Abstract
A systematic literature review of human studies relating caffeine or caffeine-rich beverages to cognitive decline reveals only 6 studies that have collected and analyzed cognition data in a prospective fashion that enables study of decline across the spectrum of cognition. These 6 studies, in general, evaluate cognitive function using the Mini Mental State Exam and base their beverage data on FFQs. Studies included in our review differed in their source populations, duration of study, and most dramatically in how their analyses were done, disallowing direct quantitative comparisons of their effect estimates. Only one of the studies reported on all 3 exposures, coffee, tea, and caffeine, making comparisons of findings across studies more difficult. However, in general, it can be stated that for all studies of tea and most studies of coffee and caffeine, the estimates of cognitive decline were lower among consumers, although there is a lack of a distinct dose response. Only a few measures showed a quantitative significance and, interestingly, studies indicate a stronger effect among women than men.
Introduction
As life expectancies increase, the specter of inevitable cognitive decline looms more menacingly among older adults. Unfortunately, we know of no cognition enhancer that reliably and repeatedly counteracts age-associated cognitive decline (1). In the quest for knowledge on slowing cognitive decline in man, randomized controlled studies are unlikely to be undertaken. Therefore, there is a need to rely on animal studies and observational epidemiologic studies of natural experiments in man to provide knowledge on the relationship between beverage consumption and decline. Animal studies do suggest that chronic caffeine consumption may prevent cognitive decline in male rats (2). Caffeine-treated rats given 5 mg/(kg · d) for 6 mo outperformed a tap water control group on maze-based cognition tests conducted 2–3 wk after caffeine withdrawal. The caffeinated rats demonstrated measurably greater dendritic length of 4th and 5th order branches, total dendritic length, and spine density in distal dendritic branches in the basal dendrites of CA1 pyramidal neurons, 1 of 3 layers of pyramidal neurons within the hippocampus. Substances other than caffeine in tea and coffee might also affect cognition. Theanine, e.g., an amino acid that crosses the blood-brain barrier and found only in tea and mushrooms (3, 4), is one of these substances experimentally studied in relation to cognition in man (5–7).
Cross-sectional human epidemiologic studies have reported better cognitive function among self-selected coffee or tea drinkers for many years (8). In northern Europe, global cognition scores among community-dwelling, nondemented elderly in Dublin and Norway were positively correlated with tea intake (9, 10). Asian studies of tea and cognition have also reported less cognitive impairment among former tea drinkers, as evidenced in a study of 90–108 y olds living in western China (8) and ≥70 y olds in Japan (11). Such cross-sectional studies, however, are vulnerable to multiple biases, including the possibility that the drinking behavior may be a result of cognitive status instead of causally related.
To overcome these limitations, study in humans requires cohorts of representative healthy populations in whom cognition has been measured at baseline and repeatedly over time using the same assessment tool. Comparisons of change in function over time between consumers and nonconsumers and the relationship of change to dose can then be modeled. Challenges will still exist due to the fact that people with the greatest decline are more likely to drop out of the cohort and because results can be confounded by the fact that drinkers of tea or coffee differ from nondrinkers. In addition, when people switch their beverage after the onset of illness, it can appear causal.
This report summarizes the cohort studies of tea, coffee, and caffeine intake and cognitive decline in man. It highlights the many challenges in such studies, including testing demented elderly, missing values, loss to follow-up, many potential confounding variables, and changes in exposure categorization for reasons that might be related to the outcomes of interest.
Materials and Methods
Search strategy
Potential eligible studies were identified through an electronic search of the database PubMed from dates up until March 2012. The search used the following terms to identify the risk exposure (coffee OR caffeine OR tea) combined with terms to determine the outcomes of interest [cognit* AND (declin* OR deteriorat*)]. There were no language restrictions on the search. We screened titles, keywords, and abstracts of the citations obtained from the database. If deemed appropriate for our study, a full copy of the article was obtained for further assessment.
We included studies with a cohort design that addressed the relation between caffeine consumption through coffee and/or tea regardless of assessment of other dietary sources of caffeine.
Articles that were reviews or did not study humans were excluded. Articles in which caffeine was not studied were excluded. Also, articles in which cognitive decline was not measured or measured through Alzheimer’s disease or dementia were excluded as well. Because the focus of this paper is on the prevention of age-related cognitive decline, and analyses that use dichotomous outcomes ignore decline within the normal range and are insensitive to further decline among those determined demented, such publications have not been included in this review. Lastly, studies that were cross-sectional rather than cohort were additionally excluded.
