Abstract
Adipose tissue can be regarded as a multidepot organ responsible for metabolic homeostasis by managing sophisticated energy transactions as well as by producing bioactive molecules that regulate insulin sensitivity and immune and vascular responses. Chronic nutrient excess expands adipose tissue, and concomitant variations in its cellular and matrix remodeling can affect the extent of the metabolic dysfunction that is associated with obesity. Preadipocytes, also termed adipose progenitor cells, play a pivotal role in determining whether a dysfunctional hypertrophic state arises as opposed to a hyperplastic process in which mature adipocytes remain relatively responsive. Obesity is associated with infiltration of macrophages, and these immune cells have been shown to communicate with preadipocytes to influence how they differentiate, survive, and proliferate. Understanding macrophage–preadipocyte interactions and their effect on adipose remodeling mechanisms may identify potential therapeutic molecular targets to improve adipose tissue function, even in the face of obesity.
Introduction
White adipose tissue is a complex, multidepot organ whose function is vital for metabolic and vascular health. It serves as a sophisticated bank for energy currency, building up reserves from incoming postprandial calories in the form of triacylglycerol and releasing supplies when needed during fasting or exercise as fatty acids. In addition, bioactive molecules produced and secreted by adipose tissue influence diverse physiological parameters including appetite, energy expenditure, insulin sensitivity, vascular function, immunity, and coagulation (1).
Obesity, that is, the excessive accumulation of adipose tissue, is associated with poor health outcomes due to several metabolic and cardiovascular complications, such as type 2 diabetes and myocardial infarction. A proinflammatory process in adipose tissue causing insulin resistance is thought to underlie many of these obesity-associated disorders. This dysfunctional state may be intricately tied to defective cellular turnover and remodeling of adipose tissue, including infiltration of macrophages, during nutrient excess (2).
Adipose tissue is composed of distinct cell types in addition to adipocytes. Adipose progenitor cells are thought to reside within the adipose tissue stromal compartment. Those cells, which are farther along the continuum of commitment to the adipocyte lineage, are often called preadipocytes, whereas labels such as adipose progenitor or stem cells are often used to indicate less committed cells that exhibit some degree of multipotency toward other mesodermal fates, but there is some overlap in this usage in the literature (3).
The abundance of preadipocytes and their adipogenic capacity are important variables that influence the architecture and operational status of expanding adipose tissue in the obese. This review focuses on white adipose tissue preadipocytes and, in particular, on their novel interactions with macrophages as related to adipose tissue form and function in obesity.
Current state of knowledge
Adipose cellularity: hypertrophy versus hyperplasia
Owing to technological advances in cell biology studies in the 1960s, attention was drawn to the cellular nature of adipose tissue, and the concepts of adipose tissue hyperplasia (increased adipocyte number) versus hypertrophy (increased adipocyte size) in obesity were introduced (4). This paradigm has been invigorated by recent studies clearly documenting adipocyte turnover in humans, that is, adipocyte loss counterbalanced by adipocyte formation through the differentiation of preadipocytes. Depending on variables such as the methodology used and the metabolic profile of the population, adipocyte turnover rates in humans have been calculated to be 10%/y, using carbon 14 dating of adipocyte genomic DNA (5), to as high as 58–105%/y based on 2H2O labeling of newly synthesized adipocyte DNA (6). The higher rate was thought to be influenced to a minor degree by the inadvertent minor presence of stromal vascular cells in the sample, suggesting that preadipocytes turn over more rapidly than adipocytes. Adipocyte number can increase in nonobese humans in response to overfeeding for ∼8 wk, depending on the adipose depot (7).
Human experimental data are emerging that support the concept of a preadipocyte deficit. Adipocyte formation rates are decreased in subjects with adipose tissue hypertrophy (8). There are fewer adipose progenitor cells, based on CD90 positivity, in adipose tissue obtained from obese versus lean subjects (9). Using a different strategy to identify preadipocytes, another group also reported a reduction in preadipocyte number in obesity (10). A diminished ability of subcutaneous preadipocytes to differentiate has been linked to central obesity (11). Reduced adipogenic gene expression in adipose tissue of obese adolescents with versus without insulin resistance has been documented (12). A relative waning of adipogenic reserve also may occur with age (13).
