Abstract
Seventeen vitamin K–dependent proteins have been identified to date of which several are involved in regulating soft-tissue calcification. Osteocalcin, matrix Gla protein (MGP), and possibly Gla-rich protein are all inhibitors of soft-tissue calcification and need vitamin K–dependent carboxylation for activity. A common characteristic is their low molecular weight, and it has been postulated that their small size is essential for calcification inhibition within tissues. MGP is synthesized by vascular smooth muscle cells and is the most important inhibitor of arterial mineralization currently known. Remarkably, the extrahepatic Gla proteins mentioned are only partly carboxylated in the healthy adult population, suggesting vitamin K insufficiency. Because carboxylation of the most essential Gla proteins is localized in the liver and that of the less essential Gla proteins in the extrahepatic tissues, a transport system has evolved ensuring preferential distribution of dietary vitamin K to the liver when vitamin K is limiting. This is why the first signs of vitamin K insufficiency are seen as undercarboxylation of the extrahepatic Gla proteins. New conformation-specific assays for circulating uncarboxylated MGP were developed; an assay for desphospho-uncarboxylated matrix Gla protein and another assay for total uncarboxylated matrix Gla protein. Circulating desphospho-uncarboxylated matrix Gla protein was found to be predictive of cardiovascular risk and mortality, whereas circulating total uncarboxylated matrix Gla protein was associated with the extent of prevalent arterial calcification. Vitamin K intervention studies have shown that MGP carboxylation can be increased dose dependently, but thus far only 1 study with clinical endpoints has been completed. This study showed maintenance of vascular elasticity during a 3-y supplementation period, with a parallel 12% loss of elasticity in the placebo group. More studies, both in healthy subjects and in patients at risk of vascular calcification, are required before conclusions can be drawn.
Introduction
In vertebrates, all extracellular body fluids are supersaturated with respect to calcium and phosphate, resulting in a tendency for spontaneous calcium phosphate precipitation, which is often expressed as the calcium × inorganic phosphate product (1). Potent inhibitors of calcium salt precipitation and crystallization are therefore essential for survival, and consequently a broad range of low and high molecular weight inhibitors are found in the circulation. Low molecular weight inhibitors include pyrophosphate and citrate, whereas the most potent inhibitors are small and medium-sized proteins.
Fetuin-A is a 48-kD protein synthesized in the liver and secreted into the circulation; it is the most important systemic inhibitor of soft-tissue calcification. Transgenic fetuin-A–deficient mice display a severe diffuse systemic calcification phenotype with punctuate calcified lesions in most tissues (2). Besides fetuin-A, a number of small vitamin K–dependent proteins have been discovered acting as potent calcification inhibitors. Examples are osteocalcin (OC), also known as bone Gla protein, matrix Gla protein (MGP), and possibly also the newly discovered Gla-rich protein (GRP), which are all proteins between 5 and ∼10 kD. In contrast to fetuin-A, these proteins are local inhibitors of calcification, i.e., they are synthesized in the tissues in which they exert their function. OC is synthesized by the osteoblasts in bone, and transgenic OC-deficient mice show increased bone mineral content (3). MGP is primarily synthesized by chondrocytes and vascular smooth muscle cells, and MGP-deficient mice show impaired growth (resulting from excessive growth plate calcification) and massive calcification of the arterial tunica media (4). An interesting question is why fetuin-A alone is unable to stop calcification of these tissues. Recently, a hypothesis was presented that provided a possible answer to this question. In their paper, Price et al. (5) demonstrate that with its molecular mass of 48 kD, fetuin-A is too large to penetrate the luminal space within collagen and elastin fibrils. But if no other inhibitors are present, these fibrils will rapidly calcify. Only calcification inhibitors that are small enough to penetrate the collagen and elastin fibrils, i.e., OC and MGP, will be able to move freely into the fibril and prevent mineral growth inside. This explains why the elastin fibrils are prone to calcification, especially during vitamin K insufficiency, and demonstrates the vital importance of vitamin K in the prevention of soft-tissue calcification. In this paper, we review the current knowledge in this field, especially with respect to the role of MGP in the regulation of soft-tissue calcification, which may have implications for the risk of cardiovascular and other diseases.
Current status of knowledge
Vitamin K status and insufficiency
Animal models.
OC was the first Gla protein discovered not to be involved in blood coagulation and also the first Gla protein found to be synthesized outside the liver (6–8). Remarkably, the molecular role of OC has remained obscure for >30 y. In vitro experiments with purified OC showed that it is a strong inhibitor of calcium salt precipitation from supersaturated solutions (9). OC-deficient animals show increased bone size and high bone mineral content (3). Therefore, OC was described as a negative regulator of bone growth. Only after the hypothesis of mineralization by inhibitor exclusion was put forward did it become clear how OC may contribute to the controlled deposition of hydroxyapatite in bone (5).
In contrast to OC, the phenotype of MGP-deficient mice immediately showed the function of MGP (4). MGP−/− animals were smaller than their heterozygous littermates, resulting from calcification of the epiphysis that blocked longitudinal growth. Moreover, all large arteries rapidly calcified, resulting in death at 6–8 wk after birth. Tissue-specific expression of MGP in the vascular smooth muscle cells completely prevented arterial calcification; hepatic MGP expression did result in high circulating MGP levels, but not in the prevention of artery calcification (10). These data clearly showed that MGP is a local inhibitor of calcification and thus complementary to the systemic action of fetuin-A.