Results
Studies selected
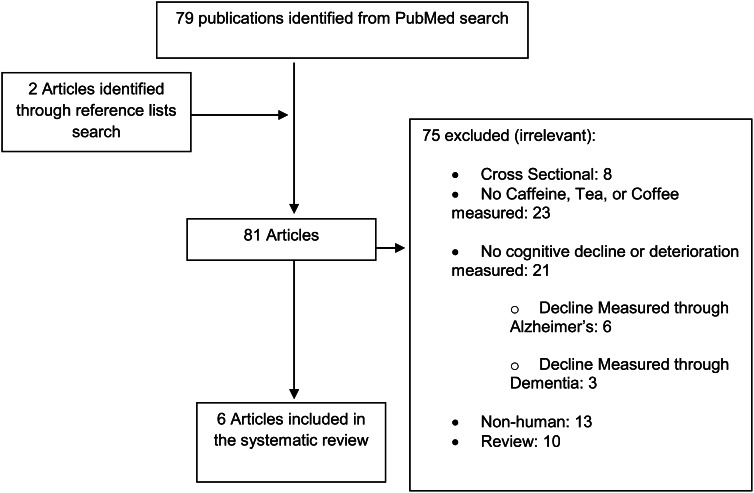
As seen in Figure 1, our search revealed 81 articles, 79 through the PubMed search and 2 through reference list searches. Of these, 75 were excluded for the following reasons: 10 were review articles; 23 did not measure caffeine, tea, or coffee as a variable; 21 did not measure for cognitive decline as an outcome; 13 were not human studies; and 8 were cross-sectional studies.
Figure 1.
Search strategy.
Dietary assessment
Usual dietary intake was assessed at baseline FFQs in all of the studies except Santos (4). Participants were asked to indicate how often they had consumed specific foods and beverages during the past year, including tea and coffee. The questionnaires differed in their range of options, the most narrow being ordinal categories that truncated intake at “almost every day” (3) and the most frequent being >10 cups/d (12). One cup was ~250 mL.
Caffeine consumption
Total caffeine consumption for participants was estimated by multiplying their reported frequency of tea and coffee consumption by the respective estimates of caffeine content; this can be seen in Table 1. The amount each study used per cup, with few exceptions (13, 14), was 30 mg caffeine/cup of tea and 85 mg caffeine/cup of coffee (3, 8, 12, 15). The Ritchie Study used 50 mg caffeine/cup of tea and 100 mg caffeine/cup of coffee (13). The Santos study used Food Processor Plus, where a standard cup of coffee has 63 mg caffeine, a standard cup of tea has 47 mg caffeine, and caffeine intake from other sources, including soft drinks and chocolate, are included (14).
Table 1.
Method of exposure assessment for beverages and caffeine
| Caffeine conversion formula |
|||
| Reference | Range of intake | Coffee/cup1 | Tea/cup |
| mg caffeine | |||
| (12) | 0, 1–3, 4–6, 7–10, >10 (cups of tea or coffee/d) | 85 | 30 |
| (15) | 0, 1, 2, 3, 4, >4 (cups of coffee/d) | 85 | N/A |
| (13) | 0–1, 2–3, >3 (caffeine units/d) | 100 | 50 |
| (8) | <1 cup/wk, <1, 1–2, 3–5, 6–9, >10 (cups of tea or coffee/d) | N/A | N/A |
| (14) | <22, 22–62, >62 (mg caffeine/d) | 63 | 47 |
| (3) | 0, 0.14, 0.46, 2.5, 5 (cups of tea or coffee/wk) | 85 | 30 |
1 cup ≈ 250 mL.
Cognitive assessments
Commonly used in studies of global cognitive function as well as in clinical practice, the 30-point scale Mini Mental State Exam (MMSE),3 which takes ∼10 min to administer, was the most frequent outcome measure across studies (16, 17). It includes modules on orientation to time and place, registration and repeating prompts, attention, and calculation, recall, language, phrase repetition, and complex commands skills expressed through drawing. It has been modified to a 100-point scale for greater information capture in the Modified Mini Mental State (3MS) examination (17). Only one study used the more extensive 3MS examination (17). In addition, the Benton Visual Retention Test and the Isaacs Set tests were employed in one study (13). Another (12) employed a Visual Verbal Learning Test, Motor Choice Reaction Test, Concept Shifting Test, Letter Digit Substitution Test, Fluency Test, and Stroop Color Word Test to test different aspects of cognition but did not use the MMSE or 3MS.