Therefore, it is pertinent to consider how adipose tissue expands to store energy in the setting of a long-term positive caloric imbalance. One current model proposes that hyperplastic growth, generating healthy functional smaller adipocytes derived from responsive and abundant preadipocytes, will boost overall storage capacity while maintaining healthy adipose tissue function. This concept is consistent with the metabolically healthy, or insulin-sensitive, obese phenotype (14). If adipogenic capacity is constrained, either due to an inadequate number of preadipocytes or attenuated differentiation capacity, hypertrophic growth of existing adipocytes will dominate, yielding enlarged swollen adipocytes predisposed to inflammation and insulin resistance (4, 15, 16). These dysfunctional adipocytes will release chemokines that attract circulating monocytes to infiltrate adipose tissue where they become macrophages, further contributing to the production of pro-inflammatory cytokines with deleterious paracrine and endocrine effects (1, 2, 17). Other immune cells (neutrophils, eosinophils, mast cells, T cells, B cells) also move to, or reside within, adipose tissue, but are beyond the scope of this review.
Hypertrophic growth of adipose tissue is also less effective in providing adequate storage capacity for the excess energy. As a consequence, fatty acids are redirected to the liver, skeletal muscle, and other organs. The accumulation of ectopic fat and its byproducts disrupts cellular functions in those locations and contributes to insulin resistance (16).
Given the influential role of preadipocytes in determining how adipose tissue remodels during adipose tissue accumulation including the accompanying macrophage immigration, it is timely to review recent advances in preadipocyte biology and to consider how macrophages may alter preadipocyte responses.
Origin of preadipocytes
Adipose tissue is composed of mature adipocytes (∼50% of cells), as well as stromal cells made up of adipose progenitor cells, preadipocytes, fibroblasts, immune cells, endothelial cells, and smooth muscle cells (18, 19). It has been a challenge to precisely define the preadipocyte cellular component, but recent studies have taken exciting steps toward distinguishing the molecular characteristics of preadipocytes in vivo.
Using mice, one group performed lineage analysis to identify proliferating adipose progenitors that exhibited early commitment to the adipose lineage. These cells, located in the mural cell compartment of adipose tissue vasculature, express PPARγ and platelet-derived growth factor (PDGF) receptor β, as well as Sca1 and CD34; they are also negative for the immune/endothelial markers CD45, CD105, TER119, and Mac-1 (20). Another group isolated 2 populations of adipocyte progenitors from mouse adipose tissue stroma (depleted of endothelial and hematopoietic cells, i.e., CD31−, CD45−, and Ter119−) on the basis of stem cell surface markers: lin−:CD29+:CD34+:Sca-1+ and either CD24+ or CD24− (21). They accounted for 0.08% or 53.5% of the stromal cells, respectively. Although both cell types could form adipocytes in vitro, only CD24+ cells developed into adipocytes when placed in a lipodystrophic mouse model.
In humans, characterization of the adipose progenitor cells has been more difficult and inconsistent. Studies have identified a population of human adipose tissue stromal cells (CD34+: CD31−) that display adipogenic capacity (22, 23). Another group described human adipose tissue progenitors that were CD73+:CD90+:CD105+:CD16−:CD34−:CD45− (24). The reasons for these variations are still unclear. A comprehensive review of the pattern of these markers in adipose cells has been published (3).
These mouse and human studies are consistent with the view of adipocyte progenitors as residing within adipose tissue stroma. However, some still consider the possibility that these cells may be derived from circulating bone marrow progenitor cells that home to adipose tissue (25).
Preadipocyte cell culture models
Cell culture models of preadipocytes have facilitated cellular and molecular investigations of proliferation, differentiation, and survival. They were developed over a long time span, from both mouse and human tissues, and each has their own strengths and weaknesses. A recent review provides a comparative overview of these systems (26).
C3H10T1/2 cells were derived from mouse embryos and are characterized by their multipotency with respect to being able to differentiate into myocytes, osteocytes, chondrocytes, and adipocytes. Bone morphogenic protein 2 and 4 activate intracellular signaling pathways in these cells that commit them to the adipose lineage in vitro and when implanted subcutaneously into athymic mice (27, 28).
3T3-F442A and 3T3-L1 are committed preadipocytes, and, like C3H10T1/2 cells, were derived from mouse embryos: 3T3-F442A preadipocytes differentiate in culture and form fat pads when implanted in athymic mice (29, 30). 3T3-L1 preadipocytes are believed to be less mature than their F442A counterparts. The molecular basis for this is their elevated expression of Wnt-10b (31). Downregulation of Wnt-10b or blockade of its action is required to permit them to differentiate. This occurs in response to adipogenic inducers in vitro, or after in vivo implantation into athymic mice, with the use of dominant-negative (e.g., TC4 mutant) genetic strategies to block Wnt action (31).
The 3T3-L1 and 3T3-F442A mouse models have been very useful for understanding the importance and regulation of the early proliferative phase required for activation of adipogenic genes called mitotic clonal expansion (MCE)4. MCE moves preadipocytes from confluence-induced cell cycle arrest through 1–2 rounds of limited cell division, followed by an exit from the cell cycle and induction of the differentiation program (32).
