Abstract
In 2001, the US Food and Nutrition Board concluded that there were insufficient data with which to establish a RDA for vitamin K, in large part because of a lack of robust endpoints that reflected adequacy of intake. Knowledge of the relative bioavailability of multiple vitamin K forms was also poor. Since then, stable isotope methodologies have been applied to the assessment of the bioavailability of the major dietary form of vitamin K in its free state and when incorporated into a plant matrix. There is a need for stable isotope studies with enhanced sensitivity to expand knowledge of the bioavailability, absorption, disposition, and metabolism of different molecular forms of vitamin K. Another area for future research stems from evidence that common polymorphisms or haplotypes in certain key genes implicated in vitamin K metabolism might affect nutritional requirements. Thus far, much of this evidence is indirect via effects on warfarin dose requirements. In terms of clinical endpoints, vitamin K deficiency in early infancy continues to be a leading cause of intracranial bleeding even in developed countries and the reasons for its higher prevalence in certain Asian countries has not been solved. There is universal consensus for the need for vitamin K prophylaxis in newborns, but the effectiveness of any vitamin K prophylactic regimen needs to be based on sound nutritional principles. In contrast, there is still a lack of suitable biomarkers or clinical endpoints that can be used to determine vitamin K requirements among adults.
Introduction
In many countries, concepts of setting human requirements for micronutrients, including vitamin K, have evolved over the years according to changing scientific knowledge and a greater awareness of the need for statistically valid methods for their assessment. In addition, there has been increasing recognition of the variety of applications (governmental and commercial) to which such dietary recommendations are put. The US Dietary Reference Intakes for vitamin K were last considered in 2001 (1), at which time the committee felt that there were insufficient data to set a RDA and instead set an AI5 based on representative dietary intake data from healthy individuals. This decision contains the logic that virtually any diet is adequate to prevent clinically significant changes in coagulation tests that could pose a risk of bleeding, which is the only clearly defined health risk of vitamin K insufficiency. The exception is that the intakes of vitamin K in a very small proportion of exclusively breast-fed infants do not always protect against bleeding and therefore the AI from birth to 6 mo assumes that newborns have also received vitamin K prophylaxis (1). Although the committee did recognize the promising development of biomarkers, such as ucOC, there were no data that had shown a causal link between ucOC (which to some degree is omnipresent in the healthy population) and a reproducible bone health outcome measure. Therefore, in the absence of a suitable endpoint that reflected adequacy of intake for a defined health outcome, the committee decided that a requirement could not be established.
Another problem in establishing robust recommendations was the lack of detailed knowledge of the bioavailability of vitamin K from foods as well as how different K vitamins are transported, taken up by cells, utilized, metabolized, and excreted, all considerations that are central to any consideration of dietary requirements. In particular, there was a huge knowledge gap relating to the sources and metabolism of MK. The aim of this review is to assess our current concepts of metabolism and assess how this knowledge might help to inform future considerations of human requirements of vitamin K.
Current status of knowledge
Intestinal absorption and bioavailability
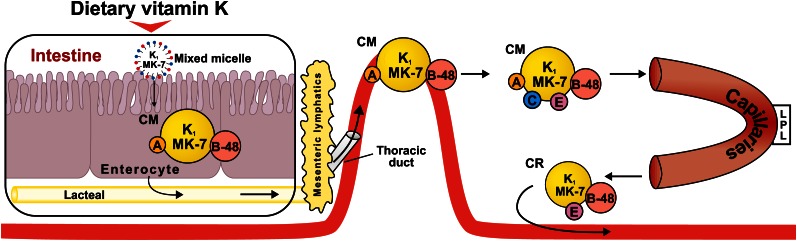
The intestinal absorption of vitamin K follows a well-established pathway that applies to most dietary lipids, which includes bile salt- and pancreatic-dependent solubilization, uptake of mixed micelles into the enterocytes, the packaging of dietary lipids into CM, and their exocytosis into the lymphatic system (2). A simplified diagram of how this absorption process relates to phylloquinone (vitamin K1) and MK-7 is shown in Figure 1.
Figure 1.
Intestinal absorption of dietary phylloquinone (K1) and MK-7. In the intestinal lumen, K1 and MK-7 are incorporated into mixed micelles comprising bile salts, the products of pancreatic lipolysis, and other dietary lipids. Mixed micelles are taken up by intestinal enterocytes of the small intestine and are incorporated into nascent CM, which have apoA and apoB-48 on their surface. CM are secreted from within the intestinal villi into the lymphatic capillaries (lacteals), which join larger lymphatic vessels and empty into the blood circulation via the thoracic duct. In the bloodstream, CM acquire apoC and apoE from HDL. CM enter the capillary beds of peripheral tissues where they lose much of their TG cargo through the action of LPL, at the same time losing apoA and C. The resulting CR that reenter the circulation are smaller and have a central lipid core with surface apoB-48 and apoE. CM, chylomicron; LPL, lipoprotein lipase; MK, menaquinone.
Bioavailability of a nutrient is defined as the rate and extent to which the nutrient is absorbed and becomes available to the site of activity (3). In most diets, green leafy vegetables are the major source of phylloquinone, followed by certain phylloquinone-rich plant oils or fats that are widespread in many food products (4). One common but limited method for assessing bioavailability is to measure the AUC of plasma measurements during the absorption phase. Gijsbers et al. (5) reported that the 10-h AUC for plasma phylloquinone from raw spinach alone was 4% of that from a detergent-solubilized phylloquinone (Konakion). In contrast, spinach consumed with butter improved the relative bioavailability 3-fold (5). In a similar study, mean 9-h AUC values from raw or cooked broccoli, raw spinach, and romaine lettuce ingested with a standard test meal ranged from 9 to 28% of that from a Konakion tablet but did not significantly differ from each other (6). One disadvantage of these 2 studies (6) is that they were small and absorption efficiencies from foods were compared to drug formulations of phylloquinone. In a larger, well-controlled residential study (7), the difference between the bioavailability of phylloquinone from a vegetable matrix (raw broccoli) compared to an unbound form (fortified oil) was much less apparent, with 24–h AUC values for the broccoli diet representing ~60% of those from the oil diet.
In the last 10 y, methods have been developed to study the bioavailability of tracer amounts of phylloquinone by stable isotope technology (8–13). This technique combined with compartmental modeling has obvious advantages of specificity and sensitivity and is currently expanding our knowledge of the absorption, disposition, and metabolism of phylloquinone in humans. In particular, the ability to label phylloquinone with deuterium or 13C during the growth of plants allows the study of the bioavailability of phylloquinone under conditions that closely replicate the normal digestive process. Compartmental analysis of the plasma distribution of 13C-labeled phylloquinone after consumption of isotopically labeled uncooked kale (with 30 g oil) gave a mean bioavailability of phylloquinone of only 5% (14). This is much lower than the absorption efficiency of ~80% for free [3H]-radiolabeled phylloquinone as measured by balance studies (15).
Jones et al. (12) reported that the absorption of a physiological dose (20 μg) of free 13C-phylloquinone varied according to the type of accompanying meal that had been formulated according to 3 dietary clusters identified in the UK. Significantly more phylloquinone tracer was absorbed when consumed with heart-healthy (termed cosmopolitan) or animal-based foods than with the meal pattern characterized by fast foods (termed convenience meal). The convenience meal contained a similar fat concentration compared to the other meal patterns but was 2-fold higher in PUFA than the other meals, suggesting that differences in vitamin K absorption might be explained by PUFA rather than total fat. In a second part of the same study, Jones et al. (12) measured the bioavailability of phylloquinone from within the food matrix of their 3 dietary cluster test meals relative to the 13C-labeled tracer. Their results showed that the absorption of phylloquinone from the convenience meal (in which 80% of phylloquinone was in the oil phase) was >3-fold greater than the absorption from the other meal patterns, in which 80–90% of the phylloquinone was present in a vegetable matrix (12). This finding is in accordance with the greater absorption efficiency of phylloquinone from fortified oil compared to broccoli (7).
There is currently little information of the bioavailability of MK either from dietary sources to which they are minor contributors or from the bacterial flora of the gut. A comparative study of the plasma time curves of synthetic phylloquinone, MK-4, and MK-9 in healthy males given equivalent oral doses (2 μmol) showed that the peak concentrations (and AUC) for MK-4 and MK-9 were <20% of those for phylloquinone (16). The reasons for the lower AUC for MK-4 and MK-9 are presently unknown but could be due to a faster absorption and clearance (likely for MK-4, which peaked early) and a less efficient absorption (likely for MK-9). The gut microflora has long been appreciated as a potentially large but unknown source of long-chain MK. The question of bioavailability of microfloral MK has been reviewed in detail elsewhere (17, 18) with a consensus that they do contribute to vitamin K nutrition but to a degree that is less important than was considered up until the 1980s.
Lipoprotein transport in the blood
Early studies using radiolabeled phylloquinone showed that after intestinal absorption, vitamin K first appears in lymph (19) and then enters the blood stream associated with CM (20). Later investigations of lipoprotein transport after the administration of unlabeled (16, 21) or stable isotope-labeled phylloquinone (9) showed that the majority of phylloquinone was associated with TRL during the postprandial phase of absorption. When healthy volunteers were given unlabeled phylloquinone (~100–3000 μg) with a fat-rich meal, the concentrations of phylloquinone peaked in plasma at 6 h, at which time the proportion of phylloquinone in the TRL fraction ranged from 75 to 90% (16, 21). The remaining phylloquinone was approximately equally distributed between LDL and HDL, with lesser amounts in the IDL fraction (16, 21). A consistent finding was that phylloquinone peaked in plasma and the TRL fraction ~3 h later than the TG present in the test meal (16, 21). A similar phenomenon was seen for plasma retinyl esters after administration of vitamins A (22) and E (23). Several hypotheses were forwarded to explain this relative delay of vitamin K compared to TG (21). Apart from a delayed absorption at the intestinal absorption level, one possibility is that when CM undergo lipolysis in the capillary endothelium (catalyzed by LPL), the resulting CR retain their phylloquinone cargo to a much greater extent than TG. Another possibility is that CR taken up by the liver are resecreted with VLDL, which have an increased cargo of phylloquinone relative to TG (21). However, the fractionation methods used in these studies did not differentiate between different classes of TRL comprising CM and CR of intestinal origin and VLDL secreted by the liver. One difference between phylloquinone and vitamins A and E is that in the fasting state, >70% of phylloquinone remains associated with TRL (9, 16, 21), whereas this proportion falls to ~40% for retinyl esters (22) and to ~20 and 30% for α-and γ-tocopherols, respectively (23).