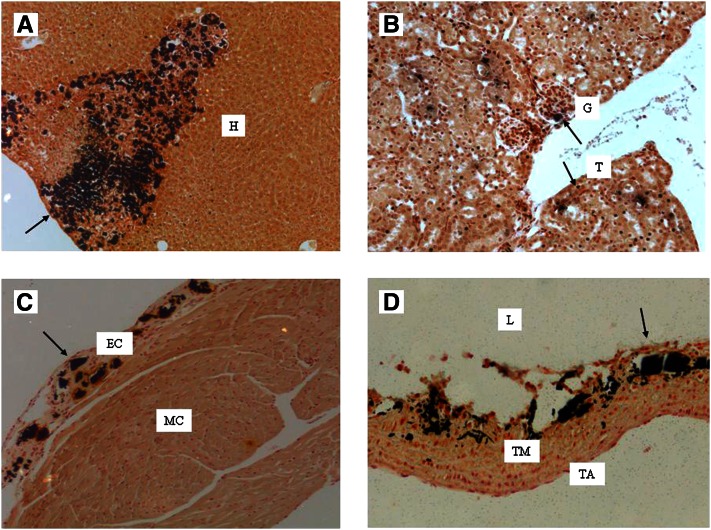
The effects of MGP deficiency could be mimicked by feeding rodents a diet containing a mixture of warfarin and phylloquinone. The warfarin + phylloquinone model is based on the preferential distribution of phylloquinone to the liver. By combining a high dose of warfarin with a relatively low dose of phylloquinone, hepatic synthesis of blood coagulation factors can be maintained at a level that prevents bleeding, and severe vitamin K deficiency will be evoked in all extrahepatic tissues. The phenotype of this model is comparable to that of the MGP-deficient mouse showing calcification of the epiphysis and severe arterial calcifications. Initially, the reported model required daily injections with phylloquinone (11, 12), but in our laboratory, a regimen was developed in which both warfarin and phylloquinone could be given in the diet (13). The calcifications (visualized in black by von Kossa staining) in a mouse on the warfarin + phylloquinone diet are shown in Figure 1. Next to arterial calcifications, severe calcifications were found in other tissues, such as the liver, kidney, and heart.
Figure 1.
Calcification induced by the warfarin + phylloquinone regimen. Calcifications (visualized by von Kossa staining) induced by feeding a DBA2 mouse warfarin (3 mg/g) and phylloquinone (1.5 mg/g) for 6 wk. A, liver; B, kidney; C, heart; D, aorta. The arrows indicate areas of calcifications. TA, tunica adventitia; EC, endocardium; H, hepatocytes; G, glomerulus; L, lumen; MC, myocardium; T, tubulus; TM, tunica media. (C. Vermeer et al., VitaK, Maastricht University, the Netherlands, unpublished results).
Human equivalents of animal models.
MGP deficiency in humans is known as the Keutel syndrome, a rare autosomal recessive disease that was first described in 1971 (14). Affected subjects exhibit severe cartilage calcifications, brachytelephalangism, peripheral pulmonary artery stenosis, hearing loss, and facial abnormalities (i.e., midface hypoplasia and a depressed nasal bridge). Keutel syndrome was linked to mutations in the gene coding for MGP leading to the absence or nonfunctional forms of MGP (15, 16). A striking difference with MGP-deficient animals is that effects on the vasculature are less prominent, and an explanation may be that GRP forms a backup system for MGP in humans and not in mice. GRP was discovered in the cartilage of sturgeons (17), but is also found in mammals, including humans (18). Its function has not yet been established unequivocally, but seems to be related to calcification inhibition as well (19).
Human equivalents of the warfarin + phylloquinone animals are patients using oral anticoagulants [also known as vitamin K antagonists (VKA)], including warfarin, acenocoumarol, and phenprocoumon. These drugs are widely prescribed to subjects with an increased thrombosis risk and are often taken for many years or lifelong. Obviously, their mode of action is systemic, and all vitamin K-dependent calcification inhibitors (including OC, MGP, and GRP) will remain uncarboxylated and thus inactive during VKA treatment. The first study demonstrating strongly increased calcification of the aortic valves in patients taking oral anticoagulants (20) has been confirmed by many other researchers. Koos et al. (21) demonstrated that subjects taking anticoagulants had a significantly higher degree of arterial and aortic valve calcification than control subjects. In a case report, Hristova et al. (22) described a kidney transplant recipient exhibiting massive arterial calcification after the initiation of warfarin therapy. Distal subcutaneous necrosis ultimately led to the patient’s death. Rennenberg et al. (23) showed rapid calcification of the femoral artery when treating patients with oral anticoagulants, with an OR of 8.5 for calcification in patients compared with controls. Similarly, patients taking oral anticoagulants showed significantly increased levels of coronary calcification (24). From these and other data, we conclude that the use of VKA results in decreased carboxylation of MGP and probably also GRP, which may form a strong risk factor for calcification of the vasculature and heart valves.
Triage theory.
Preferential distribution of phylloquinone to the liver is consistent with the triage theory proposed by McCann and Ames (25). Nature ensures that at suboptimal supply, vitamins and minerals are primarily used for functions required for short-term survival. Because carboxylation of the most essential Gla proteins is localized in the liver and that of the less essential Gla proteins in the extrahepatic tissues, a transport system has evolved ensuring preferential targeting to the liver to preserve coagulation when dietary vitamin K is inadequate. Only at hepatic vitamin K sufficiency, particularly the long-chain menaquinones, are transported to extrahepatic tissues. This is why the first signs of vitamin K insufficiency are seen as incomplete carboxylation of extrahepatic Gla proteins. McCann and Ames concluded that long-term micronutrient insufficiencies are a risk factor for the development of a wide variety of age-related diseases, such as osteoporosis, cardiovascular disease (CVD), and cancer.