Statistical analysis
In most cases, linear mixed models examine the association between baseline beverage consumption and the change in cognitive test scores over time. Logistic regression models were applied and RRs reported in 3 of the studies, one on tea and coffee intake combined, one on tea and coffee separately, and one on caffeine derived from these 2 sources. Two other studies reported the regression coefficients of change in score decline with consumption after adjustment for confounding variables, as listed in Table 2. All models were adjusted for age at baseline, gender, educational attainment, and health conditions. Some had additional factors such as field center for multi-centered studies, diabetes, hypertension, current smoking, history of stroke, history of coronary heart disease, Center for Epidemiologic Studies Depression Scale score (18), and APOEε4 allele carrier status.
Table 2.
Population characteristics and outcome measures for selected studies1
| Reference | (12) | (15) | (13) | (8) | (14) | (3) |
| Participants, n | 1376 | 676 men | 7017 (2820 men, 4197 women) | 1438 | 309 | 4809 (2722 women, 2077 men) |
| Mean age, y (follow-up duration, y) | Varied (6) | 76.1 ± 4.15 (10) | Men, 73.6 ± 5.3; women, 73.8 ± 5.2 (3.47) | ±55 (1.3) | ± 65 (4) | ≥65+ (7.9) |
| Mean consumption | 340–550 mg caffeine/d | 2.2 cups coffee/d | Men, 176 mg caffeine/d; women, 186 mg caffeine/d | Black/oolong tea consumption: | Women: 32.2 mg caffeine/d | 0.95 cups coffee/d; |
| 67–70% <1 cup/wk; | (10.7–78.8); | 0.57 cups tea/d | ||||
| 12–15% >1 cup/wk < 1 cup/d; | Men: 33.2 mg caffeine/d | |||||
| 18–19% ≥1 cup/d | (9.5–78.8) | |||||
| Coffee consumption: | ||||||
| 26.5% <1 cup/wk; | ||||||
| 8% >1 cup/wk <1 cup/d; | ||||||
| 65.3% ≥1 cup/d | ||||||
| Evaluation of exposure | FFQ | Finland, Italy: self-administered FFQ; Netherlands: FFQ and cross check of dietary history for the past 2–4 wk | Standardized interview administered by research collaborators | Modified FFQ | FFQ | FFQ |
| Outcomes assessed | VVLT, MCRT, CST, LDST, Fluency, Stroop | ΔMMSE | Decline defined as 6-point reduction on the Isaacs; 2-point reduction on the Benton and MMSE | ΔMMSE ≥1 | ΔMMSE ≥2 | 3MS measured annually; beginning 1996, TICS, IQCODE |
| Results | VVLT sum trials 1–5: 0.49 | Coffee consumption: | Relative rate for 1–2 vs. 0 mg caffeine; | Men: | Men: | Modeled IRT adjusted difference in MMSE |
| Delayed: 0.52 | 0 cups/d = ΔMMSE (−2.6 pts) | for 200–300 mg caffeine; | Coffee consumption: | Relative rate (95% CI) | Men: | |
| MCRT | 1 cup/d = (−1.4 pts) | for >300 vs. 0 mg caffeine: | Occasionally vs. rarely: | Adjusted caffeine (mg) | Tea | |
| SR-ini: 0.40 | 2 cups/d = (−1.3 pts) | Men: | OR = 1.13 (0.49–2.61); | <22: 1 [reference] | 5–10×/y: 0.18 [−0.15, 0.51] | |
| SR-mov: 0.47 | 3 cups/d = (−0.6 pts) | Change in Isaacs ≤ −6: | ≥1 cup/d vs. rarely: | 22–62: 0.91 (0.43–1.91) | 1–3×/mo: 0.13 [−0.17, 0.42] | |
| CR-ini: 0.47 | 4 cups/d = (−1.1 pts) | 0.92 (0.73–1.17) | OR = 1.31 (0.71–2.43) | >62: 0.65 (0.27–1.54) | 1–4×/wk: 0.20 [−0.08, 0.48] | |
| CR-mov: 0.51 | >4 cups/d = (−1.6 pts) | 1.08 (0.85–1.40) | Black/oolong tea consumption: | Caffeine (mg) | ≥5×/wk: 0.10 [−0.18, 0.38] | |
| CST | 1.18 (0.87–1.59) | Occasionally vs. rarely: | <75: 1 [reference] | P = 0.67 | ||
| CST-A: 0.57 | Change in Benton ≤2: | OR = 0.18 (0.07–0.49)2* | ≥75: 0.66 (0.29–1.47) | Coffee | ||
| CST-C: 0.46 | 0.99 (0.8–1.24) | 1 cup/d vs. rarely: | Women: | 5–10×/y: 0.01 [−0.39, 0.42] | ||