The use of deuterium or 13C-labeled vegetables has enabled the study of the plasma kinetics and transport of phylloquinone at dietary intakes (70–400 μg) and within a food matrix that mimics the physiological situation (8–10, 13, 14). After vegetable digestion, maximal plasma tracer phylloquinone concentrations tended to occur later than that for free phylloquinone (20), with peak times ranging from 6 to 10 h, and then rapidly decreased (8–10, 13, 14). After ingestion of deuterated collard greens, the maximal enrichment of plasma and TRL phylloquinone with deuterium was 88 and 89%, respectively, was reached at 6 h, and was no longer detectable in plasma after 72 h (9). The findings that the TRL fraction was the major carrier of physiologically presented phylloquinone (with later transfer of low amounts to LDL and HDL) parallels the findings with pharmacological doses (16, 21). To date, no comparable studies using stable isotopes have been reported for any of the MK.
Mechanism of cellular uptake of vitamin K
Uptake of vitamin K by the liver.
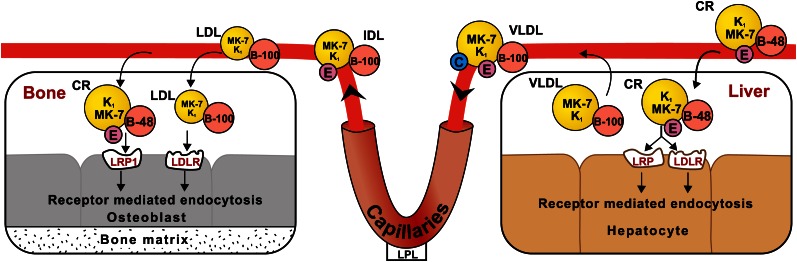
There have been no direct studies of the mechanism of hepatic uptake of dietary vitamin K, but in common with the general principles of fat absorption, it may be inferred that the majority of vitamin K is delivered to the liver within the CR generated during the postprandial phase of intestinal absorption (Fig. 1). The uptake process is complex and involves different apoproteins on the surface of lipoproteins, cell surface low-affinity binding sites of HSPG, and high-affinity lipoprotein receptors that mediate internalization of the lipoprotein particles (24). A simplified scheme of the postulated mechanism of vitamin K tissue uptake is shown in Figure 2. One important receptor component is apoE, a 34-kDa protein that is produced and secreted by many cell types, including hepatocytes, and is a constituent of both TRL and HDL (25). The primary relevance of apoE to the transport and cellular internalization of vitamin K is that it acts as a ligand that facilitates high-affinity binding of lipoproteins to the LDLR and other members of the LDLR family, which include the LRP and the VLDLR. It is the acquisition by CR of apoE from HDL and from cells within the immediate environment of CR that facilitates their binding to members of the LDLR family located on the surface of the same target cells by a process that has been described as secretion-capture (26, 27). The binding and subsequent internalization of CR by the liver is facilitated by an interaction with cell surface HSPG (26). In the case of the liver, the apoE-directed secretion-capture removal of CR takes place in the space of Disse (28). Although there is no direct proof, it is assumed that the same hepatic uptake mechanism accounts for the rapid removal of phylloquinone from the circulation. The rapid uptake of phylloquinone by the liver is supported by the rapid appearance of radiolabeled excretory metabolites in urine (29) and the bile (30) after the administration of tracer doses of [3H]-phylloquinone by the oral or parenteral routes, respectively.
Figure 2.
Uptake of phylloquinone (K1) and MK-7 by liver and by bone. It is likely that a major fraction of dietary K1 and MK-7 is delivered to the liver and bone within the CR, which have apoB-48 and apoE on their surface. CR can interact with cell surface lipoprotein receptors (e.g., LDLR and LRP) and are taken up by target cells by receptor-mediated endocytosis. There is evidence that K1 and especially MK-7 are incorporated into LDL through the hepatic export of VLDL (bearing surface apoB-100, C, and E) followed by their subsequent dilapidation in capillaries to VLDL remnants (IDL) and further catabolism to LDL (bearing surface apoB-100). It is known that human osteoblasts express the LDLR and LRP1 and that LRP1 plays a predominant role in the uptake of CR by osteoblasts, including the ability to utilize K1-rich CR for γ-carboxylation of osteocalcin. CR, chylomicron remnant; LDLR, LDL receptor; LRP, LDL receptor-related protein; MK, menaquinone.
Uptake of phylloquinone by bone.
Much less is known about the general molecular mechanisms of how lipoproteins deliver lipids and fat-soluble vitamins to extrahepatic tissues such as bone, although there are now studies that have specifically addressed the question of how phylloquinone is delivered to osteoblasts (Fig. 2). The importance of this area for vitamin K centers on knowledge that bone matrix contains several Gla proteins [e.g., osteocalcin, matrix Gla protein (MGP), Gla-rich protein, and gas 6] that require vitamin K for their function (31) and by findings that undercarboxylated species of osteocalcin and MGP normally circulate in healthy people (32).
Cell culture studies by Newman et al. (33) showed that both primary osteoblasts and osteoblast-like cells could internalize phylloquinone from all the major lipoprotein fractions in which the order of efficiency of uptake was LDL > TRL > HDL. For reasons already outlined, TRL are likely to be physiologically the most important carrier particle for phylloquinone. The mechanism of cellular uptake of TRL-associated phylloquinone was shown to be dependent on both HSPG and apoE, implying the presence of receptors of the LDLR family on the surface of osteoblasts (33). The results suggested that mature osteoblasts can effectively take up vitamin K directly from lipoproteins delivered to them in the blood. In support of this concept are findings that the fraction of circulating ucOC rapidly decreases in response to dietary phylloquinone supplementation in healthy volunteers (34).
In 2005, Niemeier et al. (35) showed that human osteoblasts expressed the lipoprotein receptors LDLR, LRP1, and, to a lesser degree, the VLDLR. Immunohistochemical analysis of sections of normal human bone showed that LRP1 was strongly expressed by osteoblasts and marrow stromal cells. Studies of the uptake of labeled, human-derived CR in the presence and absence of specific inhibitors and stimulators suggested that LRP1 was the predominant CR receptor on human osteoblasts. In common with liver cells, uptake of CR by osteoblasts was shown to involve receptor-mediated endocytosis, which was stimulated by exogenous apoE and LPL (35). The relevance of this mechanism to phylloquinone uptake was demonstrated by evidence that human-derived CR isolated after a phylloquinone-rich meal had the capacity to stimulate γ-carboxylation of osteocalcin in osteoblasts in vitro within 1–4 h (35). This in vitro uptake of CR into osteoblasts assessed by a functional marker of vitamin K status was also stimulated by exogenous apoE and LPL (35).
Quantitative and mechanistic aspects of the delivery of intestinally derived lipoproteins to extrahepatic tissues, including bone, were addressed in a series of animal studies by Hussain et al. (36–38). Like the liver, bone marrow has direct access to circulating blood and in several species acts as a rapid and major metabolic sink for the uptake of both CM and CR (38). For example, the uptake of TRL by the bone marrow in marmosets (a new world primate and, by inference, a model for humans) accounted for 20–50% of the amounts taken up by the liver (38). The cells responsible for the uptake of CM by the bone marrow were the perisinusoidal macrophages (36–38). Because osteocalcin, in common with other noncartilaginous proteins, is synthesized by the osteoblasts, vitamin K needs to be transported to these anabolic cells so that osteocalcin can undergo γ-glutamyl carboxylation. Kohlmeier et al. (39) reasoned that stromal and mesenchymal stem cells, which are obligatory precursors of all bone-forming cells, may acquire vitamin K while residing within the bone marrow and be a source of osteoblastic vitamin K. Later studies by Niemeier et al. (40) showed that fluorescently labeled CR localized with the sinusoidal endothelial cells in bone and phylloquinone-enriched CR could also be directly taken up and utilized by osteoblasts in vivo for the γ-carboxylation of osteocalcin. The quantitative importance of osteoblasts to the removal of CR from the circulation was shown by the finding that the amount of radiolabeled CR taken up into femoral cortical bone was similar to that taken up by the bone marrow. Importantly, in the context of vitamin K, the uptake of CR by osteoblasts occurred within 20 min of their injection. The authors proposed that just as in the liver, sinusoidal endothelial cells serve as a docking site to concentrate CR in the bone marrow. This would then be followed by transport through the endothelial fenestrae to the subendothelial space where CR are enriched with osteoblast-derived apoE, interact with osteoblast receptors, and are internalized (40). The final proof that osteoblasts can rapidly utilize phylloquinone-loaded CR was the finding of a small but significant increase in the γ-carboxylated fraction of circulating osteocalcin within 4 h after injection of the CR (40).
Lipoprotein transport and cellular uptake of MK.
To date, very little is known of the intestinal absorption and metabolism of MK in humans, although some characteristics of the plasma transport of MK-4, MK-7, and MK-9 were delineated in 2 studies by Schurgers et al. (16, 41).
