Abstract
Sustained periods of negative energy balance decrease body mass due to losses of both fat and skeletal muscle mass. Decreases in skeletal muscle mass are associated with a myriad of negative consequences, including suppressed basal metabolic rate, decreased protein turnover, decreased physical performance, and increased risk of injury. Decreases in skeletal muscle mass in response to negative energy balance are due to imbalanced rates of muscle protein synthesis and degradation. However, the underlying physiological mechanisms contributing to the loss of skeletal muscle during energy deprivation are not well described. Recent studies have demonstrated that consuming dietary protein at levels above the current recommended dietary allowance (0.8 g·kg−1·d−1) may attenuate the loss of skeletal muscle mass by affecting the intracellular regulation of muscle anabolism and proteolysis. However, the specific mechanism by which increased dietary protein spares skeletal muscle through enhanced molecular control of muscle protein metabolism has not been elucidated. This article reviews the available literature related to the effects of negative energy balance on skeletal muscle mass, highlighting investigations that assessed the influence of varying levels of dietary protein on skeletal muscle protein metabolism. Further, the molecular mechanisms that may contribute to the regulation of skeletal muscle mass in response to negative energy balance and alterations in dietary protein level are described.
Introduction
The consequences of negative energy balance on total body and skeletal muscle mass are well established. In general, total body mass decreases in response to sustained periods of negative energy balance, and the proportion of body mass loss is ~75% adipose tissue and 25% fat-free mass (FFM)5 (1). Although the predominant change in body composition is the loss of body fat, which may be beneficial, the concomitant decrease in skeletal muscle mass may negatively affect metabolic processes, muscular function, and physical performance. In overweight and obese individuals attempting to lose weight, decreases in muscle mass may down-regulate metabolic processes, such as protein turnover and basal metabolic rate, thus compromising healthy weight management (2, 3). Healthy, normal-weight individuals such as athletes and military personnel may also undergo periods of negative energy balance resulting from dietary energy restriction, increased energy expenditure, or the combined effects of both. Decreased FFM in this population may be of greater concern, decreasing physical performance and increasing susceptibility to injury.
Popular strategies to attenuate muscle loss during negative energy balance include nutritional interventions that provide dietary protein in excess of the current recommended dietary allowance (RDA) (0.8 g·kg−1·d−1), as several studies have described a potential muscle-sparing effect, consequent to consuming higher protein diets (4–7). Although these benefits of consuming higher protein diets are becoming evident, the physiological mechanisms by which increased protein intake confers protection against the loss of skeletal muscle mass in response to negative energy balance are not well described.
The objective of this article is to provide a contemporary analysis of the available literature regarding the effects of energy restriction on skeletal muscle mass, with an emphasis on studies assessing the influence of varying levels of dietary protein on skeletal muscle protein metabolism. Experimental models that use a variety of applied and basic molecular biological techniques to characterize the skeletal muscle proteolytic responses to manipulations in energy and dietary protein intake are described. Further, this article reviews molecular mechanisms that may contribute to the regulation of skeletal muscle mass in response to negative energy balance and explores the cellular properties by which dietary protein may conserve skeletal muscle integrity.
Negative energy balance and dietary protein intake: effects on FFM, nitrogen balance, and protein turnover
Energy balance and dietary protein intake are critical factors that contribute to the regulation of skeletal muscle mass by influencing whole-body and skeletal muscle protein metabolism (8–10). In a recent systematic review of publications from 1993 to 2009, Weinheimer et al. (1) reported that in more than half of the studies reviewed, energy restriction induced weight loss of 5–10% of the initial body mass. More than one fourth of this change in total body mass was a result of decreases in FFM. Layman et al. (5) demonstrated a greater retention of FFM and loss of body fat in overweight women adhering to a hypoenergetic diet (7113 kJ/d or 1700 kcal/d) than those consuming higher levels of dietary protein (1.6 g·kg−1·d−1) compared with those who consumed the RDA for protein (0.8 g·kg−1·d−1).
The catabolic nature of negative energy balance and the protective effect of dietary protein were also demonstrated in postmenopausal obese women who consumed diets ranging from 0.5 to 1.5 g protein·kg−1·d−1 for 20 wk (11). In that study, the extent of muscle loss in response to negative energy balance was proportional to dietary protein intake. Specifically, all volunteers lost FFM (−1.4 kg and −4.3 kg in the high- and low-protein groups, respectively). However, the percentage of total weight loss due to decreases in FFM was significantly lower for those women consuming high- (17.3%) versus low- (37.5%) protein diets. Others have also demonstrated benefits of consuming higher protein diets during prolonged periods of negative energy balance, with consistent reports documenting the attenuation of the loss of FFM after weight loss (4, 6). Taken together, these investigations indicate that a certain degree of lean mass protection is gleaned from the consumption of a higher protein diet during prolonged periods of energy deficit.
