Abstract
Osteocalcin originates from osteoblastic synthesis and is deposited into bone or released into circulation, where it correlates with histological measures of bone formation. The presence of 3 vitamin K-dependent γ carboxyglutamic acid residues is critical for osteocalcin’s structure, which appears to regulate the maturation of bone mineral. In humans, the percentage of the circulating osteocalcin that is not γ-carboxylated (percent ucOC) is used as a biomarker of vitamin K status. In contrast, when ucOC is not corrected for total osteocalcin, the interpretation of this measure is confounded by osteoblastic activity, independent of vitamin K. Observational studies using percent ucOC have led to the conclusion that vitamin K insufficiency leads to age-related bone loss. However, clinical trials do not provide overall support for the suggestion that vitamin K supplementation of the general population will reduce bone loss or fracture risk. More recently, results from in vitro and in vivo studies using animal models indicate that ucOC is an active hormone with a positive role in glucose metabolism. By inference, vitamin K, which decreases ucOC, would have a detrimental effect. However, in humans this hypothesis is not supported by the limited data available, nor is it supported by what has been established regarding osteocalcin chemistry. In summary, the specific function of osteocalcin in bone and glucose metabolism has yet to be elucidated.
Introduction
In 1972, the fundamental discovery of the highly specialized calcium-binding amino acid, Gla6, as the mechanism for the activation of the vitamin K-dependent clotting factors (1), sparked the curiosity of investigators searching for calcium-binding proteins that could regulate mineralized tissues. In 1974, 2 groups independently isolated a small vitamin K-dependent protein from bone that contained 3 Gla residues and named it Bone Gla Protein (2) and osteocalcin (3). Osteocalcin, which is now the universally accepted name, was the first protein to be identified as bone specific, is synthesized by the bone-forming osteoblasts, and accumulates in the bone matrix bound to mineral. The detection of small amounts of osteocalcin in circulation was a major breakthrough for the clinical bone field, as clear and well-documented relationships to bone formation became established with age, circadian rhythms, high bone turnover states, and metabolic bone diseases (4). Despite many in vitro and in vivo studies in animal models over the past 3 decades, osteocalcin’s specific function remains puzzling.
Current status of knowledge
Osteocalcin
The primary function of the skeleton is to provide the mechanical support of the body, respond to outside mechanical forces or molecular signals, and function as a reservoir for normal mineral metabolism. The skeleton undergoes continuous remodeling (turnover) of bone with removal of old bone by osteoclasts and replacement with new bone by osteoblasts. In the steady state, this “coupling" of bone formation and resorption maintains bone mass. After growth ceases, any unbalance can lead to bone loss. In addition to the cells, bone consists of mineral, proteins, and other macromolecules (e.g., sugars and lipids). The mineral phase of bone is principally composed of crystalline HAP and accounts for 60–70% of the weight of bone. The organic phase of bone is comprised of collagen (90%) and other smaller matrix proteins, glycoproteins, and proteoglycans (5). Osteocalcin is one of the most abundant matrix proteins found in bones and the only matrix protein synthesized exclusively there (6).
Osteocalcin’s major feature is the presence of 3 Gla residues. 1H 2D NMR and X-ray crystallography studies define a 3-dimensional structure containing 3 helical regions, a C-terminal hydrophobic core, and an unstructured N terminus. All 3 Gla residues are found in the first helical region and interact with the inter-calcium spacings in the HAP lattice. The C terminus extends outward and would be accessible to neighboring cells as well as endogenous proteinases (7–9).
Many early studies pointed to osteocalcin’s biological function as being dictated by this structure, suggesting that osteocalcin could affect the growth or maturation of the mineral phase. Support for this came from in vitro and in vivo studies that showed that the growth of the HAP crystal was inhibited by very low concentrations of osteocalcin (3). Furthermore, osteocalcin first appears coincident with the onset of mineralization in utero, and increases in the protein occur in concert with HAP deposition during skeletal growth and with mineral maturation (10, 11). Its structure is also consistent with many reports of osteocalcin C-terminal peptides having chemotactic activity to osteoclast precursors and supports a role for osteocalcin in bone remodeling. The subcutaneous implantation of osteocalcin-deficient devitalized bone shows a decreased recruitment of progenitor cells to the particles and a decrease in the number of differentiated multinucleated osteoclast-like cells surrounding the bone particles (12–14). Other studies also show that the distribution of osteocalcin in human osteons (the basic unit of structure of compact bone) changes with sex and age, and local reductions of osteocalcin in bone are associated with reduced cortical remodeling (15).
Osteocalcin was the first bone protein to be knocked out in mice, but surprisingly, there was no overt skeletal consequence of the loss of osteocalcin (16). The mice exhibited only a small increase in cortical thickness and mechanical strength, which were apparent in the mice only after the age of 6 mo. There was some suggestion, however, of a more rapid loss of bone in ovariectomized female mice lacking the osteocalcin gene than in their normal counterparts. Bone mineralization was not affected, but further analyses of crystal properties showed less remodeled mineral in the cortices of knock-out mice, indicating that osteocalcin is required to stimulate bone mineral maturation (17).
Circulating osteocalcin
Serum osteocalcin is correlated with bone formation and osteoblast number as determined by histomorphometry and calcium kinetics (18–20) and is used as a marker of bone formation. Support for this comes from studies in rats showing that circulating osteocalcin originates from new bone synthesis rather than the breakdown of bone (21). In humans, however, various forms of osteocalcin circulate. Osteoblastic synthesis contributes the majority of circulating osteocalcin. Smaller fragments derived from the action of cathepsin K and matrix metalloproteinases during bone resorption have also been identified and encompass the mid-molecular region (22–24). These fragments are rapidly cleared by the kidneys. Most current commercial assays are 2-site ELISA, which do not detect the smaller fragments, and results from these assays are an indicator of bone formation. Some single antibody assays have limited ability to detect these fragments in serum, but urine-based mid-molecular assays detect these fragments and are a measure of bone resorption (25, 26). Understanding the specificity of an assay is critical when interpreting results.
ucOC as a measure of vitamin K nutrition
Vitamin K is a post-translational cofactor for the γ-carboxylation of vitamin K-dependent proteins, including osteocalcin. Osteocalcin biosynthesis is regulated by hormones and growth factors but not by vitamin K. Instead, vitamin K influences the degree of carboxylation of osteocalcin. In rats treated with high-dose warfarin (vitamin K-antagonist) plus high-dose vitamin K, adequate blood clotting is maintained, but osteocalcin is not γ-carboxylated, suggesting that the liver sequesters vitamin K at the expense of bone (27). In most species, all 3 Gla sites are fully carboxylated, but osteocalcin in human bone and serum is incompletely carboxylated and variations in carboxylation status are due to differences in vitamin K intake (28). The assessment of the degree of circulating ucOC has been shown to be the most sensitive measure of vitamin K nutrition in humans and is responsive to conditions of dietary vitamin K depletion, repletion, and supplementation (29–31).
