Abstract
Gas6 (growth arrest-specific 6) belongs structurally to the family of plasma vitamin K-dependent proteins. Gas6 has a high structural homology with the natural anticoagulant protein S, sharing the same modular composition. Interestingly, despite the presence of a γ-carboxyglutamic acid domain in its structure, no role in the coagulation cascade has been identified for gas6. Gas6 has been shown to be involved in vascular homeostasis and more precisely is involved in proliferation, apoptosis, efferocytosis, leukocyte migration, and sequestration and platelet aggregation. It is also involved in the activation of different cell types, from platelets to endothelial and vascular smooth muscle cells. Thus, it has been shown to play a role in several pathophysiological processes such as atherosclerosis, cancer, and thrombosis. Interestingly, studies using gas6 null mice highlighted that gas6 may represent a novel potential target for anticoagulant therapy, because these animals are protected from lethal venous thromboembolism without excessive bleeding. However, the mechanism in thrombus occurrence remains to be further explored. In the present review, we will focus on the role of gas6 in innate immunity, atherosclerosis, thrombosis, and cancer-related events.
Introduction
Gas6 is a VKD5 protein that was discovered in 1988 through the screening of genes whose expression was upregulated in growth arrest embryonic mouse fibroblast (1). The gene was sequenced in 1993 (2) and shares 44% homology with protein S, another VKD protein. Despite its structural similarity, gas6 tissue expression is clearly different from that of protein S. Whereas protein S is mainly expressed in the liver and by EC, gas6 is more widely expressed and has been found in the lung, heart, kidney, intestine, EC, bone marrow, VSMC, and monocytes and at very low levels in the liver (2–4). More recently, using an ELISA-based method, gas6 was detected in human plasma (5). Its concentration is quite low (∼20–50 μg/L) and is much lower than the concentration of the VKD proteins involved in hemostasis, whose concentrations vary from 10 nmol/L for factor VII to 1.5 μmol/L for prothrombin. Moreover, protein S and gas6 functions are clearly different. As reviewed by Castoldi et al. (6), protein S is an anticoagulant protein and plays a role in thrombin generation; in contrast, no role in thrombin generation has been found for gas6. Gas6 seems to exert a pleiotropic role in a wide range of cell types. It was identified in growth-arrested cells, thus suggesting its involvement in cellular homeostasis. Gas6 plays a role in leukocyte sequestration and migration, platelet aggregation and hematopoiesis, proliferation, apoptosis, and phagocytosis and is generally associated with conditions of injury, inflammation, and repair. The role of gas6 in innate immunity, vascular biology, and cancer will be the focus of the present review.
Current status of knowledge
Structure of gas6
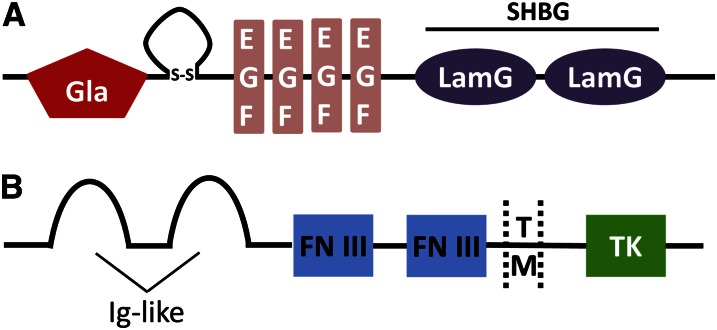
Gas6 is a multidomain protein with a molecular weight of 75 kDa. At the N terminus, gas6 contains a domain of amino acids containing Gla that is referred to as the Gla domain. The Gla domain confers to VKD proteins the ability to bind to anionic phospholipids at the cell surface (7). In resting cells, anionic phospholipids are localized in the inner leaflet of the plasma membrane. Exposition of anionic phospholipids at the cell surface is a feature of cell injury, activation, and apoptosis. In such conditions, these lipids provide a docking platform for the binding of VKD proteins and their activity (8). Consequently, Gla domains target gas6 to apoptotic or activated cells that occur in a wide range of pathologies. The Gla domain is followed by a loop maintained by a disulfide bridge. Contrary to the protein S loop, the gas6 loop seems to be insensitive to the action of serine proteases. In the case of protein S, this loop is essential for its interaction with activated protein C (9) and is inactivated by thrombin or factor Xa-specific cleavage (10, 11). The primary structure of gas6 after the loop contains 4 epidermal growth factor domains, 2 of them containing calcium-binding consensus sequences, followed by a SHBG-like domain. This domain contains 2 subdomains with a similar structure to the globular modules of laminin A (LamG). This type of globular structure is usually found in proteins interacting with heparin sulfates, steroids, or integrins (Fig. 1A).
Figure 1.
Gas6 and TAM receptor structure. (A) Gas6 is composed of, from N to C terminus, a Gla domain, a loop maintained by a disulfide bridge, 4 EGF domains, and 2 LamG subdomains containing the SHBG domain. (B) TAM receptors are composed of 2 Ig-like domains, 2 FN III domains, a TM, and a TK domain. EGF, epidermal growth factor; FN III, fibronectin type III-like; SHBG, sex hormone binding globulin; TAM, Tyro3, Axl, and Mer; TK, tyrosine kinase; TM, transmembrane domain.
