Abstract
The role of vitamin K in the nervous system has been somewhat neglected compared with other physiological systems despite the fact that this nutrient was identified some 40 y ago as essential for the synthesis of sphingolipids. Present in high concentrations in brain cell membranes, sphingolipids are now known to possess important cell signaling functions in addition to their structural role. In the past 20 y, additional support for vitamin K functions in the nervous system has come from the discovery and characterization of vitamin K–dependent proteins that are now known to play key roles in the central and peripheral nervous systems. Notably, protein Gas6 has been shown to be actively involved in cell survival, chemotaxis, mitogenesis, and cell growth of neurons and glial cells. Although limited in number, studies focusing on the relationship between vitamin K nutritional status and behavior and cognition have also become available, pointing to diet and certain drug treatments (i.e., warfarin derivatives) as potential modulators of the action of vitamin K in the nervous system. This review presents an overview of the research that first identified vitamin K as an important nutrient for the nervous system and summarizes recent findings that support this notion.
Introduction
Vitamin K is classically known for its role as a cofactor of the γ-glutamyl carboxylase (GGCX)3 enzyme involved in the biological activation of a family of proteins, the vitamin K–dependent proteins (VKDPs). One of these, Gas6, was discovered in 1993 and has been closely associated with the nervous system (1, 2). However, a role for vitamin K in this system that well predates the discovery of Gas6 is that in sphingolipid synthesis, a class of lipids present in high concentrations in both neuronal and glial cell membranes (3). This action of vitamin K was indeed characterized >40 y ago by the team of Meir Lev and is assumed to be distinct from that of the carboxylase function. In a series of publications that spanned from 1971 through 1996, Lev and colleagues (4, 5) provided evidence that vitamin K modulates the activities of key enzymes of the sphingolipid biosynthetic pathway and hence their synthesis and metabolism.
Research conducted in more recent decades has generated data that confirm the relevance of vitamin K in the nervous system and cognition. Notably, a strong relationship has been established between the K vitamers and sphingolipids in the brain and their modulation by nutritional status. Important findings have also been obtained with respect to the cell signaling actions of protein Gas6 in both the central and peripheral nervous systems. New knowledge has been gained about menaquinone-4 (MK-4), the principal K vitamer in brain, which could have far-reaching effects in the brain and other components of the nervous system. Finally, a limited number of studies have provided evidence of a relationship between vitamin K status, behavior, and cognition.
Vitamin K distribution in brain
Reports published in the past 2 decades confirmed the presence of vitamin K in the brain. However, although in the majority of extrahepatic tissues, vitamin K is present as phylloquinone (K1) and MK-4, vitamin K in the brain occurs predominantly as MK-4 (6, 7). When investigated in 6- and 21-mo-old rats, MK-4 was found to represent >98% of total vitamin K in the brain, irrespective of age (8, 9). In a detailed assessment of the anatomic distribution of vitamin K, MK-4 was present in all brain regions, although concentrations differed according to regions. Specifically, MK-4 was observed in highest concentrations in the midbrain and pons medulla and in lowest concentrations in the cerebellum, olfactory bulb, thalamus, hippocampus, and striatum (8).
Concentrations of MK-4 in the brain have also been shown to be affected by sex and age. In a study conducted in Brown Norway rats, MK-4 levels in the cortex and cerebellum were higher in female than in male rats despite similar diets, and concentrations decreased between 12 and 24 mo of age (7). Vitamin K concentrations in the brain are also influenced by diet in a manner that reflects intake. When investigated in female Sprague-Dawley rats that had been fed diets containing low (80 μg/kg diet), adequate (500 μg/kg diet), or high (2000 μg/kg diet) amounts of phylloquinone for 5 mo, MK-4 tissue concentrations from the high phylloquinone-containing diet rats were on average 8 and 3 times higher than those for the low and adequate phylloquinone-containing diet rats, respectively (8).
Vitamin K and sphingolipids
Sphingolipids are a group of complex lipids present in all mammalian cells where they are major components of cell membranes. They are present in particularly high concentrations in cells of the central and peripheral nervous systems with the major sphingolipids consisting of ceramide, sphingomyelin, cerebroside, sulfatide, and ganglioside (8). As discussed below, certain sphingolipids are highly correlated with MK-4 in the brain. A simplified scheme of the sphingolipid biosynthetic pathways is presented in Fig. 1.
Figure 1.
A simplified scheme of sphingolipid pathways. CerDase, ceramidase; CerS, ceramide synthase; GalCeramidase, galactosylceramidase; GalCer sulfotransferase, galactosyl sulfotransferase; Gal-T1, galactosyltransferase; GluCeramidase, glucosylceramidase; GluCer synthase, glucosylceramide synthase; Sial-T1, lactosylceramide synthase; SM synthase, sphingomyelin synthase; SMase, sphingomyelinase. Adapted with permission from (9).
Initially appreciated for their structural role, sphingolipids are now viewed as key players in important cellular events such as proliferation, differentiation, senescence, cell–cell interaction, and transformation (10). Furthermore, research conducted in recent years linked alterations in sphingolipid metabolism to the aging process (11) and neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (12, 13).
As indicated previously, evidence supporting a role for vitamin K in sphingolipid metabolism is largely based on the scientific legacy of Meir Lev’s group. In an initial report published in Nature in 1958 (14), vitamin K (note: all in vitro studies used an aqueous form of phylloquinone as the source of vitamin K), was shown to act as a growth factor for the rumen strain Bacteroides melaninogenicus (also known as Fusiformis nigrescens). This obligate vitamin K requirement for bacterial growth was later found to be linked to cell membrane homeostasis. When cultured in a medium devoid of vitamin K, cells grew as filaments (i.e., elongated cells), were more fragile when subjected to shaking with glass beads, and tended to autoagglutinate when placed in buffer. Growth yield under vitamin K–deficient conditions was also greatly affected, with vitamin K–deficient cultures yielding ∼80% lower bacteria weight than those grown from vitamin K–replete conditions (15). Elucidation of the role of vitamin K at the membrane level was provided in 2 subsequent reports in which it was shown that vitamin K is required for sphingolipid synthesis. The bacteria strain B. melaninogenicus is unusual in that it contains sphingolipids in addition to other phospholipids, a rare feature among bacteria. Using B. melaninogenicus cells that had been cultured in conditions of vitamin K deficiency, addition of vitamin K significantly increased the incorporation of 32P into ceramide phosphorylethanolamine and ceramide phosphorylglycerol but had no effect on other phospholipids such as phosphatidylethanolamine and phosphorylglycerol. Further experiments showed that synthesis of ceramide phosphorylethanolamine and ceramide phosphorylglycerol was stimulated shortly after the addition of vitamin K to the cell medium and before an increase in general cell metabolism. Incorporation of 32P into these compounds was also found to be linear (16, 17).