Two methods have been used to measure serum ucOC: the HAP binding assay (32, 33) and direct determination by an ELISA specific for the uncarboxylated form (Takara, Shuzo). The HAP binding assay is based on the lower affinity of uncarboxylated OC or ucOC for HAP compared to fully carboxylated OC. Osteocalcin is measured in serum and in the supernatant after incubation with HAP. This method requires using an immunoassay that detects both carboxylated and ucOC equivalently. Results are compared to a control group matched for the same total osteocalcin (34). Total and ucOC are highly correlated (r = 0.9), so to control for osteoblastic synthesis, levels are reported as a fraction of total osteocalcin (i.e., percent ucOC). This method has been used extensively and has been shown to provide discrimination between patients with and without warfarin treatment (34) or vitamin K supplementation (35). When analyzed by immunoaffinity-electrospray ionization mass spectral analysis, preliminary results show that 40–50% of osteocalcin in circulation is not fully carboxylated in free-living older adults not receiving any form of vitamin K supplementation (S. Booth, unpublished data).
The direct ELISA for Glu-type OC from Takara is specific for fully uncarboxylated OC. It cannot be used in conjunction with total osteocalcin assays from other sources to determine percent ucOC because of differences in standardization and antibody regional specificity. However, a combination kit that measures fully carboxylated osteocalcin (Gla-type) in parallel with Glu-type can provide an estimate of the percent ucOC. Neither assay recognizes undercarboxylated osteocalcin (Takara product details). Nevertheless, there is a good correlation between the HAP binding assay and the ELISA measurements (r ∼ 0.8) (C. Gundberg, unpublished results). The ELISA method displays the same high correlation between total (Glu + Gla) and Glu-OC (36) as is observed between ucOC and total osteocalcin with the HAP binding assay.
It is worth noting that rodents consuming commercially available rodent chow have low levels of ucOC. Rodents have sufficient vitamin K for all tissue functions due to coprophagy and gut synthesis of vitamin K (S. Booth, unpublished data). Furthermore, most rodent chows are fortified with large quantities of menadione (37), a precursor for MK-4, which supports γ-carboxylation of osteocalcin, whereas humans rely on a diet rich in green vegetables and plant oils to maintain adequate vitamin K (38). As such, rodent models may have limited utility for extrapolation to the study of ucOC function in humans.
Evidence for a role of carboxylated osteocalcin in bone health
There has been long-standing interest in the potential role of vitamin K in the prevention of osteoporotic fractures based on its role in carboxylation of osteocalcin. Given the widespread availability of assays for ucOC and its validation as a marker of vitamin K status, this biomarker has been used extensively as a measure of vitamin K’s role in bone health, as reviewed elsewhere (39).
A plethora of observational studies was published that examined the association of ucOC and various outcomes for bone health, including BMD, qualitative ultrasound of the heel, and hip fracture risk (39). Many of these studies measured ucOC but did not account for total osteocalcin. Although it appeared that the preponderance of evidence suggested a vitamin K-dependent association between osteocalcin and measures of bone, especially in the elderly, the high correlation between ucOC and total osteocalcin may have confounded the findings, because total osteocalcin is a robust marker of bone formation, independent of nutrient effects. Therefore, it is difficult to differentiate the osteoblastic effect of osteocalcin from the role of the vitamin K-dependent γ-carboxylation in these studies.
Vitamin K’s role as an enzyme cofactor is a post-translational event and theoretically should not have any impact on total osteocalcin, which derives from osteoblastic synthesis. However, if vitamin K’s role in the carboxylation of osteocalcin indirectly increased osteoblastic activity, changes in total osteocalcin would indirectly suggest benefits to bone formation in response to vitamin K. Indeed, there were reports that total osteocalcin increased (40), decreased (29, 41, 42), or remained unchanged (30) in response to vitamin K supplementation. In one study, total osteocalcin increased in response to a combination of vitamins D and K but did not change in response to vitamin K supplementation alone (43). These divergent findings may reflect a changing equilibrium between bone-bound and circulating osteocalcin as it attains greater γ-carboxylation in response to vitamin K supplementation. Others have suggested an age effect, with a response of total osteocalcin to vitamin K supplementation limited to postmenopausal women (44). Some studies examined the effects of manipulating dietary vitamin K on bone resorption markers, which in conjunction with markers of bone formation, such as total osteocalcin, provide a measure of the coupling of bone formation and resorption. In one study, there was a response in both bone formation and resorption markers to vitamin K supplementation among female athletes (45), whereas others have reported no change (29, 43). A modest response of both total osteocalcin and N-telopeptide cross links (a marker of bone resorption) to short-term vitamin K depletion and repletion among young adults was observed when dietary intakes of all other nutrients known to influence bone were controlled for (42). However, sensitivity and specificity of the individual markers vary and there is considerable inter-laboratory variation in bone turnover marker values. The current lack of standardization may have considerable impact on the ability to compare changes in bone formation or resorption in response to treatments among studies (46). Therefore, it is plausible that the equivocal findings of the effect of vitamin K supplementation on bone turnover reported in the literature reflect these inherent measurement variables in markers.
As reviewed elsewhere (39, 47), when only those studies that reported the associations between percent ucOC and various bone outcomes were considered, the majority reported that a high percent ucOC was associated with greater risk of low BMD (48, 49). Although compelling, it was not known if these associations were confounded by a generalized poor nutritional status in these study populations. Food sources of vitamin K are primarily limited to green leafy vegetables and plant oils, which are markers of healthy diets and predictive of healthy lifestyles (50, 51). Therefore, the epidemiologic evidence that suboptimal vitamin K could have an adverse effect on BMD and increase the risk of bone fracture needed to be supported by RCT to evaluate the protective role of vitamin K in prevention of bone loss.