| Interference: 0.09 | 1.11 (0.88–1.40) | OR = 0.60 (0.29–1.25) | Relative rate (95% CI) | 1–3×/mo: 0.01 [−0.39, 0.39] | ||
| LDST | 0.92 (0.69–1.23) | Women: | Adjusted caffeine (mg) | 1–4×/wk: 0.01 [−0.33, 0.35] | ||
| Number correct: 0.82 | Change in MMSE ≤2: | Coffee consumption: | <22: 1 [reference] | ≥5×/wk: 0.03 [−0.20, 0.26] | ||
| Fluency | 1.02 (0.81–1.28) | Occasionally vs. rarely: | 22–62: 0.65 (0.37–1.17) | P = 0.99 | ||
| Number correct: 0.48 | 1.00 (0.79–1.27) | OR = 0.97 (0.55–1.71); | >62: 0.49 (0.24–0.97)* | Caffeine | ||
| Stroop | 1.19 (0.89–1.59) | ≥1 cup/d vs. rarely: | Caffeine (mg) | Quintile 2: 0.24 [−0.06, 0.53] | ||
| Card I: 0.58 | Women: | OR = 1.03 (0.70–1.51) | <75: 1 [reference] | Quintile 3: 0.12 [−0.17, 0.41] | ||
| Card III: 0.62 | Change in Isaacs ≤ −6: | Black or oolong tea consumption: | ≥75: 0.47 (0.22–0.99)* | Quintile 4: 0.32 [0.01, 0.63] | ||
| Interference: 0.39 | 0.94 (0.77–1.13) | Occasionally vs. rarely: | Women: | |||
| 0.85 (0.70–1.04) | OR = 0.67 (0.45–1.01); | Tea | ||||
| 0.67 (0.53–0.85)* | 1 cup/d vs. rarely: | 5–10×/y: 0.20 [−0.13, 0.51] | ||||
| Change in Benton ≤2: | OR = 0.38 (0.23–0.63)* | 1–3×/mo: 0.27 [0.00, 0.54] | ||||
| 0.96 (0.79–1.16) | Longitudinal association with cognitive decline by tea intake | 1–4×/wk: 0.38 [0.12, 0.62] | ||||
| 1.00 (0.83–1.22) | Men: | ≥5×/wk: 0.21 [−0.03, 0.45] | ||||
| 0.82 (0.65–1.03) | None = 1 | P = 0.07 | ||||
| Change in MMSE ≤2: | Low = 0.73 (0.40,1.32) | Coffee | ||||
| 0.97 (0.81–1.17) | Medium = 0.72 (0.38–1.32) | 5–10×/y: 0.44 [0.07, 0.80] | ||||
| 0.89 (0.73–1.08) | High = 0.75 (0.31–1.79) | 1–3×/mo: 0.54 [0.20, 0.89] | ||||
| 0.91 (0.73–1.14) | P = 0.383 | 1–4×/wk: 0.14 [−0.20, 0.48] | ||||
| Women: | ≥5×/wk: 0.32 [0.13, 0.51] | |||||
| None = 1 | P = 0.002 | |||||
| Low = 0.74 (0.40, 1.32) | Caffeine | |||||
| Med = 0.82 (0.53, 1.26) | Quintile 2: 0.45 [0.20, 0.69] | |||||
| High = 0.36 (0.14, 0.91)* | Quintile 3: 0.38 [0.11, 0.65] | |||||
| P = 0.047 | Quintile 4: 0.53 [0.23, 0.81] | |||||
| Quintile 5: 0.48 [0.23, 0.74] | ||||||
| Control of confounding | Age, gender, education, smoking, alcohol, health, housing tenure, occupation | Age, education, country, smoking, alcohol, and physical exercise | Age, education, center. Additionally for Benton and Isaacs tests in women only: hypertension, smoking, alcohol, diabetes, BMI, depression, cardiovascular disease | Age, gender, education, BMI, depression, APOEε4, hypertension, diabetes, cardiovascular diseases, smoking, alcohol, baseline MMSE score, physical activity, food habits | Age, education, BMI, hypertension, diabetes, smoking, alcohol | Age, race, education, field center, history of stroke, congestive heart disease, diabetes, hypertension, smoking, depression, APOEε4 |
| Methods used | Multiple hierarchal linear regression | Mixed longitudinal random coefficient model | Multivariate mixed models and multivariate adjusted logistic regression | Hierarchal logistic regression model | Poisson regression | Linear mixed models used to estimate rates of change and IRT used to model scores |
CI does not include 1. Benton, Benton Visual Retention Test; CR, choice reaction; CST, Concept Shifting Test; Fluency, Fluency Test; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; IRT, Item Response Theory; Isaacs, Isaacs Set Test; LDST, Letter Digit Substitution Test; MCRT, Motor Choice Reaction test; MMSE, Mini Mental Status Exam; 3MS, Modified Mini Mental Status Exam; SR, simple reaction; Stroop, Stroop Color Word Test; TICS, Telephone Interview for Cognitive Status; VVLT, Visual Verbal Learning Test.