As depicted in Figure 1, the initial step of sphingolipid biosynthesis is the condensation of serine with palmitoyl-CoA to form the intermediate, 3-ketodihydrosphingosine (3-KDS). Building on their previous studies, the group undertook to determine whether vitamin K was involved in this initial step. Specifically, they assessed the activity of the enzyme 3-KDS synthase (also known as serine palmitoyltransferase) using 1 labeled substrate and determining its incorporation into the final product. It was observed that B. melaninogenicus that had been grown in vitamin K–depleted medium had low synthase activity compared with bacteria grown in vitamin K–supplemented medium. Addition of vitamin K to cell-free extracts did not increase enzyme activity in a significant manner, suggesting that vitamin K did not act as a coenzyme for the synthase. In contrast, adding vitamin K to intact cells resulted in a rapid induction of the enzyme, an effect that was abolished when cultures were exposed to inhibitors of protein (puromycin) and RNA (rifampicin) synthesis. These latter results strongly suggested that vitamin K induction of 3-KDS synthase was through de novo synthesis of the enzyme (18). Furthermore, the notion that vitamin K acted independently of its role in the carboxylation reaction was supported by the fact that synthase activity was not affected by warfarin treatment in vitro (5).
In 1988, the group extended their work in bacteria to rodent models and showed that mice treated with a vitamin K antagonist resulted in an alteration in the brain sphingolipids. Specifically, when 16-d-old mice were administered sodium warfarin (10 mg/kg body weight on alternate days) for 12 d, brain microsomes from these animals showed a 19% reduction in 3-KDS synthase activity. Treatment with warfarin for 2 wk also resulted in a significant reduction in brain sulfatides (42%) and, to a lesser extent, in sphingomyelin (17%) and cerebrosides (12%). When a subgroup of these mice were treated with phylloquinone (1 mg/mouse, 3 d), 3-KDS synthase activity was normalized, whereas sulfatide levels increased by 33% and gangliosides returned to the levels of control mice. In contrast, sphingomyelin and cerebrosides continued to decrease after administration of phylloquinone. Sulfatide synthesis was then directly examined by determining the incorporation of [35S]-sulfate into sulfatides and was found to increase by 52% after phylloquinone treatment. These results suggested that the cerebroside pool may have been used for sulfatide synthesis (19).
The apparent preferential inhibition of sulfatide synthesis after warfarin treatment prompted additional studies targeting the sulfotransferase enzyme, the enzyme responsible for sulfatide synthesis. Treatment of mice with warfarin (10 mg/kg body weight on alternate days) for 10 d resulted in a significant reduction (45%) in sulfotransferase activity in the brain but not in spleen and kidney, suggesting a specific effect of warfarin in the brain. Supplementation of warfarin-treated mice with phylloquinone (1 mg/mouse, 3 d) resulted in the recovery of sulfotransferase activity. A stimulatory effect of phylloquinone on sulfotransferase activity was also observed in control mice, but in this case, it was accompanied by a concurrent stimulation of arylsulfatase activity, the sulfatide degrading enzyme. This dual effect of vitamin K on biosynthetic and catabolic enzymes suggested that the vitamin could have a role in the regulation of the sulfatide biosynthetic pathway in the brain (20). The mechanism by which vitamin K modulated the activity of sulfotransferase enzyme was addressed in a subsequent study in which it was shown that phylloquinone (or menadione) + orthophosphate can partially fulfill the requirement of the enzyme for ATP. The involvement of phosphate in this reaction led to the suggestion that vitamin K contributed to the phosphorylation of the enzyme (21, 22).
In the final report by the group, sulfotransferase activity and sulfatides were investigated with respect to vitamin K status as modulated by diet (23). Similarly to what had been observed after warfarin treatment, vitamin K deficiency induced through diet had deleterious effects on sulfatide production. Specifically, when 21-d-old male Swiss mice were fed a vitamin K–deficient diet for 7 d, brain sulfatide concentrations were 21% lower than those of mice fed a vitamin K–replete diet. Furthermore, dietary vitamin K modulated sulfatide metabolism in nondeficiency states. Feeding Sprague-Dawley rats an excess of vitamin K for 7 and 14 d resulted in 26% and 31% greater sulfotransferase activity and 15% and 18% greater brain concentrations of sulfatides, respectively, compared with controls. The stimulatory effect of vitamin K on enzyme activity and sulfatide levels was observed with either phylloquinone or MK-4 as a source of vitamin K.
In the following years, work was conducted focusing on the relationship between dietary vitamin K and sphingolipids in the brain. In a study from our group referred to earlier (8) in which 6-mo-old female Sprague-Dawley rats had been fed a low, adequate, or high phylloquinone-containing diet since weaning, MK-4 was found to be positively correlated with sulfatides and sphingomyelin and negatively correlated with gangliosides, the strength of the relationships decreasing as a function of phylloquinone intake. These results suggested that when phylloquinone was present in the diet in limited amounts, MK-4 preferentially accumulated in highly myelinated regions. Furthermore, the strong negative correlation observed between MK-4 and gangliosides suggested a potential modulatory role for vitamin K in the general sphingolipid biosynthetic pathway. In this group of rats, diet did not influence sphingolipid concentrations in the brain, a finding that may have been due to the duration of diet exposure. However, when investigated in rats that had been exposed to the different diets throughout their lives, dietary phylloquinone altered brain sphingolipid concentrations in a significant manner. Compared with rats from the adequate and high groups, those that had received the diet containing low amounts of phylloquinone throughout their lives (20 mo) had significantly higher concentrations of ceramides in their hippocampus and lower concentrations of gangliosides in their pons medulla and midbrain (Fig. 2). Interestingly and as discussed in a later section, this sphingolipid profile was associated with cognitive impairment (9). It should be mentioned that positive correlations between MK-4 and sulfatide concentrations have also been observed in the hippocampus and cortex of 12- and 24-mo-old male Fisher 344 rats, supporting the tight association between the K vitamer and this sphingolipid in the brain (24).