Primary diploid stromal preadipocytes are isolated from adipose tissue, most commonly through collagenase digestion and size filtration/centrifugation, and placed into culture (33). Depending on conditions, the presence of immune cells and/or endothelial cells can be a concern if not monitored. Sorting of the stromal cells according to the surface markers described above may improve the homogeneity of the population, but as discussed above, a standardized sorting protocol is not yet in sight for human preadipocytes (3).
Unlike mouse preadipocyte cell line models, human stromal preadipocytes do not undergo MCE in culture (34). The precise explanation for this is not known. It has been speculated that these cells represent a more advanced stage of maturation, having already proceeded through MCE in vivo. Human preadipocytes lose their capacity for differentiation after several cell passages, and the required access to ongoing fresh supplies of these cells is a limitation of this valuable experimental human cell model.
A human preadipocyte cell line free of tumor origin has been described. Wabitsch et al. (35) established a new strain of stromal cells from adipose tissue of an infant with Simpson-Golabi-Behmel syndrome (SGBS) and found that the cells could proliferate markedly without losing their adipogenic capacity. However, the precise genetic abnormality in these cells has not been identified.
Preadipocytes and differentiation
With the use of these experimental models, mechanistic insights into the differentiation process ensued. Many of the in vitro molecular findings have been confirmed with in vivo evidence. There is a structured sequence of gene regulation that comprises the adipogenic program. The identity and interconnection between a variety of adipogenic transcription factors has been reviewed and includes CCAAT/enhancer binding protein family members and PPARγ (36–38). Acting in concert, they are responsible for the generation of the lipid synthesizing/storage machinery that forms the mature adipocyte and allows for the operational coupling of insulin signal transduction to metabolic responses such as glucose transport.
The early phase of differentiation requires modifications to the extracellular matrix (ECM) and cytoskeleton (2, 39). In general, there are a downregulation of fibrillar collagens and fibronectin and an increase in laminar proteins. In addition to changes in expression of the matrix proteins, overall remodeling includes a role for ECM degradation enzymes such as tissue inhibitors of metalloproteinases and matrix metalloproteinases (MMP). If these ECM changes are perturbed, adipose tissue development and function can be perturbed. For example, loss of MT1-MMP (MMP14) inhibits adipogenesis in mice (40). Pathological fibrosis in adipose tissue leading to dysfunction also occurs in the absence of fibroblast growth factor 1 (41). On the other hand, removal of collagen VI, which is increased in adipose tissue in obesity, results in healthier adipocytes (42). As discussed in the following, altered ECM is a component of adipose tissue inflammation and macrophage infiltration.
Cell surface signal transduction by insulin-like growth factor (IGF)-1 or insulin is required for adipogenesis. Important roles for the signaling network downstream of these factors have been reported, including insulin receptor substrate, phosphoinositide 3-kinase (PI3K), Akt, mammalian target of rapamycin, S6 kinase 1, eukaryotic initiation factor 4 binding protein 1, and Forkhead box O (43–49). In particular, a mutated Akt2 was identified in a human kindred with diabetes and lipodystrophy (50).
Preadipocytes and survival
In addition to studies on the adipogenic fate of preadipocytes, investigations concerning their survival responses have also been of interest. Survival capacity may be relevant with respect to the factors governing the size of the preadipocyte pool in vivo. The 3T3-L1 preadipocyte model is very susceptible to apoptosis induced by growth factor withdrawal (serum deprivation). Apoptotic resistance develops as they differentiate, and this correlates with the induction of anti-apoptotic proteins, such as neuronal apoptosis inhibitor protein and Bcl-2 (51–53). Insulin/IGF-1 survival signaling molecules have been delineated in the 3T3-L1 preadipocyte model. There is a requirement for PI3K that can be overcome by exogenous addition of its lipid product, PI(3,4,5)P3 (54). The MEK-ERK1/2 pathway may also operate as part of the 3T3-L1 preadipocyte survival response (55).
Studies on human primary stromal preadipocytes show lower levels of apoptosis than 3T3-L1 preadipocytes in response to serum withdrawal (56–58). A depot-dependent property was observed, with a higher degree of apoptotic sensitivity to serum withdrawal in omental versus subcutaneous abdominal human preadipocytes (57, 59). This anatomic depot-related pattern is consistent with observations of regional differences in mouse and human preadipocyte differentiation responses (60, 61).
