement of biomarkers in NHANES. Am J Clin Nutr. 2011;94:337S–42S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diniz BS, Yassuda MS, Nunes PV, Radanovic M, Forlenza OV. Mini-mental State Examination performance in mild cognitive impairment subtypes. Int Psychogeriatr. 2007;19:647–56 [DOI] [PubMed] [Google Scholar]
- 58.Kalmijn S, Launer LJ, Lindemans J, Bots ML, Hofman A, Breteler MM. Total homocysteine and cognitive decline in a community-based sample of elderly subjects: the Rotterdam Study. Am J Epidemiol. 1999;150:283–9 [DOI] [PubMed] [Google Scholar]
- 59.Budge MM, de Jager C, Hogervorst E, Smith AD. Total plasma homocysteine, age, systolic blood pressure, and cognitive performance in older people. J Am Geriatr Soc. 2002;50:2014–8 [DOI] [PubMed] [Google Scholar]
- 60.Duthie SJ, Whalley LJ, Collins AR, Leaper S, Berger K, Deary IJ. Homocysteine, B vitamin status, and cognitive function in the elderly. Am J Clin Nutr. 2002;75:908–13 [DOI] [PubMed] [Google Scholar]
- 61.Robbins MA, Elias MF, Budge MM, Brennan SL, Elias PK. Homocysteine, type 2 diabetes mellitus, and cognitive performance: The Maine-Syracuse Study. Clin Chem Lab Med. 2005;43:1101–6 [DOI] [PubMed] [Google Scholar]
- 62.Dufouil C, Alperovitch A, Ducros V, Tzourio C. Homocysteine, white matter hyperintensities, and cognition in healthy elderly people. Ann Neurol. 2003;53:214–21 [DOI] [PubMed] [Google Scholar]
- 63.Feng L, Ng TP, Chuah L, Niti M, Kua EH. Homocysteine, folate, and vitamin B-12 and cognitive performance in older Chinese adults: findings from the Singapore Longitudinal Ageing Study. Am J Clin Nutr. 2006;84:1506–12 [DOI] [PubMed] [Google Scholar]
- 64.Ravaglia G, Forti P, Maioli F, Muscari A, Sacchetti L, Arnone G, Nativio V, Talerico T, Mariani E. Homocysteine and cognitive function in healthy elderly community dwellers in Italy. Am J Clin Nutr. 2003;77:668–73 [DOI] [PubMed] [Google Scholar]
- 65.Wright CB, Lee HS, Paik MC, Stabler SP, Allen RH, Sacco RL. Total homocysteine and cognition in a tri-ethnic cohort: the Northern Manhattan Study. Neurology. 2004;63:254–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hin H, Clarke R, Sherliker P, Atoyebi W, Emmens K, Birks J, Schneede J, Ueland PM, Nexo E, Scott J, et al. Clinical relevance of low serum vitamin B12 concentrations in older people: the Banbury B12 study. Age Ageing. 2006;35:416–22 [DOI] [PubMed] [Google Scholar]
- 67.Hooshmand B, Solomon A, Kareholt I, Rusanen M, Hanninen T, Leiviska J, Winblad B, Laatikainen T, Soininen H, Kivipelto M. Associations between serum homocysteine, holotranscobalamin, folate and cognition in the elderly: a longitudinal study. J Intern Med. 2012;271:204–12 [DOI] [PubMed] [Google Scholar]
- 68.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A., 3rd High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82:627–35 [DOI] [PubMed] [Google Scholar]
- 69.Clarke R, Birks J, Nexo E, Ueland PM, Schneede J, Scott J, Molloy A, Evans JG. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr. 2007;86:1384–91 [DOI] [PubMed] [Google Scholar]
- 70.Mooijaart SP, Gussekloo J, Frolich M, Jolles J, Stott DJ, Westendorp RG, de Craen AJ. Homocysteine, vitamin B-12, and folic acid and the risk of cognitive decline in old age: the Leiden 85-Plus study. Am J Clin Nutr. 2005;82:866–71 [DOI] [PubMed] [Google Scholar]