Figure 2.
Sphingolipid concentrations in brain regions of 20-mo-old rats fed a low, adequate, or high phylloquinone diet since weaning. Values are mean ± SEM, n = 4–6. *Bracketed means differ, P < 0.05 (ANOVA followed by Tukey’s post hoc test). CB, cerebellum; PM, pons medulla; MB, midbrain; HIP, hippocampus; STR, striatum. Adapted with permission from (9).
These more recent studies that confirm the modulation of brain sphingolipids by vitamin K nutritional status, extend the work of Lev’s group and underscore the potentially far-reaching effect of vitamin K in brain function given the key role of these lipids in cell-signaling functions.
Vitamin K–dependent proteins in the nervous system
Two VKDPs have been closely associated with the nervous system, namely, Gas6 and, to a lesser extent, protein S.
Gas6
Discovered in 1993, Gas6, hence named after it was found to be the product of the growth arrest–specific gene 6, is a secreted protein (75 kDa) that contains 11–12 carboxyglutamic acid (Gla) residues. It is structurally related to the vitamin K–dependent anticoagulation factor protein S, with which it shares 44% amino acid homology (1). Gas6 binds and activates the receptor tyrosine kinases of the Tyro3, Axl, and Mer (TAM) family, a function that is dependent on the presence of Gla residues. As for all VKDPs, Gas6 undergoes a post-translational transformation of its glutamic acid residues into Gla in a reaction catalyzed by the vitamin K–dependent GGCX (25, 26). Synthesis of Gla residue in the nervous system is expected because the GGCX is highly expressed in this system. Using in situ hybridization techniques, prominent expression of the enzyme was observed in the periventricular neuroepithelium of the central nervous system (CNS) during development, its expression persisting in the brain during adulthood. The GGCX is also highly expressed in the gray matter of the spinal cord during early and mid gestation (27). Support for GGCX activity in the brain was provided in an earlier report based on the vitamin K–dependent incorporation of 14CO2 in brain microsomes (28).
Using biochemical and histological techniques, a detailed analysis of the distribution of Gas6 in the rat CNS was conducted (2). At early stages of development, Gas6 expression in the rat embryo is mostly confined to non-neuronal tissues, with expression becoming more generalized in late embryonic stages and remaining high during adulthood. In the adult rats, Gas6 is expressed in the cerebral cortex, piriform cortex, hippocampus (areas CA1, CA3, and the dentate gyrus), thalamic and hypothalamic structures, midbrain, and cerebellum where it is found at high levels in the Purkinje neurons and deep cerebellar nuclei. Gas6 is also detected in motor neurons at embryonic d 14. In an independent study, expression of Gas6 mRNA was found to be particularly marked in the large neurons of the dorsal root ganglia and in the neurons of the ventral horn of the spinal cord (29). Gas6 expression was also investigated in adulthood as a function of age. Using rat synaptosomes from the striatum, hippocampus, and frontal cortex of 6-, 12-, and 24-mo-old Fisher 344 rats, Gas6 was shown to decrease in a tissue-specific manner. Decreased expression was most dramatic in the frontal cortex, with levels in 24-mo-old rats being >84% lower than 6-mo-old rats, whereas in the striatum and hippocampus, the age-associated decrease was on the order of 55% (30).
In the nervous system, Gas6 has been involved in chemotaxis, mitogenesis, cell growth, and myelination. These actions have all been linked to the ability of the protein to bind the TAM receptors and induce their phosphorylation (25). Specifically, Gas6 has been shown to prevent gonadotropin-releasing hormone neurons from undergoing serum deprivation–induced apoptosis, an effect mediated by the extracellular signal–regulated (ERK) and the serine/threonine protein kinase (Akt), the latter being a downstream component of the phosphatidylinositol 3-kinase (PI3K) signaling pathway (31). Gas6/Axl signaling has also been shown to contribute to the migration of the gonadotropin-releasing hormone neurons from the olfactory bulb to the hypothalamus via signaling to the p38 mitogen-activated protein kinase (MAPK), an essential step for these cells’ function in ensuring sexual maturation during development (32). A prosurvival effect of Gas6 has also been observed in hippocampal neurons (33) through the activation of MAPK and PI3K signaling pathways and their downstream effectors (34). Using primary cultures of cortical and hippocampal neurons, Prieto et al. (34) provided evidence that the Gas6-mediated activation of MAPK results in the recruitment of ERK, 90-kDa ribosomal protein S6 kinase, and cAMP response element-binding protein, whereas Gas6 activation of PI3K leads to the phosphorylation of Akt and the subsequent recruitment of mammalian target of rapamycin and 70-kDa ribosomal protein S6 kinase (P70S6K). Protein Gas6 has also been shown to rescue cortical neurons form amyloid β protein–induced apoptosis. A hallmark of AD (35), the addition of Gas6 to primary cultures of rat cortical neurons prevented cell apoptosis by inhibiting Ca2+ influx and reducing amyloid β protein–induced apoptotic features such as condensation of chromatin and fragmentation of DNA (36). Finally, Gas6 has been shown to protect cortical neurons form phospholipase A2-IIA–induced apoptosis (37).
In addition to its well-documented actions in neurons, Gas6 also modulates glial cell functions, notably oligodendrocytes, Schwann cells, and microglia. Oligodendrocytes and Schwann cells are the cells responsible for myelinating the neurons in the central and peripheral nervous systems, respectively, and play essential roles in the transmission of the nervous impulses. Microglia are the primary immune effectors of the CNS and are at the core of tissue homeostasis and repair (38). As macrophages, they accomplish important phagocytic functions, removing pathogens, cellular debris, and apoptotic cells that accumulate over time. In addition, microglial cells secrete cytotoxic substances such as cytokines and reactive oxygen species that they use to protect the brain from infectious organisms. However, an overstimulation of microglia can lead to cytotoxicity and result in damage of local cells (39).