Gas6-receptor interactions
Gas6 is the ligand for the TAM family of receptors, which is composed of 3 members: Tyro3, Axl, and Mer (12, 13). Axl has the highest affinity for gas6 followed by Tyro3 and Mer (14). These receptors belong to the large family of type I transmembrane receptor tyrosine kinase. Their extracellular domain contains 2 Ig-like domains, which are characteristic of adhesion molecules, followed by 2 fibronectin type III-like motifs. The cytoplasmic tail contains a tyrosine kinase domain (Fig. 1B). Axl was first identified in 1991 as a product of a transforming gene in a T-cell leukemia cell line and was named Axl from the Greekword “anexelekto,” meaning uncontrolled (15). Axl is a 140-kDa protein ubiquitously expressed. Independently of its ligand gas6, it has been shown that Axl has a transforming activity and its intracellular domain is able to induce tumors in nude mice (16). Moreover, it seems to be overexpressed in a wide variety of human cancers (17). In addition to its role in cancer, several studies have suggested a role in cell adhesion for Axl due to its extracellular domain, independently of the tyrosine kinase domain (18, 19). However, the functional relevance of Axl adhesion potency remains to be explored. Tyro3 was identified by several groups in 1994 and is predominantly expressed in the brain and central nervous system. Mer was identified in 1994 and is almost exclusively expressed in the monocytic cell lineage. Contrary to Axl, there is little data concerning a possible role of Tyro3 and Mer in cancer. Important insights concerning the biologic functions of the TAM receptors come from the study of triple knock-out mice (20), where Tyro3, Axl, and Mer are all deleted, suggesting a redundancy in receptor function. These mice were viable but presented several degenerative troubles related to an inefficient phagocytosis of apoptotic cells, leading to several abnormalities in spermatogenesis and immunity.
To date, many studies have found an activation of PI3K following gas6/Axl binding (21). PI3K/Akt activations are required for the antiapoptotic function of gas6 in several cell types such as EC, VSMC, fibroblasts, chrondrocytes, oligodendrocytes, neurons, and several cancer cells (22–31). Activation of Akt leads to the inactivation of Bad, a proapoptotic mediator, and to an increase of the antiapoptotic protein Bcl-2 by an NFκB-dependent mechanism (31–34). Interestingly, C1-TEN, a phosphatase with a similar structure as PTEN, a PI3K/Akt inhibitor, has been shown to bind to Axl. However, the biologic relevance of this interaction remains to be explored (35). Src is also involved in gas6-mediated survival or mitogenic effect and a docking site has been identified for Src on Axl (21, 36). Axl also provides a docking platform for the adaptor protein Grb2, which might be involved in the Ras-mediated ERK1/2 activation (36, 37). The Ras/ERK1/2 pathway is essential for mediating gas6 mitogenic activity. More recently, Gallicchio et al. (38) have highlighted a crosstalk between the gas6/Axl pathway and vascular endothelial growth factor type 2 that signals through the SHP-2 phosphatase. PLCγ has also been shown to interact with TAM, in the process of efferocytosis, inducing cytoskeletal rearrangements (39, 40). Finally, other signaling molecules have been suggested to be involved in gas6 signaling such as the stress-activated protein kinase/c-Jun NH2-terminal kinase (JNK/SAPK), p38, and the janus kinase-signal transducer and activator of transcription (JAK/STAT) pathways (33, 41, 42).
The role of the Gla domain in the regulation of gas6-induced receptor activation is very intriguing and still a matter of debate. In fact, several studies have demonstrated the importance of the Gla domain in gas6 function. Gla domain-deleted gas6 is still able to bind Axl in solution (43). Nevertheless, Nagata et al. (14) have shown that decarboxylated gas6 is unable to stimulate Axl activation in CHO cells. The Gla domain is also required for a gas6-mediated mitogenic effect on VSMC (44), mesangial cells (42), and the growth of cardiac fibroblasts (45). More recently, Hasanbasic et al. (46) showed that γ carboxylation is required for gas6-mediated EC survival as well as Akt phosphorylation. Consequently, the gas6 Gla domain seems to be required for signaling pathway activation but is not necessary for gas6 binding to Axl, which is mediated by the SHBG domain. How gas6 Gla domain affects Axl and downstream signaling pathways remains unclear and requires further investigation.
Gas6/Axl in innate immunity
In vivo studies using deficient mice for the TAM receptors highlight the involvement of gas6 receptors in immunity. After birth, these mice show an abnormal growth of peripheral lymphoid organs such as the spleen and lymph nodes and demonstrate a delayed clearance of apoptotic cells. Moreover, they develop a wide spectrum of autoimmune diseases (20, 47). They develop a lupus-like syndrome and present high levels of circulating auto-antibodies against DNA, collagen, and phospholipids. The Mer receptor seems to be the most important TAM receptor in the regulation of innate immunity. For example, LPS administration in Mer-deficient mice induces an excessive production of TNFα leading to endotoxic shock (48). These data are reinforced by in vitro studies showing that the activation of murine APC as well as the production of TNFα, IL-6, and IL-1 in monocytes/macrophages was inhibited by recombinant gas6, probably by a negative regulation of the inflammation response induced by Toll-like receptors (49–51). Gas6/TAM interactions also inhibited activation of dendritic cells (52). Biochemical analyses showed that the TAM receptors act as negative regulators for Toll-like receptors 3, 6, and 9 in dendritic cells. Interestingly, TAM receptor expression is detected on APC from both peripheral blood and tissue but is not found on granulocytes and lymphocytes. Consequently, the activation state of lymphocytes in TAM-deficient mice is probably due to a lack of an inhibitory signal from APC. The above data highlight that gas6 might act as an antiinflammatory mediator by regulating the clearance of apoptotic cells and innate immunity.
However, these data are counteracted by in vivo analysis showing that gas6 promotes inflammation. Tjwa et al. (53) have shown that gas6 amplified EC activation and promoted recruitment of platelets and leukocytes on EC surface as well as leukocyte extravasation through a P-selectin–dependent mechanism. These observations were confirmed by the fact that leukocyte recruitment in the neointima is decreased after vascular injury in Axl-deficient mice (54). Moreover, gas6 null mice appear to be protected against inflammation in models such as sepsis or vasculitis (53). More recently, gas6 has been also shown to be proinflammatory in a model of chronic liver disease in gas6 null mice (55).