- 71.De Jager CA, Hogervorst E, Combrinck M, Budge MM. Sensitivity and specificity of neuropsychological tests for mild cognitive impairment, vascular cognitive impairment and Alzheimer's disease. Psychol Med. 2003;33:1039–50 [DOI] [PubMed] [Google Scholar]
- 72.Narayan SK, Saxby BK, Firbank MJ, O'Brien JT, Harrington F, McKeith IG, Hansrani M, Stansby G, Ford GA. Plasma homocysteine and cognitive decline in older hypertensive subjects. Int Psychogeriatr. 2011;23:1607–15 [DOI] [PubMed] [Google Scholar]
- 73.Gallagher D, Mhaolain AN, Coen R, Walsh C, Kilroy D, Belinski K, Bruce I, Coakley D, Walsh JB, Cunningham C, et al. Detecting prodromal Alzheimer's disease in mild cognitive impairment: utility of the CAMCOG and other neuropsychological predictors. Int J Geriatr Psychiatry. 2010;25:1280–7 [DOI] [PubMed] [Google Scholar]
- 74.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Hyperhomocysteinemia associated with poor recall in the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2001;73:927–33 [DOI] [PubMed] [Google Scholar]
- 75.Prins ND, Den Heijer T, Hofman A, Koudstaal PJ, Jolles J, Clarke R, Breteler MM. Homocysteine and cognitive function in the elderly: the Rotterdam Scan Study. Neurology. 2002;59:1375–80 [DOI] [PubMed] [Google Scholar]
- 76.Elias MF, Sullivan LM, D'Agostino RB, Elias PK, Jacques PF, Selhub J, Seshadri S, Au R, Beiser A, Wolf PA. Homocysteine and cognitive performance in the Framingham offspring study: age is important. Am J Epidemiol. 2005;162:644–53 [DOI] [PubMed] [Google Scholar]
- 77.Clark MS, Guthrie JR, Dennerstein L. Hyperhomocysteinemia is associated with lower performance on memory tasks in post-menopausal women. Dement Geriatr Cogn Disord. 2005;20:57–62 [DOI] [PubMed] [Google Scholar]
- 78.Schafer JH, Glass TA, Bolla KI, Mintz M, Jedlicka AE, Schwartz BS. Homocysteine and cognitive function in a population-based study of older adults. J Am Geriatr Soc. 2005;53:381–8 [DOI] [PubMed] [Google Scholar]
- 79.Nurk E, Refsum H, Tell GS, Engedal K, Vollset SE, Ueland PM, Nygaard HA, Smith AD. Plasma total homocysteine and memory in the elderly: the Hordaland Homocysteine Study. Ann Neurol. 2005;58:847–57 [DOI] [PubMed] [Google Scholar]
- 80.Elias MF, Robbins MA, Budge MM, Elias PK, Brennan SL, Johnston C, Nagy Z, Bates CJ. Homocysteine, folate, and vitamins B6 and B12 blood levels in relation to cognitive performance: the Maine-Syracuse study. Psychosom Med. 2006;68:547–54 [DOI] [PubMed] [Google Scholar]
- 81.Miller JW, Green R, Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN. Homocysteine and cognitive function in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2003;78:441–7 [DOI] [PubMed] [Google Scholar]
- 82.de Lau LM, Refsum H, Smith AD, Johnston C, Breteler MM. Plasma folate concentration and cognitive performance: Rotterdam Scan Study. Am J Clin Nutr. 2007;86:728–34 [DOI] [PubMed] [Google Scholar]
- 83.Green R, Miller JW. Vitamin B12 deficiency is the dominant nutritional cause of hyperhomocysteinemia in a folic acid-fortified population. Clin Chem Lab Med. 2005;43:1048–51 [DOI] [PubMed] [Google Scholar]
- 84.Joy S, Kaplan E, Fein D. Speed and memory in the WAIS-III Digit Symbol–Coding subtest across the adult lifespan. Arch Clin Neuropsychol. 2004;19:759–67 [DOI] [PubMed] [Google Scholar]
- 85.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests. New York: Oxford University Press; 2006 [Google Scholar]
- 87.Wallesch CW, Curio N, Galazky I, Jost S, Synowitz H. The neuropsychology of blunt head injury in the early postacute stage: effects of focal lesions and diffuse axonal injury. J Neurotrauma. 2001;18:11–20 [DOI] [PubMed] [Google Scholar]