In recent years, studies have provided evidence of an implication of the Gas6-dependent activation of TAM receptors in the survival of glial cells and modulation of microglial phenotype. Using primary cultures of oligodendrocytes from human fetal spinal cord, Shankar et al. (40) showed that addition of Gas6 to the medium significantly enhanced oligodendrocyte survival and protected them from apoptosis, an action mediated by the PI3K pathway. They subsequently confirmed the prosurvival effect of Gas6 in growth factor (insulin)-depleted cultures and provided evidence that Gas6 could protect oligodendrocytes from TNF-α–mediated toxicity through activation of the Axl receptor and the PI3K/Akt signaling pathway (41).
More recently, the action of Gas6 in oligodendrocytes was investigated in the cuprizone-induced demyelination model, a well-established model of multiple sclerosis. Treatment with this copper chelator results in focal demyelination of the corpus callosum and in the recruitment of microglia before and during demyelination (42). When Gas6 knockout mice (Gas6−/−) were subjected to a cuprizone challenge for 3 wk, the animals were found to present alterations in both myelination and microglial activation. Specifically, the absence of Gas6 was associated with decreased oligodendrocyte survival, greater cell loss, fewer myelinated axons, and a reduction in overall myelination. In an in vitro study, Gas6 was shown to decrease expression of inflammatory cytokine TNF-α after an LPS challenge, suggesting an anti-inflammatory role for Gas6 (39). These results are in line with those of Grommes et al. (43), who, using a murine microglia cell line, observed reduced expression of the proinflammatory mediators inducible nitric oxide synthase and interleukin-1β in Gas6-treated cells after LPS treatment. Importantly, recent reports point to a modulatory role for Gas6 during remyelination. Addition of Gas6 to a culture of oligodendrocyte precursor cells resulted in an increased number of myelin basic protein–positive segments in a dose-dependent manner. In contrast, the absence of Gas6 as investigated in cuprizone-treated Gas6−/− mice was associated with a lower number of oligodendrocytes and reduced levels of myelination 4 wk post-treatment. However, the degree of myelination was not different between groups after 10 wk, suggesting that Gas6 delayed myelination but did not ultimately prevent the process (44). It should be mentioned that in these studies, Gas6−/− mice did not present neurological anomalies before treatment, a phenotype reported by others (45). Similar positive effects of Gas6 on myelin repair were also observed in cuprizone-treated C57B16J mice administered Gas6 directly into the corpus callosum by an osmotic mini-pump. Brains of Gas6-treated mice assessed 14 d post-treatment were characterized by increased clearance of cellular and myelin debris and remyelination, increased axonal survival and integrity, and increased maturation of oligodendrocyte progenitor cells. Noteworthy, these effects of Gas6 on remyelination were not associated with an activation of microglial cells (46). Interestingly, a growth-promoting effect of Gas6 on glia has also been observed in Schwann cells, the oligodendrocyte cell type equivalent in the peripheral nervous system. When added to a serum-free defined culture of adult human Schwann cells, Gas6 was shown to stimulate mitogenesis (i.e., thymidine incorporation) and cell growth via phosphorylation of Axl and Tyro-3 receptors and subsequent stimulation of ERK (29).
Collectively, results gathered in the past 15 y clearly established Gas6 as an important regulator of cell survival and growth and of the myelination process.
Protein S
Initially discovered for its role in blood coagulation as a cofactor of protein C (47), protein S is expressed in the brain, although to a much lesser extent than Gas6. In the adult nervous system, expression of the protein has been observed in the locus coeruleus and the choroid plexus (2) as well as in astrocytes (48). In the rabbit brain, protein S mRNA has been detected in pyramidal neurons of the cortex and hippocampus and in granule neurons of the dentate gyrus (49). Protein S mRNA has also been shown to be upregulated in response to nerve injury (48) and has been detected in certain glioblastoma and neuroblastoma cell lines (50).
Research pertaining to the role of protein S in the nervous systems is significantly more limited than that for the parent protein Gas6. Nonetheless, available data point to specific actions for protein S in this system. Like Gas6, protein S is a ligand for the TAM receptors, and this association has been linked to some of its actions. Functionally, protein S has been shown to offer neuronal protection during ischemic/hypoxic injury, both in vivo and in vitro. In an in vivo model of stroke, protein S was found to significantly reduce brain infarction and edema volumes and to improve postischemic cerebral blood flow in treated mice. Protein S treatment was also associated with less fibrin deposition and infiltration with neutrophils and fewer apoptotic neurons, an effect that was also confirmed in cultured neurons. Finally, protein S was associated with improved motor neurological scores in treated mice (51). In a subsequent study, protein S was shown to protect neurons from N-methyl-d-aspartate–induced toxicity and apoptosis via the Tyro3-PI3K-Akt pathway, via the sex hormone–binding globulin domain of the protein (52).
Although limited in scope, data collected thus far suggest that protein S has the potential to protect the brain and the nervous system through its antithrombotic functions and its signaling-mediated neuroprotective actions. This is in line with the severe thrombotic and necrotic phenotypes that have been observed in the brains of mice lacking protein S (53).