Gas6/Axl in the vasculature
Strong evidence suggested that gas6/Axl signaling is important in the vasculature. Axl is involved in the integrity of the vasculature and its expression is upregulated at the site of vascular injury, suggesting a role for Axl in vascular remodeling (56). In 1995, Nakano et al. (3) were the first to demonstrate that gas6, isolated from conditioned media of VSMC, potentiated the growth response of VSMC treated with angiotensin II. Shortly thereafter, Melaragno et al. (24) found that Axl expression increased after balloon injury of rat carotid arteries. Axl and gas6 expression were temporally correlated with neointima formation. In cultured VSMC, Axl expression and activation were induced by thrombin and angiotensin II (56). Activation of Axl has also been shown to be partially independent of gas6 in VSMC treated with H2O2 (57). More recently, the same group demonstrated that gas6 increased reactive oxygen species production and Axl association to nonmuscle myosine IIB in VSMC (58). In another study, in VSMC, gas6/Axl interactions lead to activation of different signaling pathways depending on glucose concentration. In conditions of high glucose, gas6/Axl induces ERK1/2. A role for gas6/Axl has also been described in flow-induced vascular remodeling. Genetic deletion of Axl significantly attenuated intima-media thickening of the mouse carotid by increasing cellular apoptosis and altering vascular inflammation. An important finding was that the gas6/Axl pathway regulated the function of several cell types in the vessel wall and appeared to act in an autocrine/paracrine fashion. Remodeled carotids from Axl−/− mice had significantly altered inflammatory responses compared with Axl+/+ mice. Specific alterations included relatively more monocytes than VSMC, decreased macrophages and neutrophils in the intima, and increased neutrophils in the adventitia (54). Finally, gas6/Axl interactions have been shown to play a role in vascular calcifications. Axl was found to be specifically expressed by confluent pericytes and its expression was downregulated in postconfluent cultures containing mineralized nodules. Pericytes also secrete gas6 and the inhibition of activity of endogenous Axl in pericytes enhanced their differentiation along the osteogenic pathway (59). Statins have been found to prevent apoptosis of aortic smooth muscle cells by restoring the gas6/Axl pathway, thus inhibiting calcification (60).
Gas6/Axl in atherosclerosis
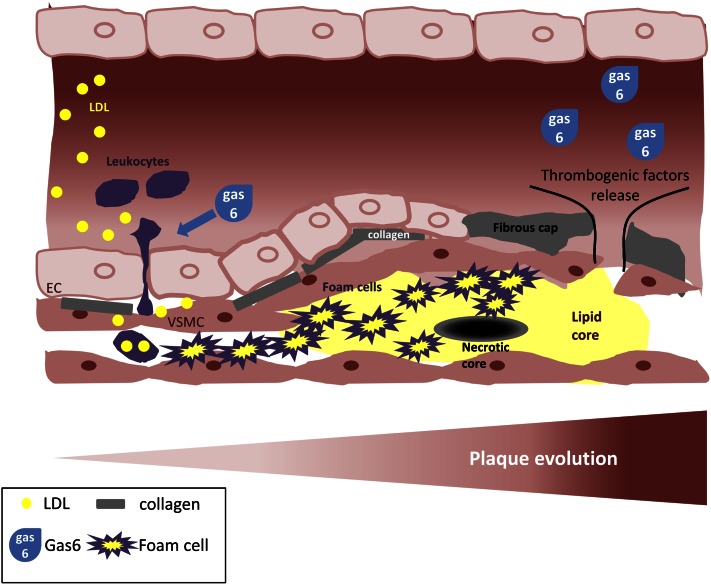
Several lines of evidence, mostly obtained in mouse models, suggest that gas6 may play a role in atherosclerosis. As mentioned above, gas6 promotes survival (61) and migration (62) of VSMC and was reported to be overexpressed, along with its receptor Axl, in rat arterial neointima following balloon injury, with a time correlation between gas6 and Axl expressions, and neointima formation (56). Thus, gas6 can promote VSMC survival and accumulation within the atherosclerotic plaque as well as vascular remodeling, which leads to a more stable plaque with a strengthened fibrous cap. Gas6 can also promote apoptotic cell clearance (63), and the Mer receptor has been shown to participate in the phagocytic clearance of cholesterol-induced apoptotic murine cells (64). Double inactivation of Mer and either Ldlr (65) or ApoE (66) in mice resulted in an acceleration of atherosclerosis with accumulation of apoptotic cells in the plaques and an increase of the necrotic core size. Thus, the gas6/Mer axis may also be beneficial for the plaque by reducing local inflammation caused by the accumulation of apoptotic cells, which itself leads to the formation of the necrotic core (67). However, other murine models showed a detrimental effect of gas6 on atherosclerosis development. Indeed, gas6 can promote recruitment and extravasation of leukocytes to the arterial wall, thus enhancing plaque inflammation (53). Finally, double knock-out mice for gas6 and ApoE exhibited more stable and less inflammatory plaques, containing more smooth muscle cells and collagen and fewer macrophages compared to ApoE−/− mice (68). These results suggested a protective role of gas6 deletion against atherosclerosis. Fewer data are available in human atherosclerosis plaque, but 2 recent studies have examined the expression of gas6 and its receptors in human atherosclerosis plaques. Results from Hurtado et al. showed that gas6 was not differentially expressed in carotids without atherosclerosis compared to carotids with advanced atherosclerosis (69). At the same time, Axl and Tyro3, which could be the receptors for gas6 in the context of the vessel wall, are downregulated in advanced human carotid plaques. The only TAM receptor whose expression increased in human carotid plaques was Mer, recapitulating data found in animal models of atherosclerosis deficient for Mer (65, 66). A very recent study from Clauser et al. (70) reported increased expression of gas6 and receptor Axl in VSMC and showed that gas6 is also able to reduce the in vitro expression of proinflammatory molecules TNFα and intercellular adhesion molecule-1 by VSMC. These discrepancies might be explained by the different stages (advanced vs. uncomplicated) of human atherosclerotic plaque between the 2 studies. (Fig. 2)
Figure 2.
Role of gas6 in atherosclerosis pathogenesis. Initially, gas6 promotes inflammation by increasing leukocytes extravasation. Afterwards, when an atherosclerotic plaque ruptures, thrombogenic factors, including gas6, are released in the bloodstream leading to thrombus formation.