In the last decade, multiple RCT have been conducted to test the hypothesis that supplemental vitamin K will slow progression of bone loss in older adults. At least 5 have focused on phylloquinone, the primary dietary form, as summarized in Table 1. The primary outcome for these trials was the change in hip BMD in response to phylloquinone supplementation compared to no phylloquinone supplementation in a calcium- and vitamin D-supplemented study population. All studies reported a reduction in the percent ucOC in response to phylloquinone supplementation (52–56), and several (55, 56) reported that bone turnover, including total osteocalcin measures, did show a transient increase in the first 6–12 mo among the vitamin D, calcium, and vitamin K-supplemented group but returned to baseline levels by the end of the study. However, only the Maastricht study reported a beneficial effect of vitamin K supplementation on bone loss at the hip (54). This observed protective effect of phylloquinone (54) has not been replicated since and may be indicative of a study group that has unique characteristics, because in y 3 only, the group that received calcium and vitamin D supplementation had substantial bone loss that was equivalent to that in the placebo group. Overall, the RCT summarized in Table 1 varied in the duration and dose of the phylloquinone supplementation, so while some have been criticized for their short duration (56) or low phylloquinone dose (52), these potential limitations did not appear to explain the overall null findings. These data also bring into question the utility of percent ucOC as a robust marker of bone health, because percent ucOC consistently decreased in the phylloquinone-supplemented groups among the published RCT, with no concomitant effects on rates of bone loss at the hip. Instead, these data suggest that in the general population, phylloquinone supplementation does not confer an additional protective effect against age-related bone loss among older adults who are also receiving calcium and vitamin D supplementation.
Table 1.
Summary of RCT studying the effect of phylloquinone (K1) supplementation on bone loss at the hip1
| Study | C vs. T | Duration (mo) | Change in hip BMD | Reference |
| Tufts (M+F; 60–80 y) | C: vitamin D + Ca T: vitamin D + Ca + 500 μg K1 | 36 | No difference | (53) |
| Maastricht (F; 50–60 y) | C: placebo T1: vitamin D + Ca T2: vitamin D + Ca + 1000 μg K1 | 36 | T < C; P < 0.05 | (54) |
| Wisconsin (F; >55 y) | C: vitamin D + Ca T: vitamin D + Ca + 1000 μg K1 | 12 | No difference | (56) |
| UK Bones and vitamins (F; >60 y) | C: placebo T1: vitamin D + Ca + 200 μg K1 T2: 200 μg K1 | 24 | No difference | (52) |
| ECKO (F; 40–82 y) | C: vitamin D + Ca T: vitamin D + Ca + 5000 μg K1 | 24 | No difference | (55) |
BMD, bone mineral density; C, control; RCT, randomized, double-masked, controlled clinical trial; T, treatment.
Although these studies were designed prior to 2011 when the dietary requirements for vitamin D were increased (57), it is important to recognize that the majority of these studies were designed to measure any potential additive effect vitamin K has to calcium and vitamin D supplementation in slowing bone loss. Only the UK Bones and Vitamins study (52) had a comparison group that was not supplemented with calcium and vitamin D and hence could measure how vitamin K supplementation alone would influence bone loss in a vitamin D and calcium-deplete environment. This study did report a protective effect of phylloquinone supplementation in bone loss at the radius but not at any other measured anatomical sites, including the hip (52). Unfortunately, the BMD of the radius was not measured in any other study, so it is not known if this was a spurious finding or a true beneficial effect. Similarly, the ECKO study, which used a daily dose of 5 mg phylloquinone in the treatment group for up to 48 mo, reported a protective effect of phylloquinone supplementation in all-cause fracture risk (55). However, this observation was not supported when the authors considered only osteoporotic-related fractures. None of the published RCT for vitamin K have been designed using fracture risk as a primary outcome and the authors of the ECKO study acknowledged that they did not have sufficient statistical power; hence, their conclusions on the putative protective effect of vitamin K on fracture risk need to be interpreted with caution (55). The RCT approach to the study of nutrient supplementation on health outcomes has been questioned and merits consideration in the interpretation of studies of phylloquinone supplementation on bone loss. As posited by Morris et al. (58), the RCT study design targets study participants at the lower end of optimal intake of a particular nutrient given that the exclusion and inclusion criteria for selection favors overall healthy individuals. The benefits of short-term additional nutrient intake relative to a lifetime exposure in such individuals will have limited impact on the improvement in function, such as change in hip BMD. It is plausible that individuals with a chronic marginal intake of phylloquinone would have a measurable improvement in change of hip BMD in response to phylloquinone supplementation. An RCT that targets individuals with a consistently high percent ucOC and/or conditions that are at greater risk of vitamin K deficiency, such as patients with cystic fibrosis (59) or fat malabsorption (60), needs to be conducted to address this concern about RCT study design.
MK are another form of vitamin K that have been implicated in bone health and have been demonstrated to reduce the percent ucOC in response to supplementation (3–97). The majority of studies have focused on MK-4, a form of vitamin K that is endogenously produced from either menadione or phylloquinone (61). In these studies, MK-4 is given therapeutically in daily doses of 45 mg, which is unattainable through diet. A thorough meta-analysis of the studies conducted up to 2006 reported an overall benefit of MK-4 supplementation on reducing fracture risk (62). However, as the authors explained, the studies included in the meta-analysis were limited geographically, had a mixture of co-supplementation with other nutrients and medications, and did not necessarily have concealed randomization schemes. Furthermore, the reported reduction risk associated with MK-4 was greater than those reported for bisphosphonates (potent bone antiresorptives) such that the authors cautioned interpretation of the findings (62). A more recent meta-analysis was conducted that combined findings of studies that used either phylloquinone or MK supplementation (63). The authors concluded that there were modest treatment effects with vitamin K, but the results needed to be interpreted with caution. Of concern with this recent meta-analysis was the number of errors in the published tables describing the studies that were included in the analysis. Errors included wrong sample sizes and incomplete grouping for which the studies were statistically designed to compare. Furthermore, multiple forms of vitamin K were combined in the analysis (63), which assumes equal bioavailability and transport. More recently, it was reported in a study of 4378 women that MK-4 combined with calcium supplementation did not provide additional protection against fracture risk compared to calcium supplementation alone (64). In addition, recent RCT that focused on MK-4 supplementation reported no beneficial effect on progression of bone loss (56, 65). Another form of vitamin K, MK-7, was recently approved by the European Food Safety Authority as safe to add to food products (66), which has generated interest in its potential role in bone health through the carboxylation of osteocalcin. Similar to other forms of vitamin K, including phylloquinone, MK-7 supplementation consistently reduces percent ucOC (67). However, in a 12-mo study of 334 women, MK-7 supplementation did not change loss in BMD at any anatomical site (68). Findings from longer term RCT using MK-7 supplementation have yet to be reported.