Other actions of vitamin K in the brain
In addition to the literature relative to the VKDPs discussed previously, there is evidence to suggest that the K vitamers have actions of their own. Whether these actions have any relationship to sphingolipid metabolism remains to be determined. In a report by Tsang et al. (54), both phylloquinone and MK-4 were shown to promote neurite outgrowth on PC12D cells in the presence of nerve growth factor, an action mediated by the protein kinase A and MAPK signaling pathways. Furthermore, both K vitamers led to an increase in nerve growth factor–induced acetylcholinesterase activity. These results are in line with those of a previous study in which phylloquinone and MK-4 vitamers were observed to promote survival of different neuronal cell types (cortex, hippocampus, striatum) during the later stages of embyogenesis. Furthermore, treating the culture systems with warfarin had no effect on the survival-promoting effects of vitamin K (55). Recently, MK-4 and, to a lesser extent, phylloquinone were shown to prevent glutathione depletion–mediated oxidative injury as defined by free radical accumulation and cell death, in primary cultures of oligodendrocyte precursors and immature fetal cortical neurons (56). Addition of warfarin to the cell cultures had no effect on the protective effect of vitamin K, suggesting that this action of MK-4 was independent of the VKDPs. In a more recent report, this group provided evidence that the protective effect of MK-4 against oxidative cell death was, at least in part, through inhibition of enzyme 12-lipoxygenase (57). Finally, the neuroprotective effect of MK-4 was recently confirmed in the model of methylmercury-induced cell death, an experimental model associated with a significant decrease in intracellular glutathione (58). In this study, as in the one by Li et al. (56), the beneficial effect of MK-4 was not associated with the restoration of intracellular glutathione nor did it involve obvious direct antioxidant activity by the vitamer.
Vitamin K and cognition
Studies that have examined the relationship between vitamin K and behavior and cognition are very limited. In humans, fetal exposure to warfarin derivatives during the first trimester of pregnancy has long been known to result in a wide range of physical anomalies collectively referred to as warfarin embryopathy. This syndrome includes optic atrophy, dilation of the cerebral ventricles, blindness, microencephaly, and mental retardation (59, 60). More recently, our group published a detailed analysis of phylloquinone intake of 31 community-dwelling patients in the early stages of AD and compared them with 31 age- and sex-matched cognitively intact controls. Mean phylloquinone intake was significantly lower in patients (63 ± 90 μg/d vs. 139 ± 233 μg/d), even after adjusting for energy intake. Vegetables, fats, and fruits contributed >70% of total phylloquinone intake in both groups, and green vegetables, the main source of vitamin K, contributed 33% and 49% to the total intake in patients and controls, respectively. This lower consumption of green vegetables in participants with AD explained their overall lower vitamin K intake (61). In an earlier study involving 100 women with AD and 100 age-matched, community-dwelling controls, plasma phylloquinone levels were found to be significantly lower in patients than in controls. Furthermore, among AD patients, serum concentration of phylloquinone correlated positively with cognition based on the Mini-Mental State Examination and negatively with the uncarboxylated (inactive) form of the VKDP osteocalcin (62). Clearly the relationship between vitamin K status and cognitive abilities needs to be further investigated. Notably, and despite the methodological challenges that such studies entail, it would be important to determine the long-term effect of warfarin therapy on cognitive abilities. A potent anti–vitamin K agent, warfarin is widely prescribed for the prophylaxis and treatment of thromboembolic conditions such as deep venous thrombosis, pulmonary embolism, atrial fibrillation, and cardiac valve replacement (63). As individuals treated with warfarin are in a relative state of vitamin K deficiency, they could be at higher risk of cognitive problems based on the actions of vitamin K in the nervous system.
Animal studies pertaining to cognition are also very limited. In a study by Cocchetto et al. (64), vitamin K deficiency induced by eating a vitamin K–deficient diet or receiving warfarin treatment was associated with hypoactivity in rats. When assessed with the open-field paradigm, locomotor activity of vitamin K−deficient rats was 25% lower than in controls, and warfarin treatment was associated with a shift from more to less exploratory behavior. In these rats, cognitive abilities as assessed with the radial arm maze, were not altered by vitamin K status. More recently, our group reported that lifetime consumption of a diet low in phylloquinone resulted in increased cognitive deficits in old age.
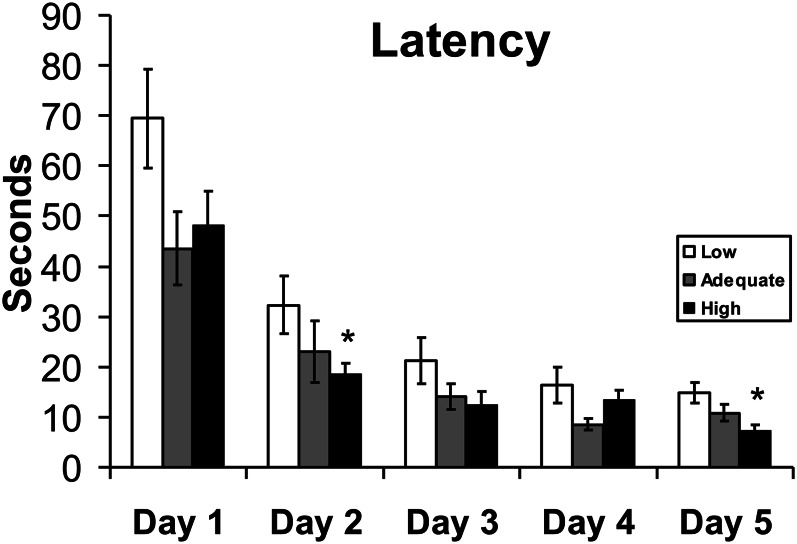
Specifically, rats were subjected to the Morris water maze, a test in which animals must learn to locate a submerged platform by using visual cues. Learning performance is based on the time (latency) needed for the animal to find the platform. When subjected to this paradigm, 20-mo-old rats that were fed a diet low in phylloquinone since weaning were found to acquire spatial learning more slowly (i.e., longer latencies) than rats fed adequate or high phylloquinone diets (Fig. 3). Importantly, we determined that learning impairment in the old rats was not related to anxiety or motor or visual deficits. Interestingly, a diet low in phylloquinone had no significant impact on cognition at 6 and 12 mo of age, suggesting that vitamin K is particularly important to brain function in the more vulnerable aging state. Of note is that these vitamin K–induced cognitive alterations with age were associated with higher concentrations of ceramides in the hippocampus and lower gangliosides in the pons medulla and midbrain (9).
Figure 3.
Performance in the Morris water maze of 20-mo-old rats fed a low, adequate, or high phylloquinone diet since weaning (n = 9–12). Values are mean ± SEM, latencies from low group differ from those of adequate (P = 0.054) and high (P < 0.05) groups. Adapted with permission from (9).