As an outcome measure, one study provided information only on the r square of a linear regression model before and after inclusion of caffeine (and showed no change in explained variance) (12). In almost all studies, the referent group consisted of nonconsumers of both beverages.
Two cohorts were identified that had the potential for reporting on cognitive decline, but because their analyses were limited to determination of dementia and our goal was to discover factors determining the rate of decline, rather than decline, they were not included (19, 20). This is because in these 2 studies from Canada, as much as a 20% decline in cognition would be ignored as long as doing so set the individual beyond the dementia cutoff.
Item response theory
The problem with global cognitive tests is that individual test items do not have difficulty levels evenly distributed across the ability spectrum (21). For example, a 5-point drop in score from 100 to 95 represents a much greater cognitive decline than a 5-point drop in individuals with moderate functioning from 80 to 75. This uneven distribution of item difficulties has been shown to cause biased estimates of rates of decline (21). An increasingly popular and validated approach to minimize this bias is the use of item response theory (IRT) to score global cognition data to establish linearity in the scale (22, 23). Because analyses using the IRT scores provide a more statistically robust estimate of the associations evaluated, such results are more likely to estimate the true underlying association. Unfortunately, only one study reported IRT adjustments (3). That group used Samejima’s (24, 25) graded response model to estimate item variables from the baseline time point with Parscale software (26).
Survivor bias
Bias can result from participants experiencing greater cognitive decline being less likely to participate in the in-person visits (27). In one study (3), nonparticipation on the assessment of cognitive function over time was addressed by incorporated data from the 2 alternative cognitive assessments administered by telephone, using a regression model based on those methods (27) and imputing scores for participants.
Characteristics of the studies
The 6 longitudinal cohort studies that met the inclusion criteria for evaluating the effects of tea or coffee on cognitive decline were published between 2003 and 2011 (3, 8, 12–15) and are summarized in Table 1. Four of the studies were conducted in Europe [Netherlands (12, 15), Finland (15), Italy (15), France (13), and Portugal (14)]. One study was conducted on a cohort from China (8) and the other was from the United States (3). The smallest study included 309 participants and the largest cohort included 7017 participants (13). Four of the studies stratified results for men and women (3, 8, 13, 14); one study was conducted on a male cohort (15) and one study did not distinguish between men and women in their results (12). Follow-up ranged from a mean of 1.3 y (8) to 10 y (15). Consumption varied within a range of 30 mg/wk caffeine (1 cup of tea) to 550 mg/d caffeine. Two studies analyzed coffee and tea consumption separately (3, 8). One study investigated only coffee (15).