- 88.Sweeney JE, Slade HP, Ivins RG, Nemeth DG, Ranks DM, Sica RB. Scientific investigation of brain-behavior relationships using the Halstead-Reitan Battery. Appl Neuropsychol. 2007;14:65–72 [DOI] [PubMed] [Google Scholar]
- 89.Almkvist O. Cognitive syndrome(s) in preclinical and clinical vascular dementia. Int Psychogeriatr. 2003;15: Suppl 1:127–31 [DOI] [PubMed] [Google Scholar]
- 90.Graham NL, Emery T, Hodges JR. Distinctive cognitive profiles in Alzheimer's disease and subcortical vascular dementia. J Neurol Neurosurg Psychiatry. 2004;75:61–71 [PMC free article] [PubMed] [Google Scholar]
- 91.Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, D'Agostino RB, DeCarli C. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63:246–50 [DOI] [PubMed] [Google Scholar]
- 92.Kado DM, Karlamangla AS, Huang MH, Troen A, Rowe JW, Selhub J, Seeman TE. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful Aging. Am J Med. 2005;118:161–7 [DOI] [PubMed] [Google Scholar]
- 93.van den Kommer TN, Dik MG, Comijs HC, Jonker C, Deeg DJ. Homocysteine and inflammation: predictors of cognitive decline in older persons? Neurobiol Aging. 2010;31:1700–9 [DOI] [PubMed] [Google Scholar]
- 94.Garcia A, Zanibbi K. Homocysteine and cognitive function in elderly people. CMAJ. 2004;171:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tangney CC, Tang Y, Evans DA, Morris MC. Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline. Neurology. 2009;72:361–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ravaglia G, Forti P, Maioli F, Zanardi V, Dalmonte E, Grossi G, Cucinotta D, Macini P, Caldarera M. Blood homocysteine and vitamin B levels are not associated with cognitive skills in healthy normally ageing subjects. J Nutr Health Aging. 2000;4:218–22 [PubMed] [Google Scholar]
- 97.Bernard MA, Nakonezny PA, Kashner TM. The effect of vitamin B12 deficiency on older veterans and its relationship to health. J Am Geriatr Soc. 1998;46:1199–206 [DOI] [PubMed] [Google Scholar]
- 98.Lindeman RD, Romero LJ, Koehler KM, Liang HC, LaRue A, Baumgartner RN, Garry PJ. Serum vitamin B12, C and folate concentrations in the New Mexico elder health survey: correlations with cognitive and affective functions. J Am Coll Nutr. 2000;19:68–76 [DOI] [PubMed] [Google Scholar]
- 99.Morris MS, Selhub J, Jacques PF. Vitamin B-12 and folate status in relation to decline in scores on the Mini-Mental State Examination in the Framingham Heart Study. J Am Geriatr Soc. Epub 2012. Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Durga J, van Boxtel MP, Schouten EG, Bots ML, Kok FJ, Verhoef P. Folate and the methylenetetrahydrofolate reductase 677C→T mutation correlate with cognitive performance. Neurobiol Aging. 2006;27:334–43 [DOI] [PubMed] [Google Scholar]
- 101.Kang JH, Irizarry MC, Grodstein F. Prospective study of plasma folate, vitamin B12, and cognitive function and decline. Epidemiology. 2006;17:650–7 [DOI] [PubMed] [Google Scholar]
- 102.Jelicic M, Jonker C, Deeg DJ. Effect of low levels of serum vitamin B12 and folic acid on cognitive performance in old age: a population-based study. Dev Neuropsychol. 2001;20:565–71 [DOI] [PubMed] [Google Scholar]