Although in this study, the vitamin K–induced cognitive impairment was associated with alterations in sphingolipid metabolism, other vitamin K–related parameters could have contributed to the observed deficits. In light of their signaling actions through TAM receptors, both Gas6 and protein S would have the potential to influence cognition, a low vitamin K status leading to undercarboxylation of the proteins, and their suboptimal functions. Similarly, a low vitamin K status could limit the protective effects of MK-4 observed in conditions of glutathione-mediated oxidative stress as discussed earlier. Finally, suboptimal vitamin K status could interfere with the protective actions of MK-4 in inflammation. There is indeed growing evidence that MK-4 offers protection against inflammation, this K vitamer having been shown to limit the production of cytokines and other related substances after inflammatory challenges, both in vitro and in vivo (65–67). All these vitamin K–related parameters should be investigated in future studies focusing on the role of dietary vitamin K in cognition.
Conclusion
Research conducted in the past 10 y has lent additional support to the role of vitamin K in sphingolipid metabolism and its modulation by vitamin K status. The discovery of protein Gas6 and the characterization of its signaling actions in neurons and the various glial cell types have also shed light on other mechanisms through which vitamin K can influence the nervous system. Research on these proteins needs to be pursued to deepen our understanding of their mode of action and their modulation by nutritional status. Similarly, future efforts should aim at further characterizing the emerging actions of vitamin K with respect to oxidative injury and inflammation because these could have wide-ranging implications, notably with respect to the aging state and a number of neurodegenerative diseases. Finally, research on the role of vitamin K in cognition needs to be pursued and could benefit from experimental models of cognitive vulnerability or premature cognitive decline.
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Supported by the Canadian Institutes of Health Research.
Author disclosures: G. Ferland, no conflicts of interest.
Abbreviations used: AD, Alzheimer’s disease; Akt, serine/threonine protein kinase; CNS, central nervous system; ERK, extracellular signal-regulated kinase; Gas6−/−, Gas6 knockout mice; GGCX, γ-glutamyl carboxylase; Gla, γ-carboxyglutamic acid; 3-KDS, 3-ketodihydrosphingosine; MAPK, mitogen-activated protein kinase; MK-4, menaquinone-4; PI3K, phosphatidylinositol 3-kinase; VKDP, vitamin K–dependent protein; TAM, Tyro3, Axl, and Mer.
Literature Cited
- 1.Manfioletti G, Brancolini C, Avanzi G, Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol. 1993;13:4976–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prieto AL, Weber JL, Tracy S, Heeb MJ, Lai C. Gas6, a ligand for the receptor protein-tyrosine kinase Tyro-3, is widely expressed in the central nervous system. Brain Res. 1999;816:646–61 [DOI] [PubMed] [Google Scholar]
- 3.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50: Suppl:S91–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lev M. Sphingolipid biosynthesis and vitamin K metabolism in Bacteroides melaninogenicus. Am J Clin Nutr. 1979;32:179–86 [DOI] [PubMed] [Google Scholar]
- 5.Lev M, Sundaram S. Modulation of glycosphingolipid synthesis by vitamin K depletion in bacteria and brain. In: Suttie JW, editor. Current Advances in Vitamin K Research: Proceedings of the 17th Steenbock Symposium. Madison: University of Wisconsin-Madison; 1988 [Google Scholar]
- 6.Thijssen HH, Drittij-Reijnders MJ. Vitamin K distribution in rat tissues: dietary phylloquinone is a source of tissue menaquinone-4. Br J Nutr. 1994;72:415–25 [DOI] [PubMed] [Google Scholar]
- 7.Huber AM, Davidson KW, O'Brien-Morse ME, Sadowski JA. Tissue phylloquinone and menaquinones in rats are affected by age and gender. J Nutr. 1999;129:1039–44 [DOI] [PubMed] [Google Scholar]
- 8.Carrié I, Portoukalian J, Vicaretti R, Rochford J, Potvin S, Ferland G. Menaquinone-4 concentration is correlated with sphingolipid concentrations in rat brain. J Nutr. 2004;134:167–72 [DOI] [PubMed] [Google Scholar]
- 9.Carrié I, Portoukalian J, Rochford J, Ferland G. Life-long low phylloquinone intake is associated with cognitive impairments in old rats. J Nutr. 2011;141:1495–501 [DOI] [PubMed] [Google Scholar]
- 10.Zeidan YH, Hannun YA. Translational aspects of sphingolipid metabolism. Trends Mol Med. 2007;13:327–36 [DOI] [PubMed] [Google Scholar]
- 11.Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:2070–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jana A, Hogan EL, Pahan K. Ceramide and neurodegeneration: susceptibility of neurons and oligodendrocytes to cell damage and death. J Neurol Sci. 2009;278:5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posse de Chaves E, Sipione S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010;584:1748–59 [DOI] [PubMed] [Google Scholar]
- 14.Lev M. Apparent requirement for vitamin K of rumen strains of Fusiformis nigrescens. Nature. 1958;181:203–4 [DOI] [PubMed] [Google Scholar]
- 15.Lev M. Vitamin K deficiency in Fusiformis nigrescens. I. Influence on whole cells and cell envelope characteristics. J Bacteriol. 1968;95:2317–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lev M, Milford AF. Vitamin K stimulation of sphingolipid synthesis. Biochem Biophys Res Commun. 1971;45:358–62 [DOI] [PubMed] [Google Scholar]