Hormonal function of osteocalcin
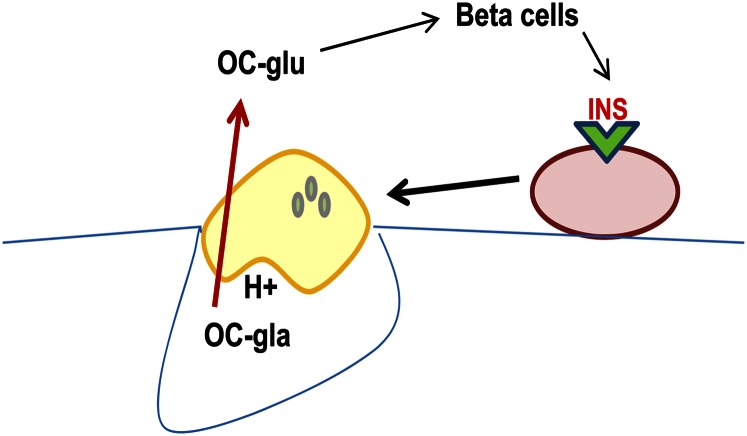
In 2007, the research group headed by Gerard Karsenty and Patricia Ducy reported that the osteocalcin knockout mice were obese, had elevated glucose and lipid concentrations, and reduced insulin levels and glucose tolerance (69). It is well known that osteoblasts respond to numerous hormonal signals and secrete factors that affect other cell populations both within bone and in other organ systems. In this regard, a novel paradigm was proposed in which an endocrine loop, mediated by osteocalcin, links the osteoblast to the islet and adipocyte (70). However, the unexpected finding was that the hormonal form of osteocalcin was uncarboxylated and the carboxylated form was inactive. Additionally, an osteotesticular protein tyrosine phosphatase, OST-PTP, was involved in the activation of osteocalcin. Although it was initially thought that OST-PTP was involved in the regulation of the enzymes of the vitamin K cycle, this proved not to be the case. Rather, OST-PTP dephosphorylates the insulin receptor in osteoblasts, leading to inhibition of insulin signaling there. In a series of studies, the Karsenty group showed that insulin signaling in osteoblasts limits production of osteoprotegrin, an inhibitor of osteoclast maturation (71, 72). This facilitates osteoclast bone resorption, producing an acid environment that decarboxylates, and hence activates, intact osteocalcin (Fig. 1). However, these results are at odds with current knowledge regarding both cellular activity of the osteoclast and the kinetics of osteocalcin decarboxylation,
Figure 1.
INS signaling in osteoblasts activates osteoclast bone resorption that requires an acid environment. The low pH (H+) converts fully carboxylated osteocalcin (OC-gla) to an undercarboxylated form (OC-glu) and activates it for a putative role in glucose homeostasis. Gla, γ carboxyglutamic acid; Glu, glutamic acid; INS, insulin.
Resorption involves a process whereby the osteoclast attaches to the bone and forms a tight seal. Dissolution of inorganic material occurs by secretion of HCl into the resorptive compartment via a vacuolar H+ATPase. The organic matrix is then degraded by proteolytic enzymes, internalized, transcytosed, and excreted into the extracellular space. The osteoclast forms resorption pits and continuously clears the mineral and matrix as the excavated site is deepened and the osteoclast moves along the surface of the bone (73, 74). Ivaska et.al. (75) cultured human osteoclasts on bovine bone and observed osteocalcin in the medium by 48 h. Initially, a small amount of intact osteocalcin was released during acidification followed by enzymatically produced fragments of the protein. These observations are consistent with clinical studies that show that urinary osteocalcin fragments are associated with bone resorption (76). Decarboxylation of osteocalcin, on the other hand, requires drying the protein from a solution of 50 mmol/L HCl and heating the dry acidified protein in vacuo at 110°C for 3 h (77). When fully carboxylated osteocalcin was incubated for 48 h at pH 4.5 and 37°C, conditions similar to that found in the resorption lacuna, we found no decarboxylation of the protein (C. Gundberg unpublished data).
Human studies
In recent years, the relationship between diabetes and bone mass has received considerable scientific attention. Bone mass is generally lower in patients with type 1 diabetes but higher than normal in those with type 2 diabetes, especially when obesity is considered. Poor diabetic control, low insulin and IGF-1 levels, low bone formation, altered vitamin D metabolism, and biomechanical forces have all been implicated in the pathogenesis of changes in bone mass and fracture risk in diabetes (78–80). Various bone active factors are expressed by both adipocytes and β-cells (81) and insulin is a very potent anabolic agent for bone with direct mitogenic effect on osteoblasts (82). The possibility that a specific osteoblast product could be a target for treatment of metabolic disease is of considerable interest.