In the first study, 6 healthy volunteers simultaneously ingested 2-μmol doses of phylloquinone, MK-4, and MK-9 (16). The serum and lipoprotein time course profiles of MK-4 and MK-9 showed distinct differences both from each other and from phylloquinone, as did the AUC values during the postprandial phase. Whereas both serum phylloquinone and MK-9 peaked at 4 h, the serum peak of MK-4 was earlier at 2 h and coincided with the peak of TG from the test meal. In the early phase of absorption (0–4 h), MK-4 and MK-9 behaved in the same way as phylloquinone in being predominately associated with TRL, although the absolute concentrations of MK-4 and MK-9 in the TRL fraction were less than one-fifth of that for phylloquinone. One difference between MK-4 and MK-9 was that at later times, MK-4 transferred to LDL and then to HDL, whereas MK-9 was found only with LDL, with high concentrations being maintained for up to 50 h (16). It should be noted that because MK-4 and MK-9 are normally undetectable in plasma (unlike phylloquinone), the lipoprotein distributions found in this study would have reflected the metabolism of the original administered dose. In the second study, Schurgers et al. (41) examined the plasma disappearance kinetics of MK-7, which is a constituent of the Japanese fermented food natto, but is otherwise a minor component of most diets. When MK-7 was given as a single oral dose, it was found that its delayed plasma clearance kinetics resembled that of MK-9 in their earlier study (16). By analogy to MK-9, it seems likely that the prolonged half-life of MK-7 results from its association with the LDL fraction. This second publication (41) also contained a crossover study in which healthy volunteers were fed daily equimolar (0.22 μmol) doses of either phylloquinone or MK-7 for 40 d. This showed that serum concentrations of phylloquinone and MK-7 reached a plateau after 3 and 20 d, respectively, with MK-7 attaining serum concentrations that were 7- to 8-fold higher than those for phylloquinone. These higher concentrations of MK-7 were associated with a greater tissue uptake and biological activity in bone as evidenced by an increased proportion of serum γ-carboxylated osteocalcin that plateaued after 3 d in the phylloquinone arm but continued to rise for at least 40 d in the MK-7 arm (41). The hypothesis that vitamin K carried with LDL can be delivered to osteoblasts is supported by previous findings that LDL labeled with [3H]-phylloquinone are readily internalized by osteoblasts in cell culture (33) and that the LDLR is expressed by osteoblasts (35). Certainly, this would explain the greater biological efficacy of MK-7 compared to phylloquinone in stimulating γ-carboxylation of osteocalcin in healthy volunteers (41).
The greater bioactivity of MK-7 compared to phylloquinone also extended to coagulation factor synthesis in the liver as shown in a crossover study in volunteers who had been stabilized on the vitamin K antagonist, acenocoumarol (41), and were then given increasing oral doses of either phylloquinone or MK-7 without alteration of the antagonist dose. The decreases in the international normalized ratio in each study arm revealed that on a molar basis, MK-7 was 3 to 4 times more potent than phylloquinone as an antidote to vitamin K antagonists (41).
Inter-individual variation in response to vitamin K from the diet
Of all the fat-soluble vitamins, vitamin K (as phylloquinone) has the largest intra-:inter-individual variation ratios for diet and fasting plasma concentrations, even after accounting for effects of food matrices (42). An unpredictable intra-individual variability in phylloquinone bioavailability has been noted in most absorption studies (5, 11, 12, 14), suggesting the need to account for nondietary factors. Men and women do not appear to have significant differences in vitamin K bioavailability (6, 7, 11, 12, 14), whereas age may be a nondietary factor influencing response (7, 34). In 2 residential studies (7, 34), plasma phylloquinone AUC values after fortified meals were significantly higher in older adults compared to younger adults, but this age effect disappeared when plasma phylloquinone concentrations were adjusted for the higher TG concentrations of older people (7). No difference between younger and older adults was seen in the response of the functional marker percent ucOC to dietary intervention (34). The large range of bioavailability of phylloquinone from isotopically labeled kale of 1–14% (CV 100%) would be expected to reflect to some degree inter-individual variations in the ability to digest the plant matrix (14). On the other hand, the AUC of pure tracer 13C-labeled phylloquinone also showed a high variability even when taken with the same standardized meal (12). The reported wide range of bioavailability of 2–26% for the absorption of free ring-D4 labeled phylloquinone is difficult to interpret because of the unexpectedly low bioavailability in this study that might reflect the form in which the dose was given or the absence of a test meal (11). There are no studies that have examined nondietary sources of variability in response to MK intake. Shea et al. (43) recently reported that vitamin K status, as estimated by plasma phylloquinone and serum percent ucOC, is not significantly heritable but is associated with nongenetic factors, such as TG, age, and smoking status. However, to date, there are few studies that have systematically explored the associations between individual genetic polymorphisms and biochemical measures of vitamin K status.
Effect of polymorphisms of apo E on vitamin K metabolism
ApoE occurs as 3 isoforms (apoE2, E3, and E4) that differ by single amino acid substitutions at positions 112 and 158. The allele frequencies in populations are 60–70% for APOE3, 15–20% for APOE4, and 5–10% for APOE2.
Genetic variations in APOE are to known to influence lipoprotein transport and cellular uptake both in vivo and in vitro. Using the rate of removal of retinyl ester-labeled CR as a marker of intestinally derived TRL, Weintraub et al. (44) reported that clearance rates from fast to slow followed the order of alleles E4 > E3 > E2 and others have reported a delayed clearance in E2 carriers (45, 46). Associations with APOE genotype have been reported for endogenous phylloquinone concentrations and the functional marker percent ucOC, but the results lack consensus. A study in renal patients on maintenance hemodialysis reported that plasma phylloquinone was related to APOE genotype in the order of E2 > E3 > E4 (47). This would be the order predicted if fasting phylloquinone was solely dependent on the rate of clearance of intestinally derived TRL. A later study in a much larger population of hemodialysis patients did not show any dependence of fasting phylloquinone concentrations on APOE phenotypes, although patients with an APOE4 allele had a significantly higher circulating percent ucOC, suggesting a lower vitamin K status of bone (48).
Studies to find out whether APOE-vitamin K relationships exist in healthy populations have also proved contradictory. In a study of healthy older adults living in the UK or China, Yan et al. (49) reported that participants from the UK with an APOE4 allele (E3/4 + E4/4) had significantly higher fasting phylloquinone concentrations than those with either E2/3 or E3/3 genotypes. Chinese participants with APOE4 also showed significantly lower levels of ucOC (adjusted for total osteocalcin) than those with either E2/3 or E3/3, suggesting a higher bioavailability of vitamin K to bone in APOE4 participants (49). Opposite findings to the UK-Chinese study (49) were recently reported in a U.S. study of an older, primarily Caucasian, population in which individuals with the APOE2/3 genotype had higher serum phylloquinone concentrations than those with other genotypes (50). In this study, there was no association of APOE polymorphisms with percent ucOC (50).
APOE genotype and relative delivery of vitamin K to liver and bone.
Based on relationships of fasting phylloquinone to APOE genotype in hemodialysis patients, Kohlmeier et al. (39, 47) postulated that the APOE genotype was a significant determinant of vitamin K availability to extrahepatic tissues such as bone. The basis of this hypothesis is that the delivery of vitamin K to bone is less efficient than to the liver, as evidenced in part by the fact that in healthy individuals, hepatic Gla coagulation proteins are fully γ-carboxylated, whereas bone Gla proteins such as osteocalcin are only partially γ-carboxylated. This suggested that the liver and bone might compete for available amounts of circulating vitamin K carried by lipoproteins. The original postulate (39, 47) was that if the APOE4 allele promoted faster hepatic uptake of TRL-vitamin K, there might be a sparing effect on the delivery and therefore availability of vitamin K to bone, with opposite effects for the APOE2 allele. At the cellular level, there is in vitro evidence that apoE4 is the most effective isoform in stimulating [3H]-phylloquinone-labeled TRL uptake into osteoblasts (33) and the defective binding of apoE2 to LDLR is well described (51, 52).
The hypothesis that the APOE4 genotype accelerates the hepatic uptake of phylloquinone was based on 2 assumptions, first that retinyl esters are markers of intestinally derived TRL and second that fasting concentrations of phylloquinone reflect only the rate of clearance of intestinally derived TRL. Both these assumptions are open to question. The specificity of the vitamin A fat-loading test for measuring clearance of intestinally derived TRL (44) has been questioned, because as postprandial lipemia progresses, increasing proportions of retinyl esters are carried by LDL (22). A similar transfer of 30–40% of circulating phylloquinone to LDL and HDL is also seen in the later postprandial phase (16, 21). Furthermore, when apoB-48 and apoB-100 were employed as more specific markers of intestinally and hepatically derived TRL, respectively (Figs. 1 and 2), Bergeron and Havel (53) found that rather than enhancing the clearance of CM, CR, and VLDL, the APOE4 allele impaired their clearance.
Several investigators have also sought to find out whether APOE polymorphisms affected the dose requirements of vitamin K antagonists. The main working hypothesis was that if the APOE4 allele enhanced hepatic uptake of vitamin K (39, 47), the warfarin dose would be expected to be higher for APOE4 carriers than for other genotypes. The results have been mixed and often population dependent. For the APOE4 allele, some studies have shown the expected increased warfarin dose requirement (54, 55), but other studies have shown a lower requirement (56, 57). For the APOE2 allele, a lower warfarin dose requirement has been reported (58). Usually, the contribution of APOE genotype to warfarin dose requirements is small.
In summary, the results of the effect of APOE genotype on vitamin K metabolism suggest that the hypothesis of apoE-dependent mechanisms that lead to liver and bone competing with each other for available circulating vitamin K is too simplistic. Of note is the conflicting evidence that individuals with the APOE4 allele either had the lowest (47) or highest (49) phylloquinone concentrations. Another interpretative problem is that the effect of the APOE4 allele on the clearance of intestinally derived TRL is itself contradictory, being either accelerated (44) or delayed (53) according to how this is measured. The associations of APOE genotypes with functional markers such as percent ucOC are also inconsistent. Whatever the modulating role of apoE in the delivery and uptake by bone, several studies have shown correlations between circulating concentrations of phylloquinone (59), MK-7 (41), and TG (39, 47) and the γ-carboxylation status of osteocalcin. Although larger population studies may help to clarify any genetic influences of apoE on vitamin K metabolism, detailed studies measuring the clearance of TRL-bound phylloquinone in the same way as has been carried out for vitamin A are also warranted.
Interconversion of phylloquinone to MK-4
A potential complication to any consideration of the relationship of vitamin K metabolism to human requirements is a pathway by which phylloquinone can be converted in the body to MK-4. Although the existence of this pathway has been known since the 1950s, the biochemistry of this transformation has remained elusive. A recent advance is the identification of a novel human prenyltransferase (UBIAD1) that Nakagawa et al. (60) proposed not only catalyzed the well-described prenylation of precursor menadione to MK-4 but might also be responsible for the initial side chain cleavage of phylloquinone or other MK. Before UBIAD1 had been implicated in this interconversion, Thijssen et al. (61) had presented evidence that menadione is a physiological human metabolite that could be measured in the urine. Daily excretion of menadione was of the order of 5 μg, but this was increased when exogenous doses of phylloquinone, MK-7, or MK-4 were given by the oral route but not when given by the s.c. route, despite the latter being efficiently released into the plasma compartment. It was previously shown that this conversion of phylloquinone to MK-4 could occur in germ-free rats; this together with the human findings of the dependence on the oral route and the rapid appearance of menadione in the urine pointed to the possibility that the release of menadione may have occurred during intestinal absorption within the enterocyte (61). The extent of the conversion to MK-4 was estimated to range from 5 to 25% of the ingested phylloquinone. If this is true, it would obviously have implications to our current understanding of the human requirements for phylloquinone, which hitherto have always been considered independently of the possibility of its conversion to MK-4. The same is true for MK such as MK-7, which was also shown to release urinary menadione (61).