- 17.Lev M, Milford AF. Effect of vitamin K depletion and restoration on sphingolipid metabolism in Bacteroides melaninogenicus. J Lipid Res. 1972;13:364–70 [PubMed] [Google Scholar]
- 18.Lev M, Milford AF. The 3-ketodihydrosphingosine synthetase of Bacteroides melaninogenicus: induction by vitamin K. Arch Biochem Biophys. 1973;157:500–8 [DOI] [PubMed] [Google Scholar]
- 19.Sundaram KS, Lev M. Warfarin administration reduces synthesis of sulfatides and other sphingolipids in mouse brain. J Lipid Res. 1988;29:1475–9 [PubMed] [Google Scholar]
- 20.Sundaram KS, Lev M. Regulation of sulfotransferase activity by vitamin K in nouse brain. Arch Biochem Biophys. 1990;277:109–13 [DOI] [PubMed] [Google Scholar]
- 21.Sundaram KS, Lev M. Vitamin K and phosphate mediated enhancement of brain sulfotransferase activity. Biochem Biophys Res Commun. 1990;169:927–32 [DOI] [PubMed] [Google Scholar]
- 22.Sundaram KS, Lev M. Purification and activation of brain sulfotransferase. J Biol Chem. 1992;267:24041–4 [PubMed] [Google Scholar]
- 23.Sundaram KS, Fan JH, Engelke JA, Foley AL, Suttie JW, Lev M. Vitamin K status influences brain sulfatide metabolism in young mice and rats. J Nutr. 1996;126:2746–51 [DOI] [PubMed] [Google Scholar]
- 24.Crivello NA, Casseus SL, Peterson JW, Smith DE, Booth SL. Age- and brain region-specific effects of dietary vitamin K on myelin sulfatides. J Nutr Biochem. 2010;21:1083–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW, Hunt RW, Trail G, Clogston C, Toso RJ, et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature. 1995;373:623–6 [DOI] [PubMed] [Google Scholar]
- 26.Tanabe K, Nagata K, Ohashi K, Nakano T, Arita H, Mizuno K. Roles of gamma-carboxylation and a sex hormone-binding globulin-like domain in receptor-binding and in biological activities of Gas6. FEBS Lett. 1997;408:306–10 [DOI] [PubMed] [Google Scholar]
- 27.Romero EE, Velazquez-Estades LJ, Deo R, Schapiro B, Roth DA. Cloning of rat vitamin K-dependent gamma-glutamyl carboxylase and developmentally regulated gene expression in postimplantation embryos. Exp Cell Res. 1998;243:334–46 [DOI] [PubMed] [Google Scholar]
- 28.de Boer-van den Berg MA, Thijssen HH, Vermeer C. The in vivo effects of acenocoumarol, phenprocoumon and warfarin on vitamin K epoxide reductase and vitamin K-dependent carboxylase in various tissues of the rat. Biochim Biophys Acta. 1986;884:150–7 [DOI] [PubMed] [Google Scholar]
- 29.Li R, Chen J, Hammonds G, Phillips H, Armanini M, Wood P, Bunge R, Godowski PJ, Sliwkowski MX, Mather JP. Identification of Gas6 as a growth factor for human Schwann cells. J Neurosci. 1996;16:2012–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsaioun KI, Denisova NA, Obin M, Joseph J. Novel growth factor Gas6, phosphatidylserine and their age-related changes in the rat brain. Neurosci Res Commun. 2000;26:113–22 [Google Scholar]
- 31.Allen MP, Zeng C, Schneider K, Xiong X, Meintzer MK, Bellosta P, Basilico C, Varnum B, Heidenreich KA, Wierman ME. Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt. Mol Endocrinol. 1999;13:191–201 [DOI] [PubMed] [Google Scholar]
- 32.Allen MP, Linseman DA, Udo H, Xu M, Schaack JB, Varnum B, Kandel ER, Heidenreich KA, Wierman ME. Novel mechanism for gonadotropin-releasing hormone neuronal migration involving Gas6/Ark signaling to p38 mitogen-activated protein kinase. Mol Cell Biol. 2002;22:599–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funakoshi H, Yonemasu T, Nakano T, Matumoto K, Nakamura T. Identification of Gas6, a putative ligand for Sky and Axl receptor tyrosine kinases, as a novel neurotrophic factor for hippocampal neurons. J Neurosci Res. 2002;68:150–60 [DOI] [PubMed] [Google Scholar]
- 34.Prieto AL, O'Dell S, Varnum B, Lai C. Localization and signaling of the receptor protein tyrosine kinase Tyro3 in cortical and hippocampal neurons. Neuroscience. 2007;150:319–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagami T, Ueda K, Asakura K, Sakaeda T, Nakazato H, Kuroda T, Hata S, Sakaguchi G, Itoh N, Nakano T, et al. Gas6 rescues cortical neurons from amyloid beta protein-induced apoptosis. Neuropharmacology. 2002;43:1289–96 [DOI] [PubMed] [Google Scholar]
- 37.Yagami T, Ueda K, Asakura K, Okamura N, Sakaeda T, Sakaguchi G, Itoh N, Hashimoto Y, Nakano T, Fujimoto M. Effect of Gas6 on secretory phospholipase A(2)-IIA-induced apoptosis in cortical neurons. Brain Res. 2003;985:142–9 [DOI] [PubMed] [Google Scholar]
- 38.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8 [DOI] [PubMed] [Google Scholar]
- 39.Binder MD, Cate HS, Prieto AL, Kemper D, Butzkueven H, Gresle MM, Cipriani T, Jokubaitis VG, Carmeliet P, Kilpatrick TJ. Gas6 deficiency increases oligodendrocyte loss and microglial activation in response to cuprizone-induced demyelination. J Neurosci. 2008;28:5195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shankar SL, O'Guin K, Cammer M, McMorris FA, Stitt TN, Basch RS, Varnum B, Shafit-Zagardo B. The growth arrest-specific gene product Gas6 promotes the survival of human oligodendrocytes via a phosphatidylinositol 3-kinase-dependent pathway. J Neurosci. 2003;23:4208–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shankar SL, O'Guin K, Kim M, Varnum B, Lemke G, Brosnan CF, Shafit-Zagardo B. Gas6/Axl signaling activates the phosphatidylinositol 3-kinase/Akt1 survival pathway to protect oligodendrocytes from tumor necrosis factor alpha-induced apoptosis. J Neurosci. 2006;26:5638–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grommes C, Lee CY, Wilkinson BL, Jiang Q, Koenigsknecht-Talboo JL, Varnum B, Landreth GE. Regulation of microglial phagocytosis and inflammatory gene expression by Gas6 acting on the Axl/Mer family of tyrosine kinases. J Neuroimmune Pharmacol. 2008;3:130–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Binder MD, Xiao J, Kemper D, Ma GZ, Murray SS, Kilpatrick TJ. Gas6 increases myelination by oligodendrocytes and its deficiency delays recovery following cuprizone-induced demyelination. PLoS ONE. 2011;6:e17727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lutgens E, Tjwa M, Garcia de Frutos P, Wijnands E, Beckers L, Dahlbäck B, Daemen MJ, Carmeliet P, Moons L. Genetic loss of Gas6 induces plaque stability in experimental atherosclerosis. J Pathol. 2008;216:55–63 [DOI] [PubMed] [Google Scholar]