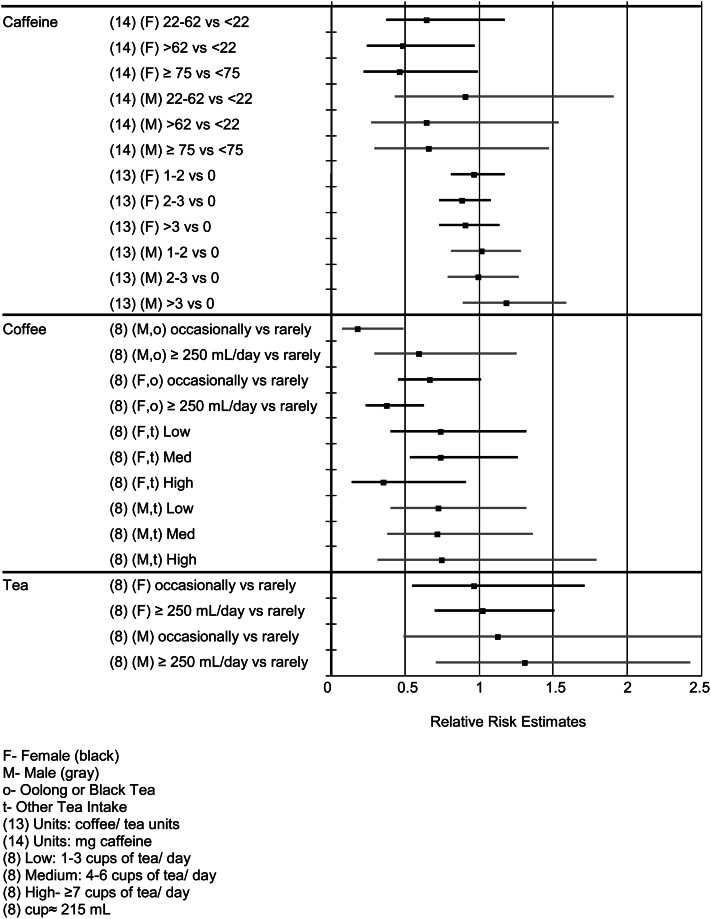
Three studies reported ORs with dose stratification (3, 8, 13) and are illustrated in Figure 2. The other studies reported R2 values (12) or relative changes in MMSE scores (15) or conducted Poisson regression to estimate the risk associated with caffeine consumption (14).
Figure 2.
Relative risks of cognitive decline as a function of caffeine, tea, or coffee in the studies that stratified by gender.
Age and education were controlled for in all studies. Five studies controlled for smoking, sex, and a number of health variables (i.e., physical exercise, diabetes, BMI, depression, cardiovascular disease, hypertension, history of stroke, etc.).
Tree diagrams of the RR results for those studies that conducted logistic regressions are presented in Figure 2. The study of Santos et al. (14) showed a significant reduction in cognitive decline for caffeine consumption; however, although the ORs for the study of Ritchie et al. (13) were <1.0, only a few reached significance and those were only in women. The study of Ng et al. (8) also showed a significant effect of tea in reducing cognitive decline only when high tea consumption was compared with no consumption in women. None of their coffee estimates were indicative of a protective effect (8).
Given these findings, a meta-analysis could not be conducted. There are not enough studies on a single exposure (tea or coffee or caffeine in total) and there was heterogeneity in both the exposure measures, (tea, coffee, caffeine) and the different outcomes uses (MSE, 3ME, and other indicators) that unfortunately make this impossible.
Discussion
Caffeine, present in coffee, tea, soft drinks, energy drinks, and chocolate, is known to enhance information processing speed, attention, and reaction time in humans (14). Cognitive effects of caffeine have been related to antagonism of A1 adenosine receptors in the hippocampus and cortex (28–30). A meta-analysis of caffeine and cognitive decline conducted by Santos et al. (14) reported a nonsignificant RR of 0.98 (0.87–1.11).
Across the 6 studies, we found modestly reduced rates of cognitive decline over median follow-up times ranging from 1.3 to 10 y. Reductions in cognitive decline were not linearly related to frequency of consumption. Caffeine consumption was also associated with slower cognitive decline among men and women in Portugal (14); however, no association could be made from another study with a reduction in cognitive decline in men and women in France (13).
Both of the cohorts studying tea suggest a reduction in cognitive decline among female tea drinkers (3, 8). The Singapore Longitudinal Ageing Studies cohort followed 1408 Chinese men and women drinking black and oolong tea briefly for 1–2 y (8). The Cardiovascular Health Study included 4809 Americans followed for almost 8 y (3).
Two longitudinal studies of cognitive decline and coffee consumption showed less decline among consumers but no dose response (3, 12). Another showed no relationship (8, 31). These findings reflect those seen in studies of coffee and dementia: isolated significant findings but without demonstrating a dose-response relationship (15, 32). In the U.S. study, an association was found between coffee drinking and somewhat attenuated rates of cognitive decline in women (3).
The limitations of the studies include differences in ranges of intakes that prohibit direct comparisons with doses of coffee, tea, and total caffeine intakes estimated across studies of cognitive decline. The low ceilings of intake allowed by some assessment tools result in conservative assessments of intake that are likely to underestimate the true population consumption and mask the effects of heavy daily consumption.
Another concern is that caffeine is also underestimated. The questionnaires did not allow differentiation of caffeinated sodas at baseline. Therefore, this source of caffeine was not added to the total weekly caffeine consumption and contributions from chocolate and other caffeinated sources were not included.