Vitamin K-epoxide cycle
The vitamin K-epoxide cycle is pivotal to both the function of vitamin K and to the conservation of the microsomal cellular stores of vitamin K. The cycle encompasses both the glutamyl γ-carboxylation step of vitamin K-dependent proteins and the recovery of the cofactor vitamin K quinol that is oxidized to vitamin K epoxide metabolite as a consequence of γ-carboxylation (62, 63). It involves 2 major integral-membrane proteins: γ-glutamyl carboxylase (GGCX) and vitamin K epoxide reductase (VKOR). Evidence suggests that both these enzymes are part of a concerted mechanism for the efficient oxidation and reduction of membrane-bound vitamin K (64). In a nutritional context, the fact that cells possess the machinery for the efficient recycling of vitamin K consumed during γ-carboxylation can be regarded as a local salvage pathway to preserve limited stores of vitamin K. This is illustrated clinically in infants born with a defective VKOR who often present with severe coagulopathy and/or skeletal defects (65).
The VKOR protein contains 163 amino acid residues, of which subunit 1 (VKORC1) is essential for VKOR enzymatic activity (66). Several common polymorphisms have been reported within the VKORC1 gene and have been attributed to the inter-individual variability in warfarin dose (67). Similarly, the GGCX gene exhibits common polymorphisms that have been linked to variability in warfarin dose, although the data are less consistent compared to VKORC1 (68–70). It is assumed that VKORC1 and GGCX polymorphisms that have been reported to influence vitamin K recycling in the liver are also likely to have parallel effects on extrahepatic tissues. Indeed, VKORC1 polymorphisms have been associated with risk of vascular disease and the vitamin K functional marker percent ucOC in a Chinese cohort (71). The GGCX rs699664 single nucleotide polymorphism that causes a single nucleotide substitute of glutamine to arginine is not associated with warfarin variability but has been proposed to increase γ-carboxylation of osteocalcin, resulting in higher BMD (72). In a study of community-dwelling elderly who were participating in a vitamin K randomized clinical trial, there were significant cross-sectional associations between measures of vitamin K status (plasma phylloquinone and/or percent ucOC) and polymorphisms of both VKORC1 and GGCX (73). However, there were no detectable genotype effects on the response to 3 y of phylloquinone supplementation. This may have been in part a consequence of the overall health of the study participants and, hence, lack of phenotypic responses, a common phenomenon in randomized clinical trials (74).
Catabolism of vitamin K
Humans excrete phylloquinone and MK by a common degradative pathway whereby the polyisoprenoid side chain is first shortened to 2 major carboxylic acid metabolites with 7- and 5-carbon side chains, respectively; the metabolites are then conjugated, mainly with glucuronic acid, and excreted in the bile and urine (75, 76). The enzymological details of the biochemical pathway have not yet been studied in any detail, but by analogy to similar isoprenoids, the side chain is thought to be metabolized by an initial ω-hydroxylation followed by a progressive side-chain shortening via the β-oxidation pathway (76). Recently, searches for genetic polymorphisms of cytochrome P450 that might influence warfarin dosage led to the discovery of a DNA variant (rs2108622; V433M) of cytochrome P450 4F2 (CYP4F2) that caused an increase in warfarin dose requirements in a European-American cohort of patients (77). In dose-response terms, patients with 2 TT alleles required ~1 mg/d more warfarin than patients with 2 CC alleles (77). Based on knowledge that CYP4F2 participates in the ω-hydroxylation of tocopherols, Caldwell et al. (77) speculated that CYP4F2 might also hydroxylate the isoprenoid side chain of vitamin K. Support for this hypothesis was subsequently obtained by Alan Rettie’s group, who showed that recombinant CYP4F2 could catalyze the conversion of phylloquinone to a novel oxidized metabolite tentatively identified as the previously predicted ω-hydroxylation product of phylloquinone (78). The significance of the V433M polymorphism was shown by findings that human liver microsomes obtained from carriers of this variant had a reduced ability to oxidize phylloquinone and lower protein concentrations of CYP4F2 (78). The reason why carriers of the CYP4F2 V433M allele required higher doses of warfarin could therefore be explained by invoking their lower capacity to metabolize vitamin K, leading to presumed higher hepatic concentrations. Although there is as yet no direct proof, one predictive outcome relevant to the setting of human requirements is that carriers of this V433M variant should require lower dietary intakes of vitamin K compared to noncarriers to maintain an equivalent vitamin K status. One possible demographic impact of this polymorphism to vitamin K requirements is that the minor allele frequency for CYP4F2 is ~30% in whites and Asians but only 7% in blacks (77).
One notable feature of vitamin K metabolism compared to other fat-soluble vitamins is that the most abundant dietary form, phylloquinone is poorly retained in the body. Although there are limited data, early studies with [3H]-labeled phylloquinone indicated that ~60–70% of doses in the range of 45–1000 μg of phylloquinone were excreted within 5 d (15). These high losses may give added importance to a gene such as CYP4F2 that influences the ability of the liver to catalyze the synthesis of the initial ω-hydroxylation product in the catabolic pathway. The recent development of methods for measuring endogenous concentrations of these urinary metabolites has enabled their measurement in 24-h collections in response to varying intakes of phylloquinone diets in a dietary controlled residential environment (79). The results showed that urinary output of vitamin K metabolites significantly declined by ~20% during 15 d of dietary restriction (11 μg/d) and increased rapidly on a repletion diet (206 μg/d). A potential advantage of measuring urinary excretion is that the catabolic pathway is common to all K vitamins so that the excretion of the 2 terminal metabolites represents turnover and losses of the total body pool of vitamin K (79). As such, it represents a global biomarker of vitamin K metabolism with the caveat that a larger proportion of dietary vitamin K is excreted in the bile (15).
Nutritional deficiency syndromes and human requirements
Despite the many efforts in recent years to demonstrate the disease-preventing roles of the plethora of Gla proteins that are present in the human body, the only disease outcome of clinical concern that can be shown to specifically relate to a nutritional deficiency of vitamin K in otherwise healthy individuals occurs during early infancy. This is a bleeding disorder previously known as hemorrhagic disease of the newborn and now renamed VKDB (80). Perhaps to this definition of a condition being caused by an acute deficiency should be added the rare but now fairly well-characterized cases of severe vitamin K maternal deficiency during pregnancy that result in infants being born with a condition known as chondrodysplasia punctata (81, 82). Less clear is the relationship of vitamin K status with chronic diseases such as osteoporosis and cardiovascular disease for which vitamin K research has centered on the possible health roles of osteocalcin and MGP, respectively (31). More recently, there has been interest in the possible role of osteocalcin and vitamin K status in glucose homeostasis (31).
Vitamin K deficiency bleeding in early infancy
There is a narrow window from birth to 6 mo of life when the human infant is exposed to a small but potentially life-threatening risk of bleeding (80). In late onset VKDB, with a peak incidence between 3 and 8 wk, infants typically present with intracranial bleeding, which may result in death or permanent neurological damage. The major nutritional risk is to those infants who are exclusively breastfed. Greer et al. (83) measured phylloquinone intakes in a North American cohort of exclusively breast-fed infants over 3 consecutive 24-h periods at 2, 12, and 26 wk and reported daily intakes that averaged 0.55, 0.74, and 0.56 μg, respectively, and ranged from 0.1 to 2.1 μg. When expressed per kilogram body weight, the average daily intakes at 6 and 12 wk were 9.3 and 8.3 μg/kg, respectively, which represent about one-tenth of the intakes of healthy adults. Formula-fed infants are protected from VKDB, because phylloquinone contents of milk formulas are typically 50-fold higher than human milk, providing average daily intakes of ~50 μg of phylloquinone (83). Greer et al. (83) found that this huge disparity in intakes was mirrored in plasma phylloquinone, with mean concentrations ranging from 0.29 to 0.53 nmol/L (0.13–0.24 μg/L) in breast-fed infants and from 9.8 to 13.3 nmol/L (4.4–6.0 μg/L) in formula-fed infants.
While the generally low, weight-adjusted intakes of phylloquinone in breast-fed infants account for the much higher prevalence of VKDB in breast-fed infants as a group, knowledge of the precipitating factors that trigger VKDB in an individual infant are less well understood. In some infants, underlying pathologies that cause cholestasis with resultant malabsorption of vitamin K may be identified, but in a substantial proportion no predisposing factor is found (80).
Geographical variation of VKDB.
Epidemiological studies of the incidence of late VKDB have indicated that China, Japan, and countries within Southeast Asia such as Thailand and Vietnam have higher rates of VKDB than in the rest of the world. This is most accurately exemplified by comparing the incidence of VKDB between countries that have published data from nationally representative surveillance programs such as those carried out in the UK, The Netherlands, Germany, Switzerland, Australia, New Zealand, and Japan (80). In other countries such as Thailand, China, and Vietnam, regional surveys have also yielded valuable incidence data of VKDB. Care needs to be taken in comparing incidence data because of the different methodologies and case definitions. Where acceptable criteria for international comparisons have been met, the incidence of late VKDB in infants not given vitamin K prophylaxis has been shown to be ~5 cases/105 births in Western European countries compared to 11 and 72/105 births in Japan and Thailand, respectively (80).
Concepts of vitamin K requirements during early infancy
The AI for infants aged 0–6 mo is based on an average daily intake of milk of 0.78 L and an average phylloquinone concentration in human milk of 2.5 μg/L (1). This gives an AI of 2.0 μg/d after rounding. The weak link in this calculation as a precise AI value lies with the fairly wide variations in the reported concentrations of phylloquinone in breast milk, for which the largest component contributing to variability is likely to be caused by methodological differences rather than to differences between the populations studied. Thus, the data used for this calculation (1) included 4 individual studies from 2 groups in the US, one of which reported average concentrations in mature breast milk that ranged from 0.86 to 1.17 μg/d (83), and the other group reported average concentrations that were much higher and ranged from 2.31 to 3.15 μg/d (84, 85). Notably, the nonoverlapping, quite tight range of average values for mature milk reported by each laboratory were obtained over 4 similar time points taken over 6 mo. Hence, using the lower values for breast milk concentrations, which also agree more closely with European values (86), the estimated vitamin K intake would be ~0.5 μg/d and using the higher values, ~2.5 μg/d.