Nitrogen balance methodology is widely used as a holistic assessment of protein balance, allowing one to gain valuable insight regarding the relationship between energy status, dietary protein, and skeletal muscle mass. In general, when energy intake is sufficient to meet energy demand, increasing the protein content of the diet imparts no added influence on nitrogen retention (12). However, increasing dietary protein intake may offset the increase in nitrogen excretion and negative nitrogen balance that generally occurs during periods of energy deficiency (13, 14). For example, nitrogen balance and basal metabolic rate were preserved in premenopausal women who consumed a higher protein diet (1.4 g·kg−1·d−1) during a 10-wk period of negative energy balance induced by dietary restriction coupled with a modest increase in physical activity (15). In a second study, Pikosky et al. (16) demonstrated negative nitrogen balance in healthy young volunteers in response to a 7-d period of negative energy balance (−4184 kJ/d or −1000 kcal/d) elicited solely by an increase in aerobic-type physical activity when protein was consumed at levels similar to the current RDA (0.9 g·kg−1·d−1). However, doubling dietary protein intake (1.8 g·kg−1·d−1) abrogated the increased nitrogen excretion and resultant negative nitrogen balance that occurred after the 7-d energy deficit. Again, these results indicate that lean body mass may be defended in response to negative energy balance by consuming a diet that provides protein at levels above the RDA, regardless of whether the energy deficit is caused by diet or physical activity.
Although these studies demonstrate a protective effect of consuming high levels of dietary protein during periods of negative energy balance, they fail to elucidate the physiological mechanisms for that effect. Because changes in skeletal muscle mass are likely due to imbalanced rates of protein synthesis and breakdown, amino acid tracer techniques have been used to assess the whole-body and skeletal muscle protein metabolic responses to varying levels of dietary protein and energy intakes (3, 14, 17–20).
In general, acute periods of negative energy balance associated with fasting result in increased whole-body proteolysis, amino acid oxidation, and nitrogen excretion, which become less pronounced and plateau over an extended period of time as the body adapts to conserve energy and protein reserves (e.g., muscle protein) (3, 17, 21, 22). For example, Nair et al. (17) reported a significant up-regulation of whole-body proteolysis and oxidation after a 72-h fast; however, longer duration studies observed a reversal of this response, as whole-body proteolysis and protein synthesis were decreased by 20% after a 4-wk period of negative energy balance in overweight adults consuming the RDA for dietary protein (3). The down-regulation of protein turnover was proportional to the loss of FFM, which accounted for nearly 25% of the total body mass lost. Recent experimental evidence from Campbell et al. (19) confirms these findings because whole-body protein synthesis and proteolysis were decreased in postmenopausal overweight women who consumed 1.0 g protein·kg−1·d−1 during a 13-wk period of moderate negative energy balance (−2092 kJ/d or −500 kcal/d). Together, these data suggest that whole-body protein turnover, an energy-requiring process, is down-regulated in response to sustained energy deficit, perhaps to conserve endogenous protein stores when dietary protein intake is equivalent to the current RDA.
Consuming a high-protein diet may also contribute to the regulation of muscle mass by maintaining whole-body protein turnover in response to either acute or prolonged periods of negative energy balance (16, 23–25). Pikosky et al. (16) assessed whole-body protein turnover in response to a 7-d physical activity–induced energy deficit in young healthy volunteers consuming dietary protein at either 0.9 g·kg−1·d−1 or 1.8 g·kg−1·d−1. Although nitrogen balance was maintained in volunteers consuming protein at levels more than twice the RDA, no differences in whole-body protein turnover were observed between groups. Friedlander et al. (23) also reported the maintenance of whole-body protein turnover in healthy young men consuming 1.2 g protein·kg−1·d−1 after a 3-wk 40% energy deficit. Although whole-body protein turnover measurements suggested that consuming dietary protein at RDA levels was adequate, nitrogen balance and resting metabolic rate were lower in response to energy deficiency, which corresponded to a significant decrease in FFM. It is important to note that the whole-body protein turnover assessments were performed in subjects under fasted conditions in these studies; it is possible that the positive effects of dietary protein on whole-body protein turnover may be observed only during the fed state (26). Further, it is important to recognize that although skeletal muscle accounts for ~50–75% of total body protein stores, it only contributes to 30–45% of whole-body protein turnover (27). As such, extrapolating findings from whole-body protein turnover studies to represent the skeletal muscle protein metabolic response to negative energy balance may not be appropriate. Studies directly assessing skeletal muscle protein turnover in response to negative energy balance and varying levels of protein intake are limited.
Only 3 studies investigated the direct skeletal muscle protein metabolic response to negative energy balance (18–20). Pasiakos et al. (18) demonstrated that a 10-d moderate energy deficit (−2092 kJ/d or −500 kcal/d) resulted in a 19% decrease in fasting skeletal muscle protein synthesis (weight maintenance: 0.074 ± 0.01%/h vs. energy deficit: 0.06 ± 0.01%/h) in physically active adults who consumed dietary protein at 1.5 g·kg−1·d−1. Other studies failed to confirm an energy deficit–induced impairment of skeletal muscle protein synthesis (19, 20). Campbell et al. (19) reported increased fasting muscle protein synthesis (weight maintenance: 0.04 ± 0.01%/h vs. energy deficit: 0.11 ± 0.01%/h) after a 13-wk modest energy deficit (−2092 kJ/d or −500 kcal/d) in overweight postmenopausal women consuming 1.0 g protein·kg−1·d−1. In contrast, Villareal et al. (20) observed no change in fasting muscle protein synthesis, although postprandial muscle protein synthesis increased (increase in muscle protein synthesis above fasting values: 0.033 ± 0.01%/h) in older adults (60–85 y) after a 3-mo period of negative energy balance (−2092–3138 kJ/d or −500–750 kcal/d). A combination of factors likely contributed to the discordant skeletal muscle protein turnover data observed across studies, including variations in experimental design, dietary interventions, and study populations. For example, Campbell et al. (19) assessed muscle protein synthesis after the 13-wk energy deficit 3 d after reestablishing energy balance (+2092 kJ/d or +500 kcal/d), which may have directly contributed to the dramatic increase in muscle protein synthesis. Further, Villareal et al. (20) assessed muscle kinetics in older obese adults using [5,5,5-2H5]-leucine, whereas Pasiakos et al. (18) characterized muscle protein synthesis in young, physically active adults using [2H5]-phenylalanine. Despite the apparent discrepancies in study populations and methodologies, these data suggest that energy restriction may elicit a down-regulation in muscle protein synthesis in the early stages of negative energy balance, perhaps representing an adaptive mechanism to conserve energy and protein reserves.
