Abstract
The vitamin K–dependent carboxylase uses vitamin K oxygenation to drive carboxylation of multiple glutamates in vitamin K–dependent proteins, rendering them active in a variety of physiologies. Multiple carboxylations of proteins are required for their activity, and the carboxylase is processive, so that premature dissociation of proteins from the carboxylase does not occur. The carboxylase is unique, with no known homology to other enzyme families, and structural determinations have not been made, rendering an understanding of catalysis elusive. Although a model explaining the relationship of oxygenation to carboxylation had been developed, until recently almost nothing was known of the function of the carboxylase itself in catalysis. In the past decade, discovery and analysis of naturally occurring carboxylase mutants has led to identification of functionally relevant residues and domains. Further, identification of nonmammalian carboxylase orthologs has provided a basis for bioinformatic analysis to identify candidates for critical functional residues. Biochemical analysis of rationally chosen carboxylase mutants has led to breakthroughs in understanding vitamin K oxygenation, glutamate carboxylation, and maintenance of processivity by the carboxylase. Protein carboxylation has also been assessed in vivo, and the intracellular environment strongly affects carboxylase function. The carboxylase is an integral membrane protein, and topological analysis, coupled with biochemical determinations, suggests that interaction of the carboxylase with the membrane is an important facet of function. Carboxylase homologs, likely acquired by horizontal transfer, have been discovered in some bacteria, and functional analysis of these homologs has the potential to lead to the discovery of new roles of vitamin K in biology.
Introduction
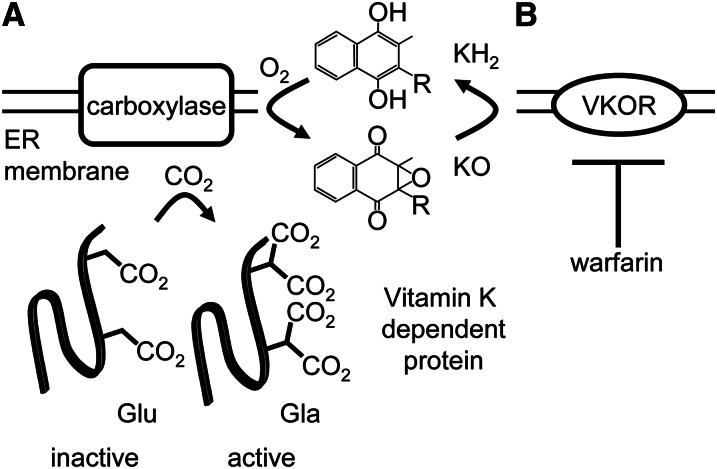
The vitamin K–dependent (VKD)3 carboxylase is a bifunctional enzyme that catalyzes the oxygenation of vitamin K hydroquinone, resulting in formation of vitamin K epoxide, in parallel with the carboxylation of multiple glutamate (Glu) residues in vitamin K–dependent proteins, with resulting formation of γ-carboxyglutamate (Gla) (Fig. 1A). The carboxylase, an integral membrane protein, is localized at the endoplasmic reticulum (ER) membrane, where lumenal VKD protein carboxylation takes place as part of the protein secretion pathway. For some time, the only known VKD proteins were involved in coagulation, undergoing carboxylation primarily in the liver. However, the carboxylase appears to be ubiquitous, and a growing number of VKD proteins have been discovered with involvement in an array of physiologies beyond hemostasis such as apoptosis, calcium homeostasis, signal transduction, and growth control. Currently, known VKD proteins comprise a family of 16 members (Table 1), and the carboxylase is capable of recognizing this family of proteins and no others. The carboxylase is autosomal and has no homology to any known enzyme families, so that functional comparisons with related enzymes are not possible. The reactions catalyzed by the carboxylase are also each unique in biochemistry, making an understanding of this enzyme both critical and rewarding.
Figure 1.
The vitamin K cycle supports vitamin K–dependent (VKD) protein carboxylation. A, The carboxylase uses oxygenation of vitamin K hydroquinone (KH2) to drive multiple carboxylation of VKD proteins in the endoplasmic reticulum (ER) lumen, conferring activity on them. B, The vitamin K epoxide produced by the carboxylase is reduced by vitamin K epoxide reductase (VKOR) to regenerate vitamin K hydroquinone, which is required for continual carboxylase activity. VKOR is inhibited by warfarin, so that vitamin K hydroquinone levels are decreased, impairing carboxylase activity and in turn decreasing the levels of active VKD proteins including those involved in hemostasis. Gla, γ-carboxyglutamate; Glu, glutamate.
Table 1.
Known VKD proteins are involved in many physiologies1
| VKD proteins involved in hemostasis | VKD proteins with nonhemostatic function |
| Prothrombin | Matrix Gla protein |
| Factor VII | Osteocalcin |
| Factor IX | TMG 1 |
| Factor X | TMG 2 |
| Protein Z | TMG 3 |
| Protein S | TMG 4 |
| Protein C | Gla-rich protein |
| Gas6 | |
| Periostin |
Proteins C and S have both hemostatic and nonhemostatic function. Gla, γ-carboxyglutamate; TMG, transmembrane Gla protein; VKD, vitamin K–dependent.
Multiple Glu carboxylations are required for the activity of VKD proteins. Gla formation results in creation of a cluster of bidentate ligands with calcium binding affinity. In the presence of calcium, Gla clusters form a binding network involving multiple calcium ions with a number of different ultimate functional consequences. Many VKD proteins involved in coagulation undergo a conformational change on calcium binding, exposing a membrane-insertion domain that targets the proteins to sites of vascular injury. Other VKD proteins are involved in the regulation of tissue calcification or are part of a structural lattice involving calcium, and in some cases the ultimate functional consequence of VKD protein carboxylation is not yet understood.
The importance of VKD protein carboxylation to function is the basis for anticoagulation therapy with warfarin. Carboxylase activity requires vitamin K hydroquinone, and continued supply of this substrate in vivo is provided by recycling of the vitamin K epoxide product by vitamin K epoxide reductase (VKOR) (Fig. 1B). Warfarin targets and inactivates VKOR, resulting in a decrease in vitamin K hydroquinone available to the carboxylase. Carboxylase activity is thus indirectly decreased during warfarin therapy, and VKD proteins, including those involved in coagulation, are not carboxylated to the required extent, impairing their procoagulant activity. However, the effects of warfarin therapy on other physiologies affected by VKD protein carboxylation are unclear, highlighting the importance of understanding the details of the function of the carboxylase.
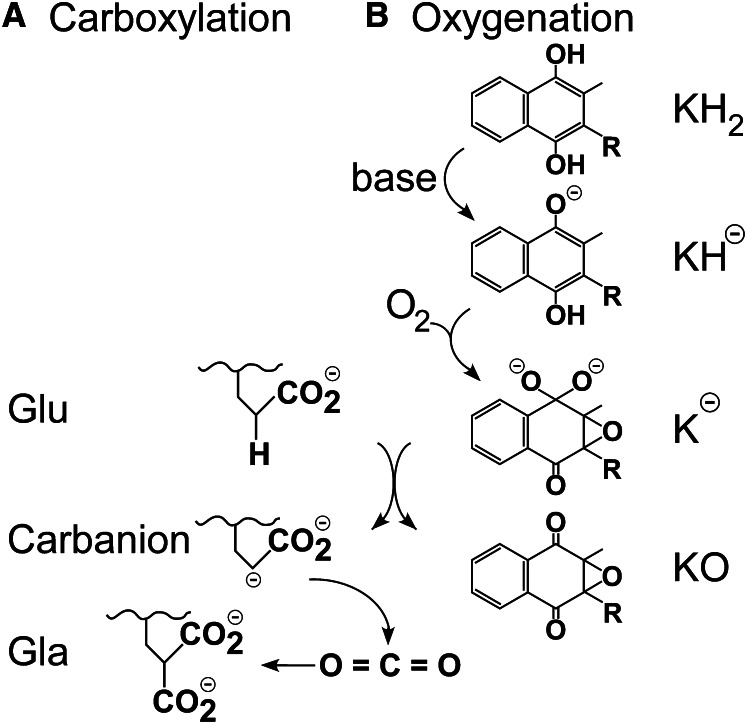
Although the significance of the Glu carboxylation activity has always been understood, that of vitamin K oxygenation was initially unclear. However, analysis of Glu carboxylation led to a proposed role for vitamin K oxygenation and a major breakthrough in understanding the carboxylase. Use of short Glu-containing peptide substrate analogs established that Glu underwent deprotonation at the γ carbon, leading to reaction of CO2 at that site to form Gla (Fig. 2A), as opposed to a mechanism involving abstraction of a hydrogen radical (1, 2). γ Carbon deprotonation would be extremely difficult, however, requiring action of a base far stronger than any possible enzyme active site residue. In a series of elegant, although nonenzymatic, experiments using a vitamin K model compound and 18O-labeled oxygen, Dowd et al. (3, 4) established a pathway for formation of the observed vitamin K epoxide product and showed that an intermediate on this pathway was a powerful base that could perform Glu deprotonation (Fig. 2B). Dowd et al. (3) then went on to perform similar experiments with the carboxylase, and the results of the analysis of the 18O-labeled vitamin K epoxide that was produced were consistent with their mechanism. Their proposed pathway, wherein the enzyme uses energy available from the oxygenation of vitamin K to perform a very difficult deprotonation and thus drive carboxylation is known as the Dowd model, and essentially all subsequent work on the mechanism of the carboxylase has been interpreted in light of this mechanism.
Figure 2.
Glutamate (Glu) deprotonation and carboxylation is accomplished by formation of a strong vitamin K base. A, Glu deprotonation, as opposed to radical formation, was established by experiments with Glu peptide analogs. Carboxylation could either occur in a stepwise process as shown, in which formation of a fully negatively charged carbanion is followed by reaction with CO2 to form γ-carboxyglutamate (Gla), or in a concerted process in which deprotonation and carboxylation are simultaneous and a free, high-energy carbanion is not formed. B, Deprotonation of vitamin K hydroquinone (KH2) by a weak active site base activates the quinone ring for reaction with oxygen, leading to formation of a very strong base species such as K− as shown. This strong base deprotonates Glu, resulting in formation of the vitamin K epoxide product (KO).
Although the Dowd model established the significance of vitamin K oxygenation, nothing was yet known about how the carboxylase itself contributes to catalysis. However, the studies of Dowd et al. (3, 4) established that vitamin K hydroquinone must be deprotonated for reaction with oxygen. Thus, the Dowd model requires that the carboxylase furnish a weak base residue in the active site to initiate vitamin K oxygenation. Subsequent steps on the oxygenation pathway do not appear to require catalysis, so that no further role of the enzyme before formation of the strong vitamin K base species is suggested by the Dowd model.