The extrahepatic Gla proteins OC and MGP, for which conformation-specific tests have been developed, exhibit substantial undercarboxylation in subjects not taking vitamin K supplements. Recently, a conformation-specific test for uncarboxylated GRP was described, showing that this protein also partially occurs in a noncarboxylated form (M. Herfs, E. Smit, C. Viegas, S. Simes, C. Vermeer, VitaK, Maastricht University, the Netherlands, unpublished results). At this time, there is no example of an extrahepatic Gla protein demonstrated to be fully carboxylated in nonsupplemented healthy subjects. We conclude that the Western diet contains insufficient vitamin K to ascertain the requirements of extrahepatic tissues, such as bone and the vascular wall.
MGP, vitamin K status, and calcification
MGP exists as various distinct species according to its state of phosphorylation and/or carboxylation, including phosphorylated carboxylated MGP (cGMP), phosphorylated ucMGP (p-ucMGP), desphospho-carboxylated matrix Gla protein (dp-cMGP), and desphospho-uncarboxylated matrix Gla protein (dp-ucMGP). Unfortunately, there are currently no assays available to measure each individual circulating MGP species or even the total circulating MGP pool. An overview of published papers in which circulating MGP species—dp-ucMGP, dp-cMGP, total uncarboxylated matrix Gla protein (t-ucMGP)—have been used to explore their potential diagnostic utility is given in the following (see also TABLES 1 and 2).
Table 1.
Overview of associations between circulating MGP (dp-ucMGP, dp-cMGP, t-ucMGP) and calcification1
| Population, age | MGP species | MGP categories | Calcification score | Statistics |
| 96 HT patients, 53 y2 | t-ucMGP(nmol/L) | 4361 ± 1111 | Vascular calcification (scored as present/not present by renal angiography) | Correlation analysis (P = 0.164) |
| 438 adults, 68 y (32) | dp-ucMGP (pmol/L) | 237 (52–330) 403 (335–462) 524 (464–599) 826 (604–2994) | 149 ± 50 263 ± 48 243 ± 49 235 ± 47 (CAC) | ANCOVA (P = 0.55) |
| 188 dialysis patients, 59 y (29) | dp-cMGP (pmol/L) | 12% lower levels in high score group | 0–6 (low) 7–15 (high) Total calcification (pelvis + hands + carotids) | t test (P = 0.011) |
| 36 HT patients, 53 y (30) | t-ucMGP (nmol/L) | 3471 (2031–4260) 4708 (4351–5215) 6126 (5416–9603) | 145 (0–1546) 111 (1–3866) 36 (0–5951) (CAC) | Kruskal-Wallis (NS) |
| 19 OAC patients, 48 y (23) | dp-ucMGP (pmol/L) | 1439 ± 481 | Femoral artery calcification | Correlation analysis (r = 0.59, P < 0.001), MV regression analysis3 (P < 0.05, β = 0.68) |
| 107 CKD patients, 67 y (31) | dp-ucMGP (pmol/L) | ⩽921 >921 | 2.4 ± 2.9 4.4 ± 3.1 (AC) | t test (P < 0.001) |
| 615 CVD patients without DM, 68 y (36) | t-ucMGP (nmol/L) | 3287 ± 1178 | Mitral annular calcification | Logistic regression (OR = 0.64, P < 0.001; MV4 OR = 0.73, P = 0.03) |
| 221 CVD patients with DM, 68 y (36) | t-ucMGP (nmol/L) | 3287 ± 1178 | Mitral annular calcification | Logistic regression (OR = 1.29, P = 0.08; MV4 OR = 1.89, P = 0.001) |
| 191 AVD patients, 71 y (35) | t-ucMGP (nmol/L) | 320 400 | 1800 (OAC use) 400 (no OAC use) (CAC ) | ND |
| 40 HD patients,67 y (34) | t-ucMGP (nmol/L) | 237 ± 66 174 ± 46* 171 ± 66** | ≤103.0 (low) 103.1–600.0 ≥600.1 (CAC) | Fisher exact (*P = 0.022 and **P = 0.021 compared with low CAC score) |
AC, aortic calcification; AVD, aortic valve disease; CAC, coronary artery calcification; CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus 2; dp-cMGP, desphospho-carboxylated matrix Gla protein; dp-ucMGP, desphospho-uncarboxylated matrix Gla protein; HD, hemodialysis; HT, hypertensive; MGP, matrix Gla protein; MV OR, multivariable adjusted OR; ND, not determined; OAC, oral anticoagulant; t-ucMGP, total uncarboxylated matrix Gla protein; ucMGP, uncarboxylated matrix Gla protein.
R. Rennenberg, L. Sckungers, B. Van Varik, E. Magdeleyns, C. Vermeer, P. de Leeuw, A. Kroon. Maastricht University Medical Center, Maastricht, the Netherlands, unpublished results.
Adjusted for age, fasting glucose, LDL cholesterol, estimated creatinine clearance, and calcium phosphate product.
Adjusted for age, sex, race, BMI, hypertension, smoking, blood pressure, albumin, total cholesterol, HDL, C-reactive protein, and epidermal growth factor receptor.
Table 2.