Human studies to date suggest an association between circulating osteocalcin and insulin sensitivity, but most do not measure other bone formation makers (e.g., pro-peptide of type I collagen) to control for osteocalcin as a measure of osteoblast activity or for the role of the osteoblast in mediating insulin’s actions in bone. Studies are thus far unable to define the form of osteocalcin responsible, because they have been primarily cross-sectional (83, 84), have not measured all forms of osteocalcin (i.e., ucOC, carboxylated osteocalcin, and total osteocalcin) (83–86), and/or were not designed to address the impact of carboxylation of osteocalcin on insulin sensitivity (85, 87). By inference, if the ucOC form is the active hormonal form that confers beneficial glucose control, then of concern is the notion that vitamin K intake is detrimental to diabetic control because of its role in the γ-carboxylation of osteocalcin. On the contrary, some evidence suggests that vitamin K may have a beneficial effect on fasting measures of insulin resistance. Higher vitamin K intake was associated with reduced insulin resistance and risk of type 2 diabetes (88, 89). Furthermore, 1 wk supplementation of vitamin K improved acute insulin response after an oral glucose load in young men with low vitamin K status at the baseline (90). Vitamin K supplementation for 3 y has a beneficial effect on insulin resistance in older men (91). In contrast, others did not find any effect after 1 y of treatment (92). It is possible that vitamin K supplementation may have had a positive role on vitamin K-dependent processes, separate from osteocalcin, which may be involved in any putative effect of vitamin K on glucose homeostasis. The vitamin K-dependent carboxylase is expressed in the pancreas, and both prothrombin and Gas6 have been identified there (93). Furthermore, vitamin K may have novel functions in addition to its classic role as a cofactor for γ-carboxylation.
Warfarin increases the proportion of ucOC released by the osteoblast. Treatment of rodents with warfarin results in a shift of serum OC from its carboxylated to an uncarboxylated form within 3 h. An early study showed that large doses of warfarin given to rats for 15 d had no effect on glucose handling (94). In humans, there is only one report of hypoglycemia associated with warfarin embryopathy (95). However, the infant had neonatal hepatopathy, which may have impaired hepatic glucose production independent of ucOC. No other reports of hypoglycemia exist in an otherwise large body of literature of case studies of warfarin embryopathy. To date, there are no reported studies regarding the effect of adult warfarin therapy on glucose homeostasis. There are, however, known interactions between warfarin and drugs used for diabetic control. The hypoglycemic agent acarbose can increase the absorption of warfarin, resulting in an increase in prothrombin time, but there is no effect on glucose status. Because both are metabolized by CYP2C9, there is a substantial warfarin-sulfonylurea drug interaction that can lead to hypoglycemia and an increase in the international normalized ratio, a standard measure of coagulation (96–98). No consequences of warfarin’s inhibition of the vitamin K cycle on glucose homeostasis have been reported.
Conclusions and future directions
Despite the many in vitro and in vivo studies using animal models, osteocalcin’s specific function remains controversial. This uncertainty regarding its function has been a hindrance to the interpretation of changes in the uncarboxylated form used in observational and clinical trials as a biomarker of vitamin K status in bone. Unlike rodents, which have been the recent animal model of choice for vitamin K studies, the majority of humans do not have fully carboxylated circulating osteocalcin. The observation that the majority of apparently healthy humans have circulating ucOC raises the question regarding the optimal proportion of osteocalcin that needs to be γ-carboxylated for human health. Historically, it was assumed that the goal was to fully γ-carboxylate osteocalcin and there was interest in the optimal dose of vitamin K to reduce the percent ucOC to zero. Among the RCT that provided up to 4 y of vitamin K supplementation to individuals with established risk factors for age-related bone loss, the reduction in percent ucOC observed among the vitamin K-supplemented individuals did not confer a concomitant improvement in bone loss. It is plausible that future clinical trials that target individuals with a chronic marginal intake of vitamin K, and hence very high percent ucOC, may demonstrate a beneficial response in bone loss in response to vitamin K supplementation. However, percent ucOC appears to be a relative measure of vitamin K status in bone, but not a biomarker of bone health, and caution should be taken in the interpretation of relative changes in percent ucOC when discussing the role of vitamin K in bone health.
A novel paradigm was recently proposed in which an endocrine loop, mediated by osteocalcin, links the osteoblast to the islet and the adipocyte. Based on in vivo and in vitro studies using a rodent model, it appeared that the uncarboxylated form of osteocalcin is the active form. The mechanism proposed is inconsistent with the current understanding about the kinetics of osteocalcin decarboxylation and the cellular activity of the osteoclast, and more research is clearly required to fully understand the role of osteocalcin in the regulation of glucose metabolism. The importance of carboxylation of osteocalcin in glucose metabolism in humans is even more puzzling, because vitamin K is responsible for the γ-carboxylation of osteocalcin and hence by inference would be detrimental to glucose metabolism as proposed by this novel paradigm. It is apparent that the species-specific differences in osteocalcin preclude extrapolation of the findings in rodents to the study of diabetes in humans. Therefore, despite the evidence for a role of osteocalcin in regulation of glucose metabolism in in vitro and in vivo animal models, the evidence in humans remains largely inconclusive.
Acknowledgments
All authors have read and approved the final manuscript.
Footnotes
Supported by the USDA Agricultural Research Service under Cooperative Agreement no. 58-1950-7-707 (S.L.B.) and NIH AR38460 and P30 DK04735 (C.M.G.) and 5R37DE012528 (J.B.L.). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Author disclosures: C. M. Gundberg, J. B. Lian, and S. L. Booth, no conflicts of interest.
Abbreviations used: BMD, bone mineral density; Gla, γ carboxyglutamic acid; Glu, glutamic acid; HAP, hydroxyapatite; MK, menaquinone; RCT, randomized, double-masked, controlled clinical trial; ucOC, undercarboxylated osteocalcin.