- 103.Tangney CC, Aggarwal NT, Li H, Wilson RS, Decarli C, Evans DA, Morris MC. Vitamin B12, cognition, and brain MRI measures: a cross-sectional examination. Neurology. 2011;77:1276–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garcia AA, Haron Y, Evans LR, Smith MG, Freedman M, Roman GC. Metabolic markers of cobalamin deficiency and cognitive function in normal older adults. J Am Geriatr Soc. 2004;52:66–71 [DOI] [PubMed] [Google Scholar]
- 105.Morris MC, Evans DA, Bienias JL, Tangney CC, Hebert LE, Scherr PA, Schneider JA. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol. 2005;62:641–5 [DOI] [PubMed] [Google Scholar]
- 106.Reynolds EH. Folic acid and the prevention of neural tube defects. Folate has potential to cause harm. BMJ. 1995;311:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palca J. Folic acid. Agencies split on nutrition advice. Science. 1992;257:1857. [DOI] [PubMed] [Google Scholar]
- 108.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5:949–60 [DOI] [PubMed] [Google Scholar]
- 109.Morris MS, Jacques PF, Rosenberg IH, Selhub J. doi: 10.3945/ajcn.2009.28671. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr. 2010 ;91:1733–44. Epub 2010 Mar 31. [DOI] [PubMed] [Google Scholar]
- 110.Clarke R, Sherliker P, Hin H, Molloy AM, Nexo E, Ueland PM, Emmens K, Scott JM, Evans JG. Folate and vitamin B12 status in relation to cognitive impairment and anaemia in the setting of voluntary fortification in the UK. Br J Nutr. 2008;100:1054–9 [DOI] [PubMed] [Google Scholar]
- 111.Fioravanti M, Ferrario E, Massaia M, Cappa G, Rivolta G, Grossi E, et al. Low folate levels in the cognitive decline of elderly patients and the efficacy of folate as a treatment for improving memory deficits. Arch Gerontol Geriatr. 1998;26:1–13 [DOI] [PubMed] [Google Scholar]
- 112.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354:2764–72 [DOI] [PubMed] [Google Scholar]
- 113.Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369:208–16 [DOI] [PubMed] [Google Scholar]
- 114.Walker JG, Batterham PJ, Mackinnon AJ, Jorm AF, Hickie I, Fenech M, Kljakovic M, Crisp D, Christensen H. Oral folic acid and vitamin B-12 supplementation to prevent cognitive decline in community-dwelling older adults with depressive symptoms–the Beyond Ageing Project: a randomized controlled trial. Am J Clin Nutr. 2012;95:194–203 [DOI] [PubMed] [Google Scholar]
- 115.Kelly P, McPartlin J, Goggins M, Weir DG, Scott JM. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr. 1997;65:1790–5 [DOI] [PubMed] [Google Scholar]
- 116.Food and Nutrition Board IoM. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin and choline. Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- 117.Eussen SJ, de Groot LC, Clarke R, Schneede J, Ueland PM, Hoefnagels WH, van Staveren WA. Oral cyanocobalamin supplementation in older people with vitamin B12 deficiency: a dose-finding trial. Arch Intern Med. 2005;165:1167–72 [DOI] [PubMed] [Google Scholar]
- 118.Rajan S, Wallace JI, Brodkin KI, Beresford SA, Allen RH, Stabler SP. Response of elevated methylmalonic acid to three dose levels of oral cobalamin in older adults. J Am Geriatr Soc. 2002;50:1789–95 [DOI] [PubMed] [Google Scholar]
- 119.Pfeiffer CM, Schleicher RL, Johnson CL, Coates PM. Assessing vitamin status in large population surveys by measuring biomarkers and dietary intake--two case studies: folate and vitamin D. Food Nutr Res. 2012;56. Epub 2012 Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kang JH, Cook N, Manson J, Buring JE, Albert CM, Grodstein F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr. 2008;88:1602–10 [DOI] [PMC free article] [PubMed] [Google Scholar]