Overview of associations between circulating MGP (dp-ucMGP, dp-cMGP, t-ucMGP) and mortality1
| Population, age | MGP species | MGP categories | Statistics, CV mortality | Statistics, all-cause mortality |
| 179 chronic HF patients, 56 y (28) | dp-ucMGP (pmol/L) | −2 SD −1 SD +1 SD +2 SD | Kaplan-Meier (P = 0.001) MV Cox regression2 (HR = 5.62, P = 0.001) | Kaplan-Meier (P = 0.002) |
| 188 dialysis patients, 59 y (29) | dp-cMGP (pmol/L) | <6139 >6139 | Kaplan-Meier (P = 0.003)MV Cox regression3 (HR = 2.74, P = 0.015) | Kaplan-Meier (P = 0.008) MV Cox regression3 (HR = 2.16, P = 0.027) |
| 147 AS patients, 74 y (27) | dp-ucMGP (pmol/L) | <950 >950 | ND | Kaplan-Meier (P < 0.001) MV Cox regression4 (HR = 7.29, P = 0.002) |
| dp-cMGP (pmol/L) | <2400 >2400 | ND | Kaplan-Meier (P < 0.001), MV analysis (NS) | |
| 833 CVD patients, 67 y (36) | t-ucMGP (nmol/L) | <2757 (ref) 2757–3649 >3649 | Kaplan-Meier (NS5) MV Cox regression6 (HR = 0.65, NS5) | Kaplan-Meier (P < 0.05) MV Cox regression6 (HR = 0.48, P < 0.05) |
| 107 CKD patients, 67 y (31) | dp-ucMGP (pmol/L) | ≤921 >921 | ND | Kaplan-Meier (P = 0.006) MV Cox regression7 (HR = 1.57, NS) |
AS, aortic stenosis; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; dp-cMGP, desphospho-carboxylated matrix Gla protein; dp-ucMGP, desphospho-uncarboxylated matrix Gla protein; HF, heart failure; MV, multivariate; ND, not determined; t-ucMGP, total uncarboxylated matrix Gla protein.
Adjusted for New York Heart Association functional class II–IV, etiology, previous myocardial infarction, creatinine, C-reactive protein, N-terminal pro–B-type natriuretic peptide, warfarin, aspririn use.
Adjusted for age, calcium, phosphate, high-sensitivity C-reactive protein.
Adjusted for age, sex, BMI, epidermal growth factor receptor, N-terminal pro–B-type natriuretic peptide, C-reactive protein, hypertension, diabetes mellitus, left ventricular ejection fraction.
Significant when modeled continuously (instead of tertile-based modeling).
Adjusted for age, sex, race, waist-to-hip ratio, smoking, hypertension, diabetes mellitus, blood pressure, epidermal growth factor receptor, total cholesterol, HDL cholesterol, C-reactive protein, ejection fraction, peak exercise capacity, use of aspirin, β-blockers, statins, anticoagulants.
Adjusted for age, calculated propensity score.
dp-ucMGP.
Uncarboxylated species of MGP have been detected in the healthy population and may be a useful marker of vascular vitamin K status and disease. The most robust assay available to date is that for circulating MGP lacking both posttranslational modifications, i.e., serine phosphorylation and γ-glutamate carboxylation. This fraction of total circulating MGP is dp-ucMGP. As for uncarboxylated OC, changes in systemic vitamin K status resulting from the use of vitamin K supplementation or vitamin K antagonists are reflected in circulating levels of dp-ucMGP. Circulating dp-ucMGP levels were shown to correlate with age in healthy adults, with significantly higher levels in the elderly (65 y and older) (26). Case-control studies showed higher dp-ucMGP values among patients at risk of vascular calcification, including those with rheumatoid arthritis, aortic valve disease, aortic stenosis (AS), heart failure (HF), chronic kidney disease (CKD), and patients taking VKA (26–30). Extremely high dp-ucMGP values were measured in dialysis patients with median dp-ucMGP levels that were 3–5 times higher than in the corresponding healthy controls. It was further shown in 107 CKD patients that plasma dp-ucMGP levels increased progressively with CKD stage (31). High circulating dp-ucMGP could reflect a low dietary intake of vitamin K, resulting in lower inhibition of artery calcification.
In CKD patients, increased dp-ucMGP levels (>921 pmol/L) were associated with aortic calcification and overall mortality (31). The association with mortality was, however, lost after correction for age and other confounders, such as CKD stage, hemoglobin, and the aortic calcification score. In this patient group, patients on VKA therapy presented significantly higher levels of dp-ucMGP. A separate study in 19 VKA patients found a significant correlation between plasma dp-ucMGP levels and femoral artery calcification (30). The relationship between dp-ucMGP and vascular calcification could not be confirmed in a recently studied cohort of healthy elderly (32). Elevated levels of dp-ucMGP (>950 pmol/L) were also associated with overall mortality (adjusted HR >7) in 147 AS patients (27). Again, a positive association was found between the use of VKA and dp-ucMGP levels. In the non-VKA users in this patient group, circulating dp-ucMGP was significantly correlated with neurohormonal and hemodynamic markers of HF severity. Plasma dp-ucMGP levels were shown to increase with disease severity, as assessed by clinical, neurohormonal, and hemodynamic measurements, in a separate cohort of 179 HF patients (28). Moreover, high levels of dp-ucMGP were associated with mortality due to progression of HF (adjusted HR >5). Also in this cohort, VKA use influenced plasma dp-ucMGP levels. In conclusion, all published data show that dp-ucMGP is inversely correlated with vitamin K intake/status, and in most studies, this marker is also inversely correlated with survival and life expectancy.
dp-cMGP.