The carboxylase is a complex enzyme with a number of additional requirements beyond deprotonation of vitamin K hydroquinone. The carboxylase must provide an appropriate active site environment, ensuring that the strong vitamin K base is not protonated by water instead of Glu by exclusion of water from the active site. The carboxylase can also promote Glu deprotonation in a number of ways to support the action of the strong vitamin K base. Finally, as discussed previously, multiple Glu residues must be carboxylated on a single VKD protein for activity, and the carboxylase must provide a mechanism to ensure that VKD protein carboxylation achieves the threshold required for activity. Ultimately, proper control of the extent of VKD protein carboxylation is therefore as critical as oxygenation and carboxylation activity to VKD protein function.
Understanding the role of the carboxylase in catalysis has been especially challenging because the difficulties associated with large, integral membrane proteins have precluded direct structural determinations. In the absence of structural information, 2 approaches involving biochemical analysis have each led to major advances in understanding carboxylase function. The first source of information has been the discovery of several naturally occurring carboxylase mutants, which has in some cases led to defining the role of the mutated residues and to mapping of functional domains in the carboxylase. The second source of information has been rational creation and analysis of mutants. The discovery of carboxylase orthologs in nonmammalian organisms in recent years has been crucial in this regard. Functionally important amino acids must be conserved in active orthologs, and combining biochemical information with bioinformatics has been highly successful at identifying critical amino acids and demonstrating their role in catalysis. Thus, in recent years, understanding of the enzymology of the carboxylase has progressed from knowing only the chemical transformations undergone by the substrates to identifying regions of the carboxylase that interact with substrates, identifying residues that are involved in catalysis, and establishing the mechanism by which the carboxylase facilitates both of the linked reactions and maintains processive VKD protein carboxylation.
Current status
VKD protein–carboxylase interaction.
Understanding the interaction of the carboxylase with its substrates is especially important because of the requirement for complete carboxylation of multiple Glu residues in VKD proteins and the flexible yet specific nature of protein interaction in which 16 known VKD proteins must be carboxylated while all other proteins are excluded (Table 1). Specific protein binding is accomplished by carboxylase interaction with a VKD protein sequence, which is usually an 18-residue N-terminal propeptide that is removed in the Golgi apparatus after carboxylation and transit from the ER. Propeptides do not themselves undergo carboxylation and are thus bound at an exosite rather than the carboxylation active site. VKD protein propeptides show significant sequence variation; however, 3 residues are highly conserved, and substitution of any of these strongly impairs carboxylase binding (5). The affinities of the propeptide sequences alone, lacking the remainder of the VKD protein, for the carboxylase have been studied by direct measurement of the association/dissociation rates of fluorescently labeled peptides by anisotropy (6) and by determination of inhibition constants of propeptides for substrates containing a propeptide-linked Glu-containing sequence (7). Interestingly, the affinity of the propeptides for the carboxylase, except osteocalcin, varies from ∼3 to 300 nmol/L, a range of 2 orders of magnitude that reveals highly distinct interaction of VKD proteins with the carboxylase. The propeptide from osteocalcin contains a substitution for 2 of the 3 conserved residues and has a binding affinity of >0.5 mmol/L, and such poor propeptide binding affinity raises the question of how osteocalcin binding is accomplished.
The presence of a high-affinity carboxylase-binding propeptide sequence has implications for how extensive carboxylation of VKD proteins is accomplished. Two potential mechanisms for multiple Glu residue carboxylations exist: a distributive mechanism in which each VKD protein binds and dissociates from the carboxylase many times before Glu carboxylation is complete, or a processive mechanism in which all Glu residues are carboxylated as a result of 1 binding event. The carboxylase was shown to be processive in a challenge assay in which carboxylation of a preformed carboxylase/VKD complex was complete before carboxylation of an exogenous truncated VKD protein was initiated (8) and also by mass spectral analysis of peptide products (9). The tight binding conferred by propeptide sequences is likely to be critical to enforcing processivity.
Although the interaction of VKD protein propeptides and the carboxylase has been the subject of much study, another binding determinant was discovered when a region of the carboxylase was found to exhibit binding affinity for the mature forms (i.e., lacking propeptide) of prothrombin and factor IX (10). The existence of a second propeptide-independent VKD protein binding site, denoted vitamin K protein–interacting sequence (VKS), has manifold implications. Because propeptide binding varies widely among VKD proteins, a second site could allow proteins with weakly interacting propeptides to compete for carboxylase binding with other proteins exhibiting strongly bound propeptides. This could be especially important for osteocalcin because its propeptide binds only very weakly. VKD propeptides are found adjacent to Glu residues that undergo carboxylation, and propeptide binding thus holds these Glu residues in close proximity to the carboxylase. A second site of interaction could increase the rate of carboxylation by further restricting the mobility of Glu residues so that they are held near the active site, whereas regions of VKD proteins that do not undergo Glu residue carboxylation are not localized to the active site. The VKS motif is conserved in vertebrate carboxylases, and mutation of several conserved residues results in impairment of carboxylase activity with a small Glu-containing substrate. However, mutation of a conserved lysine-arginine dyad in this region did not affect the activity of either vitamin K oxygenation or Glu carboxylation in short peptides, raising the question of whether this dibasic pair contributes to processivity, the remaining critical property of the carboxylase. This hypothesis is particularly attractive because Glu carboxylation results in an increasing negative charge on protein substrates, and subsequent interaction with positively charged amino acids could neutralize the resulting charge density and/or contribute to triggering an active protein release mechanism.
The interactions of Glu at the carboxylation active site do not appear to contribute strongly to binding but are critical to carboxylase function. Short Glu-containing peptides that lack a linked propeptide exhibit Michaelis constants of ∼3 mmol/L, several orders of magnitude higher than propeptide dissociation constants (11). Weak Glu binding has been considered to be indicative of the lack of importance of interactions between the carboxylase and Glu undergoing carboxylation. However, short peptide inhibitors that differ only by the replacement of a methyl group for the labile proton have inhibition constants of ∼50 μmol/L, 100-fold lower than the Michaelis constants of the corresponding substrates (12). Inhibition constants are superior to Michaelis constants as a measurement of true binding affinity, especially for an enzyme as kinetically complex as the carboxylase. Thus, significant binding energy is present between Glu and the carboxylase active site.
An important property of the carboxylase is that it exhibits tight coupling or a 1:1 stoichiometry of vitamin K oxygenation and Glu carboxylation (13). Although the Dowd model clearly explains the dependence of Glu carboxylation on vitamin K oxygenation, it cannot explain the observation that the carboxylase is regulated such that oxygenation does not occur in the absence of Glu-containing substrates. Both free propeptides alone and short Glu-containing peptides can potentiate vitamin K oxygenation, so that interactions at the propeptide binding site and carboxylation active site each regulate coupling (13). Thus, enforcement of coupling is one example of the importance of interactions of Glu at the carboxylase active site.
Vitamin K–carboxylase interaction.
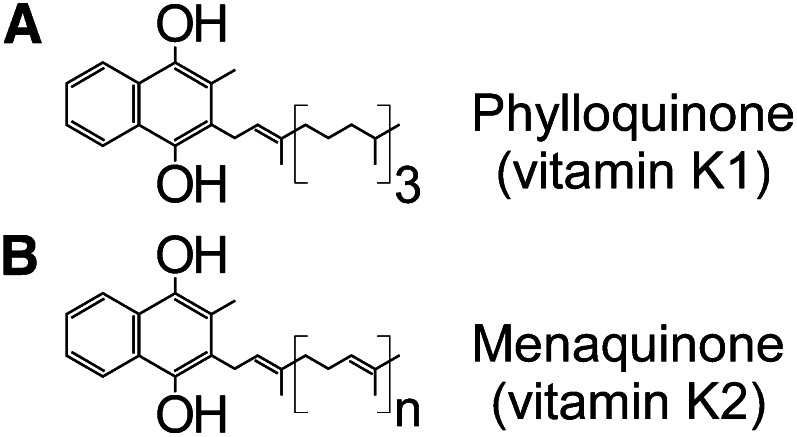
The issue of vitamin K interaction with the carboxylase is of especial interest because vitamin K exists as a family of vitamers with identical quinone cores but with variation in the length and saturation of the long hydrophobic isoprenoid tail (Fig. 3). Although the carboxylase appears to be ubiquitous in tissue, the relative levels of vitamin K family members in different tissues vary widely (14, 15). Additionally, expression of VKD proteins varies in different tissues, so that although carboxylation takes place in all tissues, the set of VKD proteins to be carboxylated is distinct. The conserved quinone nucleus confers catalytic competence for oxygenation on all family members, so that the variable tails might effect differential anchoring of vitamin K forms in the ER membrane where the carboxylase is bound and/or differential binding of vitamin K forms to the carboxylase. Cooperativity of vitamin K binding and truncated VKD proteins has been observed in vitro (16), as has similar catalytic activity with phylloquinone and various menaquinone forms (17). Thus, although all phylloquinone and menaquinone forms are competent for carboxylation, they may have differential cooperativity with VKD proteins. These observations raise the question of whether altered levels of vitamin K forms in different tissues is related to the requirement of carboxylation of distinct sets of VKD proteins in different tissues.
Figure 3.
Vitamin K exists as a family of vitamers. A, Phylloquinone (or vitamin K-1) consists of a quinone ring that confers vitamin K oxygenation activity and a long hydrophobic tail consisting of 4 isoprenoid units with a single double bond. B, Menaquinone (or vitamin K-2) retains the same quinone ring functionality but varies in the composition of the hydrophobic tail. Several menaquinone forms are known with different numbers of isoprenoid units, and each unit contains a double bond. The isoprenoid tails are not involved in oxygenation chemistry but could serve to bind the vitamers to the endoplasmic reticulum membrane where the carboxylase and VKOR are localized.
CO2 and O2 interaction with the carboxylase.
Interactions of the carboxylase with CO2 and O2 have generally not been studied, and almost nothing is known about the sites of binding of or functional interactions with these substrates. It is known that the presence of CO2 is not required for vitamin K oxygenation to take place; thus, CO2 is not a determinant of the coupling that is enforced by the carboxylase (18). An issue that has received little recent attention is that the carboxylase is almost unique among enzymes in exhibiting dioxygenase activity while having no known cofactors or metals that are required for activity (19). The lack of such a requirement raises the question of whether vitamin K oxygenation takes place with the triplet ground state of molecular oxygen, which is symmetry forbidden and therefore a high-energy process, or whether the carboxylase uses an unknown mechanism to provide the singlet excited state of oxygen. This issue has only been addressed computationally; Davis et al. (20) found that the Dowd pathway was energetically feasible but assumed that singlet oxygen was available, whereas Silva and Ramos (21) calculated that the Dowd pathway was feasible even with triplet oxygen. Dowd himself never addressed this question, and his oxygenation experiments with a model of vitamin K did not require inclusion of a provider of singlet oxygen. If the carboxylase is in fact a dioxygenase that uses triplet oxygen, it is almost alone among enzymes in doing so.