As with all dietary exposures, measurement error is inevitable. Individuals poorly remember their usual consumption of foods and beverages. This concern is in addition to the potential effect of unmeasured confounders and residual confounding that is inevitable in studies of this nature.
Another concern is that consumption behavior was considered only at baseline. Ideally, repeat measures of consumption would be used to characterize individuals. Baseline values are useful only if there is a relative stability in consumption over time among elder individuals.
Aside from the differences in exposure assessment, the handling of the outcome variables also differed tremendously across studies. The variety in modeling approaches and consideration of missing values and handling of item response bias was inconsistent and inadequately addressed in numerous studies.
Given these constraints, the relatively consistent effect for women at a very low frequency of consumption and the lack of a linear dose-response relationship was surprising. The lack of a linear dose-response relationship suggests that some other factor or factors associated with consumption and nonconsumption of these beverages may explain the associations found.
Conclusion
Tea or coffee consumption may somewhat attenuate the rate of cognitive decline in women as may caffeine consumption. The lack of a linear dose-response relationship remains a concern. Whether these findings reflect underlying measurement error in the exposure assessment, fluctuations in behavior, a true biological threshold, or basic differences between consumers and nonconsumers requires further investigation. The mounting evidence of a possible protective role of tea, coffee, or caffeine against neurodegenerative disorders seems to indicate there is a correlation with a reduction in cognitive decline. Hopefully, in the future, more sensitive outcomes such as findings from neuro-imaging studies in which participants were asked to drink coffee or tea and how this has affected their brain functional MRI activation will become available from experimental data.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: IRT, Item Response Theory; MMSE, Mini Mental State Exam; 3MS, Modified Mini Mental State.
Literature Cited
- 1.Riedel WJ, Jolles J. Cognition enhancers in age-related cognitive decline. Drugs Aging. 1996;8:245–74 [DOI] [PubMed] [Google Scholar]
- 2.Vila-Luna S, Cabrera-Isidoro S, Vila-Luna L, Juarez-Diaz I, Bata-Garcia JL, Alvarez-Cervera FJ, Zapata-Vazquez RE, Arankowsky-Sandoval G, Heredia-Lopez F, Flores G, et al. Chronic caffeine consumption prevents cognitive decline from young to middle age in rats, and is associated with increased length, branching, and spine density of basal dendrites in CA1 hippocampal neurons. Neuroscience. 2012;202:384–95 [DOI] [PubMed] [Google Scholar]
- 3.Arab L, Biggs ML, O'Meara ES, Longstreth WT, Crane PK, Fitzpatrick AL. Gender differences in tea, coffee, and cognitive decline in the elderly: the cardiovascular health study. J Alzheimers Dis. 2011;27:553–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumanyika SK, Tell GS, Shemanski L, Martel J, Chinchilli VM. Dietary assessment using a picture-sort approach. Am J Clin Nutr. 1997;65 Suppl 4:S1123–9 [DOI] [PubMed] [Google Scholar]
- 5.Tsushida T, Takeo T. Zinc, copper, lead and cadmium contents in green tea. J Sci Food Agric. 1977;28:255–8 [DOI] [PubMed] [Google Scholar]
- 6.Einöther SJ, Martens VE, Rycroft JA, De Bruin EA. L-theanine and caffeine improve task switching but not intersensory attention or subjective alertness. Appetite. 2010;54:406–9 [DOI] [PubMed] [Google Scholar]
- 7.Giesbrecht T, Rycroft JA, Rowson MJ, De Bruin EA. The combination of L-theanine and caffeine improves cognitive performance and increases subjective alertness. Nutr Neurosci. 2010;13:283–90 [DOI] [PubMed] [Google Scholar]
- 8.Ng TP, Feng L, Niti M, Kua EH, Yap KB. Tea consumption and cognitive impairment and decline in older Chinese adults. Am J Clin Nutr. 2008;88:224–31 [DOI] [PubMed] [Google Scholar]
- 9.Chin AV, Robinson DJ, O'Connell H, Hamilton F, Bruce I, Coen R, Walsh B, Coakley D, Molloy A, Scott J, et al. Vascular biomarkers of cognitive performance in a community-based elderly population: the Dublin Healthy Ageing study. Age Ageing. 2008;37:559–64 [DOI] [PubMed] [Google Scholar]