It is generally agreed that the vitamin K coagulation status of the healthy newborn infant is brittle. This conclusion is based on both tissue analyses of K vitamins and on sensitive functional assays such as PIVKA-II that can detect undercarboxylated species of factor II well before there are any changes in global coagulation assays such as the prothrombin time. Whereas detectable concentrations of PIVKA-II are rarely detected in healthy adults, elevated concentrations are fairly common in the cord blood of healthy newborns. The prevalence of a raised PIVKA-II in cord blood depends on both the sensitivity of the PIVKA-II method and the population being studied. The analysis of blood samples collected from 693 cord/mother pairs in Thailand showed that the prevalence of detectable PIVKA-II in cord samples was 16%, of which 1.5% had a PIVKA-II level that would have been expected to be associated with a clinically significant lengthening of the prothrombin time (87). Interestingly, 12% of mothers had a detectable PIVKA-II, but there was no association between the presence of PIVKA-II in infants and mothers (87). In the same Thai study, infants categorized as high risk according to birth weight and delivery type had a higher median detectable PIVKA-II concentration than infants categorized as normal risk as well as a higher prevalence of PIVKA-II concentrations considered to be clinically relevant (87). Analysis of dietary intakes of vitamin K in a subgroup of 106 mothers showed that those Thai mothers who had phylloquinone intakes below the US. AI for pregnancy (<90 μg/d) had a higher prevalence of functional subclinical vitamin K deficiency as measured by a detectable PIVKA-II than mothers with adequate intakes (18.8 vs. 3.3%). The median intake of phylloquinone in Thai mothers as measured by FFQ was 241 μg/d and was comparable to previously reported intakes in the US and Europe (4).
Metabolic risk factors in early infancy.
The concentrations of phylloquinone in cord blood are <0.1 nmol/L (<50 ng/L) and are generally too low to be accurately measured. However, the fact that the average maternal/neonatal cord concentration gradient of phylloquinone is within the range of 20:1 to 40:1 has led to the concept of a placental barrier to phylloquinone, which is fairly unresponsive to maternal vitamin K supplementation (17).
There is limited information of liver stores of vitamin K in fetuses and young infants, but the difference between fetal/neonatal and adult concentrations is much less than the 20- to 40-fold difference between blood concentrations. In the largest study (88), the median hepatic concentration of phylloquinone in infants at term was 2.2 pmol/g compared to a median of 12 pmol/g in adults. Concentrations of ~2–4 pmol/g were detected as early as 10 wk of gestation, suggesting that the concept of a placental barrier for phylloquinone may be overplayed. The major difference between fetal/neonatal hepatic reserves and those of adults is that whereas the long-chain MK (mainly MK 7–13) make up the majority of adult reserves (~90%), they are absent, or very low, at birth and build up slowly over several weeks (17). This slow buildup of hepatic MK would be consistent with the colonization of the neonatal gut by MK-producing bacteria, although some dietary contribution cannot be ruled out. The implication that low hepatic reserves of MK may be a major contributory factor to the brittleness of neonatal vitamin K status is enticing but presently lacks hard evidence.
Vitamin K prophylaxis for newborns.
The American Academy of Pediatrics has recommended the parenteral administration of the natural plant form phylloquinone for the prevention of VKDB since 1961 (89). Before 1961, the only vitamin K compounds available for vitamin K prophylaxis had been water-soluble derivatives of menadione that in large doses or in certain hereditary conditions were shown to be associated with hemolytic anemia, hyperbilirubinemia, and kernicterus (89). No such toxicity was found for the naturally occurring phylloquinone. The routine application of vitamin K prophylaxis to all newborns has had a checkered history (in some countries more than others) that includes the problem with toxicity of menadione derivatives, ignorance of the incidence of VKDB leading to beliefs that prophylaxis was unnecessary, and a subsequently unsubstantiated concern of an association between intramuscular phylloquinone prophylaxis and later childhood cancer (90).
In the last 20 y, and especially with incidence data gathered from active surveillance of VKDB, there has been a growing consensus that vitamin K prophylaxis is required for all newborn infants. This consensus cuts across both developed and developing countries and has been reinforced by the conclusions of expert committees (91). However, there still remains a lack of consensus of the most effective dose regimen that would protect almost all infants from VKDB. It is generally agreed that the i.m. injection of 0.5–1.0 mg of phylloquinone (phytomenadione, phytonadione) provides the best protection against late VKDB, probably because muscle acts as a depot from which the lipophilic vitamin leaches out slowly over several weeks (92). The i.m. injections are the most common route in the US, and in many states such as New York, i.m. prophylaxis is mandatory (93). In other countries such as Germany and The Netherlands, oral regimes are more popular. In the UK, the choice of prophylactic regime is made locally and has been described as chaotic (94). In the UK, there are also discreet regional differences, with the northeast region recently changing to an oral policy comprising a 1-mg oral dose at birth followed by a daily dose of 50 μg phylloquinone for 3 mo (95).
Another potential concern of some prophylactic regimens, which particularly applies to preterm infants, is the markedly supraphysiological tissue concentrations that for serum can be several 100-fold higher than normal (96). A recent study provided evidence that the combination of a raised serum vitamin K epoxide concentration with an increased proportional excretion of the 7-carbon side chain urinary metabolite may together indicate a metabolic overload of both vitamin K recycling and catabolic pathways (97). This is an example of how specific measurements of vitamin K metabolites might help to set optimal dosage guidelines for the prevention of VKDB.
Concepts of vitamin K requirements in adults
As reviewed elsewhere in this volume, vitamin K is an emerging factor in the regulation of calcification in multiple tissues, including vessel walls and cartilage (31), and the recent identification of a new enzyme (UBIAD1) involved in vitamin K metabolism provides evidence for potential novel physiological roles for vitamin K (60). Another enzyme (CYP4F2) may have a role in regulating the rate of catabolism of vitamin K. Despite these exciting developments, the gaps in knowledge regarding vitamin K metabolism and function impede the ability to establish optimal dietary recommendations for vitamin K.
To establish dietary requirements for vitamin K, it is essential to have a biomarker or clinical endpoint that reflects the consequence of vitamin K adequacy. When compared globally, dietary recommendations for vitamin K have a 2-fold range among adults (Table 1). This range reflects the lack of consensus on a suitable endpoint or biomarker of adequacy from which to base recommendations.
Table 1.
The Dietary Reference Intakes for vitamin K in adults1
| Country | Dietary Reference Intakes (μg/d, unless otherwise indicated) | |
| 19–50 y | >51 y | |
| UK | ||
| Women | 1 μg/(kg·d) | 1 μg/(kg·d) |
| Men | 1 μg/(kg·d) | 1 μg/(kg·d) |
| WHO/ Bosnia/Herzegovina/Poland | ||
| Women | 55 | 55 |
| Men | 65 | 65 |
| Belgium | ||
| Women | 50–70 | 50–70 |
| Men | 50–70 | 50–70 |
| New Zealand /Australia | ||
| Women | 60 | 60 |
| Men | 70 | 70 |
| Japan | ||
| Women | 60, 652 | 65 |
| Men | 70 | 70 |
| Germany/Switzerland/Austria | ||
| Women | 60 | 65 |
| Men | 70 | 80 |
| Croatia | ||
| Women | 65 | 65 |
| Men | 65 | 65 |
| USA/Canada/Montenegro/ Albania | ||
| Women | 90 | 90 |
| Men | 120 | 120 |
Values from Eurreca Micronutrient database (109).
60 g/d for women, aged 19–29 y, and 65 μg/d for women ≥ 30 y (110).
The best understood role of vitamin K is as an enzyme cofactor. However, dietary intakes of phylloquinone required for full or optimal γ-carboxylation of coagulation or extrahepatic Gla proteins, respectively, have not yet been determined. Coagulation proteins are the best characterized Gla proteins, and although a hemorrhagic event is the classical sign of vitamin K deficiency, frank vitamin K deficiency is extremely rare in the adult population (1). Furthermore, measurements of coagulation are insensitive measures of vitamin K status and have limited value in establishing dietary recommendations (98). Despite this, the first attempts at establishing dietary requirements were based on the amount of vitamin K that was required to restore abnormal coagulation in hospitalized elderly male patients (99). This gave rise to an initial estimate of 0.75–1.0 μg/(kg · d), which in the US was revised in the mid-1980s to 75 to 90 μg/d for women and men, respectively.
In the last 2 decades, there has been growing interest in Gla proteins in bone, specifically osteocalcin. There has been much controversy regarding the role of vitamin K in bone health, because dietary intakes of vitamin K, and concomitant changes in biomarkers of vitamin K status, are indicative of a healthy diet and lifestyle (100). Observational studies that report associations between vitamin K and bone health have been unable to isolate the effect of vitamin K from that of a healthy diet (101, 102). Critical evaluation of the randomized clinical trials, as reviewed elsewhere in this volume, does not support the use of bone outcomes, including percent ucOC, as a physiological measure from which to derive vitamin K dietary requirements.
It is now emerging that vitamin K and vitamin K-dependent proteins have potential physiological roles beyond coagulation and bone metabolism (31). Putative regulatory roles include: calcification processes in multiple tissues (31), key enzymes involved in sphingolipid metabolism (103), energy metabolism and inflammation (31), and the prevention of oxidative injury in vivo (104). However, these potential roles for vitamin K require confirmation in controlled dose-response trials, with validation of suitable biomarkers or clinical endpoints in order to determine vitamin K requirements for optimal health.