Naturally occurring carboxylase mutants.
The known importance of the carboxylase to hemostasis has led to the screening of patients with coagulation disorders for carboxylase variation and to the discovery of a number of naturally occurring carboxylase mutations, and these findings have been of great value in breaking ground for understanding carboxylase function. The first such mutant to be characterized was L394R, which was discovered in a patient homozygous for this variant and exhibited impaired substrate interaction at the Glu carboxylation active site (22, 23). Leu394 is located in a highly conserved region of the carboxylase, and further site-directed mutagenesis experiments led to the definition of this region as a Glu binding site (24) that is critical to function. A second recently discovered naturally occurring mutant is W501S, again discovered in a patient homozygous for this variant, which exhibited impaired binding to free propeptides in an in vitro assay (25, 26). In contrast, osteocalcin binding to W501S is not impaired, further supporting that the role of Trp501 is propeptide binding because the propeptide of osteocalcin is bound very weakly. In wild-type carboxylase, the presence of a propeptide sequence enhances the binding of both vitamin K and short Glu-containing peptides (27, 28). This effect was reduced in the W501S mutant, even when sufficiently high levels of propeptide were present to ensure that every mutant carboxylase was bound by propeptide. The observation of an effect on vitamin K binding is of interest because Trp501 is 1 of 3 known naturally occurring tryptophan mutations, a suspiciously high occurrence that raises the question of the importance of tryptophan motifs in vitamin K binding such as aromatic stacking interactions.
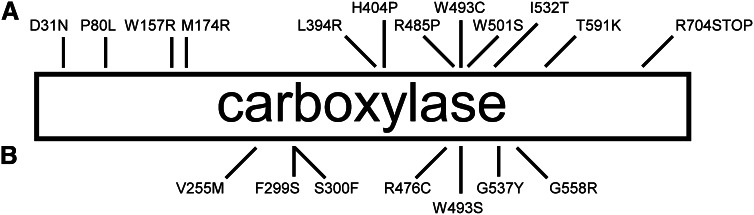
A further set of 3 carboxylase mutations was discovered in a patient with impaired hemostasis. The patient exhibited compound heterozygosity for the single mutant W157R and double mutant D31N/T591K, and each of the 3 single mutants were created and analyzed (29). The D31N mutation appeared to have no functional effect; however, the W157R variant exhibited only 10% of wild-type vitamin K oxygenation (and therefore carboxylation) activity, whereas the T591K mutant was completely inactive. Deficits of this magnitude implicate these residues as being critical to catalysis of vitamin K oxygenation, but their roles have not been elucidated in detail. Several naturally occurring mutants discovered in subjects with impaired hemostasis have not yet been analyzed (Fig. 4A) (30–32).
Figure 4.
Naturally occurring carboxylase mutants. A, Mutants discovered due to impaired hemostasis. The P80L, M174R, H404P, R485P, W493C, I532T, and R704STOP mutants have not yet been characterized. The D31N mutation appears to have no functional effect. The remaining mutants exhibit a variety of functional defects that have advanced understanding of the carboxylase. B, Mutants discovered due to the PXE-like phenotype. Only V255M and S300F have been characterized. Although the PXE-like phenotype is most prominent, mildly impaired hemostasis is generally also associated with these mutants. Interestingly, although W493C was identified due to impaired hemostasis, W493S was independently discovered due to PXE-like symptoms.
Recently, a new class of carboxylase mutants was discovered in patients whose primary phenotype is not impaired hemostasis, but rather abnormal skin folding along with other manifestations resulting from abnormal calcification of soft tissue (33, 34). This condition is nearly identical to that found in patients with pseudoxanthoma elasticum (PXE), which is caused by mutation of the abcc6 receptor, whose function is not known. The abcc6 receptor is primarily present in the liver, also the primary site of production of hemostatic VKD proteins. Knockout of the abcc6 gene in mice results in undercarboxylation of the VKD matrix Gla protein as well as tissue calcification (35). Because matrix Gla protein has been implicated in regulation of tissue calcification, these combined findings raise the question of whether the function of abcc6 is directly linked to VKD protein carboxylation. The finding of a similar phenotype resulting from carboxylase mutation represents a second genetic determinant for this disease. Elucidating the mechanism by which mutations in 2 seemingly unrelated proteins result in similar phenotypes has great potential to add to our understanding of the carboxylase and vitamin K biology.
Several carboxylase mutants have been identified in patients with PXE-like symptoms (Fig. 4B). An interesting observation is that, although the W493C mutant was discovered due to impaired hemostasis, the W493S mutant was discovered due to the PXE-like phenotype (Fig. 4A,B). The different phenotypes observed with the 2 Trp493 mutants must be manifested by differential effects on carboxylation of classes of VKD proteins and is therefore evidence of the exquisite nature and importance of carboxylase–VKD protein interaction.
Of the mutants identified in patients with the PXE-like phenotype, only V255M and S300F have been characterized (34). A patient exhibiting compound heterozygosity for these 2 mutants presented because of PXE-like symptoms and was then also found to have moderately impaired VKD coagulation factor function. Although the S300F variant has vitamin K oxygenation activity equivalent to that of wild-type, no carboxylation activity has been observed with any short peptide or VKD protein substrate. Thus, the V255M mutant must perform all VKD protein carboxylation in the affected patient. Analysis of the V255M mutant revealed that catalytic activity for both oxygenation and carboxylation was equivalent to wild type with short Glu-containing peptides in the presence of a VKD propeptide. However, removal of the VKD propeptide reduced carboxylation activity by 97% for the V255M mutant but only by 64% for wild-type (34). Subsequently, a decrease in the binding affinity for free factor X propeptide of ∼5-fold was observed (M. A. Rishavy, unpublished results). These observed differential propeptide interactions suggested that the V255M mutation might result in weakened VKD protein binding and thus in impaired processivity, so that the observed phenotype results from incomplete carboxylation of VKD proteins.
Processivity of the carboxylase.
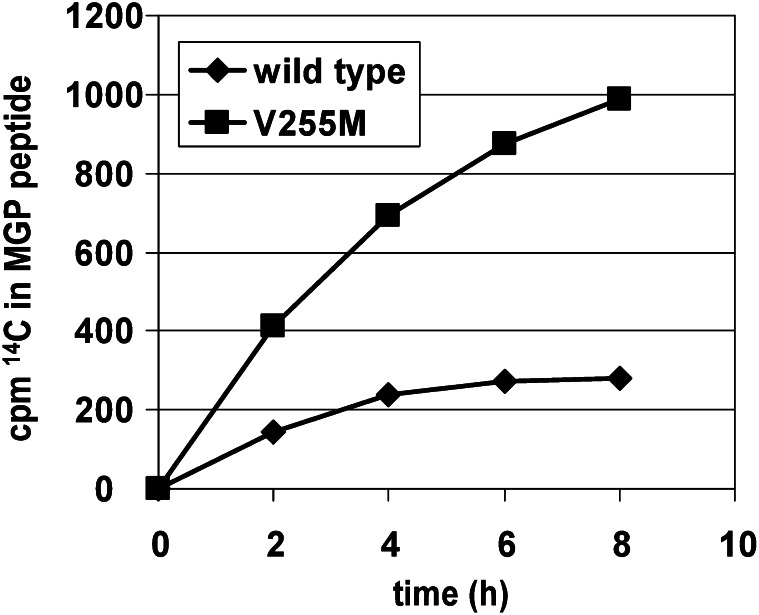
To assess processivity, assays with short Glu-containing peptides in the presence of VKD propeptides are not valid because the Glu-containing peptides are not tethered to the high-affinity binding determinant that promotes processivity and contain only 1 reactive Glu so that multiple carboxylation cannot be assessed. Indeed, short Glu-containing peptides undergo carboxylation ∼1–2 orders of magnitude faster than propeptide-linked Glu-containing peptides (9) because the former can freely exit the active site on carboxylation, whereas the latter remain bound to the carboxylase for as long as 2 h in vitro (36), blocking access of the active site to uncarboxylated peptides and decreasing the rate (Fig. 5) by preventing multiple turnovers. Accordingly, to assess processivity, the V255M mutant was assayed with 3 propeptide-linked Glu-containing substrates: the factor IX propeptide linked to the first 10 amino acids of the factor IX Gla domain, factor IX propeptide linked to the entire factor IX Gla domain, and the first 64 amino acids of matrix Gla protein (comprising all Glu residues that undergo carboxylation as well as the internal region homologous to propeptides). With each of these substrates, the carboxylase activity of V255M is ∼4-fold greater than that of wild-type (Fig. 6) (M. A. Rishavy, unpublished results), indicating that release of substrates from V255M is faster than from wild-type, allowing more cycles of binding, carboxylation, and release to occur. However, although total activity is greater with V255M, each individual substrate may be incompletely carboxylated as a result of the fast release, so that the endproduct is a VKD protein with impaired activity.
Figure 5.
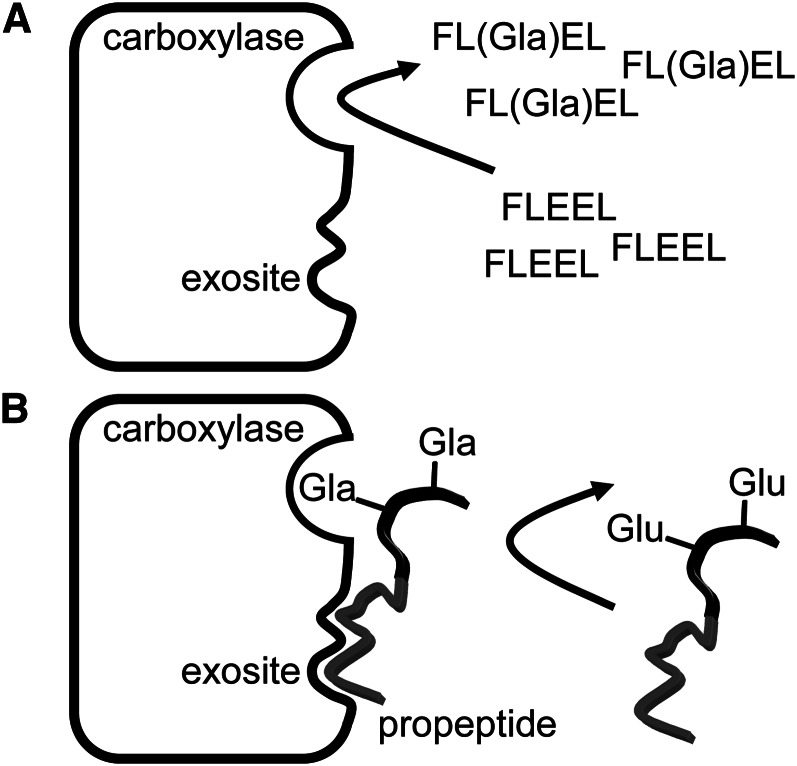
Carboxylase turnover is limited by dissociation of propeptide-containing substrates. A, Short glutamate (Glu)-containing peptides [such as the pentapeptide Phe-Leu-Glu-Glu-Leu (FLEEL) shown] that lack a propeptide have binding affinity several orders of magnitude weaker than substrates with a linked propeptide. However, in the presence of high levels of short peptides, high rates of carboxylation are observed as the peptides quickly dissociate from the carboxylase after reaction. B, Substrates containing a propeptide (in gray) are tightly bound to the carboxylase at an exosite, and thus only slowly dissociate from the carboxylase. Uncarboxylated substrate is thus unable to access the carboxylase, reducing the overall rate of carboxylation. However, lengthy residency of substrates on the carboxylase could promote processivity, providing time to complete Glu carboxylation before dissociation. Gla, γ-carboxyglutamate.