Literature Cited
- 1.Stenflo J, Fernlund P, Egan W, Roepstorff P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc Natl Acad Sci USA. 1974;71:2730–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauschka PV, Lian JB, Gallop PM. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci USA. 1975;72:3925–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price PA, Otsuka AA, Poser JW, Kristaponis J, Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci USA. 1976;73:1447–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gundberg CM. Biology, physiology, and clinical chemistry of osteocalcin. J Clin Ligand Assay. 1998;21:128–38 [Google Scholar]
- 5.Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996;17:333–68 [DOI] [PubMed] [Google Scholar]
- 6.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047 [DOI] [PubMed] [Google Scholar]
- 7.Dowd TL, Rosen JF, Li L, Gundberg CM. The three-dimensional structure of bovine calcium ion-bound osteocalcin using 1H NMR spectroscopy. Biochemistry. 2003;42:7769–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauschka PV, Carr SA. Calcium-dependent alpha-helical structure in osteocalcin. Biochemistry. 1982;21:2538–47 [DOI] [PubMed] [Google Scholar]
- 9.Hoang QQ, Sicheri F, Howard AJ, Yang DS. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003;425:977–80 [DOI] [PubMed] [Google Scholar]
- 10.Hauschka PV, Reid ML. Timed appearance of a calcium-binding protein containing gamma-carboxyglutamic acid in developing chick bone. Dev Biol. 1978;65:426–34 [DOI] [PubMed] [Google Scholar]
- 11.Lian JB, Roufosse AH, Reit B, Glimcher MJ. Concentrations of osteocalcin and phosphoprotein as a function of mineral content and age in cortical bone. Calcif Tissue Int. 1982;34 Suppl 2:S82–7 [PubMed] [Google Scholar]
- 12.Chenu C, Colucci S, Grano M, Zigrino P, Barattolo R, Zambonin G, Baldini N, Vergnaud P, Delmas PD, Zallone AZ. Osteocalcin induces chemotaxis, secretion of matrix proteins, and calcium-mediated intracellular signaling in human osteoclast-like cells. J Cell Biol. 1994;127:1149–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glowacki J, Rey C, Cox K, Lian J. Effects of bone matrix components on osteoclast differentiation. Connect Tissue Res. 1989;20:121–9 [DOI] [PubMed] [Google Scholar]
- 14.Lian JB, Tassinari M, Glowacki J. Resorption of implanted bone prepared from normal and warfarin-treated rats. J Clin Invest. 1984;73:1223–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingram RT, Park YK, Clarke BL, Fitzpatrick LA. Age- and gender-related changes in the distribution of osteocalcin in the extracellular matrix of normal male and female bone. Possible involvement of osteocalcin in bone remodeling. J Clin Invest. 1994;93:989–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52 [DOI] [PubMed] [Google Scholar]
- 17.Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23:187–96 [DOI] [PubMed] [Google Scholar]
- 18.Brown JP, Delmas PD, Arlot M, Meunier PJ. Active bone turnover of the cortico-endosteal envelope in postmenopausal osteoporosis. J Clin Endocrinol Metab. 1987;64:954–9 [DOI] [PubMed] [Google Scholar]
- 19.Brown JP, Delmas PD, Malaval L, Edouard C, Chapuy MC, Meunier PJ. Serum bone Gla-protein: a specific marker for bone formation in postmenopausal osteoporosis. Lancet. 1984;1:1091–3 [DOI] [PubMed] [Google Scholar]
- 20.Eastell R, Delmas PD, Hodgson SF, Eriksen EF, Mann KG, Riggs BL. Bone formation rate in older normal women: concurrent assessment with bone histomorphometry, calcium kinetics, and biochemical markers. J Clin Endocrinol Metab. 1988;67:741–8 [DOI] [PubMed] [Google Scholar]
- 21.Price PA, Williamson MK, Lothringer JW. Origin of the vitamin K-dependent bone protein found in plasma and its clearance by kidney and bone. J Biol Chem. 1981;256:12760–6 [PubMed] [Google Scholar]
- 22.Garnero P, Grimaux M, Demiaux B, Preaudat C, Seguin P, Delmas PD. Measurement of serum osteocalcin with a human-specific two-site immunoradiometric assay. J Bone Miner Res. 1992;7:1389–98 [DOI] [PubMed] [Google Scholar]
- 23.Gundberg CM, Weinstein RS. Multiple immunoreactive forms of osteocalcin in uremic serum. J Clin Invest. 1986;77:1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor AK, Linkhart S, Mohan S, Christenson RA, Singer FR, Baylink DJ. Multiple osteocalcin fragments in human urine and serum as detected by a midmolecule osteocalcin radioimmunoassay. J Clin Endocrinol Metab. 1990;70:467–72 [DOI] [PubMed] [Google Scholar]
- 25.Lenora J, Ivaska KK, Obrant KJ, Gerdhem P. Prediction of bone loss using biochemical markers of bone turnover. Osteoporos Int. 2007;18:1297–305 [DOI] [PubMed] [Google Scholar]
- 26.Ivaska KK, Kakonen SM, Gerdhem P, Obrant KJ, Pettersson K, Vaananen HK. Urinary osteocalcin as a marker of bone metabolism. Clin Chem. 2005;51:618–28 [DOI] [PubMed] [Google Scholar]
- 27.Cairns JR, Price PA. Direct demonstration that the vitamin K-dependent bone Gla protein is incompletely gamma-carboxylated in humans. J Bone Miner Res. 1994;9:1989–97 [DOI] [PubMed] [Google Scholar]
- 28.Price PA, Williamson MK. Effects of warfarin on bone. Studies on the vitamin K-dependent protein of rat bone. J Biol Chem. 1981;256:12754–9 [PubMed] [Google Scholar]
- 29.Binkley NC, Krueger DC, Engelke JA, Foley AL, Suttie JW. Vitamin K supplementation reduces serum concentrations of under-gamma-carboxylated osteocalcin in healthy young and elderly adults. Am J Clin Nutr. 2000;72:1523–8 [DOI] [PubMed] [Google Scholar]
- 30.Binkley NC, Krueger DC, Kawahara TN, Engelke JA, Chappell RJ, Suttie JW. A high phylloquinone intake is required to achieve maximal osteocalcin gamma-carboxylation. Am J Clin Nutr. 2002;76:1055–60 [DOI] [PubMed] [Google Scholar]
- 31.Sokoll LJ, Booth SL, O'Brien ME, Davidson KW, Tsaioun KI, Sadowski JA. Changes in serum osteocalcin, plasma phylloquinone, and urinary gamma-carboxyglutamic acid in response to altered intakes of dietary phylloquinone in human subjects. Am J Clin Nutr. 1997;65:779–84 [DOI] [PubMed] [Google Scholar]