The studies reviewed thus far highlight the catabolic nature of negative energy balance and the associated muscle-sparing effects of consuming a high-protein diet. Regardless of whether energy deficit is induced by energy restriction, increased energy expenditure, or a combination, varied measures of whole-body and skeletal muscle protein metabolism indicate that consuming dietary protein in excess of the RDA confers a level of protection for skeletal muscle integrity. The remainder of this review focuses on recent studies assessing the molecular mechanisms regulating protein synthesis and breakdown and the potential influence of amino acids as signaling molecules.
Intracellular regulation of skeletal muscle mass: effects of negative energy balance and dietary protein
A series of intracellular networks that influence the molecular regulation of muscle protein turnover likely contribute to the loss of skeletal muscle mass in response to negative energy balance. Although certain elements of these intricate signaling pathways independently modulate critical steps involved in the cellular control of skeletal muscle anabolism and proteolysis, commonality between pathways does exist. However, the finite mechanisms by which cellular signaling molecules function in concert to regulate skeletal muscle mass in response to nutritional manipulation remain to be elucidated.
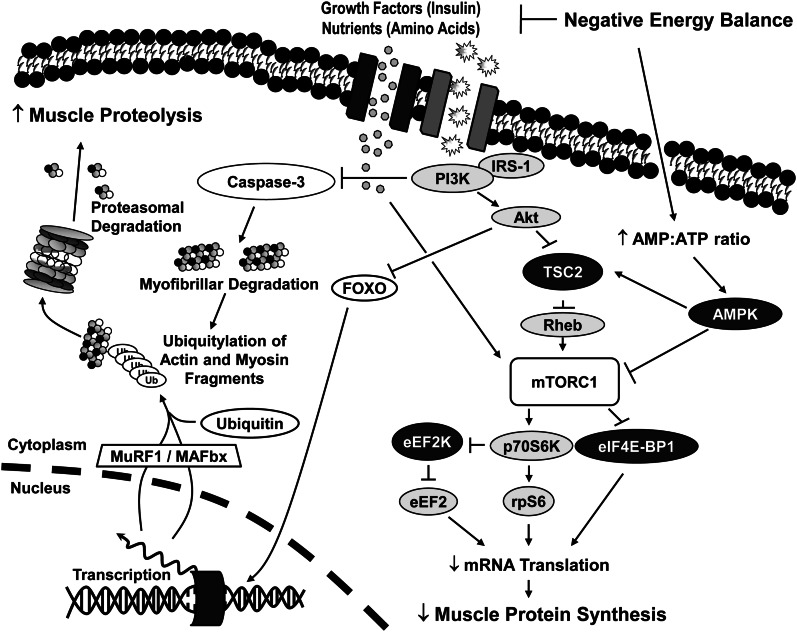
The cellular regulation of skeletal muscle protein synthesis has been well described (28, 29). Briefly, a cascade of intracellular signaling events influenced by energy status, growth factors, and nutrient availability regulate muscle protein synthesis by modifying mRNA translation initiation and elongation (18, 30). Perhaps the most important nutritionally regulated signaling component affecting mRNA translation is a multiunit protein complex termed mammalian target of rapamycin complex 1 (mTORC1), which includes the mammalian target of rapamycin (mTOR) kinase. mTORC1 is the central component of the insulin-signaling cascade [insulin/insulin-like growth factor (IGF)–phosphatidylinositol 3-kinase (PI3K) pathway) (Fig. 1) that regulates protein synthesis and mRNA translation through 2 primary mechanisms: 1) inactivation of the repressor of mRNA translation, eukaryotic translation initiation factor 4E-binding protein 1 (eIF4E-BP1), and 2) the activation of 70-kDa ribosomal protein S6 kinase. Together, changes in the phosphorylation state of these critical intracellular signaling proteins affect mRNA translation initiation and elongation, which, in turn, may directly influence the rate of protein synthesis.
Figure 1.
In response to negative energy balance, mRNA translation and muscle protein synthesis may be down-regulated as a result of decreased nutrient and growth factor availability, causing reduced mTORC1 activation. Decreased mTORC1 activation and subsequent decreases in muscle protein synthesis, coupled with increased FOXO nuclear localization, increased transcription of atrophy-related genes, with up-regulated caspase 3 activation and muscle protein ubiquitylation provide a possible mechanism contributing to skeletal muscle loss in response to periods of negative energy balance. Synthetic stimulators are depicted in gray, whereas inhibitors of synthesis are shown in black. Akt, protein kinase B; AMPK, AMP-activated protein kinase; eEF2, eukaryotic elongation factor 2; eEF2K, eukaryotic elongation factor 2 kinase; eIF4E-BP1, eukaryotic translation initiation factor 4E-binding protein 1; FOXO, forkhead box O; IRS-1, insulin receptor substrate 1; MAFbx, muscle atrogin F-box protein; mTORC1, mammalian target of rapamycin complex 1; MuRF1, muscle RING-finger protein 1; p70S6K, 70-kDa S6 kinase; PI3K, phosphatidylinositol 3-kinase; Rheb, ras homolog enriched in brain; rpS6, ribosomal protein S6; TSC, tuberous sclerosis complex; Ub, ubiquitin.