Figure 6.
The overall carboxylation rate of a peptide consisting of amino acids 1–64 of matrix γ-carboxyglutamate protein (MGP) is faster with the V255M mutant than with wild type. Almost identical results were observed with substrates containing the propeptide of factor IX linked to either the first 10 Gla domain residues of factor IX or to the entire Gla domain (data not shown). An increased carboxylation rate likely results from faster release of these substrates from V255M, which could lead to impaired processivity. Although overall turnover is faster with V255M, potentially incomplete carboxylation of vitamin K–dependent proteins would be detrimental due to loss of function. MGP, matrix gamma-carboxyglutamate protein.
The suggestion of impaired processivity with the V255M mutant has potential to reveal the functional defect of the mutant and implicate Val255 as promoting processivity, while also raising the question of why the primary observed phenotype is PXE-like tissue calcification and not a severe hemostasis defect. One possible explanation is that the effect on processivity is differential, so that carboxylase interaction with matrix Gla protein, which has been implicated in the regulation of tissue calcification (37), is more strongly affected than interaction with VKD proteins involved in hemostasis. However, the nearly identical increase in the overall carboxylation rate with the truncated forms of factor IX and matrix Gla protein with the mutant do not support this hypothesis and instead suggest that the presence of a certain percentage of undercarboxylated VKD proteins of all types may be less harmful to hemostasis than to regulation of tissue calcification.
The carboxylase sequence contains a number of occurrences of basic amino acids in close proximity, and interaction of these charged patches with VKD proteins, which contain patches of Glu that become carboxylated, was investigated by site-directed mutagenesis of individual patches to alanine (38). Interestingly, several of the mutants, including R234A/H235A, R406A/H408A, and R513A/K515A, exhibited identical carboxylation of a short Glu-containing peptide as a wild type, but when a substrate consisting of the propeptide and first 10 Gla domain amino acids of prothrombin was used, each of these mutants exhibited 3-fold faster carboxylation and 3- to 7-fold weakened binding versus wild type (38). These results are strikingly similar to those observed with the V255M mutant and suggest a role for the charged patches in maintaining processivity.
Processivity can be accomplished by either a passive mechanism, in which VKD proteins are simply bound so tightly that the release rate is much slower than the carboxylation rate, or by an active mechanism, in which carboxylation of VKD proteins is a trigger that promotes release. The disadvantage of a passive mechanism is that to ensure sufficient carboxylation, release of proteins would have to be slow enough so as to actually limit the production of VKD proteins. An active mechanism would allow release to occur on and only on carboxylation to the extent required, promoting overall production while ensuring VKD protein activity. The existence of both a propeptide binding site and a second VKD protein interacting site, as described previously, suggests that the VKD protein region undergoing carboxylation is tightly tethered near the carboxylase active site and has restricted mobility; carboxylation of several Glu residues would therefore lead to a buildup of negative-charge density that would have limited ability to delocalize. However, interaction of Gla residues with the numerous positively charged patches on the carboxylase could neutralize the developing density of negative charge, avoiding destabilization of the carboxylase–VKD protein complex. Release could then be accomplished in an active manner in at least 2 ways: interactions with sufficient numbers of carboxylase basic residues by Gla residues could trigger a conformational change that greatly reduces VKD propeptide binding or, alternatively, the number of carboxylase basic amino acids could be limited such that on complete carboxylation of a VKD protein, the last several Gla residues are not paired, resulting in a destabilizing concentration of negative charge and promoting dissociation. This hypothesis is further supported by the finding that one such carboxylase basic patch, Lys346/Arg347, is found within the region that interacts with the mature forms of VKD proteins and not with VKD protein propeptides, as discussed previously (10). Mutation of this basic pair does not affect carboxylation of short peptides, but activity with propeptide-linked substrates has not been assessed.
Autocarboxylation of the carboxylase.
An intriguing yet unexplained property of the carboxylase is that it is capable of autocarboxylation (39). Carboxylase carboxylation occurs both in vitro and in vivo, and kinetic data suggest that autocarboxylation occurs in cis. A potential role of autocarboxylation would be involvement in modulating processivity. Thus, autocarboxylation results in accumulation of negative charge on the carboxylase, which, if proximal to VKD proteins undergoing carboxylation, would destabilize the complex, promoting release. However, such a motif would likely require decarboxylase activity to regenerate the carboxylase with the requisite VKD binding affinity, and no such activity has been identified. An understanding of these phenomena has great potential for breaking new ground in the understanding of the carboxylase.
Nonmammalian carboxylases.
The carboxylase was for some time thought to occur only in vertebrates and is involved in physiologies such as hemostasis that are not relevant for many lower organisms. It has been of great interest, then, that carboxylase orthologs have been discovered in Conus and Drosophila (40, 41). The Conus and Drosophila carboxylases are fully active for both vitamin K oxygenation and carboxylation of small Glu-containing peptide substrates, and known functional regions such as the Glu-interacting region around Leu394 are conserved (although the Drosophila carboxylase lacks VKS) (10). However, the VKD substrates for Conus are distinct from mammalian VKD proteins: they are relatively short peptides with few Glu residues and their propeptides are not highly homologous, although they clearly target the peptides to the carboxylase (42). The Drosophila VKD substrates have not yet been identified and the function of protein carboxylation is therefore not known.
Carboxylase homologs have also recently been identified in a small number of bacteria, and the absence of the carboxylase in organisms on the evolutionary pathway between bacteria and mammals strongly suggests that these homologs were acquired by horizontal transfer from the genome of a host organism (43, 44). In contrast to the Conus and Drosophila carboxylases, functional regions such as the Glu binding domain are not highly conserved in bacterial homologs. Only 1 bacterial carboxylase, from Leptospira borgpetersenii, has been characterized, and it was found to have vitamin K oxygenation activity equivalent to that of human carboxylase, but no detectable carboxylation activity, consistent with the loss of known Glu-interacting regions (45). The bacterial homologs therefore may use vitamin K oxygenation for another unknown purpose rather than to effect Glu carboxylation. However, because the Leptospira homolog is an active oxygenase, it must retain amino acids that are required for this activity, and sequence alignment of the Leptospira homolog with mammalian carboxylases has been of great value for the identification of functional amino acids.
Catalysis of vitamin K oxygenation by the carboxylase.
The body of knowledge presented here has provided a basis for rational creation of carboxylase mutants, and analysis of these mutants has led to major advances in understanding the carboxylase in the past several years. The most critical advance has been the identification of the active site base that deprotonates vitamin K hydroquinone. Inactivation of the carboxylase by amino acid–specific reagents such as N-ethylmaleimide had suggested that the base might be a cysteine (11), and experiments had been performed in attempts to identify such a cysteine (46). However, subsequent mutagenesis experiments established that even mutation of all 10 carboxylase cysteines did not eliminate vitamin K oxygenation activity, overturning a long-held hypothesis that a cysteine acted as the base (47). This finding, in conjunction with further chemical inactivation experiments, suggested lysine or histidine as a candidate for the base. Alignment of the active Leptospira homolog with mammalian orthologs revealed only 3 histidines, including His160, and 1 lysine, Lys218, that were conserved. A series of mutants were created with replacement of the candidate amino acids with alanine, which has no ability to act as a base, so that replacement of the active site base should completely inactivate the carboxylase. Although all 3 histidine mutants retained wild-type vitamin K oxygenation activity, the K218A mutant was found to be completely inactive for vitamin K oxygenation and thus also for carboxylation (48).
Inactivation of the carboxylase by the K218A mutation revealed that Lys218 was critical, but did not alone establish that Lys218 deprotonates vitamin K hydroquinone. To establish that the role of Lys218 was in fact to act as a base, the K218A mutant was rescued with small amines, which can enter the active site and fill the gap created by replacement of a lysine with alanine. The ability of the amines to rescue enzyme activity could then be correlated with their properties, and it was found that rescue was dependent on the basicity of the amines, with a correction factor applied for their size (48). This result demonstrated not only that Lys218 is indispensable for catalysis, but that its role is to act as a base, and the necessity for application of a molecular volume correction revealed that the rescuing amines are indeed filling the active site gap present in the K218A mutant.