- 32.Merle B, Delmas PD. Normal carboxylation of circulating osteocalcin (bone Gla-protein) in Paget's disease of bone. Bone Miner. 1990;11:237–45 [DOI] [PubMed] [Google Scholar]
- 33.Vergnaud P, Garnero P, Meunier PJ, Breart G, Kamihagi K, Delmas PD. Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS Study. J Clin Endocrinol Metab. 1997;82:719–24 [DOI] [PubMed] [Google Scholar]
- 34.Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab. 1998;83:3258–66 [DOI] [PubMed] [Google Scholar]
- 35.Booth SL, Suttie JW. Dietary intake and adequacy of vitamin K. J Nutr. 1998;128:785–8 [DOI] [PubMed] [Google Scholar]
- 36.Kirmani S, Atkinson EJ, Melton LJ III, Riggs BL, Amin S, Khosla S. Relationship of testosterone and osteocalcin levels during growth. J Bone Miner Res. 2011;26:2212–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu X, Booth SL, Smith DE. Vitamin K contents of rodent diets: a review. J Am Assoc Lab Anim Sci. 2007;46:8–12 [PubMed] [Google Scholar]
- 38.Booth SL, Al Rajabi A. Determinants of vitamin K status in humans. Vitam Horm. 2008;78:1–22 [DOI] [PubMed] [Google Scholar]
- 39.Booth SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr. 2009;29:89–110 [DOI] [PubMed] [Google Scholar]
- 40.Knapen MH, Jie KS, Hamulyak K, Vermeer C. Vitamin K-induced changes in markers for osteoblast activity and urinary calcium loss. Calcif Tissue Int. 1993;53:81–5 [DOI] [PubMed] [Google Scholar]
- 41.Booth S, McKeown N, Lictenstein A, Morse M, Davidson K, Wood R, Gundberg C. A hydrogenated form of vitamin K: its relative bioavailability and presence in the food supply. J Food Compost Anal. 2000;13:311–7 [Google Scholar]
- 42.Booth SL, Lichtenstein AH, O'Brien-Morse M, McKeown NM, Wood RJ, Saltzman E, Gundberg CM. Effects of a hydrogenated form of vitamin K on bone formation and resorption. Am J Clin Nutr. 2001;74:783–90 [DOI] [PubMed] [Google Scholar]
- 43.Douglas AS, Robins SP, Hutchison JD, Porter RW, Stewart A, Reid DM. Carboxylation of osteocalcin in post-menopausal osteoporotic women following vitamin K and D supplementation. Bone. 1995;17:15–20 [DOI] [PubMed] [Google Scholar]
- 44.Lukacs JL, Booth S, Kleerekoper M, Ansbacher R, Rock CL, Reame NE. Differential associations for menopause and age in measures of vitamin K, osteocalcin, and bone density: a cross-sectional exploratory study in healthy volunteers. Menopause. 2006;13:799–808 [DOI] [PubMed] [Google Scholar]
- 45.Craciun AM, Wolf J, Knapen MH, Brouns F, Vermeer C. Improved bone metabolism in female elite athletes after vitamin K supplementation. Int J Sports Med. 1998;19:479–84 [DOI] [PubMed] [Google Scholar]
- 46.Seibel MJ, Lang M, Geilenkeuser WJ. Interlaboratory variation of biochemical markers of bone turnover. Clin Chem. 2001;47:1443–50 [PubMed] [Google Scholar]
- 47.Cranenburg EC, Schurgers LJ, Vermeer C. Vitamin K: the coagulation vitamin that became omnipotent. Thromb Haemost. 2007;98:120–5 [PubMed] [Google Scholar]
- 48.Knapen MH, Nieuwenhuijzen-Kruseman AC, Wouters RSME, Vermeer C. Correlation of serum osteocalcin fractions with bone mineral density in women during the first 10 years after menopause. Calcif Tissue Int. 1998;63:375–9 [DOI] [PubMed] [Google Scholar]
- 49.Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, Cupples LA, Wilson PW, Kiel DP. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab. 2004;89:4904–9 [DOI] [PubMed] [Google Scholar]
- 50.Braam L, McKeown N, Jacques P, Lichtenstein A, Vermeer C, Wilson P, Booth S. Dietary phylloquinone intake as a potential marker for a heart-healthy dietary pattern in the Framingham Offspring cohort. J Am Diet Assoc. 2004;104:1410–4 [DOI] [PubMed] [Google Scholar]
- 51.Erkkilä AT, Booth SL, Hu FB, Jacques PF, Manson JE, Rexrode KM, Stampfer MJ, Lichtenstein AH. Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. Eur J Clin Nutr. 2005;59:196–204 [DOI] [PubMed] [Google Scholar]
- 52.Bolton-Smith C, McMurdo ME, Paterson CR, Mole PA, Harvey JM, Fenton ST, Prynne CJ, Mishra GD, Shearer MJ. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res. 2007;22:509–19 [DOI] [PubMed] [Google Scholar]
- 53.Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab. 2008;93:1217–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braam LA, Knapen MH, Geusens P, Brouns F, Hamulyak K, Gerichhausen MJ, Vermeer C. doi: 10.1007/s00223-002-2084-4. Vitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of age. Calcif Tissue Int. 2003;73:21–6. [DOI] [PubMed] [Google Scholar]
- 55.Cheung AM, Tile L, Lee Y, Tomlinson G, Hawker G, Scher J, Hu H, Vieth R, Thompson L, Jamal S, et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trial. PLoS Med. 2008;5:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Binkley N, Harke J, Krueger D, Engelke J, Vallarta-Ast N, Gemar D, Checovich M, Chappell R, Suttie J. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J Bone Miner Res. 2009;24:983–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.NRC Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011 [Google Scholar]
- 58.Morris MC, Tangney CC. A potential design flaw of randomized trials of vitamin supplements. JAMA. 2011;305:1348–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dougherty KA, Schall JI, Stallings VA. Suboptimal vitamin K status despite supplementation in children and young adults with cystic fibrosis. Am J Clin Nutr. 2010;92:660–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530–47 [PubMed] [Google Scholar]
- 61.Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008;283:11270–9 [DOI] [PubMed] [Google Scholar]
- 62.Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256–61 [DOI] [PubMed] [Google Scholar]
- 63.Fang Y, Hu C, Tao X, Wan Y, Tao F. Effect of vitamin K on bone mineral density: a meta-analysis of randomized controlled trials. J Bone Miner Metab. 2011;30:60–8 [DOI] [PubMed] [Google Scholar]