In this context, the AI for vitamin K was established at 90 μg/d for women and 120 μg/d for men for the US and Canada in 2001 and was based on median phylloquinone intakes estimated from national surveys (1). The importance of MK to adequacy is unknown and has not been considered to date in forming dietary recommendations. The extent to which endogenous MK production contributes to the daily requirement for vitamin K is not known (17, 18). A subclinical deficiency of vitamin K, as indicated by sensitive biochemical measures of vitamin K status, can be created within days by limiting dietary intakes of phylloquinone without a concomitant change in gut flora or MK status, which suggests that MK are not utilized in sufficient amounts to maintain γ-carboxylation of all vitamin K-dependent proteins (34, 105, 106).
Conclusions and future directions
A decade after the US Food and Nutrition Board (1) considered vitamin K requirements, it is reasonable to ask to what extent our knowledge of nutritional concepts and metabolism of vitamin K has advanced. At that time, there had been no stable isotope studies of vitamin K and our knowledge of bioavailability was poor. Since then, there have been a number of studies in which stable isotope methodologies have been applied to the assessment of the bioavailability of phylloquinone in its free state and, more importantly, when incorporated into a plant matrix. This is still a difficult area for research because of the low tissue concentrations and the variety of molecular forms. There is a clear need for stable isotope techniques with enhanced sensitivity to be able to answer the many outstanding questions. A good illustration of the limitations of using unlabeled K vitamins is in lipoprotein transport and cellular uptake, because it is not possible to differentiate between the vitamer originating from the administration of the original dose from the stores already in the body and at later times new intakes from food sources. The potential interpretative problems include those associated with nonphysiological doses, how the bolus given might exchange with preexisting pools, and the inability to detect metabolic events such as is known for the inter-conversion of phylloquinone to MK-4. Nevertheless, some progress has been made in delineating certain features of the mode of transport of K vitamins in blood, which have included identification of their likely mode of cellular uptake by bone and the receptors involved. However, distinct differences are seen in the plasma transport and clearance rates of various molecular forms of vitamin K and these require further investigation by stable isotope techniques.
Another area for future research is the evidence that common polymorphisms or haplotypes in certain key genes implicated in vitamin K metabolism (e.g., APOE, VKOR, CYP4F2) might affect nutritional requirements. Thus far, much of this evidence is indirect via effects on warfarin dose requirements and for some (e.g., APOE), the data are inconsistent and often contradictory.
VKDB in early infancy continues to be a leading cause of intracranial bleeding even in developed countries (107) and the reasons for its higher prevalence in certain Asian countries has not been solved. Whether this susceptibility to VKDB is connected to the known lower warfarin doses required by persons of Asian ancestry and a low-warfarin–dose VKOR haplotype identified in this population (108) is worthy of further exploration. There is almost universal consensus for the need for vitamin K prophylaxis to prevent VKDB, but in many countries the method of vitamin K prophylaxis is haphazard. The effectiveness of any vitamin K prophylactic regimen needs to based on sound nutritional principles balancing underdosage against overdosage and should form part of any discussion on vitamin K requirements.
New roles for vitamin K in reducing risk of certain chronic diseases have been proposed since 2001. However, the lack of suitable biomarkers or clinical endpoints continues to limit the ability to define the optimal intake of vitamin K for adults. Similarly, as new knowledge is gained regarding the roles of multiple forms of vitamin K, it is apparent that future recommendations need to account for differences in their bioavailability. The application of stable isotope technology to future studies of relative bioavailability of phylloquinone and MK will be critical.
Acknowledgments
The authors thank Renata Gorska for her skilful artwork in the preparation of the figures. All authors have read and approved the final manuscript.
Footnotes
Supported by the USDA, Agricultural Research Service under Cooperative Agreement no. 58-1950-7-707, and the NIH (DK69341). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Author disclosures: M. J. Shearer, X. Fu, and S. L. Booth, no conflicts of interest.
Abbreviations used: AI, Adequate Intake; CM, chylomicron; CR, chylomicron remnant; Gla, γ carboxyglutamic acid; HSPG, heparin sulfate proteoglycan; LPL, lipoprotein lipase; LDLR, LDL receptor; LRP, LDL receptor-related protein; MK, menaquinone; TRL, TG-rich lipoprotein; ucOC, undercarboxylated osteocalcin; VKDB, vitamin K deficiency bleeding of the newborn; VLDLR, VLDL receptor.
Literature Cited
- 1.Food and Nutrition Board Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academies Press; 2001 [Google Scholar]
- 2.Ji Y, Li X, Tso P. Intestinal fatty acid absorption. Immununol Endocr Metab Agents Med Chem. 2009;9:60–73 [Google Scholar]
- 3.Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol. 2008;19:73–82 [DOI] [PubMed] [Google Scholar]
- 4.Booth SL, Suttie JW. Dietary intake and adequacy of vitamin K. J Nutr. 1998;128:785–8 [DOI] [PubMed] [Google Scholar]
- 5.Gijsbers BL, Jie KS, Vermeer C. Effect of food composition on vitamin K absorption in human volunteers. Br J Nutr. 1996;76:223–9 [DOI] [PubMed] [Google Scholar]
- 6.Garber AK, Binkley NC, Krueger DC, Suttie JW. Comparison of phylloquinone bioavailability from food sources or a supplement in human subjects. J Nutr. 1999;129:1201–3 [DOI] [PubMed] [Google Scholar]
- 7.Booth SL, Lichtenstein AH, Dallal GE. Phylloquinone absorption from phylloquinone-fortified oil is greater than from a vegetable in younger and older men and women. J Nutr. 2002;132:2609–12 [DOI] [PubMed] [Google Scholar]
- 8.Dolnikowski GG, Sun Z, Grusak MA, Peterson JW, Booth SL. HPLC and GC/MS determination of deuterated vitamin K (phylloquinone) in human serum after ingestion of deuterium-labeled broccoli. J Nutr Biochem. 2002;13:168–74 [DOI] [PubMed] [Google Scholar]
- 9.Erkkilä AT, Lichtenstein AH, Dolnikowski GG, Grusak MA, Jalbert SM, Aquino KA, Peterson JW, Booth SL. Plasma transport of vitamin K in men using deuterium-labeled collard greens. Metabolism. 2004;53:215–21 [DOI] [PubMed] [Google Scholar]
- 10.Fu X, Peterson JW, Hdeib M, Booth SL, Grusak MA, Lichtenstein AH, Dolnikowski GG. Measurement of deuterium-labeled phylloquinone in plasma by high-performance liquid chromatography/mass spectrometry. Anal Chem. 2009;81:5421–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones KS, Bluck LJ, Wang LY, Coward WA. A stable isotope method for the simultaneous measurement of vitamin K1 (phylloquinone) kinetics and absorption. Eur J Clin Nutr. 2008;62:1273–81 [DOI] [PubMed] [Google Scholar]
- 12.Jones KS, Bluck LJ, Wang LY, Stephen AM, Prynne CJ, Coward WA. The effect of different meals on the absorption of stable isotope-labelled phylloquinone. Br J Nutr. 2009;102:1195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurilich AC, Britz SJ, Clevidence BA, Novotny JA. Isotopic labeling and LC-APCI-MS quantification for investigating absorption of carotenoids and phylloquinone from kale (Brassica oleracea). J Agric Food Chem. 2003;51:4877–83 [DOI] [PubMed] [Google Scholar]
- 14.Novotny JA, Kurilich AC, Britz SJ, Baer DJ, Clevidence BA. Vitamin K absorption and kinetics in human subjects after consumption of 13C-labelled phylloquinone from kale. Br J Nutr. 2010;104:858–62 [DOI] [PubMed] [Google Scholar]
- 15.Shearer MJ, McBurney A, Barkhan P. Studies on the absorption and metabolism of phylloquinone (vitamin K1) in man. Vitam Horm. 1974;32:513–42 [DOI] [PubMed] [Google Scholar]
- 16.Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta. 2002;1570:27–32 [DOI] [PubMed] [Google Scholar]
- 17.Shearer MJ. Vitamin K metabolism and nutriture. Blood Rev. 1992;6:92–104 [DOI] [PubMed] [Google Scholar]
- 18.Suttie JW. The importance of menaquinones in human nutrition. Annu Rev Nutr. 1995;15:399–417 [DOI] [PubMed] [Google Scholar]
- 19.Blomstrand R, Forsgren L. Vitamin K1–3H in man. Its intestinal absorption and transport in the thoracic duct lymph. Int Z Vitaminforsch. 1968;38:45–64 [PubMed] [Google Scholar]
- 20.Shearer MJ, Barkhan P, Webster GR. Absorption and excretion of an oral dose of tritiated vitamin K1 in man. Br J Haematol. 1970;18:297–308 [DOI] [PubMed] [Google Scholar]
- 21.Lamon-Fava S, Sadowski JA, Davidson KW, O'Brien ME, McNamara JR, Schaefer EJ. Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. Am J Clin Nutr. 1998;67:1226–31 [DOI] [PubMed] [Google Scholar]
- 22.Krasinski SD, Cohn JS, Russell RM, Schaefer EJ. Postprandial plasma vitamin A metabolism in humans: a reassessment of the use of plasma retinyl esters as markers for intestinally derived chylomicrons and their remnants. Metabolism. 1990;39:357–65 [DOI] [PubMed] [Google Scholar]