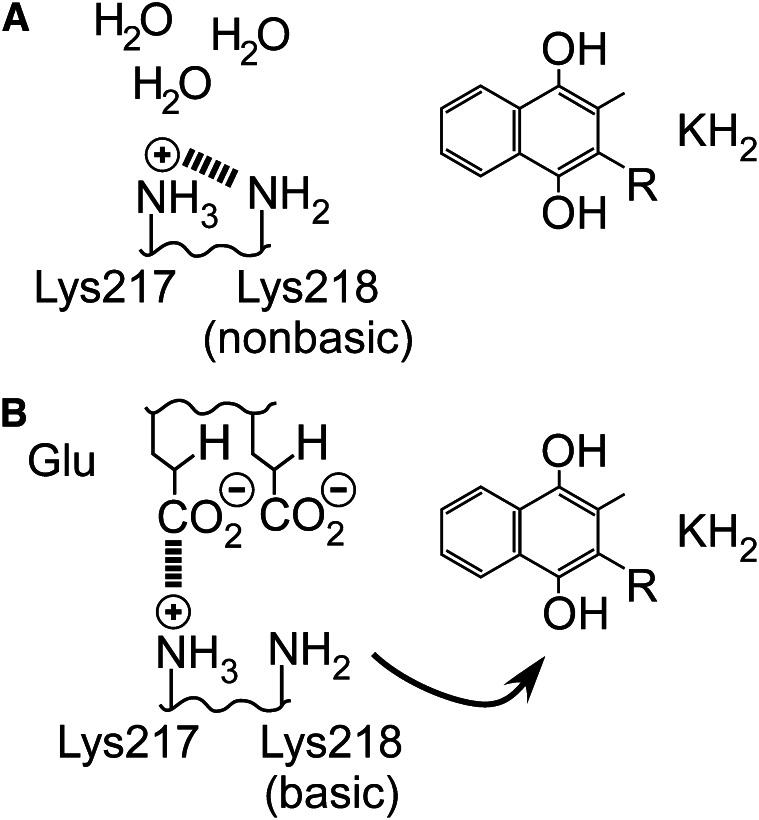
The use of a lysine as a base requires a mechanism to maintain deprotonated lysine, which would otherwise be protonated at physiological pH and unable to act as a base. Several relevant motifs are known in enzymology, but the most attractive for the carboxylase is that used by acetoacetate decarboxylase, which places another lysine immediately adjacent to the basic lysine (49). The localization of another positive charge reduces the affinity of lysine for a proton, allowing it to remain unprotonated. The carboxylase also contains a conserved lysine, Lys217, immediately adjacent to the basic lysine. Use of a lysine dyad is especially attractive for the carboxylase because it potentially explains the observation that many of the Glu residues in VKD proteins that are carboxylated form sequential pairs, whereas short peptides also show markedly higher activity when Glu pairs are present. Thus, on active site entrance, 1 Glu could interact with the positively charged Lys217, which would neutralize its charge and therefore its influence on Lys218 (Fig. 7). The carboxylase active site, which must exclude water for several reasons, would now protect Lys218 from protonation by solvent, and so instead it removes a proton from vitamin K hydroquinone. Vitamin K oxygenation would then also be impaired in the absence of Glu-containing substrates, which has been repeatedly observed (13), contributing to enforcement of coupling of oxygenation to carboxylation. Lys217 is not conserved in the Leptospira homolog, which is consistent with the loss of Glu carboxylation activity; a different mechanism must be present for modulating the basicity of Lys218 because Glu interaction does not occur.
Figure 7.
The basicity of Lys218 can be modulated by the active site environment. A, The influence of the adjacent Lys217 prevents Lys218 from becoming protonated by solvent when the active site is vacant and also prevents Lys218 from deprotonating KH2. B, When a glutamate (Glu) substrate enters the active site, any water present is displaced and excluded, and Glu interacts with Lys217, removing its influence on Lys218. The inherent basicity of Lys218 is then restored so that KH2 deprotonation takes place.
Catalysis of Glu carboxylation by the carboxylase.
Although assay of the H160A mutant revealed no effect on vitamin K oxygenation, a 10-fold decrease in Glu carboxylation along with a 5-fold weakening of CO2 binding was observed (50). This mutant was the first known example of an uncoupled carboxylase variant, in which the ratio of oxygenation to carboxylation was significantly higher than unity. His160 thus promotes catalysis of Glu carboxylation, and analysis of this mutant had great promise for shedding light on the role of the carboxylase in this reaction.
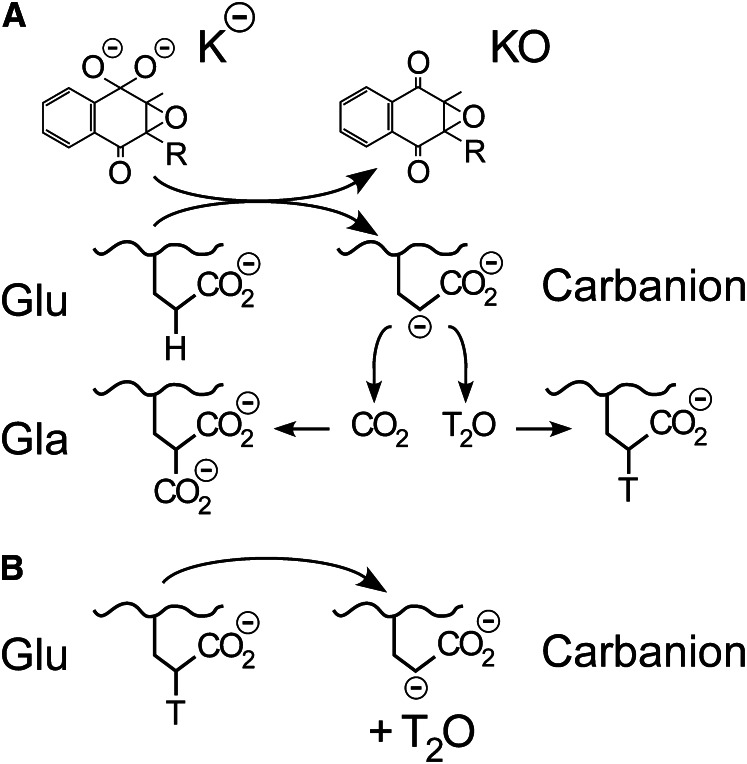
Examination of the potential mechanisms of Glu carboxylation suggested several possible catalytic roles for His160. Deprotonation of Glu by the strong vitamin K base (Fig. 2) could result in carboxylation either in a concerted manner, in which CO2 addition occurs simultaneously with deprotonation, or by a 2-step process in which a Glu carbanion intermediate is formed that then reacts with CO2. Previous experiments established that Glu deprotonation was independent of the presence of CO2 (51), which is inconsistent with a concerted mechanism and indicates a 2-step process. Additionally, in the absence of CO2, tritium incorporation from tritiated water into Glu was observed, indicating that a carbanion forms that is reprotonated by solvent when CO2 is unavailable (Fig. 8A) (2). These combined data resulted in acceptance of a 2-step mechanism of Glu carboxylation, and His160 could thus contribute to catalysis by promoting either Glu deprotonation to form a carbanion or the addition of CO2 to the carbanion.
Figure 8.
Tritium incorporation and tritium release experiments provide tools for analysis of catalysis of glutamate (Glu) carboxylation. A, Tritium incorporation experiments. In a 2-step process for carboxylation, a carbanion is formed independent of the presence of CO2. The carbanion can either then react with CO2, resulting in γ-carboxyglutamate (Gla) formation, or with tritiated water, resulting in re-formation of Glu with an incorporated tritium. In a concerted process, however, a discrete carbanion is never formed, and either carboxylation or tritium incorporation takes place simultaneously with Glu deprotonation. In this case, tritium incorporation will not be observed if water is excluded from the CO2 active site. B, Tritium release experiments. Peptides are synthesized with a tritium at the γ-carbon position, and loss of tritium from the peptide is then a direct determination of Glu deprotonation, regardless of whether a 2-step process involving a carbanion is used (as shown) or deprotonation is concerted. Thus, if a 2-step process is used, then tritium release experiments provide an assay of the first step in isolation.
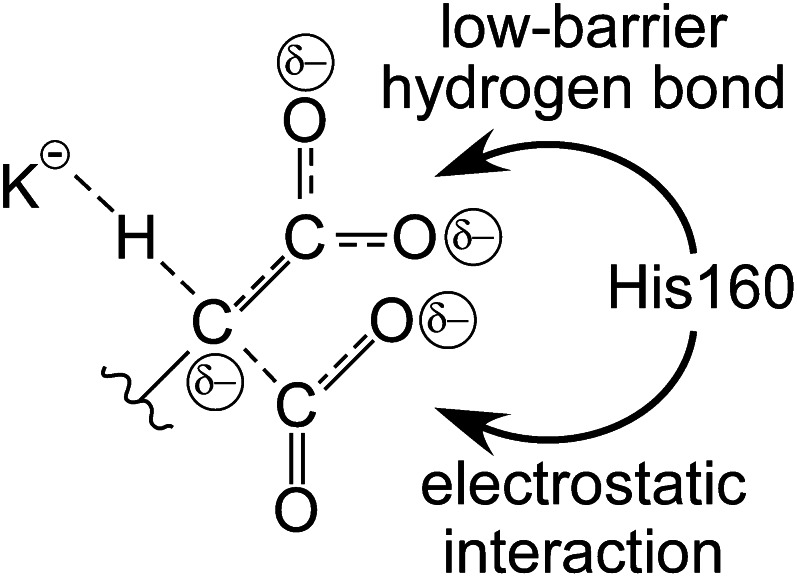
To identify the specific step in Glu carboxylation catalyzed by His160, short Glu-containing peptides with tritium at the γ carbon were synthesized and assayed for tritium release. Glu deprotonation results in labilization of tritium from the peptide (Fig. 8B), so that deprotonation can be determined directly and independently of whether the resulting carbanion undergoes carboxylation or reprotonation (Fig. 8A). His160 exhibited a 10-fold reduction in the release of tritium from Glu peptides versus wild type, indicating that His160 contributes to deprotonation of Glu by the strong vitamin K base (50). Glu deprotonation results in a negative charge appearing on the γ carbon, which can be delocalized into the γ-carboxyl group to form an aci-carboxylate resonance structure. Interaction of neutral histidines with aci-carboxylates in a number of enzymes results in the formation of a low-barrier hydrogen bond, an especially energetic interaction that can occur when the hydrogen bond donor and acceptor have closely matched pKa values. Thus, an attractive possibility for His160 catalysis of Glu deprotonation is the formation of a strong interaction with the γ-carboxyl group as a negative charge develops on Glu deprotonation.
Involvement of His160 in Glu deprotonation raised the question of the significance of the weakened CO2 binding observed with H160A. In a 2-step process, CO2 is not involved in Glu deprotonation, so that catalysis of deprotonation by His160 is inconsistent with the binding of CO2. These apparent contradictory findings prompted a re-examination of whether Glu carboxylation is in fact a concerted process, which could reconcile the conflicting observations regarding the specific role of His160. Although the previous experiments leading to acceptance of the 2-step mechanism had used microsomal preparations of the carboxylase, more recent experiments used affinity-purified carboxylase. Re-examination of the dependence of Glu deprotonation on CO2 using tritiated peptides (Fig. 8B) and wild-type enzyme revealed that with purified carboxylase, deprotonation has a profound dependence on CO2, which closely paralleled the formation of Gla (52). Further, as Glu deprotonation did not occur in the absence of CO2, tritium incorporation from tritiated water was barely detected (Fig. 8A). Thus, the accepted view of Glu carboxylation as a 2-step process has been overturned; Glu deprotonation in fact acts in concert with carboxylation and does not take place in the absence of CO2.
The conflicting determinations of the nature of the mechanism of Glu carboxylation can be reconciled by considering that the results with microsomal carboxylase are consistent with a concerted mechanism involving Glu reprotonation by water. Thus, Glu deprotonation must be supported by a species in the CO2 binding site that is capable of accepting the developing negative charge on the γ carbon, which could either be CO2, resulting in Gla formation, or water, resulting in reprotonation. However, for reasons that are not known, the purified carboxylase excludes water from the active site more completely, so that water-supported deprotonation is not observed. In contrast, with microsomal carboxylase, the CO2 site is accessible to water, so that concerted reprotonation by water can support Glu deprotonation. This interpretation is supported by the observation that cyanide, a known competitive inhibitor of CO2 (53), can support Glu deprotonation in affinity-purified carboxylase (50), acting in a manner similar to water in microsomal carboxylase.