A key intracellular signaling protein that may serve an important role in regulating the skeletal muscle response to negative energy balance is AMP–activated protein kinase (AMPK), which functions as a fuel sensor in many tissues, including skeletal muscle. It inhibits anabolic signaling pathways when cellular ATP levels are decreased and AMP levels increase in response to limited energy availability (31). More specifically, upstream mTORC1 signaling via the tuberous sclerosis complex 2 is sensitive to cellular energy status mediated through AMPK (32, 33). Inhibition of mTORC1 activity and its subsequent downstream events has been demonstrated in rat skeletal muscle in response to increased AMPK activity. This occurs primarily by AMPK-dependent phosphorylation of the tuberous sclerosis complex 2 complex and direct phosphorylation of the mTOR kinase (31, 34). To the best of our knowledge, there have been limited in vivo human studies assessing intracellular regulation of skeletal muscle protein metabolism in response to negative energy balance. In 1 study, decreased protein kinase B (Akt) and eIF4E-BP1 phosphorylation were observed after a 10-d period of modest negative energy balance (−2092 kJ/d or −500 kcal/d) in physically active adults (18). Although no changes in AMPK activity were observed, decreased intracellular signaling occurred with a concomitant decrease in skeletal muscle protein synthesis.
Although these findings are intriguing, the cellular regulation of skeletal muscle proteolysis in response to negative energy balance and manipulations in dietary protein is not well described. The 4 primary intracellular pathways that contribute to the regulation of skeletal muscle proteolysis are the autophagy/lysosomal, calpain-dependent, caspase-mediated, and ubiquitin proteasome (UP) systems. However, the available literature suggests that only 2 of these systems (caspase-mediated and UP) are major regulators of muscle mass during periods of negative energy balance. Although inflammation-induced muscle degradation resulting from autophagy/lysosomal muscle proteolysis has been well established (35), the contribution of the lysosomal system to increased muscle loss consequent to energy deficiency is only now being elucidated (36–38). Increased lysosomal activity has been demonstrated in response to short-term fasting in transgenic mice expressing the autophagy gene, microtubule-associated protein 1A/1B-light chain 3, fused with green fluorescent protein (38). However, data from other animal models demonstrate diminished lysosomal enzyme activity during extended periods of energy restriction (39). Furthermore, calcium-dependent proteolysis indicative of calpain activity has also been postulated to function in many intramuscular processes, including myogenesis, the regulation of muscle protein turnover, and the initial cleavage of the myofibril (40). However, there is limited evidence that negative energy balance sufficiently modulates calpain expression or activation to meaningfully contribute to muscle loss because a 14-d 25% energy restriction failed to promote calpain activation in physically active young women (41). With extremely limited and often conflicting data, it is not evident at this point that the autophagy/lysosomal and calpain-dependent systems significantly contribute to the degradation of human skeletal muscle during negative energy balance and, more importantly, in response to alterations in dietary protein intake.
It is well documented, however, that the UP system serves a major role in modulating skeletal muscle proteolysis (42). Additionally, the proteasome regulates cell-cycle, antigen processing through class I major histocompatibility complex molecules, gene expression, and cell signaling (43). In addition to these primary functions, there is evidence suggesting that the proteasome is involved in noncatalytic activities, including transcription regulation, DNA repair, and chromatin remodeling (44). As it relates to skeletal muscle degradation, a number of choreographed steps must occur before muscle proteins may be degraded by the proteasome itself. Myofibrils, the functional units of myocytes, composed of actin and myosin filaments spanning the length of the muscle cell, do not serve as substrate for the proteasome in their native state (45). They must first be broken down into actin and myosin monomers before they can enter the proteasome’s 20S catalytic core.
Calpains may potentially contribute to myofibrillar cleavage (46), although skeletal muscle proteolysis initiation is most likely instigated by caspase 3, a cysteine protease most notably involved in apoptosis (47). Inactive procaspase 3 is first converted to active caspase 3 via caspase 9–mediated cleavage (47). Active caspase 3 then breaks down targeted myofibrillar proteins, producing a characteristic 14-kDa actin fragment and other polypeptide segments. These cleavage products may then be tagged with ubiquitin, mediated by the ATP-dependent E1 class of ubiquitin-activating enzymes (48). Once activated, ubiquitin is conjugated to a member of the E2 class of ubiquitin-conjugating enzymes. The final step of ubiquitin ligation to the target protein is mediated by the E3 class of ubiquitin ligases. This tagged protein is then recognized by the 26S proteasome, denatured, and degraded into peptide fragments, typically 2–25 amino acids long (48).