Theoretically, this marker forms the mirror image of dp-ucMGP, and 2 studies have been published investigating the association with survival. Ueland et al. (27) found that AS patients with above the median levels of dp-cMGP had a higher unadjusted mortality rate, but in the multivariate model the significance of these associations were lost. Schlieper et al. (29) studied a cohort of 188 hemodialysis (HD) patients and found an inverse association between dp-cMGP and both cardiovascular (HR >2) and overall mortality (adjusted HR >2). Circulating dp-cMGP levels were 12% lower in the HD patents with more extensive calcifications (total calcification score of 7–15) compared with the HD patients with fewer calcifications (total calcification score of 0–6). When interpreting these data, it should be realized that the dp-cMGP assay became available only recently and has only been used on a limited scale; also its sensitivity is less than that of the dp-ucMGP assay.
t-ucMGP.
In contrast to both assays described above, the principle of the t-ucMGP assay is not that of a sandwich ELISA, but that of a competitive, single antibody assay. The fraction recognized by this assay is the sum of p-ucMGP and dp-ucMGP. Because the observed plasma levels are >1000-fold higher than those of dp-ucMGP, we assume that the t-ucMGP mainly consists of phosphorylated ucMGP species. The phosphoserines equip the molecule with strong calcium-binding groups irrespective of the Gla content, and the data observed can best be explained by assuming that the majority of this fraction has a strong affinity for vascular calcifications. This is illustrated by the fact that low t-ucMGP levels have been found in patients prone to vascular calcification, including patients with rheumatoid arthritis, dialysis patients, and patients with CVD defined as myocardial infarction, coronary artery disease, angioplasty, AS, and calciphylaxis (26, 33). In particular, dialysis and calciphylaxis patients were characterized by extremely low values; nearly all patients had values well below the healthy adult range. In healthy adults, serum t-ucMGP levels were shown to be similar among the indicated age groups: younger than 25 y, 25–65 y, and older than 65 y (26). Nevertheless, the circulating t-ucMGP value could discriminate between healthy controls and subjects with moderate to severe hypertension (R. Rennenberg et al., Maastricht University Medical Center, Maastricht, the Netherlands, unpublished results).
In studies in which arterial calcification was quantified, low circulating t-ucMGP levels were found to parallel high calcification scores, but there are insufficient data to suggest a causal relationship, for instance, by direct binding of t-ucMGP to the sites of calcification. Lower circulating t-ucMGP levels were associated with higher calcification scores in 40 HD patients (34), 191 patients with aortic valve disease (35), and >800 patients with stable CVD (36). Among the patients with stable CVD, this inverse association was limited to patients without diabetes (n = 615). The mechanism responsible for the effect modification by diabetes state could, however, not be explained. Also, more calcification was seen in 36 hypertensive patients with the lowest values of t-ucMGP, but the trend across the t-ucMGP tertiles was not significant (study 1) (30). In another hypertensive patient group (n = 96), no significant correlation was found between circulating t-ucMGP levels and the presence of calcification, as determined during renal angiography (study 2) (R. Rennenberg et al., Maastricht University Medical Center, Maastricht, the Netherlands, unpublished results). The lack of statistical significance may be explained by the small patient population (study 1) or by the atypical method used to determine calcification (study 2). In 200 healthy postmenopausal females, the inverse relationship between t-ucMGP and calcification was only found in women with clear coronary artery calcification (CAC), i.e., CAC score >0 (G. Dalmeijer et al., Julius Center for Health Sciences and Primary Care, University medical Center Utrecht, the Netherlands, unpublished results). Circulating t-ucMGP levels were significantly lower in those with CAC (n = 75) compared with those without CAC (n = 125, CAC score <10).
Like dp-ucMGP and dp-cMGP, circulating t-ucMGP was also associated with mortality risk (36). Compared with CVD patients in the lowest t-ucMGP tertile, CVD patients in the highest tertile had ∼50% lower risk of mortality in models adjusted for age, sex, and race. This association remained after adjustment for traditional CVD risk factors, kidney function, C-reactive protein, ejection fraction, peak exercise capacity, and medication use, but was limited to persons without diabetes (n = 610). The association of t-ucMGP with CVD events was in the same direction, but of lesser magnitude. When t-ucMGP was evaluated as a continuous predictor variable, each 1000-nmol/L higher t-ucMGP level was associated with a 22% lower risk of mortality and a 16% lower risk of CVD events.
Taken together, the data that have been published thus far suggest that dp-ucMGP may become a risk marker for cardiovascular disease and mortality, whereas t-ucMGP, through its calcium-binding capacity, may become a marker for vascular calcification. At present, we are developing an assay for p-cMGP, which on a theoretical basis has the highest calcium-binding capacity and would therefore be the marker of choice for monitoring vascular calcifications.
Immunohistochemical staining.