Use of a concerted mechanism for Glu deprotonation contributes to catalysis by avoidance of a full negative charge, instead dispersing the charge into the incoming CO2 moiety as it develops. The catalytic role of His160 could then involve supporting Glu deprotonation by interaction with CO2, acting to neutralize the negative charge that appears as Glu is deprotonated (Fig. 9). Alternatively, in a concerted mechanism, His160 interaction with the original γ carboxyl group results in indirect binding of CO2, which is part of the transition state of the reaction, so that the exact site of interaction of His160 remains an open question.
Figure 9.
Concerted deprotonation and carboxylation catalyzes the reaction by dispersing negative charge. As CO2 interaction is simultaneous with deprotonation, some of the negative charge developing on the γ carbon initiates bond formation with CO2, which in turn results in the appearance of negative charge on the CO2 oxygens. Additionally, the original glutamate carboxyl group can accept some negative charge from the γ carbon by formation of an aci-carboxylate structure. His160 could interact with either the aci-carboxylate moiety via a low-barrier hydrogen bond or with the negative charge on the incoming CO2 via an electrostatic interaction to effect catalysis.
Analysis of VKD protein carboxylation in vivo.
Most of our understanding of the carboxylase has been obtained through in vitro experiments; however, the carboxylase acts in vivo during protein secretion, a complex process that involves a tremendous number of factors, many of which could affect the catalytic activity of the carboxylase. A critical factor is that protein carboxylation in cells is affected by the supply of vitamin K hydroquinone to the carboxylase by VKOR (Fig. 1B). Thus, an understanding of how carboxylase activity is modulated in vivo is especially critical because of the fact that the ultimate effect of warfarin is to impair VKD protein carboxylation, leading to undercarboxylation and thus inactivity of a portion of the secreted VKD proteins. Additionally, there is interest in overexpression of VKD proteins in cells for use as therapeutic agents, but such cells must be capable of properly carboxylating these proteins. A striking example of the potential importance of the in vivo environment is that VKD protein release after carboxylation in vitro required >2 h for turnover of a single molecule in 1 case (36), a rate that is far too slow to be physiologically relevant. In contrast, the total time for carboxylation and release of factor IX in cells is ∼4 min, almost 2 orders of magnitude faster (54). This observation alone strongly suggests that intracellular factors or environment contribute to VKD protein release and are therefore intimately related to regulating processivity.
The overexpression of VKD proteins in mammalian cells by itself does not result in increased secretion of carboxylated VKD proteins, which could be due to limitation of the activity of any step of the protein carboxylation pathway (55–57). To investigate this issue, coexpression of VKD proteins with either the carboxylase or VKOR has been studied (36, 54). Coexpression of recombinant carboxylase had the interesting effect of actually decreasing secretion of carboxylated VKD protein; instead, carboxylase–VKD protein complexes accumulated in baby hamster kidney (BHK) cells (36). Saturation of intracellular factors that promote release could explain this phenomenon, and one such factor could be the presence of free, uncarboxylated VKD proteins themselves because release of VKD proteins from the carboxylase is enhanced by VKD proteins as well as their propeptide sequences alone (36). Coexpression of VKOR and VKD protein did enhance secretion of carboxylated VKD protein, but only by 2-fold, even though the expression of active VKOR was increased 14-fold (54). This result indicates that the supply of hydroquinone does contribute to limiting VKD protein carboxylation, but another step must be almost as slow and limit carboxylation when VKOR activity is enhanced. Limitation in the presence of recombinant VKOR could result from slow regeneration of VKOR, which itself must undergo reduction after each recycling of vitamin K, or from carboxylated protein release becoming the slowest step.
An interesting contrast to the observations in BHK cells was recently presented involving overexpression of human factor IX in Drosophila cells, which contain a carboxylase ortholog of unknown function that lacks the region that interacts with mature VKD proteins (58). In these cells, the secretion of factor IX was about 10-fold higher than that obtained from Chinese hamster ovary cells, which have been used in attempts to prepare VKD proteins for therapeutic use. The secreted factor IX was found to be highly active in a coagulation assay, so that processivity in the Drosophila cells under these conditions is apparently not severely impaired. Additionally, coexpression of human carboxylase slightly increased the secretion of active factor IX. It is not clear why Drosophila cells were not limited in the same manner as BHK cells, but these findings have the potential to uncover the factors that affect VKD protein carboxylation in vivo, which add further layers to the complexities of carboxylase function.
Topology of the membrane-bound carboxylase.
Although the structure of the carboxylase is not known, bioinformatics and biochemical experiments have been performed to determine what regions of the carboxylase contribute to membrane binding. Identifying membrane-spanning regions would determine the topology of the carboxylase and reveal what regions of the carboxylase are present in the ER lumen, where they can interact with VKD proteins. Thus, carboxylase residues that are implicated in function would be expected to be present in the ER lumen, especially those engaging in direct substrate binding, although complex mechanisms such as those involving global conformational changes could make cytoplasmic residues functionally relevant.
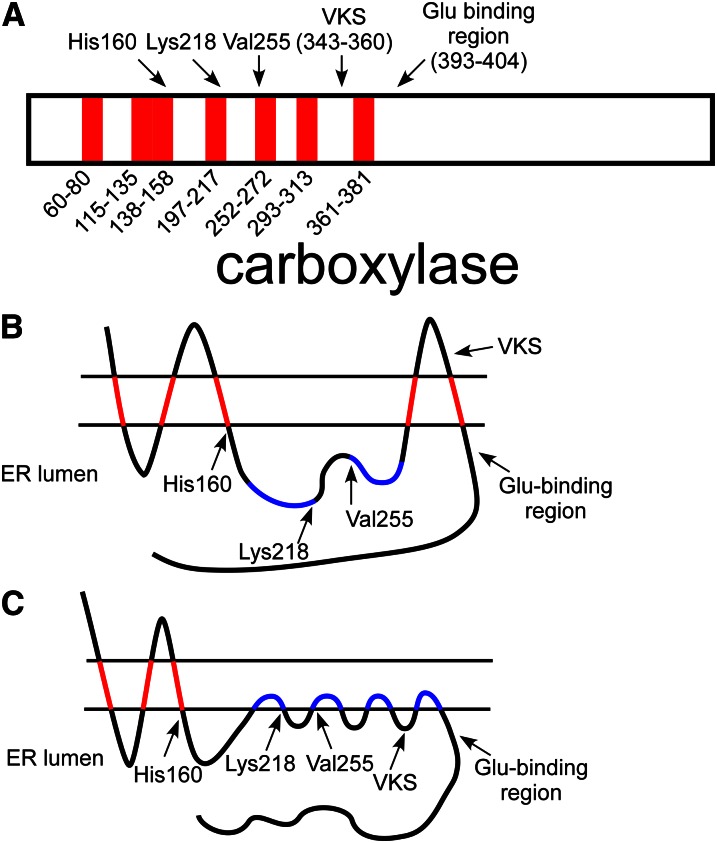
Computer analysis of the carboxylase has identified a number of candidate membrane-interacting regions in the carboxylase, which are concentrated in the N-terminal half of the enzyme (Fig. 10A). The ability of 7 candidate regions to confer membrane-binding in isolation was tested in vitro with a series of peptides containing a leader peptidase reporter tag linked to a sequence with 1 or 2 of the proposed transmembrane domains (59). Five of the 7 candidate domains were found to result in membrane association under these conditions, and, additionally, analysis of tagged full-length carboxylase established that the N-terminus is cytoplasmic, whereas the C-terminus is lumenal (which requires an odd number of transmembrane domains) (Fig. 10B). The membrane interaction of the seventh domain, in particular, is supported by the observation that expression of the C-terminal half of the carboxylase, which contains only this candidate domain, results in expression of a microsomal protein (60). However, this model places a putative transmembrane domain squarely between the conserved Glu binding region (which was established by creation of a number of site-directed mutants) and the VKS motif (which interacts directly with mature VKD proteins) (Fig. 10B). In this construct, the VKS motif is localized to the cytoplasm, across the membrane from the VKD proteins undergoing carboxylation. This apparent contradiction raises the question of whether some of the membrane-interacting regions are monotopic, i.e., they interact by shallow insertion into just 1 face of the ER membrane, instead of being transmembrane domains. Because monotopic regions do not span the membrane, carboxylase sequences immediately flanking the domain are on the same side of the membrane, allowing both of the 2 proximal carboxylase regions that interact with VKD protein substrates to be lumenal (Fig. 10C).
Figure 10.
Topological analysis of the carboxylase. A, Hydropathy analysis of the carboxylase identified 7 candidate transmembrane regions in the carboxylase sequence. Interestingly, several catalytic residues (His160, Lys218, and Val255) are found near the end of these regions. One of the candidate domains is between the vitamin K protein–interacting sequence (VKS) and the glutamate (Glu) binding region. B, An in vitro membrane insertion assay analyzed each domain in isolation and found that 5 of the candidate regions (in red) interacted with membranes, whereas 2 (in blue) did not. Analysis of full-length carboxylase found that the carboxylase N-terminus was cytoplasmic, whereas the C-terminus was lumenal, so that an odd number of transmembrane domains are required. The resulting model, however, places the VKS on the cytoplasmic side of the membrane, whereas the proteins with which this region interacts are lumenal. C, Several of the candidate transmembrane domains (e.g., those in blue) may instead be monotopic, so that they interact with membranes by shallow association with 1 face of the endoplasmic reticulum (ER) membrane. If the most C-terminal candidate region is a monotopic binding domain, then VKS and the Glu-binding region could both be present in the ER lumen for vitamin K–dependent (VKD) protein interaction. The model shown also places 3 residues known to be critical to function in the lumen at the end of membrane-binding regions, suggesting the importance of the membrane surface itself to carboxylase function.