The activities of caspase 3 and the UP system are regulated in part by the insulin/IGF-PI3K pathway (Fig. 1). In tissue culture experiments with rat myocytes, the addition of insulin to the growth medium significantly suppresses serum deprivation (starvation)–induced actin cleavage (47). However, when insulin was added to cells containing a dominant-negative (defective) PI3K, no changes were observed in actin cleavage. Data from other reports confirm these findings (49), which, taken together, clearly demonstrate the critical role of insulin in regulating caspase 3 activity through PI3K. Further, these data illustrate a potential mechanism by which skeletal muscle proteolysis is increased in response to hypoinsulinemia, a physiological characteristic of negative energy balance (18).
Dephosphorylation/inactivation of Akt may also increase the expression of UP components (50). Akt is responsible for phosphorylation of the forkhead box O (FOXO) family of transcription factors (51). However, in the absence of Akt-mediated phosphorylation, these FOXO transcription factors migrate into the nucleus and increase expression of a number of atrophy-related genes, including the muscle-specific ubiquitin ligases muscle atrogin F-box protein (MAFbx) (atrogin-1) and muscle RING-finger protein 1 (50). More specifically, addition of the PI3K inhibitor LY294002 to the growth medium of mouse muscle cells produces enhanced nuclear localization of FOXO1 and increased MAFbx mRNA transcription (52, 53). Overexpression of FOXO3A in mouse myotubes also significantly up-regulates MAFbx expression (49). As such, it appears that the insulin/IGF-PI3K pathway not only regulates myofibrillar cleavage through caspase 3, but also the ubiquitylation of the resulting protein fragments via modification of ubiquitin-ligase expression.
Limited published data detail alterations in the UP system and associated subcomponents during periods of negative energy balance and varying levels of dietary protein. Although there are studies examining these cellular pathways in certain models of protein catabolism, including trauma (54), sepsis (55), cancer (56), and denervation (57), the level of severe muscle loss in those states is not comparable to studies assessing weight loss or the effects of exercise interventions. However, 6 mo of energy restriction (−3138 kJ/d or −750 kcal/d), combined with increased physical activity, was recently demonstrated to induce increased expression of FOXO3A, muscle RING-finger protein 1, and MAFbx mRNA in previously sedentary, obese older women (58). Similarly, muscle Akt phosphorylation was decreased in healthy young adults after a 10-d period of moderate energy deprivation (−2092 kJ/d or −500 kcal/d) (18), further implying that the muscle loss observed during energy restriction may in part be due to increases in caspase 3 and UP activity. In this same study, increased enzymatic activity of the UP β5 subunit was observed that mirrored increased muscle protein breakdown (J. Carbone, S. Pasiakos, L. Vislocky, N. Rodriguez, unpublished data).
Effects of protein quality and branched-chain amino acids on the skeletal muscle response to negative energy balance
It is well established that the amino acid composition of dietary protein can influence the regulation of skeletal muscle protein turnover. Increasing branched-chain amino acid (BCAA) levels during energy restriction can support gluconeogenesis, maintain whole-body and muscle protein synthesis, and attenuate nitrogen excretion and whole-body and muscle proteolysis (59). Of particular importance is the BCAA leucine, a potent independent stimulator of muscle protein synthesis in cell culture and animal models through enhanced cellular regulation of mRNA translation (29). Human studies have demonstrated stimulation of the mTORC1 pathway, increased muscle protein synthesis (29, 60, 61), and decreased whole-body proteolysis after consumption of a leucine-containing food product during exercise (61–65). Leucine supplementation also appears to attenuate muscle proteolysis and continues to do so in the presence of the mTOR inhibitor rapamycin, acting independently of mTORC1, although dependent on functional PI3K signaling (64, 65).
Although the recommended leucine intake is currently 14 mg·kg−1·d−1 (66), the amount required to maximize the stimulation of muscle anabolic intracellular signaling may be at least 40–65 mg·kg−1·d−1 (67, 68) and even up to 7–12 g·d−1 to contribute to the preservation of muscle mass during stressors such as energy restriction (59). Leucine stimulation of anabolic pathways is decreased in the presence of sufficient essential amino acids (EAAs) (69), indicating an increased basal synthetic rate with adequate EAA ingestion, thus highlighting the importance of consuming dietary protein at levels above the current RDA during periods of energy deficit. In neonanatal pigs, supplementing a low-protein diet with leucine significantly increased muscle protein synthesis compared with a low-protein diet without additional leucine (70). Furthermore, phosphorylation states of intracellular regulators of muscle protein synthesis (mTOR, 70-kDa S6 kinase, and eIF4E-BP1) were similar in pigs that consumed a leucine-supplemented, low-protein diet or a high-protein diet without additional leucine, highlighting the potential synthetic effect resulting from increased leucine intake.
There is a paucity of current reports examining the effects of total protein intake on regulators of human muscle proteolysis, although the past 20 y provided a number of findings supporting an anticatabolic effect of leucine and BCAAs (62, 63, 71–73). In healthy men, the oral ingestion of a carbohydrate solution containing isonitrogenous amounts of either EAAs (threonine, methionine, histidine) or BCAAs resulted in BCAA suppression of whole-body proteolysis to a greater extent than EAAs (71). These data suggest that leucine, and perhaps the remaining BCAAs, may have the ability to directly influence muscle protein breakdown.
Conclusions
Decreases in FFM after periods of negative energy balance may inhibit healthy weight management and decrease skeletal muscle function and performance. Although increasing dietary protein intake, and perhaps leucine, to more than the RDA has been demonstrated to spare muscle mass, a thorough understanding of the underlying molecular mechanisms is requisite to the development of nutritional countermeasures to mitigate the detrimental effects of negative energy balance.