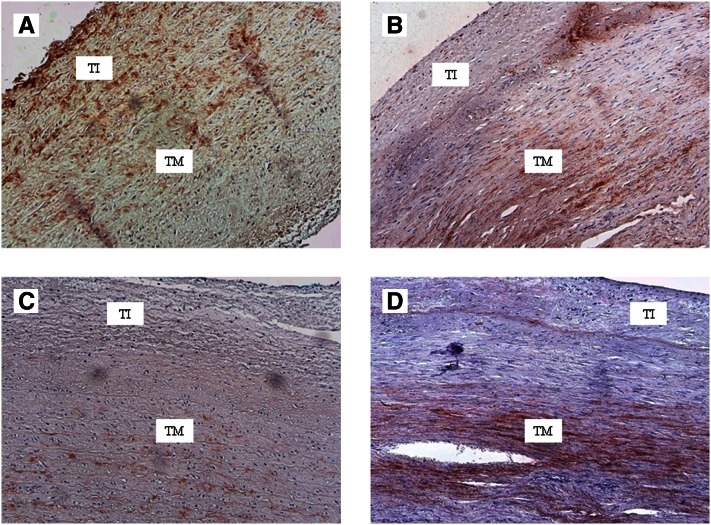
Conformation-specific anti-MGP antibodies have also been used for immunohistochemical staining of healthy and atherosclerotic vessels. In healthy arteries, there is little accumulation of either cMGP or ucMGP. In calcified atherosclerotic lesions as well as in Mönckeberg’s media sclerosis, however, both forms of MGP accumulate, probably because of increased expression. It was reported that elevated levels of calcium trigger MGP expression in cultures vascular smooth muscle cells (37). As shown in , cMGP is the most predominant form in early lesions, whereas ucMGP is mainly found around atherosclerotic lesions.
Figure 2.
Carboxylated matrix Gla protein (cMGP) and uncarboxylated matrix Gla protein (ucMGP) in the arterial wall. Early and advanced lesions in the arterial wall were stained (red) for cMGP and ucMGP. A, cMGP in early lesion. B, cMGP in advanced lesion. C, ucMGP in early lesion. D, ucMGP in advanced lesion. TI, tunica intima; TM, tunica media. (C. Vermeer et al., VitaK, Maastricht University, the Netherlands, unpublished results).
Vitamin K status and supplementation
Animal studies.
Using the warfarin + phylloquinone model for arterial calcification in rodents, Spronk et al. (13) showed that menaquinone-4 and not phylloquinone inhibited warfarin-induced arterial calcification. This is explained by the different transport and tissue distribution of menaquinones, resulting in a much more prominent protective effect of menaquinones (compared with phylloquinone) on arterial calcification. Later studies, in which the long-chain menaquinone-7 (MK-7) was used to counteract warfarin-induced calcification, showed that this form of menaquinone was even more potent than menaquinone-4. This is consistent with population-based studies in which only the long-chain menaquinones had cardioprotective effects.
Human intervention studies.
In a limited number of 3-mo clinical trials, it was demonstrated that vitamin K–containing supplements result in a strong and significant decrease of circulating dp-ucMGP in healthy adults (26, 38, 39). Thus far, most data have been obtained with MK-7; MK-7 was required in doses between 45 μg/d and 90 μg/d to find a statistically significant effect on MGP carboxylation. Based on its shorter half-life and different tissue-distribution pattern, it is to be expected that higher doses will be required for phylloquinone. Thus far, only one 3-y clinical trial has been published with clinical endpoints. In this 3-y study, 181 postmenopausal women received either phylloquinone (1 mg/d) or placebo against a background of cholecalciferol (8 μg/d). Although Young’s elasticity modulus (a marker for vascular stiffening) decreased by 12% in the placebo group, vascular characteristics remained unchanged in the vitamin K group (40).
It is well-known that CKD patients are at high risk of vascular calcification, and each year 10% of the HD population will die of cardiovascular complications. We have found that circulating dp-ucMGP levels in these patients are higher than in any other patient group, in many cases even higher than in those taking oral anticoagulants. This is suggestive of a very poor vitamin K status of the vascular wall. Obviously, this group is an ideal target for testing whether vitamin K supplements can normalize the circulating dp-ucMGP levels and whether the change in vascular vitamin K status will result in an improved clinical outcome. Westenfeld et al. (41) demonstrated in HD patients that indeed menaquinone supplements induce a dose-dependent decrease in plasma dp-ucMGP levels.
Recently, we entered into collaborative studies with the Maastricht University Medical Center (Maastricht, the Netherlands) and the RWTH Aachen University (Aachen, Germany) in which clinical endpoints will be monitored in 2 independent trials among CVD patients. In 1 trial, the effect of menaquinones (MenaQ7, 360 μg/d) on the progression of CAC is investigated in Dutch patients with established coronary artery disease. In the second trial, the effect of phylloquinone (2 mg/d) on the progression of aortic valve calcification is monitored in German patients with preexisting valve calcification. The potential of vitamin K as a modifiable risk factor is especially exciting because of its simplicity: with the aid of food supplements or fortified foods, vitamin K consumption can be increased without major changes in the dietary pattern.
CONCLUSIONS
The triage theory predicts that, with limited intake, vitamins are transported to those tissues where they are important for immediate survival of the organism. For vitamin K, this implies a preferential transport to the liver, the organ where the coagulation factors are synthesized. Without doubt, bleeding caused by an impaired hemostatic system is the most life-threatening consequence of vitamin K deficiency. This mechanism explains why vitamin K insufficiency will occur sooner and be more pronounced in extrahepatic tissue than in the liver and is consistent with the relatively high levels of circulating uncarboxylated OC and dp-ucMGP observed in healthy subjects. The question is whether the undercarboxylation (10–40%) of circulating OC, MGP, or GRP, as seen in the healthy adult population, is associated with an increased risk of age-related diseases, such as soft-tissue calcification. Only long-term vitamin K intervention studies in healthy subjects may answer this question. The very high levels of dp-ucMGP found in certain patient groups warrant studies investigating a beneficial clinical outcome of vitamin K supplementation. Notably, HD patients form an excellent model for apparent vascular vitamin K insufficiency and concomitant arterial calcification. A second target group for vitamin K supplementation studies is formed by subjects taking high doses of supplemental calcium. It has been reported that the increased calcium load is a risk factor for vascular calcification. Finally, it should be mentioned that most vitamin K intervention studies have used high doses of vitamin K (both phylloquinone and menaquinones). Benefits of increased vitamin K intake at nutritionally relevant doses in healthy subjects await the outcomes of clinical trials currently in progress.