Gas6/Axl in thrombosis
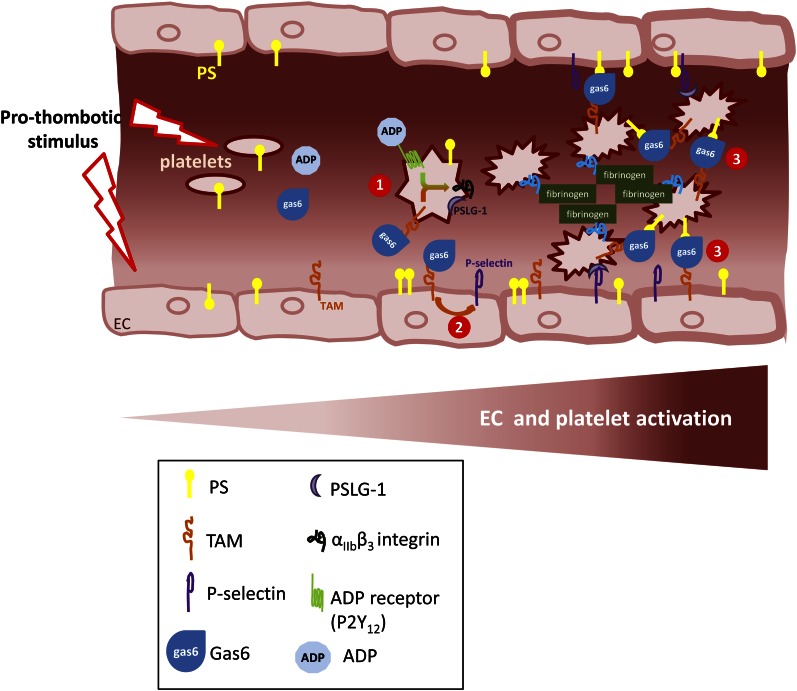
Gas6/Axl signaling is involved in venous as well as arterial disease. Gas6 null mice have been shown to be protected from lethal VTE, suggesting a prothrombotic role of gas6 (71). The mechanisms underlying this protection remain unclear even if an effect on platelets has been suggested. However, in vitro studies show only a slight effect on platelet aggregation compared to the drastic in vivo phenotype. In gas6 null mice, platelets form less dense aggregates, suggesting that gas6 is involved in thrombus stabilization, probably by acting on the αIIbβ3 integrin “outside-in” signal as well as platelet degranulation through a PI3K-dependent mechanism (72). Indeed, platelets from gas6-deficient mice show defective platelet signaling when challenged with 5 μmol/L ADP but not other platelet agonists such as thrombin, collagen, or even higher concentrations of ADP (71, 72). More recently, Cosemans et al. (73) described a synergistic effect of gas6 on the ADP pathway leading to the activation of αIIbβ3 integrin. These data highlight a crucial role for the gas6-induced TAM receptor signaling through a PI3K-dependent mechanism leading to the persistence of αIIbβ3 integrin activation and consequently thrombus integrity (Fig. 3). The clinical phenotype of gas6 null mice is quite dramatic despite this rather subtle platelet defect, suggesting other mechanisms may also be involved and that gas6 probably acts on other cellular components such as the endothelium. Indeed, it has been shown that gas6 promotes the activation of the endothelium, notably by promoting P-selectin expression (53). This latter protein is the ligand for the platelet receptor PSLG-1, thus possibly reinforcing the adhesion of the thrombus to the vascular wall. (Fig. 3)
Figure 3.
Three independent mechanisms of Gas6 in thrombus formation and stabilization. 1. Gas6 exerts a synergistic effect with ADP on “outside-in” signaling leading to the activation of αIIbβ3 integrin. 2. Gas6 induces the expression of P-selectin at the surface of the endothelium leading to the binding of platelets by P-selectin glycoprotein ligand-1-1. 3. Gas6 acts as a bridge between cells expressing PS and a TAM receptor. PS, phosphatidylserine; TAM, Tyro3, Axl, and Mer.
Finally, when cells are activated, they express phosphatidylserine, which provides a docking platform for the Gla domain of gas6. In addition, gas6 can bind to a TAM receptor by its SHBG domain. Thus, one can speculate that, because of its structure, gas6 may act as a bridge between cells expressing phosphatidylserine and cells expressing TAM receptors.
A most interesting finding is the absence of bleeding in gas6 null mice, meaning that gas6 could be a very attractive target for antithrombotic therapies. Recently, Blostein et al. (74) have shown that patients with VTE had elevated gas6 levels and that individuals with elevated gas6 had an increased risk of VTE compared to those with lower levels of gas6. Moreover, an association between allelic polymorphisms in the gas6 gene and stroke occurrence has been proposed (75).
Gas6/Axl in cancer
The first role described for gas6 is its implication in cell growth and proliferation. Thus, studies have investigated the role of gas6 in the context of cancer. Gas6/Axl signaling has been shown to regulate survival, proliferation, and migration in a variety of cells in vitro, including tumor-derived cell lines of epithelial, mesenchymal, and hematopoietic origin (15, 17). In parallel, Axl has transforming properties. Indeed, expression of a truncated version of Axl in premaligant cells is sufficient to induce tumors in mice (16). Axl is also highly expressed in human tumor cells in vitro (19, 76, 77) as well as in a large variety of primary human cancers, including leukemia (15), gastric cancer (30), colon cancer (78), breast cancer (79), ovarian cancer (80), and glioblastoma (81) among others. However, very few studies have been interested in the role of gas6 in cancer. Gas6 is overexpressed in human ovarian, endometrial, gastric, thyroid, and glioblastoma tumors (30, 80–83). In those studies, expression of gas6 was detected in tumors cells, EC, and astrocytes (80–84). More recently, Loges et al. (85) demonstrated that malignant cells promote leukocyte infiltration inside tumors by secreting gas6. Intratumoral leukocyte infiltrates vary in size, composition, and distribution and in most but not all cases they are thought to promote tumor progression via several mechanisms, including secretion of growth factors, proangiogenic cytokines, and proteases (86). In this study, the authors demonstrated that tumor growth was reduced in gas6-deficient mice compared to control mice and this was associated with a reduced proliferation rate of tumor cells. Using a bone marrow transplantation model, it was found that infiltrating leukocytes, in particular macrophages, were educated by the tumor microenvironment to produce elevated levels of gas6. Importantly, this study has also extended previous data that gas6 can be expressed by cells of the myeloid lineage (6, 87) and illustrate another level of regulation of expression that was previously not recognized.