Clearly, further study is required to assess the combined effects of negative energy balance and dietary protein intake on cellular mechanisms contributing to the regulation of skeletal muscle mass. Systematic, comprehensive studies that address changes in body composition, nitrogen balance, and whole-body and skeletal muscle protein turnover, in combination with expression and activity patterns of intracellular regulators of muscle mass, are required to identify nutritional agents (i.e., amino acids) to counteract decreases in FFM occurring in response to negative energy balance.
Acknowledgments
The authors acknowledge Dr. Andrew J. Young for his critical review in support of the development of this manuscript. All authors have read and approved the final manuscript.
Footnotes
Supported by United States Army Military Research and Material Command. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the United States Army or the Department of Defense. Any citations of commercial organizations and trade names in this report do not constitute an official United States Army endorsement of approval of the products or services of these organizations.
Author disclosures: J. W. Carbone, J. P. McClung, and S. M. Pasiakos, no conflicts of interest.
Abbreviations used: Akt, protein kinase B; AMPK, AMP–activated protein kinase; BCAA, branched-chain amino acid; EAA, essential amino acid; eIF4E-BP1, eukaryotic translation initiation factor 4E-binding protein 1; FFM, fat-free mass; FOXO, forkhead box O; IGF, insulin-like growth factor; MAFbx, muscle atrogin F-box protein; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; PI3K, phosphatidylinositol 3-kinase; RDA, recommended dietary allowance; UP, ubiquitin proteasome.
Literature Cited
- 1.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68:375–88 [DOI] [PubMed] [Google Scholar]
- 2.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318:467–72 [DOI] [PubMed] [Google Scholar]
- 3.Stein TP, Rumpler WV, Leskiw MJ, Schluter MD, Staples R, Bodwell CE. Effect of reduced dietary intake on energy expenditure, protein turnover, and glucose cycling in man. Metabolism. 1991;40:478–83 [DOI] [PubMed] [Google Scholar]
- 4.Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argyiou E, Clifton PM. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am J Clin Nutr. 2003;78:31–9 [DOI] [PubMed] [Google Scholar]
- 5.Layman DK, Erickson DJ, Painter JE, Boileau RA, Shiue H, Sather C, Christou DD. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–7 [DOI] [PubMed] [Google Scholar]
- 6.Skov AR, Toubro S, Ronn B, Holm L, Astrup A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord. 1999;23:528–36 [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez NR, Garlick PJ. Introduction to Protein Summit 2007: exploring the impact of high-quality protein on optimal health. Am J Clin Nutr. 2008;87:1551S–3S [DOI] [PubMed] [Google Scholar]
- 8.Calloway DH. Nitrogen balance of men with marginal intakes of protein and energy. J Nutr. 1975;105:914–23 [DOI] [PubMed] [Google Scholar]
- 9.Waterlow JC. Metabolic adaptation to low intakes of energy and protein. Annu Rev Nutr. 1986;6:495–526 [DOI] [PubMed] [Google Scholar]
- 10.Young VR, Yu YM, Fukagawa NK. Protein and energy interactions throughout life. Metabolic basis and nutritional implications. Acta Paediatr Scand Suppl. 1991;373:5–24 [DOI] [PubMed] [Google Scholar]
- 11.Gordon MM, Bopp MJ, Easter L, Miller GD, Lyles MF, Houston DK, Nicklas BJ, Kritchevsky SB. Effects of dietary protein on the composition of weight loss in post-menopausal women. J Nutr Health Aging. 2008;12:505–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calloway DH, Spector H. Nitrogen balance as related to caloric and protein intake in active young men. Am J Clin Nutr. 1954;2:405–12 [DOI] [PubMed] [Google Scholar]
- 13.Agus MS, Swain JF, Larson CL, Eckert EA, Ludwig DS. Dietary composition and physiologic adaptations to energy restriction. Am J Clin Nutr. 2000;71:901–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gougeon R, Hoffer LJ, Pencharz PB, Marliss EB. Protein metabolism in obese subjects during a very-low-energy diet. Am J Clin Nutr. 1992; 56(1, Suppl)249S–54S [DOI] [PubMed] [Google Scholar]
- 15.Pasiakos SM, Mettel JB, West K, Lofgren IE, Fernandez ML, Koo SI, Rodriguez NR. Maintenance of resting energy expenditure after weight loss in premenopausal women: potential benefits of a high-protein, reduced-calorie diet. Metabolism. 2008;57:458–64 [DOI] [PubMed] [Google Scholar]
- 16.Pikosky MA, Smith TJ, Grediagin A, Castaneda-Sceppa C, Byerley L, Glickman EL, Young AJ. Increased protein maintains nitrogen balance during exercise-induced energy deficit. Med Sci Sports Exerc. 2008;40:505–12 [DOI] [PubMed] [Google Scholar]
- 17.Nair KS, Woolf PD, Welle SL, Matthews DE. Leucine, glucose, and energy metabolism after 3 days of fasting in healthy human subjects. Am J Clin Nutr. 1987;46:557–62 [DOI] [PubMed] [Google Scholar]