The position of a number of naturally occurring mutants and residues known to be critical for catalysis with respect to potential membrane-interacting regions suggest that the membrane surface itself is important to carboxylase function. His160, which contributes to catalysis of Glu deprotonation (50), and Trp157, whose mutation results in impaired hemostasis (29), are both found at the end of a region predicted by computer models to interact with the membrane and found to bind to membranes by in vitro assay. Lys218, which deprotonates vitamin K hydroquinone and is indispensable to function, is located at the very end of a region predicted by several computer models to interact with membrane, although the candidate region alone did not result in membrane binding in the in vitro assay (59). This finding is of particular interest because active vitamin K forms all contain a long isoprenoid tail that could insert into the ER membrane (Fig. 3), resulting in an effective increase in the availability of vitamin K to the carboxylase as a result of this localization. Furthermore, vitamin K binding and Lys218 localization to the membrane surface would promote this required interaction, and the use of a hydrophobic membrane environment would help to exclude water from the vitamin K oxygenation site, avoiding protonation of both the Lys218 weak base and vitamin K strong base. The importance of the proposed electrostatic interactions between Lys218, Lys217, and Glu substrate are also enhanced in a hydrophobic environment. It is of interest to consider the possibility that membrane-interacting regions near the vitamin K binding site are also monotopic domains. Such a motif could result in the creation of a channel between domains, allowing entry of vitamin K, anchored in the ER membrane, to the active site while effectively excluding water. Additionally, the failure of the candidate membrane domain containing Lys218 to bind to membrane in the in vitro assay could then be due to the requirement that it interact with an adjacent monotopic domain for stable membrane binding.
The majority of naturally occurring carboxylase mutants, especially those discovered due to impaired hemostasis, are found beyond the most C-terminal proposed membrane binding domain, and almost no functionally important residues have yet been discovered in the most N-terminal 150 amino acids by any means, although several proposed membrane-interacting domains are found there. This may be an indication that the most N-terminal portion of the carboxylase does not contribute to catalysis, but rather to ER membrane localization. The lack of a known carboxylase structure greatly obscures understanding of the carboxylase, but highlights the importance of biochemical experiments as a critical source of information.
Conclusions
The advance of knowledge about carboxylase function in the past several years has seen critical findings that have for the first time begun to identify functional residues and regions and have detailed catalysis of Glu carboxylation. Equally exciting is that biochemical experiments have begun to address the issue of processivity, which is of paramount importance to the carboxylase because of the requirement for extensive carboxylation of VKD protein substrates. Although in vitro experiments have uncovered numerous aspects of function, factors that contribute to activity in vivo are not well-known, and differences between in vivo and in vitro experiments indicate that these factors are significant, especially with regard to VKD protein release. An especially interesting consideration of in vivo function is the mechanism of the exchange of vitamin K between the carboxylase and VKOR, which recycles the active form of vitamin K after oxygenation by the carboxylase. Because both enzymes and likely vitamin K as well are bound in the ER membrane, an attractive hypothesis is that the carboxylase and VKOR directly interact, perhaps by formation of a stable dimeric complex, to facilitate exchange of vitamin K forms. Direct interaction of the carboxylase with VKOR is supported by the observation that the presence of the carboxylase potentiates the ability of VKOR to interact with thioredoxin, resulting in regeneration of the VKOR vitamin K recycling activity (61). The question of carboxylase-VKOR interaction is an intriguing one that could revolutionize understanding of the vitamin K cycle.
The presence of carboxylase homologs in a number of bacteria is an open question that has not yet been addressed; indeed, it is not known whether any of these homologs have Glu carboxylation activity. The only bacterial homolog to be characterized, from Leptospira, performed vitamin K oxygenation but not carboxylation, raising the question of the function of this enzyme. The retention of His160, which contributes to stabilization of developing negative charge in mammalian carboxylases, by Leptospira suggests that this homolog uses vitamin K oxygenation to drive a different reaction involving negatively charged intermediates, and this issue has yet to be addressed. Alternatively, oxygenation of quinones that are chemically similar to vitamin K but structurally different has been observed in 2 different settings: in 1 case, the oxygenated quinone is an active antibiotic (62), whereas in the other, oxygenation is part of a biosynthetic pathway (63). Thus, bacterial carboxylase homologs may have adapted vitamin K oxygenation to other uses than to drive a second reaction. Determining the function of the bacterial carboxylase orthologs, as well as that from Drosophila, has the potential to open more new frontiers in both enzymology and vitamin K biology.
Acknowledgments
Both authors have read and approved the final manuscript.
Footnotes
Supported by funds from the National Institutes of Health (RO1 HL055666 to KLB).
Author disclosures: M. Rishavy and K. Berkner, no conflicts of interest.
Abbreviations used: BHK, baby hampster kidney; ER, endoplasmic reticulum; Gla, γ-carboxyglutamate; Glu, glutamate; PXE, pseudoxanthoma elasticum; VKD, vitamin K–dependent; VKOR, vitamin K epoxide reductase; VKS, vitamin K protein–interacting sequence.
Literature Cited
- 1.Vidal-Cros A, Gaudry M, Marquet A. Vitamin K-dependent carboxylation. Mechanistic studies with 3-fluoroglutamate-containing substrates. Biochem J. 1990;266:749–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton DL, Friedman PA. Fate of the activated gamma-carbon-hydrogen bond in the uncoupled vitamin K-dependent gamma-glutamyl carboxylation reaction. J Biol Chem. 1983;258:14084–7 [PubMed] [Google Scholar]
- 3.Dowd P, Ham SW, Naganathan S, Hershline R. The mechanism of action of vitamin K. Annu Rev Nutr. 1995;15:419–40 [DOI] [PubMed] [Google Scholar]
- 4.Dowd P, Hershline R, Ham SW, Naganathan S. Vitamin K and energy transduction: a base strength amplification mechanism. Science. 1995;269:1684–91 [DOI] [PubMed] [Google Scholar]
- 5.Furie B, Bouchard BA, Furie BC. Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood. 1999;93:1798–808 [PubMed] [Google Scholar]
- 6.Presnell SR, Tripathy A, Lentz BR, Jin DY, Stafford DW. A novel fluorescence assay to study propeptide interaction with gamma-glutamyl carboxylase. Biochemistry. 2001;40:11723–33 [DOI] [PubMed] [Google Scholar]
- 7.Stanley TB, Jin DY, Lin PJ, Stafford DW. The propeptides of the vitamin K-dependent proteins possess different affinities for the vitamin K-dependent carboxylase. J Biol Chem. 1999;274:16940–4 [DOI] [PubMed] [Google Scholar]
- 8.Stenina O, Pudota BN, McNally BA, Hommema EL, Berkner KL. Tethered processivity of the vitamin K-dependent carboxylase: factor IX is efficiently modified in a mechanism which distinguishes Gla's from Glu's and which accounts for comprehensive carboxylation in vivo. Biochemistry. 2001;40:10301–9 [DOI] [PubMed] [Google Scholar]
- 9.Morris DP, Stevens RD, Wright DJ, Stafford DW. Processive post-translational modification. vitamin K-dependent carboxylation of a peptide substrate. J Biol Chem. 1995;270:30491–8 [DOI] [PubMed] [Google Scholar]
- 10.Pudota BN, Hommema EL, Hallgren KW, McNally BA, Lee S, Berkner KL. Identification of sequences within the gamma-carboxylase that represent a novel contact site with vitamin K-dependent proteins and that are required for activity. J Biol Chem. 2001;276:46878–86 [DOI] [PubMed] [Google Scholar]
- 11.Suttie JW. Vitamin K-dependent carboxylase. Annu Rev Biochem. 1985;54:459–77 [DOI] [PubMed] [Google Scholar]
- 12.Gaudry M, Bory S, Dubois J, Azerad R, Marquet A. Vitamin K dependent carboxylation: study of diastereoisomeric gamma-methylglutamic acid containing peptidic substrates. Biochem Biophys Res Commun. 1983;113:454–61 [DOI] [PubMed] [Google Scholar]
- 13.Sugiura I, Furie B, Walsh CT, Furie BC. Propeptide and glutamate-containing substrates bound to the vitamin K-dependent carboxylase convert its vitamin K epoxidase function from an inactive to an active state. Proc Natl Acad Sci U S A. 1997;94:9069–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkner KL, Runge KW. The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis. J Thromb Haemost. 2004;2:2118–32 [DOI] [PubMed] [Google Scholar]
- 15.Booth SL, Suttie JW. Dietary intake and adequacy of vitamin K. J Nutr. 1998;12:785–8 [DOI] [PubMed] [Google Scholar]
- 16.Soute BA, Ulrich MM, Watson AD, Maddison JE, Ebberink RH, Vermeer C. Congenital deficiency of all vitamin K-dependent blood coagulation factors due to a defective vitamin K-dependent carboxylase in Devon Rex cats. Thromb Haemost. 1992;68:521–5 [PubMed] [Google Scholar]
- 17.Cheung A, Suttie JW. Synthesis of menaquinone-2 derivatives as substrates for the liver microsomal vitamin K-dependent carboxylase. Biofactors. 1988;1:61–5 [PubMed] [Google Scholar]
- 18.Larson AE, Friedman PA, Suttie JW. Vitamin K-dependent carboxylase. Stoichiometry of carboxylation and vitamin K 2,3-epoxide formation. J Biol Chem. 1981;256:11032–5 [PubMed] [Google Scholar]
- 19.Bugg TDH. Dioxygenase enzymes: catalytic mechanisms and chemical models. Tetrahedron. 2003;59:7075–101 [Google Scholar]
- 20.Davis CH, Deerfield D, 2nd, Wymore T, Stafford DW, Pedersen LG. A quantum chemical study of the mechanism of action of Vitamin K carboxylase (VKC) III. Intermediates and transition states. J Mol Graph Model. 2007;26:409–14 [DOI] [PubMed] [Google Scholar]
- 21.Silva PJ, Ramos MJ. Reaction mechanism of the vitamin K-dependent glutamate carboxylase: a computational study. J Phys Chem B. 2007;111:12883–7 [DOI] [PubMed] [Google Scholar]
- 22.Brenner B, Sánchez-Vega B, Wu SM, Lanir N, Stafford DW, Solera J. A missense mutation in gamma-glutamyl carboxylase gene causes combined deficiency of all vitamin K-dependent blood coagulation factors. Blood. 1998;92:4554–9 [PubMed] [Google Scholar]