Conclusion
Gas6 is a novel key regulator of the vascular system, because it has a role in both inflammation and thrombosis, which are related events involved in many cardiovascular diseases such as VTE and atherosclerosis. Despite some discrepancies between in vivo and in vitro data, murine models as well as preliminary data acquired in humans confer both proinflammatory and prothrombotic properties to gas6. The precise molecular mechanism underlying gas6’s effect remains to be further explored, especially in relevant cell types such as EC and VSMC. Nevertheless, all these observations suggest that gas6 should be an attractive therapeutic target in VTE and atherosclerosis. Targeting gas6, especially in the context of VTE, should reduce thrombus formation without the risk of excessive bleeding, which is the main side effect of oral anti-vitamin K inhibitors presently used in clinical settings as well as the new anticoagulants, namely the factor Xa and thrombin inhibitors, being developed. In atherosclerosis, inhibiting gas6 should stabilize the advanced plaque, thus preventing rupture and subsequent thrombus formation leading to myocardial infarction.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Supported by the Canadian Institutes of Health Research.
Author disclosures: S. Laurance, C. A. Lemarié, and M. D. Blostein, no conflicts of interest.
Abbreviations used: APC, antigen presenting cell; C1-TEN, C1 domain-containing phosphatase and tensin homolog; EC, endothelial cell; Gla, γ-carboxyglutamic acid; PI3K, phosphoinositide-3-kinase; PS, phosphatidylserine; PTEN, phosphatase and tensin homolog; SHBG, sex hormone binding globulin; TAM, Tyro3, Axl, and Mer; VKD, vitamin K dependent; VSMC, vascular smooth muscle cell; VTE, venous thromboembolism.
Literature Cited
- 1.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–93 [DOI] [PubMed] [Google Scholar]
- 2.Manfioletti G, Brancolini C, Avanzi G, Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol. 1993;13:4976–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakano T, Higashino K, Kikuchi N, Kishino J, Nomura K, Fujita H, Ohara O, Arita H. Vascular smooth muscle cell-derived, Gla-containing growth-potentiating factor for Ca(2+)-mobilizing growth factors. J Biol Chem. 1995;270:5702–5 [DOI] [PubMed] [Google Scholar]
- 4.Avanzi GC, Gallicchio M, Cavalloni G, Gammaitoni L, Leone F, Rosina A, Boldorini R, Monga G, Pegoraro L, Varnum B, et al. GAS6, the ligand of Axl and Rse receptors, is expressed in hematopoietic tissue but lacks mitogenic activity. Exp Hematol. 1997;25:1219–26 [PubMed] [Google Scholar]
- 5.Balogh I, Hafizi S, Stenhoff J, Hansson K. Dahlbäck B. Analysis of Gas6 in human platelets and plasma. Arterioscler Thromb Vasc Biol. 2005;25:1280–6 [DOI] [PubMed] [Google Scholar]
- 6.Castoldi E, Hackeng TM. Regulation of coagulation by protein S. Curr Opin Hematol. 2008;15:529–36 [DOI] [PubMed] [Google Scholar]
- 7.Stenflo J. Contributions of Gla and EGF-like domains to the function of vitamin K-dependent coagulation factors. Crit Rev Eukaryot Gene Expr. 1999;9:59–88 [PubMed] [Google Scholar]
- 8.Huang M, Rigby AC, Morelli X, Grant MA, Huang G, Furie B, Seaton B, Furie BC. Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nat Struct Biol. 2003;10:751–6 [DOI] [PubMed] [Google Scholar]
- 9.He X, Shen L, Villoutreix BO, Dahlback B. Amino acid residues in thrombin-sensitive region and first epidermal growth factor domain of vitamin K-dependent protein S determining specificity of the activated protein C cofactor function. J Biol Chem. 1998;273:27449–58 [DOI] [PubMed] [Google Scholar]
- 10.Dahlbäck B, Lundwall A, Stenflo J. Localization of thrombin cleavage sites in the amino-terminal region of bovine protein S. J Biol Chem. 1986;261:5111–5 [PubMed] [Google Scholar]
- 11.Long GL, Lu D, Xie RL, Kalafatis M. Human protein S cleavage and inactivation by coagulation factor Xa. J Biol Chem. 1998;273:11521–6 [DOI] [PubMed] [Google Scholar]
- 12.Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW, Hunt RW, Trail G, Clogston C, Toso RJ, et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature. 1995;373:623–6 [DOI] [PubMed] [Google Scholar]
- 13.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–70 [DOI] [PubMed] [Google Scholar]
- 14.Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271:30022–7 [DOI] [PubMed] [Google Scholar]
- 15.O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R III, Le Beau MM, Earp HS, Liu ET. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang QK, Boast S, de los Santos K, Begemann M, Goff SP. Transforming activity of retroviral genomes encoding Gag-Axl fusion proteins. J Virol. 1996;70:8089–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hafizi S, Dahlback B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006;273:5231–44 [DOI] [PubMed] [Google Scholar]
- 18.Bellosta P, Costa M, Lin DA, Basilico C. The receptor tyrosine kinase ARK mediates cell aggregation by homophilic binding. Mol Cell Biol. 1995;15:614–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wimmel A, Glitz D, Kraus A, Roeder J, Schuermann M. Axl receptor tyrosine kinase expression in human lung cancer cell lines correlates with cellular adhesion. Eur J Cancer. 2001;37:2264–74 [DOI] [PubMed] [Google Scholar]