- 18.Pasiakos SM, Vislocky LM, Carbone JW, Altieri N, Konopelski K, Freake HC, Anderson JM, Ferrando AA, Wolfe RR, Rodriguez NR. Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults. J Nutr. 2010;140:745–51 [DOI] [PubMed] [Google Scholar]
- 19.Campbell WW, Haub MD, Wolfe RR, Ferrando AA, Sullivan DH, Apolzan JW, Iglay HB. Resistance training preserves fat-free mass without impacting changes in protein metabolism after weight loss in older women. Obesity (Silver Spring). 2009;17:1332–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villareal DT, Smith GI, Shah K, Mittendorfer B. Effect of weight loss on the rate of muscle protein synthesis during fasted and fed conditions in obese older adults. Obesity (Silver Spring). Epub 2011 Sep 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knapik J, Meredith C, Jones B, Fielding R, Young V, Evans W. Leucine metabolism during fasting and exercise. J Appl Physiol. 1991;70:43–7 [DOI] [PubMed] [Google Scholar]
- 22.Tsalikian E, Howard C, Gerich JE, Haymond MW. Increased leucine flux in short-term fasted human subjects: evidence for increased proteolysis. Am J Physiol. 1984;247:E323–7 [DOI] [PubMed] [Google Scholar]
- 23.Friedlander AL, Braun B, Pollack M, MacDonald JR, Fulco CS, Muza SR, Rock PB, Henderson GC, Horning MA, Brooks GA, et al. Three weeks of caloric restriction alters protein metabolism in normal-weight, young men. Am J Physiol Endocrinol Metab. 2005;289:E446–55 [DOI] [PubMed] [Google Scholar]
- 24.Yang RD, Matthews DE, Bier DM, Wen ZM, Young VR. Response of alanine metabolism in humans to manipulation of dietary protein and energy intakes. Am J Physiol. 1986;250:E39–46 [DOI] [PubMed] [Google Scholar]
- 25.Hoffer LJ, Forse RA. Protein metabolic effects of a prolonged fast and hypocaloric refeeding. Am J Physiol. 1990;258:E832–40 [DOI] [PubMed] [Google Scholar]
- 26.Phillips SM. Higher protein during an energy deficit: muscle's guardian and fat's enemy? Med Sci Sports Exerc. 2008;40:503–4 [DOI] [PubMed] [Google Scholar]
- 27.Rennie MJ, Tipton KD. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu Rev Nutr. 2000;20:457–83 [DOI] [PubMed] [Google Scholar]
- 28.Stipanuk MH. Leucine and protein synthesis: mTOR and beyond. Nutr Rev. 2007;65:122–9 [DOI] [PubMed] [Google Scholar]
- 29.Pasiakos SM, McClung JP. Supplemental dietary leucine and the skeletal muscle anabolic response to essential amino acids. Nutr Rev. 2011;69:550–7 [DOI] [PubMed] [Google Scholar]
- 30.Proud CG. Amino acids and mTOR signalling in anabolic function. Biochem Soc Trans. 2007;35:1187–90 [DOI] [PubMed] [Google Scholar]
- 31.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–80 [DOI] [PubMed] [Google Scholar]
- 32.Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68:5492–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang W, Zhu Z, Thompson HJ. Modulation of the activities of AMP-activated protein kinase, protein kinase B, and mammalian target of rapamycin by limiting energy availability with 2-deoxyglucose. Mol Carcinog. 2008;47:616–28 [DOI] [PubMed] [Google Scholar]
- 34.Reiter AK, Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. Repression of protein synthesis and mTOR signaling in rat liver mediated by the AMPK activator aminoimidazole carboxamide ribonucleoside. Am J Physiol Endocrinol Metab. 2005;288:E980–8 [DOI] [PubMed] [Google Scholar]
- 35.Doyle A, Zhang G, Abdel Fattah EA, Eissa NT, Li YP. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 2011;25:99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kettelhut IC, Pepato MT, Migliorini RH, Medina R, Goldberg AL. Regulation of different proteolytic pathways in skeletal muscle in fasting and diabetes mellitus. Braz J Med Biol Res. 1994;27:981–93 [PubMed] [Google Scholar]
- 37.Lundholm K, Ekman L, Karlberg I, Edstrom S, Schersten T. Comparison of hepatic cathepsin D activity in response to tumor growth and to caloric restriction in mice. Cancer Res. 1980;40:1680–5 [PubMed] [Google Scholar]
- 38.Sandri M. Autophagy in skeletal muscle. FEBS Lett. 2010;584:1411–6 [DOI] [PubMed] [Google Scholar]
- 39.Belkhou R, Bechet D, Cherel Y, Galluser M, Ferrara M, le Maho Y. Effect of fasting and thyroidectomy on cysteine proteinase activities in liver and muscle. Biochim Biophys Acta. 1994;1199:195–201 [DOI] [PubMed] [Google Scholar]
- 40.Costelli P, Reffo P, Penna F, Autelli R, Bonelli G, Baccino FM. Ca(2+)-dependent proteolysis in muscle wasting. Int J Biochem Cell Biol. 2005;37:2134–46 [DOI] [PubMed] [Google Scholar]
- 41.Parkes SC, Belcastro AN, McCargar LJ, McKenzie D. Effect of energy restriction on muscle function and calcium stimulated protease activity in recreationally active women. Can J Appl Physiol. 1998;23:279–92 [DOI] [PubMed] [Google Scholar]
- 42.Attaix D, Taillandier D, Temparis S, Larbaud D, Aurousseau E, Combaret L, Voisin L. Regulation of ATP-ubiquitin-dependent proteolysis in muscle wasting. Reprod Nutr Dev. 1994;34:583–97 [DOI] [PubMed] [Google Scholar]
- 43.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79 [DOI] [PubMed] [Google Scholar]
- 44.Demartino GN, Gillette TG. Proteasomes: machines for all reasons. Cell. 2007;129:659–62 [DOI] [PubMed] [Google Scholar]