- 23.Meydani M, Cohn JS, Macauley JB, McNamara JR, Blumberg JB, Schaefer EJ. Postprandial changes in the plasma concentration of alpha- and gamma-tocopherol in human subjects fed a fat-rich meal supplemented with fat-soluble vitamins. J Nutr. 1989;119:1252–8 [DOI] [PubMed] [Google Scholar]
- 24.Cooper AD. Hepatic uptake of chylomicron remnants. J Lipid Res. 1997;38:2173–92 [PubMed] [Google Scholar]
- 25.Kockx M, Jessup W, Kritharides L. Regulation of endogenous apolipoprotein E secretion by macrophages. Arterioscler Thromb Vasc Biol. 2008;28:1060–7 [DOI] [PubMed] [Google Scholar]
- 26.Ji ZS, Fazio S, Lee YL, Mahley RW. Secretion-capture role for apolipoprotein E in remnant lipoprotein metabolism involving cell surface heparan sulfate proteoglycans. J Biol Chem. 1994;269:2764–72 [PubMed] [Google Scholar]
- 27.Shimano H, Namba Y, Ohsuga J, Kawamura M, Yamamoto K, Shimada M, Gotoda T, Harada K, Yazaki Y, Yamada N. Secretion-recapture process of apolipoprotein E in hepatic uptake of chylomicron remnants in transgenic mice. J Clin Invest. 1994;93:2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahley RW, Hussain MM. Chylomicron and chylomicron remnant catabolism. Curr Opin Lipidol. 1991;2:170 [Google Scholar]
- 29.Shearer MJ, Barkhan P. Studies on the metabolites of phylloquinone (vitamin K 1) in the urine of man. Biochim Biophys Acta. 1973;297:300–12 [DOI] [PubMed] [Google Scholar]
- 30.Shearer MJ, Mallinson CN, Webster GR, Barkhan P. Clearance from plasma and excretion in urine, faeces and bile of an intravenous dose of tritiated vitamin K 1 in man. Br J Haematol. 1972;22:579–88 [DOI] [PubMed] [Google Scholar]
- 31.Booth SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr. 2009;29:89–110 [DOI] [PubMed] [Google Scholar]
- 32.Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewe RB, Brandenburg VM, Bekers O, Vermeer C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost. 2010;104:811–22 [DOI] [PubMed] [Google Scholar]
- 33.Newman P, Bonello F, Wierzbicki AS, Lumb P, Savidge GF, Shearer MJ. The uptake of lipoprotein-borne phylloquinone (vitamin K1) by osteoblasts and osteoblast-like cells: role of heparan sulfate proteoglycans and apolipoprotein E. J Bone Miner Res. 2002;17:426–33 [DOI] [PubMed] [Google Scholar]
- 34.Booth SL, O'Brien-Morse ME, Dallal GE, Davidson KW, Gundberg CM. Response of vitamin K status to different intakes and sources of phylloquinone-rich foods: comparison of younger and older adults. Am J Clin Nutr. 1999;70:368–77 [DOI] [PubMed] [Google Scholar]
- 35.Niemeier A, Kassem M, Toedter K, Wendt D, Ruether W, Beisiegel U, Heeren J. Expression of LRP1 by human osteoblasts: a mechanism for the delivery of lipoproteins and vitamin K1 to bone. J Bone Miner Res. 2005;20:283–93 [DOI] [PubMed] [Google Scholar]
- 36.Hussain MM, Goldberg IJ, Weisgraber KH, Mahley RW, Innerarity TL. Uptake of chylomicrons by the liver, but not by the bone marrow, is modulated by lipoprotein lipase activity. Arterioscler Thromb Vasc Biol. 1997;17:1407–13 [DOI] [PubMed] [Google Scholar]
- 37.Hussain MM, Mahley RW, Boyles JK, Fainaru M, Brecht WJ, Lindquist PA. Chylomicron-chylomicron remnant clearance by liver and bone marrow in rabbits. Factors that modify tissue-specific uptake. J Biol Chem. 1989;264:9571–82 [PubMed] [Google Scholar]
- 38.Hussain MM, Mahley RW, Boyles JK, Lindquist PA, Brecht WJ, Innerarity TL. Chylomicron metabolism. Chylomicron uptake by bone marrow in different animal species. J Biol Chem. 1989;264:17931–8 [PubMed] [Google Scholar]
- 39.Kohlmeier M, Salomon A, Saupe J, Shearer MJ. Transport of vitamin K to bone in humans. J Nutr. 1996;126:S1192–6 [DOI] [PubMed] [Google Scholar]
- 40.Niemeier A, Niedzielska D, Secer R, Schilling A, Merkel M, Enrich C, Rensen PC, Heeren J. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 2008;43:230–7 [DOI] [PubMed] [Google Scholar]
- 41.Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109:3279–83 [DOI] [PubMed] [Google Scholar]
- 42.Booth SL, Tucker KL, McKeown NM, Davidson KW, Dallal GE, Sadowski JA. Relationships between dietary intakes and fasting plasma concentrations of fat-soluble vitamins in humans. J Nutr. 1997;127:587–92 [DOI] [PubMed] [Google Scholar]
- 43.Shea MK, Benjamin EJ, Dupuis J, Massaro JM, Jacques PF, D'Agostino RB, Sr, Ordovas JM, O'Donnell CJ, Dawson-Hughes B, Vasan RS, et al. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr. 2009;63:458–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weintraub MS, Eisenberg S, Breslow JL. Dietary fat clearance in normal subjects is regulated by genetic variation in apolipoprotein E. J Clin Invest. 1987;80:1571–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boerwinkle E, Brown S, Sharrett AR, Heiss G, Patsch W. Apolipoprotein E polymorphism influences postprandial retinyl palmitate but not triglyceride concentrations. Am J Hum Genet. 1994;54:341–60 [PMC free article] [PubMed] [Google Scholar]
- 46.Cortner JA, Coates PM, Le NA, Cryer DR, Ragni MC, Faulkner A, Langer T. Kinetics of chylomicron remnant clearance in normal and in hyperlipoproteinemic subjects. J Lipid Res. 1987;28:195–206 [PubMed] [Google Scholar]
- 47.Saupe J, Shearer MJ, Kohlmeier M. Phylloquinone transport and its influence on gamma-carboxyglutamate residues of osteocalcin in patients on maintenance hemodialysis. Am J Clin Nutr. 1993;58:204–8 [DOI] [PubMed] [Google Scholar]
- 48.Pilkey RM, Morton AR, Boffa MB, Noordhof C, Day AG, Su Y, Miller LM, Koschinsky ML, Booth SL. Subclinical vitamin K deficiency in hemodialysis patients. Am J Kidney Dis. 2007;49:432–9 [DOI] [PubMed] [Google Scholar]
- 49.Yan L, Zhou B, Nigdikar S, Wang X, Bennett J, Prentice A. Effect of apolipoprotein E genotype on vitamin K status in healthy older adults from China and the UK. Br J Nutr. 2005;94:956–61 [DOI] [PubMed] [Google Scholar]
- 50.Crosier M, Shea MK, Ordovas JM, Gundberg CM, Dawson-Hughes B, Booth SL. Apolipoprotein E genotype is a determinant of serum vitamin K, but not BMD, in older men and women. J Bone Miner Res. 2005;20:S343 [Google Scholar]
- 51.Schneider WJ, Kovanen PT, Brown MS, Goldstein JL, Utermann G, Weber W, Havel RJ, Kotite L, Kane JP, Innerarity TL, et al. Familial dysbetalipoproteinemia. Abnormal binding of mutant apoprotein E to low density lipoprotein receptors of human fibroblasts and membranes from liver and adrenal of rats, rabbits, and cows. J Clin Invest. 1981;68:1075–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weisgraber KH, Innerarity TL, Mahley RW. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982;257:2518–21 [PubMed] [Google Scholar]
- 53.Bergeron N, Havel RJ. Prolonged postprandial responses of lipids and apolipoproteins in triglyceride-rich lipoproteins of individuals expressing an apolipoprotein epsilon 4 allele. J Clin Invest. 1996;97:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimmel SE, Christie J, Kealey C, Chen Z, Price M, Thorn CF, Brensinger CM, Newcomb CW, Whitehead AS. Apolipoprotein E genotype and warfarin dosing among Caucasians and African Americans. Pharmacogenomics J. 2008;8:53–60 [DOI] [PubMed] [Google Scholar]
- 55.Kohnke H, Sorlin K, Granath G, Wadelius M. Warfarin dose related to apolipoprotein E (APOE) genotype. Eur J Clin Pharmacol. 2005;61:381–8 [DOI] [PubMed] [Google Scholar]
- 56.Visser LE, Trienekens PH, De Smet PA, Vulto AG, Hofman A, van Duijn CM, Stricker BH. Patients with an ApoE epsilon4 allele require lower doses of coumarin anticoagulants. Pharmacogenet Genomics. 2005;15:69–74 [DOI] [PubMed] [Google Scholar]
- 57.Sconce EA, Daly AK, Khan TI, Wynne HA, Kamali F. APOE genotype makes a small contribution to warfarin dose requirements. Pharmacogenet Genomics. 2006;16:609–11 [DOI] [PubMed] [Google Scholar]
- 58.Shahin MH, Khalifa SI, Gong Y, Hammad LN, Sallam MT, El Shafey M, Ali SS, Mohamed ME, Langaee T, Johnson JA. Genetic and nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet Genomics. 2011;21:130–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sokoll LJ, Sadowski JA. Comparison of biochemical indexes for assessing vitamin K nutritional status in a healthy adult population. Am J Clin Nutr. 1996;63:566–73 [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, Okuda N, Shimomura Y, Suhara Y, Okano T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 2010;468:117–21 [DOI] [PubMed] [Google Scholar]
- 61.Thijssen HH, Vervoort LM, Schurgers LJ, Shearer MJ. Menadione is a metabolite of oral vitamin K. Br J Nutr. 2006;95:260–6 [DOI] [PubMed] [Google Scholar]
- 62.Oldenburg J, Marinova M, Muller-Reible C, Watzka M. The vitamin K cycle. Vitam Horm. 2008;78:35–62 [DOI] [PubMed] [Google Scholar]
- 63.Tie JK, Stafford DW. Structure and function of vitamin K epoxide reductase. Vitam Horm. 2008;78:103–30 [DOI] [PubMed] [Google Scholar]
- 64.Wu S, Liu S, Davis CH, Stafford DW, Kulman JD, Pedersen LG. A hetero-dimer model for concerted action of vitamin K carboxylase and vitamin K reductase in vitamin K cycle. J Theor Biol. 2011;279:143–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oldenburg J, von Brederlow B, Fregin A, Rost S, Wolz W, Eberl W, Eber S, Lenz E, Schwaab R, Brackmann HH, et al. Congenital deficiency of vitamin K dependent coagulation factors in two families presents as a genetic defect of the vitamin K-epoxide-reductase-complex. Thromb Haemost. 2000;84:937–41 [PubMed] [Google Scholar]