- 20.Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–8 [DOI] [PubMed] [Google Scholar]
- 21.Goruppi S, Ruaro E, Varnum B, Schneider C. Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:4442–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Donnell K, Harkes IC, Dougherty L, Wicks IP. Expression of receptor tyrosine kinase Axl and its ligand Gas6 in rheumatoid arthritis: evidence for a novel endothelial cell survival pathway. Am J Pathol. 1999;154:1171–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goruppi S, Ruaro E, Schneider C. Gas6, the ligand of Axl tyrosine kinase receptor, has mitogenic and survival activities for serum starved NIH3T3 fibroblasts. Oncogene. 1996;12:471–80 [PubMed] [Google Scholar]
- 24.Melaragno MG, Cavet ME, Yan C, Tai LK, Jin ZG, Haendeler J, Berk BC. Gas6 inhibits apoptosis in vascular smooth muscle: role of Axl kinase and Akt. J Mol Cell Cardiol. 2004;37:881–7 [DOI] [PubMed] [Google Scholar]
- 25.Loeser RF, Varnum BC, Carlson CS, Goldring MB, Liu ET, Sadiev S, Kute TE, Wallin R. Human chondrocyte expression of growth-arrest-specific gene 6 and the tyrosine kinase receptor axl: potential role in autocrine signaling in cartilage. Arthritis Rheum. 1997;40:1455–65 [DOI] [PubMed] [Google Scholar]
- 26.Yagami T, Ueda K, Asakura K, Okamura N, Sakaeda T, Sakaguchi G, Itoh N, Hashimoto Y, Nakano T, Fujimoto M. Effect of Gas6 on secretory phospholipase A(2)-IIA-induced apoptosis in cortical neurons. Brain Res. 2003;985:142–9 [DOI] [PubMed] [Google Scholar]
- 27.Shankar SL, O'Guin K, Cammer M, McMorris FA, Stitt TN, Basch RS, Varnum B, Shafit-Zagardo B. The growth arrest-specific gene product Gas6 promotes the survival of human oligodendrocytes via a phosphatidylinositol 3-kinase-dependent pathway. J Neurosci. 2003;23:4208–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couchie D, Lafdil F, Martin-Garcia N, Laperche Y, Zafrani ES, Mavier P. Expression and role of Gas6 protein and of its receptor Axl in hepatic regeneration from oval cells in the rat. Gastroenterology. 2005;129:1633–42 [DOI] [PubMed] [Google Scholar]
- 29.Valverde P, Obin MS, Taylor A. Role of Gas6/Axl signaling in lens epithelial cell proliferation and survival. Exp Eye Res. 2004;78:27–37 [DOI] [PubMed] [Google Scholar]
- 30.Sawabu T, Seno H, Kawashima T, Fukuda A, Uenoyama Y, Kawada M, Kanda N, Sekikawa A, Fukui H, Yanagita M, et al. Growth arrest-specific gene 6 and Axl signaling enhances gastric cancer cell survival via Akt pathway. Mol Carcinog. 2007;46:155–64 [DOI] [PubMed] [Google Scholar]
- 31.Hasanbasic I, Cuerquis J, Varnum B, Blostein MD. Intracellular signaling pathways involved in Gas6-Axl-mediated survival of endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1207–13 [DOI] [PubMed] [Google Scholar]
- 32.Demarchi F, Verardo R, Varnum B, Brancolini C, Schneider C. Gas6 anti-apoptotic signaling requires NF-kappa B activation. J Biol Chem. 2001;276:31738–44 [DOI] [PubMed] [Google Scholar]
- 33.Goruppi S, Ruaro E, Varnum B, Schneider C. Gas6-mediated survival in NIH3T3 cells activates stress signalling cascade and is independent of Ras. Oncogene. 1999;18:4224–36 [DOI] [PubMed] [Google Scholar]
- 34.Lee WP, Wen Y, Varnum B, Hung MC. Akt is required for Axl-Gas6 signaling to protect cells from E1A-mediated apoptosis. Oncogene. 2002;21:329–36 [DOI] [PubMed] [Google Scholar]
- 35.Hafizi S, Alindri F, Karlsson R, Dahlback B. Interaction of Axl receptor tyrosine kinase with C1-TEN, a novel C1 domain-containing protein with homology to tensin. Biochem Biophys Res Commun. 2002;299:793–800 [DOI] [PubMed] [Google Scholar]
- 36.Braunger J, Schleithoff L, Schulz AS, Kessler H, Lammers R, Ullrich A, Bartram CR, Janssen JW. Intracellular signaling of the Ufo/Axl receptor tyrosine kinase is mediated mainly by a multi-substrate docking-site. Oncogene. 1997;14:2619–31 [DOI] [PubMed] [Google Scholar]
- 37.Fridell YW, Jin Y, Quilliam LA, Burchert A, McCloskey P, Spizz G, Varnum B, Der C, Liu ET. Differential activation of the Ras/extracellular-signal-regulated protein kinase pathway is responsible for the biological consequences induced by the Axl receptor tyrosine kinase. Mol Cell Biol. 1996;16:135–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallicchio M, Mitola S, Valdembri D, Fantozzi R, Varnum B, Avanzi GC, Bussolino F. Inhibition of vascular endothelial growth factor receptor 2-mediated endothelial cell activation by Axl tyrosine kinase receptor. Blood. 2005;105:1970–6 [DOI] [PubMed] [Google Scholar]
- 39.Nielsen-Preiss SM, Allen MP, Xu M, Linseman DA, Pawlowski JE, Bouchard RJ, Varnum BC, Heidenreich KA, Wierman ME. Adhesion-related kinase induction of migration requires phosphatidylinositol-3-kinase and ras stimulation of rac activity in immortalized gonadotropin-releasing hormone neuronal cells. Endocrinology. 2007;148:2806–14 [DOI] [PubMed] [Google Scholar]