- 23.Mutucumarana VP, Stafford DW, Stanley TB, Jin DY, Solera J, Brenner B, Azerad R, Wu SM. Expression and characterization of the naturally occurring mutation L394R in human gamma-glutamyl carboxylase. J Biol Chem. 2000;275:32572–7 [DOI] [PubMed] [Google Scholar]
- 24.Mutucumarana VP, Acher F, Straight DL, Jin DY, Stafford DW. A conserved region of human vitamin K-dependent carboxylase between residues 393 and 404 is important for its interaction with the glutamate substrate. J Biol Chem. 2003;278:46488–93 [DOI] [PubMed] [Google Scholar]
- 25.Spronk HM, Farah RA, Buchanan GR, Vermeer C, Soute BA. Novel mutation in the gamma-glutamyl carboxylase gene resulting in congenital combined deficiency of all vitamin K-dependent blood coagulation factors. Blood. 2000;96:3650–2 [PubMed] [Google Scholar]
- 26.Soute BA, Jin DY, Spronk HM, Mutucumarana VP, Lin PJ, Hackeng TM, Stafford DW, Vermeer C. Characteristics of recombinant W501S mutated human gamma-glutamyl carboxylase. J Thromb Haemost. 2004;2:597–604 [DOI] [PubMed] [Google Scholar]
- 27.Cheung A, Engelke JA, Sanders C, Suttie JW. Vitamin K-dependent carboxylase: influence of the "propeptide" region on enzyme activity. Arch Biochem Biophys. 1989;274:574–81 [DOI] [PubMed] [Google Scholar]
- 28.Knobloch JE, Suttie JW. Vitamin K-dependent carboxylase. Control of enzyme activity by the "propeptide" region of factor X. J Biol Chem. 1987;262:15334–7 [PubMed] [Google Scholar]
- 29.Darghouth D, Hallgren KW, Shtofman RL, Mrad A, Gharbi Y, Maherzi A, Kastally R, LeRicousse S, Berkner KL, Rosa JP. Compound heterozygosity of novel missense mutations in the gamma-glutamyl-carboxylase gene causes hereditary combined vitamin K-dependent coagulation factor deficiency. Blood. 2006;108:1925–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rost S, Fregin A, Koch D, Compes M, Müller CR, Oldenburg J. Compound heterozygous mutations in the gamma-glutamyl carboxylase gene cause combined deficiency of all vitamin K-dependent blood coagulation factors. Br J Haematol. 2004;126:546–9 [DOI] [PubMed] [Google Scholar]
- 31.Rost S, Geisen C, Fregin A, Seifried E, Müller CR, Oldenburg J. Founder mutation Arg485Pro led to recurrent compound heterozygous GGCX genotypes in two German patients with VKCFD type 1. Blood Coagul Fibrinolysis. 2006;17:503–7 [DOI] [PubMed] [Google Scholar]
- 32.Lunghi B, Redaelli R, Caimi TM, Corno AR, Bernardi F, Marchetti G. Novel phenotype and γ-glutamyl carboxylase mutations in combined deficiency of vitamin K-dependent coagulation factors. Haemophilia. 2011;17:822–4 [DOI] [PubMed] [Google Scholar]
- 33.Vanakker OM, Martin L, Gheduzzi D, Leroy BP, Loeys BL, Guerci VI, Matthys D, Terry SF, Coucke PJ, Pasquali-Ronchetti I, et al. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J Invest Dermatol. 2007;127:581–7 [DOI] [PubMed] [Google Scholar]
- 34.Li Q, Grange DK, Armstrong NL, Whelan AJ, Hurley MY, Rishavy MA, Hallgren KW, Berkner KL, Schurgers LJ, Jiang Q, et al. Mutations in the GGCX and ABCC6 genes in a family with pseudoxanthoma elasticum-like phenotypes. J Invest Dermatol. 2009;129:553–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q, Jiang Q, Schurgers LJ, Uitto J. Pseudoxanthoma elasticum: reduced gamma-glutamyl carboxylation of matrix gla protein in a mouse model (Abcc6−/−). Biochem Biophys Res Commun. 2007;364:208–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hallgren KW, Hommema EL, McNally BA, Berkner KL. Carboxylase overexpression effects full carboxylation but poor release and secretion of factor IX: implications for the release of vitamin K-dependent proteins. Biochemistry. 2002;41:15045–55 [DOI] [PubMed] [Google Scholar]
- 37.Price PA, Fraser JD, Metz-Virca G. Molecular cloning of matrix Gla protein: implications for substrate recognition by the vitamin K-dependent gamma-carboxylase. Proc Natl Acad Sci U S A. 1987;84:8335–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugiura I, Furie B, Walsh CT, Furie BC. Profactor IX propeptide and glutamate substrate binding sites on the vitamin K-dependent carboxylase identified by site-directed mutagenesis. J Biol Chem. 1996;271:17837–44 [DOI] [PubMed] [Google Scholar]
- 39.Berkner KL, Pudota BN. Vitamin K-dependent carboxylation of the carboxylase. Proc Natl Acad Sci U S A. 1998;95:466–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czerwiec E, Begley GS, Bronstein M, Stenflo J, Taylor K, Furie BC, Furie B. Expression and characterization of recombinant vitamin K-dependent gamma-glutamyl carboxylase from an invertebrate, Conus textile. Eur J Biochem. 2002;269:6162–72 [DOI] [PubMed] [Google Scholar]
- 41.Li T, Yang CT, Jin D, Stafford DW. Identification of a Drosophila vitamin K-dependent gamma-glutamyl carboxylase. J Biol Chem. 2000;275:18291–6 [DOI] [PubMed] [Google Scholar]
- 42.Bandyopadhyay PK, Garrett JE, Shetty RP, Keate T, Walker CS, Olivera BM. gamma -Glutamyl carboxylation: an extracellular posttranslational modification that antedates the divergence of molluscs, arthropods, and chordates. Proc Natl Acad Sci U S A. 2002;99:1264–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren SX, Fu G, Jiang XG, Zeng R, Miao YG, Xu H, Zhang YX, Xiong H, Lu G, Lu LF, et al. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature. 2003;422:888–93 [DOI] [PubMed] [Google Scholar]
- 44.Schultz J. HTTM, a horizontally transferred transmembrane domain. Trends Biochem Sci. 2004;29:4–7 [DOI] [PubMed] [Google Scholar]
- 45.Rishavy MA, Hallgren KW, Yakubenko AV, Zuerner RL, Runge KW, Berkner KL. The vitamin K-dependent carboxylase has been acquired by Leptospira pathogens and shows altered activity that suggests a role other than protein carboxylation. J Biol Chem. 2005;280:34870–7 [DOI] [PubMed] [Google Scholar]
- 46.Pudota BN, Miyagi M, Hallgren KW, West KA, Crabb JW, Misono KS, Berkner KL. Identification of the vitamin K-dependent carboxylase active site: Cys-99 and Cys-450 are required for both epoxidation and carboxylation. Proc Natl Acad Sci U S A. 2000;97:13033–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rishavy MA, Pudota BN, Hallgren KW, Qian W, Yakubenko AV, Song JH, Runge KW, Berkner KL. A new model for vitamin K-dependent carboxylation: the catalytic base that deprotonates vitamin K hydroquinone is not Cys but an activated amine. Proc Natl Acad Sci U S A. 2004;101:13732–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rishavy MA, Hallgren KW, Yakubenko AV, Shtofman RL, Runge KW, Berkner KL. Brønsted analysis reveals Lys218 as the carboxylase active site base that deprotonates vitamin K hydroquinone to initiate vitamin K-dependent protein carboxylation. Biochemistry. 2006;45:13239–48 [DOI] [PubMed] [Google Scholar]
- 49.Schmidt DE, Jr, Westheimer FH. PK of the lysine amino group at the active site of acetoacetate decarboxylase. Biochemistry. 1971;10:1249–53 [DOI] [PubMed] [Google Scholar]
- 50.Rishavy MA, Berkner KL. Insight into the coupling mechanism of the vitamin K-dependent carboxylase: mutation of histidine 160 disrupts glutamic acid carbanion formation and efficient coupling of vitamin K epoxidation to glutamic acid carboxylation. Biochemistry. 2008;47:9836–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman PA, Shia MA, Gallop PM, Griep AE. Vitamin K-dependent gamma-carbon-hydrogen bond cleavage and nonmandatory concurrent carboxylation of peptide-bound glutamic acid residues. Proc Natl Acad Sci U S A. 1979;76:3126–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rishavy MA, Hallgren KW, Berkner KL. The vitamin K-dependent carboxylase generates gamma-carboxylated glutamates by using CO2 to facilitate glutamate deprotonation in a concerted mechanism that drives catalysis. J Biol Chem. 2011;286:44821–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dowd P, Ham SW. Mechanism of cyanide inhibition of the blood-clotting, vitamin K-dependent carboxylase. Proc Natl Acad Sci U S A. 1991;88:10583–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hallgren KW, Qian W, Yakubenko AV, Runge KW, Berkner KL. r-VKORC1 expression in factor IX BHK cells increases the extent of factor IX carboxylation but is limited by saturation of another carboxylation component or by a shift in the rate-limiting step. Biochemistry. 2006;45:5587–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berkner KL. Expression of recombinant vitamin K-dependent proteins in mammalian cells: factors IX and VII. Methods Enzymol. 1993;222:450–77 [DOI] [PubMed] [Google Scholar]
- 56.Yan SC, Razzano P, Chao YB, Walls JD, Berg DT, McClure DB, Grinnell BW. Characterization and novel purification of recombinant human protein C from three mammalian cell lines. Biotechnology (N Y). 1990;8:655–61 [DOI] [PubMed] [Google Scholar]
- 57.Kaufman RJ, Wasley LC, Furie BC, Furie B, Shoemaker CB. Expression, purification, and characterization of recombinant gamma-carboxylated factor IX synthesized in Chinese hamster ovary cells. J Biol Chem. 1986;261:9622–8 [PubMed] [Google Scholar]
- 58.Vatandoost J, Zomorodipour A, Sadeghizadeh M, Aliyari R, Bos MH, Ataei F. Expression of biologically active human clotting factor IX in Drosophila S2 cells: γ-carboxylation of a human vitamin K-dependent protein by the insect enzyme. Biotechnol Prog. 2011. Sep 8 [DOI] [PubMed] [Google Scholar]
- 59.Tie J, Wu SM, Jin D, Nicchitta CV, Stafford DW. A topological study of the human gamma-glutamyl carboxylase. Blood. 2000;96:973–8 [PubMed] [Google Scholar]
- 60.Tie JK, Zheng MY, Hsiao KL, Perera L, Stafford DW, Straight DL. Transmembrane domain interactions and residue proline 378 are essential for proper structure, especially disulfide bond formation, in the human vitamin K-dependent gamma-glutamyl carboxylase. Biochemistry. 2008;47:6301–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rishavy MA, Usubalieva A, Hallgren KW, Berkner KL. Novel insight into the mechanism of the vitamin K oxidoreductase (VKOR): electron relay through Cys43 and Cys51 reduces VKOR to allow vitamin K reduction and facilitation of vitamin K-dependent protein carboxylation. J Biol Chem. 2011;286:7267–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen B, Gould SJ. Opposite facial specificity for two hydroquinone epoxidases: (3-si,4-re)-2,5-dihydroxyacetanilide epoxidase from Streptomyces LL–C10037 and (3-re,4-si)-2,5-dihydroxyacetanilide epoxidase from Streptomyces MPP 3051. Biochemistry. 1991;30:8936–44 [DOI] [PubMed] [Google Scholar]
- 63.Priest JW, Light RJ. Patulin biosynthesis: epoxidation of toluquinol and gentisyl alcohol by particulate preparations from Penicillium patulum. Biochemistry. 1989;28:9192–200 [DOI] [PubMed] [Google Scholar]