- 66.Goodstadt L, Ponting CP. Vitamin K epoxide reductase: homology, active site and catalytic mechanism. Trends Biochem Sci. 2004;29:289–92 [DOI] [PubMed] [Google Scholar]
- 67.Rettie AE, Tai G. The pharmocogenomics of warfarin: closing in on personalized medicine. Mol Interv. 2006;6:223. [DOI] [PubMed] [Google Scholar]
- 68.Shikata E, Ieiri I, Ishiguro S, Aono H, Inoue K, Koide T, Ohgi S, Otsubo K. Association of pharmacokinetic (CYP2C9) and pharmacodynamic (factors II, VII, IX, and X; proteins S and C; and gamma-glutamyl carboxylase) gene variants with warfarin sensitivity. Blood. 2004;103:2630–5 [DOI] [PubMed] [Google Scholar]
- 69.Vecsler M, Loebstein R, Almog S, Kurnik D, Goldman B, Halkin H, Gak E. Combined genetic profiles of components and regulators of the vitamin K-dependent gamma-carboxylation system affect individual sensitivity to warfarin. Thromb Haemost. 2006;95:205–11 [DOI] [PubMed] [Google Scholar]
- 70.Wadelius M, Chen LY, Downes K, Ghori J, Hunt S, Eriksson N, Wallerman O, Melhus H, Wadelius C, Bentley D, et al. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 2005;5:262–70 [DOI] [PubMed] [Google Scholar]
- 71.Wang Y, Zhang W, Zhang Y, Yang Y, Sun L, Hu S, Chen J, Zhang C, Zheng Y, Zhen Y, et al. VKORC1 haplotypes are associated with arterial vascular diseases (stroke, coronary heart disease, and aortic dissection). Circulation. 2006;113:1615–21 [DOI] [PubMed] [Google Scholar]
- 72.Kinoshita H, Nakagawa K, Narusawa K, Goseki-Sone M, Fukushi-Irie M, Mizoi L, Yoshida H, Okano T, Nakamura T, Suzuki T, et al. A functional single nucleotide polymorphism in the vitamin-K-dependent gamma-glutamyl carboxylase gene (Arg325Gln) is associated with bone mineral density in elderly Japanese women. Bone. 2007;40:451–6 [DOI] [PubMed] [Google Scholar]
- 73.Crosier MD, Peter I, Booth SL, Bennett G, Dawson-Hughes B, Ordovas JM. Association of sequence variations in vitamin K epoxide reductase and gamma-glutamyl carboxylase genes with biochemical measures of vitamin K status. J Nutr Sci Vitaminol (Tokyo). 2009;55:112–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morris MC, Tangney CC. A potential design flaw of randomized trials of vitamin supplements. JAMA. 2011;305:1348–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McBurney A, Shearer MJ, Barkhan P. Preparative isolation and characterization of the urinary aglycones of vitamin K1 (phylloquinone in man. Biochem Med. 1980;24:250–67 [DOI] [PubMed] [Google Scholar]
- 76.Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530–47 [PubMed] [Google Scholar]
- 77.Caldwell MD, Awad T, Johnson JA, Gage BF, Falkowski M, Gardina P, Hubbard J, Turpaz Y, Langaee TY, Eby C, et al. CYP4F2 genetic variant alters required warfarin dose. Blood. 2008;111:4106–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE. CYP4F2 is a vitamin K1 oxidase: an explanation for altered warfarin dose in carriers of the V433M variant. Mol Pharmacol. 2009;75:1337–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harrington DJ, Booth SL, Card DJ, Shearer MJ. Excretion of the urinary 5C- and 7C-aglycone metabolites of vitamin K by young adults responds to changes in dietary phylloquinone and dihydrophylloquinone intakes. J Nutr. 2007;137:1763–8 [DOI] [PubMed] [Google Scholar]
- 80.Shearer MJ. Vitamin K deficiency bleeding (VKDB) in early infancy. Blood Rev. 2009;23:49–59 [DOI] [PubMed] [Google Scholar]
- 81.Howe AM, Webster WS, Lipson AH, Halliday JL, Sheffield LJ. Binder's syndrome due to prenatal vitamin K deficiency: a theory of pathogenesis. Aust Dent J. 1992;37:453–60 [DOI] [PubMed] [Google Scholar]
- 82.Irving MD, Chitty LS, Mansour S, Hall CM. Chondrodysplasia punctata: a clinical diagnostic and radiological review. Clin Dysmorphol. 2008;17:229–41 [DOI] [PubMed] [Google Scholar]
- 83.Greer FR, Marshall S, Cherry J, Suttie JW. Vitamin K status of lactating mothers, human milk, and breast-feeding infants. Pediatrics. 1991;88:751–6 [PubMed] [Google Scholar]
- 84.Canfield LM, Hopkinson JM, Lima AF, Martin GS, Sugimoto K, Burr J, Clark L, McGee DL. Quantitation of vitamin K in human milk. Lipids. 1990;25:406–11 [DOI] [PubMed] [Google Scholar]
- 85.Canfield LM, Hopkinson JM, Lima AF, Silva B, Garza C. Vitamin K in colostrum and mature human milk over the lactation period–a cross-sectional study. Am J Clin Nutr. 1991;53:730–5 [DOI] [PubMed] [Google Scholar]
- 86.von Kries R, Shearer M, McCarthy PT, Haug M, Harzer G, Gobel U. Vitamin K1 content of maternal milk: influence of the stage of lactation, lipid composition, and vitamin K1 supplements given to the mother. Pediatr Res. 1987;22:513–7 [DOI] [PubMed] [Google Scholar]
- 87.Chuansumrit A, Plueksacheeva T, Hanpinitsak S, Sangwarn S, Chatvutinun S, Suthutvoravut U, Herabutya Y, Shearer MJ. Prevalence of subclinical vitamin K deficiency in Thai newborns: relationship to maternal phylloquinone intakes and delivery risk. Arch Dis Child Fetal Neonatal Ed. 2010;95:F104–8 [DOI] [PubMed] [Google Scholar]
- 88.Shearer MJ, McCarthy PT, Crampton OE, Mattock MB. The assessment of human vitamin K status from tissue measurements. In: Suttie JW, editor. Current advances in vitamin K research. New York: Elsevier; 1988. p. 437–52. [Google Scholar]
- 89.American Association of Pediatrics Report of Committee on Nutrition. Vitamin K compounds and the water-soluble analogues. Pediatrics. 1961;28:501–7 [Google Scholar]
- 90.Golding J, Greenwood R, Birmingham K, Mott M. Childhood cancer, intramuscular vitamin K, and pethidine given during labour. BMJ. 1992;305:341–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.WHO. Priority medicines for mothers and children. Geneva: WHO; 2011
- 92.Loughnan PM, McDougall PN. Does intramuscular vitamin K1 act as an unintended depot preparation? J Paediatr Child Health. 1996;32:251–4 [DOI] [PubMed] [Google Scholar]
- 93.Tulchinsky TH, Patton MM, Randolph LA, Meyer MR, Linden JV. Mandating vitamin K prophylaxis for newborns in New York State. Am J Public Health. 1993;83:1166–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Busfield A, McNinch A, Tripp J. Neonatal vitamin K prophylaxis in Great Britain and Ireland: the impact of perceived risk and product licensing on effectiveness. Arch Dis Child. 2007;92:754–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strehle EM, Howey C, Jones R. Evaluation of the acceptability of a new oral vitamin K prophylaxis for breastfed infants. Acta Paediatr. 2010;99:379–83 [DOI] [PubMed] [Google Scholar]
- 96.Clarke P. Vitamin K prophylaxis for preterm infants. Early Hum Dev. 2010;86 Suppl 1:17–20 [DOI] [PubMed] [Google Scholar]
- 97.Harrington DJ, Clarke P, Card DJ, Mitchell SJ, Shearer MJ. Urinary excretion of vitamin K metabolites in term and preterm infants: relationship to vitamin K status and prophylaxis. Pediatr Res. 2010;68:508–12 [DOI] [PubMed] [Google Scholar]
- 98.Booth SL, Al Rajabi A. Determinants of vitamin K status in humans. Vitam Horm. 2008;78:1–22 [DOI] [PubMed] [Google Scholar]
- 99.Frick PG, Riedler G, Brogli H. Dose response and minimal daily requirement for vitamin K in man. J Appl Physiol. 1967;23:387–9 [DOI] [PubMed] [Google Scholar]
- 100.Erkkilä AT, Booth SL, Hu FB, Jacques PF, Manson JE, Rexrode KM, Stampfer MJ, Lichtenstein AH. Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. Eur J Clin Nutr. 2005;59:196–204 [DOI] [PubMed] [Google Scholar]
- 101.Booth SL, Broe K, Gagnon D, Tucker K, Hannan M, McLean R, Dawson-Hughes B, Wilson P, Cupples L, Kiel D. Vitamin K intake and bone mineral density in women and men. Am J Clin Nutr. 2003;77:512–6 [DOI] [PubMed] [Google Scholar]
- 102.Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, Cupples LA, Wilson PW, Kiel DP. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab. 2004;89:4904–9 [DOI] [PubMed] [Google Scholar]
- 103.Denisova NA, Booth SL. Vitamin K and sphingolipid metabolism: evidence to date. Nutr Rev. 2005;63:111–21 [DOI] [PubMed] [Google Scholar]
- 104.Li J, Lin JC, Wang H, Peterson JW, Furie BC, Furie B, Booth SL, Volpe JJ, Rosenberg PA. Novel role of vitamin K in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci. 2003;23:5816–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Booth SL, Lichtenstein AH, O'Brien-Morse M, McKeown NM, Wood RJ, Saltzman E, Gundberg CM. Effects of a hydrogenated form of vitamin K on bone formation and resorption. Am J Clin Nutr. 2001;74:783–90 [DOI] [PubMed] [Google Scholar]
- 106.Booth SL, Martini L, Peterson JW, Saltzman E, Dallal GE, Wood RJ. Dietary phylloquinone depletion and repletion in older women. J Nutr. 2003;133:2565–9 [DOI] [PubMed] [Google Scholar]
- 107.Visser DY, Jansen NJ, Ijland MM, de Koning TJ, van Hasselt PM. Intracranial bleeding due to vitamin K deficiency: advantages of using a pediatric intensive care registry. Intensive Care Med. 2011;37:1014–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, Blough DK, Thummel KE, Veenstra DL, Rettie AE. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–93 [DOI] [PubMed] [Google Scholar]
- 109. European Micronutrient Recommendations Aligned (Eurreca) Micronutrient Database. Nutri-RecQuest. http://www.serbianfood.info/eurreca/ Last accessed: February 2, 2012.
- 110.Kamao M, Suhara Y, Tsugawa N, Uwano M, Yamaguchi N, Uenishi K, Ishida H, Sasaki S, Okano T. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J Nutr Sci Vitaminol (Tokyo). 2007;53:464–70 [DOI] [PubMed] [Google Scholar]