Acknowledgments
All authors have read and approved the manuscript.
Footnotes
Conflicts of interest: E. Theuwissen, E. Smit, and C. Vermeer, no conflicts of interest.
Abbreviations used: AS, aortic stenosis; CAC, coronary artery calcification; cGMP, carboxylated matrix Gla protein; CKD, chronic kidney disease; CVD, cardiovascular disease; dp-cMGP, desphospho-carboxylated matrix Gla protein; dp-ucMGP, desphospho-uncarboxylated matrix Gla protein; GRP, Gla-rich protein; HD, hemodialysis; HF, heart failure; MGP, matrix Gla protein; MK-7, menaquinone-7; OC, osteocalcin; t-ucMGP, total uncarboxylated matrix Gla protein; ucMGP, uncarboxylated matrix Gla protein; VKA, vitamin K antagonist.
Literature Cited
- 1.Heiss A, Eckert T, Aretz A, Richtering W, van Dorp W, Schafer C, Jahnen-Dechent W. Hierarchical role of fetuin-A and acidic serum proteins in the formation and stabilization of calcium phosphate particles. J Biol Chem. 2008;283:14815–25 [DOI] [PubMed] [Google Scholar]
- 2.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52 [DOI] [PubMed] [Google Scholar]
- 4.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81 [DOI] [PubMed] [Google Scholar]
- 5.Price PA, Toroian D, Lim JE. Mineralization by inhibitor exclusion: the calcification of collagen with fetuin. J Biol Chem. 2009;284:17092–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price PA, Otsuka AA, Poser JW, Kristaponis J, Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A. 1976;73:1447–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauschka PV, Reid ML. Vitamin D dependence of a calcium-binding protein containing gamma-carboxyglutamic acid in chicken bone. J Biol Chem. 1978;253:9063–8 [PubMed] [Google Scholar]
- 8.Hauschka PV, Lian JB, Gallop PM. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A. 1975;72:3925–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Loo PG, Soute BA, van Haarlem LJ, Vermeer C. The effect of Gla-containing proteins on the precipitation of insoluble salts. Biochem Biophys Res Commun. 1987;142:113–9 [DOI] [PubMed] [Google Scholar]
- 10.Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price PA, Williamson MK, Haba T, Dell RB, Jee WS. Excessive mineralization with growth plate closure in rats on chronic warfarin treatment. Proc Natl Acad Sci U S A. 1982;79:7734–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–7 [DOI] [PubMed] [Google Scholar]
- 13.Spronk HM, Soute BA, Schurgers LJ, Thijssen HH, De Mey JG, Vermeer C. Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated rats. J Vasc Res. 2003;40:531–7 [DOI] [PubMed] [Google Scholar]
- 14.Keutel J, Jorgensen G, Gabriel P. A new autosomal-recessive hereditary syndrome. Multiple peripheral pulmonary stenosis, brachytelephalangia, inner-ear deafness, ossification or calcification of cartilages [in German]. Dtsch Med Wochenschr. 1971;96:1676–81, passim [DOI] [PubMed] [Google Scholar]
- 15.Munroe PB, Olgunturk RO, Fryns JP, Van Maldergem L, Ziereisen F, Yuksel B, Gardiner RM, Chung E. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat Genet. 1999;21:142–4 [DOI] [PubMed] [Google Scholar]
- 16.Cranenburg EC, van Spaendonck-Zwarts KY, Bonafe L, Mittaz Crettol L, Rödiger LA, Dikkers FG, van Essen AJ, Superti-Furga A, Alexandrakis E, Vermeer C, et al. Circulating matrix gamma-carboxyglutamate protein (MGP) species are refractory to vitamin K treatment in a new case of Keutel syndrome. J Thromb Haemost. 2011;9:1225–35 [DOI] [PubMed] [Google Scholar]
- 17.Viegas CS, Simes DC, Laize V, Williamson MK, Price PA, Cancela ML. Gla-rich protein (GRP), a new vitamin K-dependent protein identified from sturgeon cartilage and highly conserved in vertebrates. J Biol Chem. 2008;283:36655–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tagariello A, Luther J, Streiter M, Didt-Koziel L, Wuelling M, Surmann-Schmitt C, Stock M, Adam N, Vortkamp A, Winterpacht A. Ucma–a novel secreted factor represents a highly specific marker for distal chondrocytes. Matrix Biol. 2008;27:3–11 [DOI] [PubMed] [Google Scholar]
- 19.Viegas CS, Cavaco S, Neves PL, Ferreira A, Joao A, Williamson MK, Price PA, Cancela ML, Simes DC. Gla-rich protein is a novel vitamin K-dependent protein present in serum that accumulates at sites of pathological calcifications. Am J Pathol. 2009;175:2288–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schurgers LJ, Aebert H, Vermeer C, Bultmann B, Janzen J. Oral anticoagulant treatment: friend or foe in cardiovascular disease? Blood. 2004;104:3231–2 [DOI] [PubMed] [Google Scholar]
- 21.Koos R, Mahnken AH, Muhlenbruch G, Brandenburg V, Pflueger B, Wildberger JE, Kuhl HP. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol. 2005;96:747–9 [DOI] [PubMed] [Google Scholar]