- 10.Nurk E, Refsum H, Drevon CA, Tell GS, Nygaard HA, Engedal K, Smith AD. Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J Nutr. 2009;139:120–7 [DOI] [PubMed] [Google Scholar]
- 11.Kuriyama S, Hozawa A, Ohmori K, Shimazu T, Matsui T, Ebihara S, Awata S, Nagatomi R, Arai H, Tsuji I. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am J Clin Nutr. 2006;83:355–61 [DOI] [PubMed] [Google Scholar]
- 12.van Boxtel MP, Schmitt JA, Bosma H, Jolles J. The effects of habitual caffeine use on cognitive change: a longitudinal perspective. Pharmacol Biochem Behav. 2003;75:921–7 [DOI] [PubMed] [Google Scholar]
- 13.Ritchie K, Carriere I, de Mendonca A, Portet F, Dartigues JF, Rouaud O, Barberger-Gateau P, Ancelin ML. The neuroprotective effects of caffeine: a prospective population study (the Three City Study). Neurology. 2007;69:536–45 [DOI] [PubMed] [Google Scholar]
- 14.Santos C, Lunet N, Azevedo A, de Mendonca A, Ritchie K, Barros H. Caffeine intake is associated with a lower risk of cognitive decline: a cohort study from Portugal. J Alzheimers Dis. 2010;20 Suppl 1:S175–85 [DOI] [PubMed] [Google Scholar]
- 15.van Gelder BM, Buijsse B, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Coffee consumption is inversely associated with cognitive decline in elderly European men: the FINE Study. Eur J Clin Nutr. 2007;61:226–32 [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76 [DOI] [PubMed] [Google Scholar]
- 17.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8 [PubMed] [Google Scholar]
- 18.Smith AK, Simon JS, Gustafson EL, Noviello S, Cubells JF, Epstein MP, Devlin DJ, Qiu P, Albrecht JK, Brass CA, et al. Association of a polymorphism in the indoleamine- 2,3-dioxygenase gene and interferon-alpha-induced depression in patients with chronic hepatitis C. Mol Psychiatry. 2012. 17:781–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyas SL, Manfreda J, Strain LA, Montgomery PR. Risk factors for Alzheimer's disease: a population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol. 2001;30:590–7 [DOI] [PubMed] [Google Scholar]
- 20.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–53 [DOI] [PubMed] [Google Scholar]
- 21.Crane PK, Narasimhalu K, Gibbons LE, Mungas DM, Haneuse S, Larson EB, Kuller L, Hall K, van Belle G. doi: 10.1016/j.jclinepi.2007.11.011. Item response theory facilitated cocalibrating cognitive tests and reduced bias in estimated rates of decline. J Clin Epidemiol. 2008;61:1018–27 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crane PK, Gruhl JC, Erosheva EA, Gibbons LE, McCurry SM, Rhoads K, Nguyen V, Arani K, Masaki K, White L. Use of spoken and written Japanese did not protect Japanese-American men from cognitive decline in late life. J Gerontol B Psychol Sci Soc Sci. 2010;65:654–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samejima F. Estimation of latent ability using a response pattern of graded scores. Psychometrika Monograph. 1969;17.
- 25.Samejima F. Graded response model. In: van der Linden WJ, Hambleton RK, editors. Handbook of modern item response theory. New York: Springer; 1997. p. 85–100. [Google Scholar]
- 26.Muraki E, Bock D. PARSCALE for Windows. Chicago: Scientific Software International; 2003. [Google Scholar]
- 27.Arnold AM, Newman AB, Dermond N, Haan M, Fitzpatrick A. Using telephone and informant assessments to estimate missing Modified Mini-Mental State Exam scores and rates of cognitive decline. The Cardiovascular Health Study. Neuroepidemiology. 2009;33:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costenla AR, Cunha RA, de Mendonca A. Caffeine, adenosine receptors, and synaptic plasticity. J Alzheimers Dis. 2010;20 Suppl 1:S25–34 [DOI] [PubMed] [Google Scholar]
- 29.Prediger RD, Batista LC, Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging. 2005;26:957–64 [DOI] [PubMed] [Google Scholar]
- 30.Rahman A. The role of adenosine in Alzheimer's disease. Curr Neuropharmacol. 2009;7:207–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laitala VS, Kaprio J, Koskenvuo M, Raiha I, Rinne JO, Silventoinen K. Coffee drinking in middle age is not associated with cognitive performance in old age. Am J Clin Nutr. 2009;90:640–6 [DOI] [PubMed] [Google Scholar]
- 32.Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16:85–91 [DOI] [PubMed] [Google Scholar]