- 40.Todt JC, Hu B, Curtis JL. The receptor tyrosine kinase MerTK activates phospholipase C gamma2 during recognition of apoptotic thymocytes by murine macrophages. J Leukoc Biol. 2004;75:705–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellosta P, Zhang Q, Goff SP, Basilico C. Signaling through the ARK tyrosine kinase receptor protects from apoptosis in the absence of growth stimulation. Oncogene. 1997;15:2387–97 [DOI] [PubMed] [Google Scholar]
- 42.Yanagita M, Arai H, Ishii K, Nakano T, Ohashi K, Mizuno K, Varnum B, Fukatsu A, Doi T, Kita T. Gas6 regulates mesangial cell proliferation through Axl in experimental glomerulonephritis. Am J Pathol. 2001;158:1423–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mark MR, Chen J, Hammonds RG, Sadick M, Godowsk PJ. Characterization of Gas6, a member of the superfamily of G domain-containing proteins, as a ligand for Rse and Axl. J Biol Chem. 1996;271:9785–9 [DOI] [PubMed] [Google Scholar]
- 44.Nakano T, Kawamoto K, Kishino J, Nomura K, Higashino K, Arita H. Requirement of gamma-carboxyglutamic acid residues for the biological activity of Gas6: contribution of endogenous Gas6 to the proliferation of vascular smooth muscle cells. Biochem J. 1997;323:387–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stenhoff J, Dahlback B, Hafizi S. Vitamin K-dependent Gas6 activates ERK kinase and stimulates growth of cardiac fibroblasts. Biochem Biophys Res Commun. 2004;319:871–8 [DOI] [PubMed] [Google Scholar]
- 46.Hasanbasic I, Rajotte I, Blostein M. The role of gamma-carboxylation in the anti-apoptotic function of gas6. J Thromb Haemost. 2005;3:2790–7 [DOI] [PubMed] [Google Scholar]
- 47.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–11 [DOI] [PubMed] [Google Scholar]
- 48.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162:3498–503 [PubMed] [Google Scholar]
- 49.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–36 [DOI] [PubMed] [Google Scholar]
- 50.Alciato F, Sainaghi PP, Sola D, Castello L, Avanzi GC. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J Leukoc Biol. 2010;87:869–75 [DOI] [PubMed] [Google Scholar]
- 51.Deng T, Zhang Y, Chen Q, Yan K, Han D. Toll-like receptor-mediated inhibition of Gas6 and ProS expression facilitates inflammatory cytokine production in mouse macrophages. Immunology. 2012;135:40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scutera S, Fraone T, Musso T, Cappello P, Rossi S, Pierobon D, Orinska Z, Paus R, Bulfone-Paus S, Giovarelli M. Survival and migration of human dendritic cells are regulated by an IFN-alpha-inducible Axl/Gas6 pathway. J Immunol. 2009;183:3004–13 [DOI] [PubMed] [Google Scholar]
- 53.Tjwa M, Bellido-Martin L, Lin Y, Lutgens E, Plaisance S, Bono F, Delesque-Touchard N, Herve C, Moura R, Billiau AD, et al. Gas6 promotes inflammation by enhancing interactions between endothelial cells, platelets, and leukocytes. Blood. 2008;111:4096–105 [DOI] [PubMed] [Google Scholar]
- 54.Korshunov VA, Mohan AM, Georger MA, Berk BC. Axl, a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circ Res. 2006;98:1446–52 [DOI] [PubMed] [Google Scholar]
- 55.Fourcot A, Couchie D, Chobert MN, Zafrani ES, Mavier P, Laperche Y, Brouillet A. Gas6 deficiency prevents liver inflammation, steatohepatitis, and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1043–53 [DOI] [PubMed] [Google Scholar]
- 56.Melaragno MG, Wuthrich DA, Poppa V, Gill D, Lindner V, Berk BC, Corson MA. Increased expression of Axl tyrosine kinase after vascular injury and regulation by G protein-coupled receptor agonists in rats. Circ Res. 1998;83:697–704 [DOI] [PubMed] [Google Scholar]
- 57.Konishi A, Aizawa T, Mohan A, Korshunov VA, Berk BC. Hydrogen peroxide activates the Gas6-Axl pathway in vascular smooth muscle cells. J Biol Chem. 2004;279:28766–70 [DOI] [PubMed] [Google Scholar]
- 58.Cavet ME, Smolock EM, Menon P, Konishi A, Korshunov VA, Berk BC. Gas6-Axl pathway: the role of redox-dependent association of Axl with nonmuscle myosin IIB. Hypertension. 2010;56:105–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collett G, Wood A, Alexander MY, Varnum BC, Boot-Handford RP, Ohanian V, Ohanian J, Fridell YW, Canfield AE. Receptor tyrosine kinase Axl modulates the osteogenic differentiation of pericytes. Circ Res. 2003;92:1123–9 [DOI] [PubMed] [Google Scholar]
- 60.Son BK, Kozaki K, Iijima K, Eto M, Kojima T, Ota H, Senda Y, Maemura K, Nakano T, Akishita M, et al. Statins protect human aortic smooth muscle cells from inorganic phosphate-induced calcification by restoring Gas6-Axl survival pathway. Circ Res. 2006;98:1024–31 [DOI] [PubMed] [Google Scholar]
- 61.Nakano T, Kawamoto K, Higashino K, Arita H. Prevention of growth arrest-induced cell death of vascular smooth muscle cells by a product of growth arrest-specific gene, gas6. FEBS Lett. 1996;387:78–80 [DOI] [PubMed] [Google Scholar]
- 62.Fridell YW, Villa J, Jr, Attar EC, Liu ET. GAS6 induces Axl-mediated chemotaxis of vascular smooth muscle cells. J Biol Chem. 1998;273:7123–6 [DOI] [PubMed] [Google Scholar]