- 22.Hristova M, van Beek C, Schurgers LJ, Lanske B, Danziger J. Rapidly progressive severe vascular calcification sparing the kidney allograft following warfarin initiation. Am J Kidney Dis. 2010;56:1158–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rennenberg RJ, van Varik BJ, Schurgers LJ, Hamulyak K, Ten Cate H, Leiner T, Vermeer C, de Leeuw PW, Kroon AA. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood. 2010;115:5121–3 [DOI] [PubMed] [Google Scholar]
- 24.Weijs B, Blaauw Y, Rennenberg RJ, Schurgers LJ, Timmermans CC, Pison L, Nieuwlaat R, Hofstra L, Kroon AA, Wildberger J, et al. Patients using vitamin K antagonists show increased levels of coronary calcification: an observational study in low-risk atrial fibrillation patients. Eur Heart J. 2011;32:2555–62 [DOI] [PubMed] [Google Scholar]
- 25.McCann JC, Ames BN. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am J Clin Nutr. 2009;90:889–907 [DOI] [PubMed] [Google Scholar]
- 26.Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewe RB, Brandenburg VM, Bekers O, Vermeer C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost. 2010;104:811–22 [DOI] [PubMed] [Google Scholar]
- 27.Ueland T, Gullestad L, Dahl CP, Aukrust P, Aakhus S, Solberg OG, Vermeer C, Schurgers LJ. Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J Intern Med. 2010;268:483–92 [DOI] [PubMed] [Google Scholar]
- 28.Ueland T, Dahl CP, Gullestad L, Aakhus S, Broch K, Skardal R, Vermeer C, Aukrust P, Schurgers LJ. Circulating levels of non-phosphorylated undercarboxylated matrix Gla protein are associated with disease severity in patients with chronic heart failure. Clin Sci (Lond). 2011;121:119–27 [DOI] [PubMed] [Google Scholar]
- 29.Schlieper G, Westenfeld R, Kruger T, Cranenburg EC, Magdeleyns EJ, Brandenburg VM, Djuric Z, Damjanovic T, Ketteler M, Vermeer C, et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol. 2011;22:387–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rennenberg RJ, de Leeuw PW, Kessels AG, Schurgers LJ, Vermeer C, van Engelshoven JM, Kemerink GJ, Kroon AA. Calcium scores and matrix Gla protein levels: association with vitamin K status. Eur J Clin Invest. 2010;40:344–9 [DOI] [PubMed] [Google Scholar]
- 31.Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G, Massy ZA. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010;5:568–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shea MK, O'Donnell CJ, Vermeer C, Magdeleyns EJ, Crosier MD, Gundberg CM, Ordovas JM, Kritchevsky SB, Booth SL. Circulating uncarboxylated matrix gla protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. J Nutr. 2011;141:1529–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cranenburg EC, Vermeer C, Koos R, Boumans ML, Hackeng TM, Bouwman FG, Kwaijtaal M, Brandenburg VM, Ketteler M, Schurgers LJ. The circulating inactive form of matrix Gla Protein (ucMGP) as a biomarker for cardiovascular calcification. J Vasc Res. 2008;45:427–36 [DOI] [PubMed] [Google Scholar]
- 34.Cranenburg EC, Brandenburg VM, Vermeer C, Stenger M, Muhlenbruch G, Mahnken AH, Gladziwa U, Ketteler M, Schurgers LJ. Uncarboxylated matrix Gla protein (ucMGP) is associated with coronary artery calcification in haemodialysis patients. Thromb Haemost. 2009;101:359–66 [PubMed] [Google Scholar]
- 35.Koos R, Krueger T, Westenfeld R, Kuhl HP, Brandenburg V, Mahnken AH, Stanzel S, Vermeer C, Cranenburg EC, Floege J, et al. Relation of circulating matrix Gla-protein and anticoagulation status in patients with aortic valve calcification. Thromb Haemost. 2009;101:706–13 [PubMed] [Google Scholar]
- 36.Parker BD, Schurgers LJ, Vermeer C, Schiller NB, Whooley MA, Ix JH. The association of uncarboxylated matrix Gla protein with mitral annular calcification differs by diabetes status: the Heart and Soul study. Atherosclerosis. 2010;210:320–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendoza FJ, Martinez-Moreno J, Almaden Y, Rodriguez-Ortiz ME, Lopez I, Estepa JC, Henley C, Rodriguez M, Aguilera-Tejero E. Effect of calcium and the calcimimetic AMG 641 on matrix-Gla protein in vascular smooth muscle cells. Calcif Tissue Int. 2011;88:169–78 [DOI] [PubMed] [Google Scholar]
- 38.Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109:3279–83 [DOI] [PubMed] [Google Scholar]
- 39.Theuwissen E, Cranenburg EC, Knapen MH, Magdeleyns EJ, Teunissen KJ, Schurgers LJ, Smit E, Vermeer C. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br J Nutr. 2012. In press. [DOI] [PubMed] [Google Scholar]
- 40.Braam LA, Hoeks AP, Brouns F, Hamulyak K, Gerichhausen MJ, Vermeer C. Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: a follow-up study. Thromb Haemost. 2004;91:373–80 [DOI] [PubMed] [Google Scholar]
- 41.Westenfeld R, Krueger T, Schlieper G, Cranenburg EC, Magdeleyns EJ, Heidenreich S, Holzmann S, Vermeer C, Jahnen-Dechent W, Ketteler M, et al. Effect of vitamin K(2) supplementation on functional vitamin K deficiency in hemodialysis patients: a randomized trial. Am J Kidney Dis. 2012;59:186–95. [DOI] [PubMed] [Google Scholar]