- 46.Tsiperson V, Li X, Schwartz GJ, Raine CS, Shafit-Zagardo B. GAS6 enhances repair following cuprizone-induced demyelination. PLoS ONE. 2010;5:e15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DiScipio AW, Burchard KW. Continuous arteriovenous hemofiltration attenuates polymorphonuclear leukocyte phagocytosis in porcine intra-abdominal sepsis. Am J Surg. 1997;173:174–80 [DOI] [PubMed] [Google Scholar]
- 48.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–70 [DOI] [PubMed] [Google Scholar]
- 49.He X, Shen L, Bjartell A, Dahlback B. The gene encoding vitamin K-dependent anticoagulant protein S is expressed in multiple rabbit organs as demonstrated by northern blotting, in situ hybridization, and immunohistochemistry. J Histochem Cytochem. 1995;43:85–96 [DOI] [PubMed] [Google Scholar]
- 50.Phillips DJ, Greengard JS, Fernandez JA, Ribeiro M, Evatt BL, Griffin JH, Hooper WC. Protein S, an antithrombotic factor, is synthesized and released by neural tumor cells. J Neurochem. 1993;61:344–7 [DOI] [PubMed] [Google Scholar]
- 51.Liu D, Guo H, Griffin JH, Fernandez JA, Zlokovic BV. Protein S confers neuronal protection during ischemic/hypoxic injury in mice. Circulation. 2003;107:1791–6 [DOI] [PubMed] [Google Scholar]
- 52.Zhong Z, Wang Y, Guo H, Sagare A, Fernandez JA, Bell RD, Barrett TM, Griffin JH, Freeman RS, Zlokovic BV. Protein S protects neurons from excitotoxic injury by activating the TAM receptor Tyro3-phosphatidylinositol 3-kinase-Akt pathway through its sex hormone-binding globulin-like region. J Neurosci. 2010;30:15521–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saller F, Brisset AC, Tchaikovski SN, Azevedo M, Chrast R, Fernandez JA, Schapira M, Hackeng TM, Griffin JH, Angelillo-Scherrer A. Generation and phenotypic analysis of protein S-deficient mice. Blood. 2009;114:2307–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsang CK, Kamei Y. Novel effect of vitamin K(1) (phylloquinone) and vitamin K(2) (menaquinone) on promoting nerve growth factor-mediated neurite outgrowth from PC12D cells. Neurosci Lett. 2002;323:9–12 [DOI] [PubMed] [Google Scholar]
- 55.Nakajima M, Furukawa S, Hayashi K, Yamada A, Kawashima T, Hayashi Y. Age-dependent survival-promoting activity of vitamin K on cultured CNS neurons. Brain Res Dev Brain Res. 1993;73:17–23 [DOI] [PubMed] [Google Scholar]
- 56.Li J, Lin JC, Wang H, Peterson JW, Furie BC, Furie B, Booth SL, Volpe JJ, Rosenberg PA. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci. 2003;23:5816–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Wang H, Rosenberg PA. Vitamin K prevents oxidative cell death by inhibiting activation of 12-lipoxygenase in developing oligodendrocytes. J Neurosci Res. 2009;87:1997–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakaue M, Mori N, Okazaki M, Kadowaki E, Kaneko T, Hemmi N, Sekiguchi H, Maki T, Ozawa A, Hara S, et al. Vitamin K has the potential to protect neurons from methylmercury-induced cell death in vitro. J Neurosci Res. 2011;89:1052–8 [DOI] [PubMed] [Google Scholar]
- 59.Pauli RM. Mechanism of bone and cartilage maldevelopment in the warfarin embryopathy. Pathol Immunopathol Res. 1988;7:107–12 [DOI] [PubMed] [Google Scholar]
- 60.Hall JG, Pauli RM, Wilson KM. Maternal and fetal sequelae of anticoagulation during pregnancy. Am J Med. 1980;68:122–40 [DOI] [PubMed] [Google Scholar]
- 61.Presse N, Shatenstein B, Kergoat MJ, Ferland G. Low vitamin K intakes in community-dwelling elders at an early stage of Alzheimer's disease. J Am Diet Assoc. 2008;108:2095–9 [DOI] [PubMed] [Google Scholar]
- 62.Sato Y, Nakamura R, Satoh M, Fujishita K, Mori S, Ishida S, Yamaguchi T, Inoue K, Nagao T, Ohno Y. Thyroid hormone targets matrix Gla protein gene associated with vascular smooth muscle calcification. Circ Res. 2005;97:550–7 [DOI] [PubMed] [Google Scholar]
- 63.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:160S–98S [DOI] [PubMed] [Google Scholar]
- 64.Cocchetto DM, Miller DB, Miller LL, Bjornsson TD. Behavioral perturbations in the vitamin K-deficient rat. Physiol Behav. 1985;34:727–34 [DOI] [PubMed] [Google Scholar]
- 65.Reddi K, Henderson B, Meghji S, Wilson M, Poole S, Hopper C, Harris M, Hodges SJ. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine. 1995;7:287–90 [DOI] [PubMed] [Google Scholar]
- 66.Moriya M, Nakatsuji Y, Okuno T, Hamasaki T, Sawada M, Sakoda S. Vitamin K2 ameliorates experimental autoimmune encephalomyelitis in Lewis rats. J Neuroimmunol. 2005;170:11–20 [DOI] [PubMed] [Google Scholar]
- 67.Ohsaki Y, Shirakawa H, Hiwatashi K, Furukawa Y, Mizutani T, Komai M. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci Biotechnol Biochem. 2006;70:926–32 [DOI] [PubMed] [Google Scholar]