- 45.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem. 1996;271:26690–7 [DOI] [PubMed] [Google Scholar]
- 46.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801 [DOI] [PubMed] [Google Scholar]
- 47.Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428 [DOI] [PubMed] [Google Scholar]
- 49.Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 2004;15:1537–45 [DOI] [PubMed] [Google Scholar]
- 50.Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve. 2006;33:155–65 [DOI] [PubMed] [Google Scholar]
- 51.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO's road. Sci STKE. 2003;(172):RE5. [DOI] [PubMed] [Google Scholar]
- 52.Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem. 2005;280:2737–44 [DOI] [PubMed] [Google Scholar]
- 53.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403 [DOI] [PubMed] [Google Scholar]
- 54.Mansoor O, Beaufrere B, Boirie Y, Ralliere C, Taillandier D, Aurousseau E, Schoeffler P, Arnal M, Attaix D. Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci U S A. 1996;93:2714–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiao G, Hobler S, Wang JJ, Meyer TA, Luchette FA, Fischer JE, Hasselgren PO. Sepsis is associated with increased mRNAs of the ubiquitin-proteasome proteolytic pathway in human skeletal muscle. J Clin Invest. 1997;99:163–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bossola M, Muscaritoli M, Costelli P, Grieco G, Bonelli G, Pacelli F, Rossi Fanelli F, Doglietto GB, Baccino FM. Increased muscle proteasome activity correlates with disease severity in gastric cancer patients. Ann Surg. 2003;237:384–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urso ML, Chen YW, Scrimgeour AG, Lee PC, Lee KF, Clarkson PM. Alterations in mRNA expression and protein products following spinal cord injury in humans. J Physiol. 2007;579:877–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wohlgemuth SE, Lees HA, Marzetti E, Manini TM, Aranda JM, Daniels MJ, Pahor M, Perri MG, Leeuwenburgh C, Anton SD. An exploratory analysis of the effects of a weight loss plus exercise program on cellular quality control mechanisms in older overweight women. Rejuvenation Res. 2011;14:315–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Layman DK. Protein quantity and quality at levels above the RDA improves adult weight loss. J Am Coll Nutr. 2004; 23(6, Suppl)631S–6S [DOI] [PubMed] [Google Scholar]
- 60.Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care. 2008;11:222–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pasiakos SM, McClung HL, McClung JP, Margolis LM, Andersen NE, Cloutier GJ, Pikosky MA, Rood JC, Fielding RA, Young AJ. Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am J Clin Nutr. 2011;94:809–18 [DOI] [PubMed] [Google Scholar]
- 62.Hoffer LJ, Taveroff A, Robitaille L, Hamadeh MJ, Mamer OA. Effects of leucine on whole body leucine, valine, and threonine metabolism in humans. Am J Physiol. 1997;272:E1037–42 [DOI] [PubMed] [Google Scholar]
- 63.Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol. 1992;263:E928–34 [DOI] [PubMed] [Google Scholar]
- 64.Nakashima K, Ishida A, Yamazaki M, Abe H. Leucine suppresses myofibrillar proteolysis by down-regulating ubiquitin-proteasome pathway in chick skeletal muscles. Biochem Biophys Res Commun. 2005;336:660–6 [DOI] [PubMed] [Google Scholar]
- 65.Sadiq F, Hazlerigg DG, Lomax MA. Amino acids and insulin act additively to regulate components of the ubiquitin-proteasome pathway in C2C12 myotubes. BMC Mol Biol. 2007;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.FAO/WHO/UNU Energy and Protein Requirements: Report of an FAO/WHO/UNU Expert Consultation. WHO Tech Rept Ser 1985;724. [PubMed] [Google Scholar]
- 67.Kurpad AV, Raj T, El-Khoury A, Kuriyan R, Maruthy K, Borgonha S, Chandukudlu D, Regan MM, Young VR. Daily requirement for and splanchnic uptake of leucine in healthy adult Indians. Am J Clin Nutr. 2001;74:747–55 [DOI] [PubMed] [Google Scholar]
- 68.Young VR, Borgonha S. Nitrogen and amino acid requirements: the Massachusetts Institute of Technology amino acid requirement pattern. J Nutr. 2000;130:1841S–9S [DOI] [PubMed] [Google Scholar]
- 69.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010;140:1970–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murgas Torrazza R, Suryawan A, Gazzaneo MC, Orellana RA, Frank JW, Nguyen HV, Fiorotto ML, El-Kadi S, Davis TA. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J Nutr. 2010;140:2145–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrando AA, Williams BD, Stuart CA, Lane HW, Wolfe RR. Oral branched-chain amino acids decrease whole-body proteolysis. JPEN J Parenter Enteral Nutr. 1995;19:47–54 [DOI] [PubMed] [Google Scholar]
- 72.Louard RJ, Barrett EJ, Gelfand RA. Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin Sci. 1990;79:457–66 [DOI] [PubMed] [Google Scholar]
- 73.Louard RJ, Barrett EJ, Gelfand RA. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism. 1995;44:424–9 [DOI] [PubMed] [Google Scholar]