- 64.Inoue T, Fujita T, Kishimoto H, Makino T, Nakamura T, Sato T, Yamazaki K. Randomized controlled study on the prevention of osteoporotic fractures (OF study): a phase IV clinical study of 15-mg menatetrenone capsules. J Bone Miner Metab. 2009;27:66–75 [DOI] [PubMed] [Google Scholar]
- 65.Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int. 2007;18:963–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.European Food Safety Authority Scientific opinion on principles for deriving and applying dietary reference values. EFSA J. 2010;8:1458 [Google Scholar]
- 67.Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109:3279–83 [DOI] [PubMed] [Google Scholar]
- 68.Emaus N, Gjesdal CG, Almas B, Christensen M, Grimsgaard AS, Berntsen GK, Salomonsen L, Fonnebo V. Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos Int. 2010;21:1731–40 [DOI] [PubMed] [Google Scholar]
- 69.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA. 2008;105:5266–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Bruning JC, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salo J, Lehenkari P, Mulari M, Metsikko K, Vaananen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276:270–3 [DOI] [PubMed] [Google Scholar]
- 74.Nesbitt SA, Horton MA. Trafficking of matrix collagens through bone-resorbing osteoclasts. Science. 1997;276:266–9 [DOI] [PubMed] [Google Scholar]
- 75.Ivaska KK, Hentunen TA, Vaaraniemi J, Ylipahkala H, Pettersson K, Vaananen HK. Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J Biol Chem. 2004;279:18361–9 [DOI] [PubMed] [Google Scholar]
- 76.Kumm J, Ivaska KK. Rohtla K, Vaananen K, Tamm A. Urinary osteocalcin and other markers of bone metabolism: the effect of risedronate therapy. Scand J Clin Lab Invest. 2008;68:459–63 [DOI] [PubMed] [Google Scholar]
- 77.Poser JW, Price PA. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J Biol Chem. 1979;254:431–6 [PubMed] [Google Scholar]
- 78.Lowe H, Shane E. Osteoporosis. 3rd ed New York: Elsevier, Inc; 2008 [Google Scholar]
- 79.Schwartz AV. Diabetes mellitus: does it affect bone? Calcif Tissue Int. 2003;73:515–9 [DOI] [PubMed] [Google Scholar]
- 80.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes: a meta-analysis. Osteoporos Int. 2007;18:427–44 [DOI] [PubMed] [Google Scholar]
- 81.Reid IR. Relationships between fat and bone. Osteoporos Int. 2008;19:595–606 [DOI] [PubMed] [Google Scholar]
- 82.Ogata N, Chikazu D, Kubota N, Terauchi Y, Tobe K, Azuma Y, Ohta T, Kadowaki T, Nakamura K, Kawaguchi H. Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J Clin Invest. 2000;105:935–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prats-Puig A, Mas-Parareda M, Riera-Perez E, Gonzalez-Forcadell D, Mier C, Mallol-Guisset M, Diaz M, Bassols J, de Zegher F, Ibanez L, et al. Carboxylation of osteocalcin affects its association with metabolic parameters in healthy children. Diabetes Care. 2010;33:661–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94:827–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernández-Real JM, Izquierdo M, Ortega F, Gorostiaga E, Gomez-Ambrosi J, Moreno-Navarrete JM, Fruhbeck G, Martinez C, Idoate F, Salvador J, et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J Clin Endocrinol Metab. 2009;94:237–45 [DOI] [PubMed] [Google Scholar]
- 86.Hwang YC, Jeong IK, Ahn KJ, Chung HY. The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab Res Rev. 2009;25:768–72 [DOI] [PubMed] [Google Scholar]
- 87.Shea MK, Gundberg CM, Meigs JB, Dallal GE, Saltzman E, Yoshida M, Jacques PF, Booth SL. Gamma-carboxylation of osteocalcin and insulin resistance in older men and women. Am J Clin Nutr. 2009;90:1230–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beulens JW. van der A DL, Grobbee DE, Sluijs I, Spijkerman AM, van der Schouw YT. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care. 2010;33:1699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshida M, Booth SL, Meigs JB, Saltzman E, Jacques PF. Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am J Clin Nutr. 2008;88:210–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakamoto N, Nishiike T, Iguchi H, Sakamoto K. Possible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levels. Clin Nutr. 2000;19:259–63 [DOI] [PubMed] [Google Scholar]
- 91.Yoshida M, Jacques PF, Meigs JB, Saltzman E, Shea MK, Gundberg C, Dawson-Hughes B, Dallal G, Booth SL. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care. 2008;31:2092–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kumar R, Binkley N, Vella A. Effect of phylloquinone supplementation on glucose homeostasis in humans. Am J Clin Nutr. 2010;92:1528–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stenberg LM, Nilsson E, Ljungberg O, Stenflo J, Brown MA. Synthesis of gamma-carboxylated polypeptides by alpha-cells of the pancreatic islets. Biochem Biophys Res Commun. 2001;283:454–9 [DOI] [PubMed] [Google Scholar]
- 94.Bailey CJ, Atkins TW, Matty AJ. Effect of sodium warfarin of plasma insulin estimations. Gen Pharmacol. 1976;7:63–5 [DOI] [PubMed] [Google Scholar]
- 95.Hetzel PG, Glanzmann R, Hasler PW, Ladewick A, Buhrer C. Coumarin embryopathy in an extremely low birth weight infant associated with neonatal hepatitis and ocular malformations. Eur J Pediatr. 2006;165:358–60 [DOI] [PubMed] [Google Scholar]
- 96.Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, Crowther M, Wells PS. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095–106 [DOI] [PubMed] [Google Scholar]
- 97.Scheen AJ. Drug interactions of clinical importance with antihyperglycaemic agents: an update. Drug Saf. 2005;28:601–31 [DOI] [PubMed] [Google Scholar]
- 98.Wilke RA, Berg RL, Vidaillet HJ, Caldwell MD, Burmester JK, Hillman MA. Impact of age, CYP2C9 genotype and concomitant medication on the rate of rise for prothrombin time during the first 30 days of warfarin therapy. Clin Med Res. 2005;3:207–13 [DOI] [PMC free article] [PubMed] [Google Scholar]