- 63.Ishimoto Y, Ohashi K, Mizuno K, Nakano T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem. 2000;127:411–7 [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Gerbod-Giannone MC, Seitz H, Cui D, Thorp E, Tall AR, Matsushima GK, Tabas I. Cholesterol-induced apoptotic macrophages elicit an inflammatory response in phagocytes, which is partially attenuated by the Mer receptor. J Biol Chem. 2006;281:6707–17 [DOI] [PubMed] [Google Scholar]
- 65.Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A, et al. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1429–31 [DOI] [PubMed] [Google Scholar]
- 66.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009;50 Suppl:S382–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lutgens E, Tjwa M, Garcia de Frutos P, Wijnands E, Beckers L, Dahlback B, Daemen MJ, Carmeliet P, Moons L. Genetic loss of Gas6 induces plaque stability in experimental atherosclerosis. J Pathol. 2008;216:55–63 [DOI] [PubMed] [Google Scholar]
- 69.Hurtado B, Munoz X, Recarte-Pelz P, Garcia N, Luque A, Krupinski J, et al. Expression of the vitamin K-dependent proteins GAS6 and protein S and the TAM receptor tyrosine kinases in human atherosclerotic carotid plaques. Thromb Haemost. 2011;105:873–82 [DOI] [PubMed] [Google Scholar]
- 70.Krueger T, Westenfeld R, Ketteler M, Schurgers LJ, Floege J. Vitamin K deficiency in CKD patients: a modifiable risk factor for vascular calcification? Kidney Int. 2009;76:18–22 [DOI] [PubMed] [Google Scholar]
- 71.Angelillo-Scherrer A, de Frutos P, Aparicio C, Melis E, Savi P, Lupu F, Arnout J, Dewerchin M, Hoylaerts M, Herbert J, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–21 [DOI] [PubMed] [Google Scholar]
- 72.Angelillo-Scherrer A, Burnier L, Flores N, Savi P, DeMol M, Schaeffer P, Herbert JM, Lemke G, Goff SP, Matsushima GK, et al. Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J Clin Invest. 2005;115:237–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cosemans JM, Van Kruchten R, Olieslagers S, Schurgers LJ, Verheyen FK, Munnix IC, Waltenberger J, Angelillo-Scherrer A, Hoylaerts MF, Carmeliet P, et al. Potentiating role of Gas6 and Tyro3, Axl and Mer (TAM) receptors in human and murine platelet activation and thrombus stabilization. J Thromb Haemost. 2010;8:1797–808 [DOI] [PubMed] [Google Scholar]
- 74.Blostein MD, Rajotte I, Rao DP, Holcroft CA, Kahn SR. Elevated plasma gas6 levels are associated with venous thromboembolic disease. J Thromb Thrombolysis. 2011;32:272–8 [DOI] [PubMed] [Google Scholar]
- 75.Muñoz X, Sumoy L, Ramirez-Lorca R, Villar J, de Frutos PG, Sala N. Human vitamin K-dependent GAS6: gene structure, allelic variation, and association with stroke. Hum Mutat. 2004;23:506–12 [DOI] [PubMed] [Google Scholar]
- 76.Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P, Avanzi GC. Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J Cell Physiol. 2005;204:36–44 [DOI] [PubMed] [Google Scholar]
- 77.van Ginkel PR, Gee RL, Shearer RL, Subramanian L, Walker TM, Albert DM, Meisner LF, Varnum BC, Polans AS. Expression of the receptor tyrosine kinase Axl promotes ocular melanoma cell survival. Cancer Res. 2004;64:128–34 [DOI] [PubMed] [Google Scholar]
- 78.Craven RJ, Xu LH, Weiner TM, Fridell YW, Dent GA, Srivastava S, Varnum B, Liu ET, Cance WG. Receptor tyrosine kinases expressed in metastatic colon cancer. Int J Cancer. 1995;60:791–7 [DOI] [PubMed] [Google Scholar]
- 79.Meric F, Lee WP, Sahin A, Zhang H, Kung HJ, Hung MC. Expression profile of tyrosine kinases in breast cancer. Clin Cancer Res. 2002;8:361–7 [PubMed] [Google Scholar]
- 80.Sun W, Fujimoto J, Tamaya T. Coexpression of Gas6/Axl in human ovarian cancers. Oncology. 2004;66:450–7 [DOI] [PubMed] [Google Scholar]
- 81.Hutterer M, Knyazev P, Abate A, Reschke M, Maier H, Stefanova N, Knyazeva T, Barbieri V, Reindl M, Muigg A, et al. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14:130–8 [DOI] [PubMed] [Google Scholar]
- 82.Ito T, Ito M, Naito S, Ohtsuru A, Nagayama Y, Kanematsu T, Yamashita S, Sekine I. Expression of the Axl receptor tyrosine kinase in human thyroid carcinoma. Thyroid. 1999;9:563–7 [DOI] [PubMed] [Google Scholar]
- 83.Sun WS, Fujimoto J, Tamaya T. Coexpression of growth arrest-specific gene 6 and receptor tyrosine kinases Axl and Sky in human uterine endometrial cancers. Ann Oncol. 2003;14:898–906 [DOI] [PubMed] [Google Scholar]
- 84.Ito M, Nakashima M, Nakayama T, Ohtsuru A, Nagayama Y, Takamura N, Demedchik EP, Sekine I, Yamashita S. Expression of receptor-type tyrosine kinase, Axl, and its ligand, Gas6, in pediatric thyroid carcinomas around chernobyl. Thyroid. 2002;12:971–5 [DOI] [PubMed] [Google Scholar]
- 85.Loges S, Schmidt T, Tjwa M, van Geyte K, Lievens D, Lutgens E, Vanhoutte D, Borgel D, Plaisance S, Hoylaerts M, et al. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood. 2010;115:2264–73 [DOI] [PubMed] [Google Scholar]
- 86.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44 [DOI] [PubMed] [Google Scholar]
- 87.Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178:5635–42 [DOI] [PubMed] [Google Scholar]