Serum deprivation alone is not adequate to induce considerable human preadipocyte apoptosis. A more effective trigger of human preadipocyte death consists of serum deprivation in association with cycloheximide and with death ligands such as CD95 and TNF-α (62, 63). TNF-α can induce cell death by activating caspase-8 or promote cell survival by activating nuclear factor-κB, whereas cycloheximide reduces levels of cellular FLICE-like inhibitory protein, a negative regulator of apoptosis that acts as an endogenous brake on caspase 8 activation (58, 64).
Little has been reported on human preadipocyte survival signaling. A recent report based on SGBS preadipocytes implicates Akt2 in this response (46). The reduced sensitivity of human preadipocytes to apoptosis has been suggested to result from autocrine activation of prosurvival IGF-1 receptor signaling (63).
Preadipocytes and proliferation
The size of the preadipocyte pool within adipose tissue is not only influenced by survival, but also by proliferation. Subconfluent preadipocyte proliferation is distinct from MCE and its specialized pattern of cell cycle regulation (65). Proliferation of preadipocytes is stimulated by a variety of molecules, including growth factors such as PDGF, ligands of α2-adrenergic receptors, lysophosphatidic acid, thyroid-stimulating hormone, and endothelial cell factors (66–69). Regional anatomic variation in mouse and human preadipocyte proliferation rates has been observed, as well as differences linked to lean versus obese states (70, 71).
Investigations of antiproliferative conditions have also been considered. These include cytokines, such as IL-4, that inhibit PDGF-stimulated 3T3-L1 preadipocyte proliferation, a 5-phosphoinositide lipid phosphatase mutant that reduces 3T3-L1 proliferation in response to PDGF, certain prostaglandins, and reactive oxygen species (ROS) (72–75).
Preadipocyte–macrophage interactions
Lean adipose tissue contains ∼10% resident macrophages existing in a noninflamed M2-type state (1). Obesity is accompanied by the infiltration of circulating blood monocytes into adipose tissue, producing an abundant population of inflamed M1-type macrophages, making up as much as 50–60% of adipose tissue stromal cells. This process of macrophage infiltration is associated with adipocyte hypertrophy (76). Therefore, macrophages might influence preadipocytes by altering their differentiation, survival, and/or proliferation responses.
Adipogenesis
Constant et al. (77) reported that medium conditioned by mouse or human macrophage lines [macrophage-conditioned medium (MacCM)] inhibited adipogenesis of mouse 3T3-L1 and human preadipocytes. These observations were extended by Lacasa et al. (78), who demonstrated the antiadipogenic effect of secreted factors from bacterial LPS-activated human monocyte–derived and adipose tissue–derived macrophages. A subsequent series of studies by these groups and others, described in the following, continues to add more information to this intercellular dialogue. Several macrophage-secreted factors have been proposed to contribute to the antiadipogenic activity, including TNF-α, IL-1β, and Wnt-5a.
Immunoneutralization of TNF-α from LPS-activated human MacCM resulted in a statistically significant but numerically very small attenuation of the antiadipogenic effect, as assessed by the adipogenic marker aP2 (78). A larger effect of that immunoneutralization was the reduction in preadipocyte inflammation, as assessed by IL-6 levels. This suggests a potential uncoupling of the antiadipogenic from the proinflammatory effect induced by the TNF-α in the MacCM on the preadipocytes.
In other work, the antiadipogenic effect of mouse adipose tissue macrophages was attenuated by growth hormone, and this correlated with the amount of IL-1β that they produce (79). However, these investigators did not directly address experimentally whether IL-1β was required for the antiadipogenic effect.
Wnt-5a was found to be expressed in human adipose tissue macrophages and in circulating human monocytes (80). Evidence of release from macrophages, i.e., the presence of Wnt-5a protein in MacCM, was demonstrated using mouse J774 macrophages. Immunoneutralization of Wnt-5a in the J774-MacCM reversed the antiadipogenic effect on mouse 3T3-L1 preadipocytes. Whether this intercellular communication operates within human models remains to be seen.
Another strategy to understand the paracrine effect of macrophages on preadipocytes has been to delineate the signaling networks required for the antiadipogenic action of MacCM. Human THP-1 monocytes inhibit 3T3-L1 adipocyte differentiation and acutely stimulate ERK1/2 phosphorylation. Pharmacological inhibition of the MEK-ERK1/2 pathway with PD98059 partially blocked the antiadipogenic effect of THP-1-MacCM (81). In the context of human THP-1-MacCM and human primary adipose progenitor cells, the antiadipogenic effect was associated with IKKβ phosphorylation and activation of NF-κB (82). Activation of NF-κB in differentiating human preadipocytes exposed to conditioned medium generated from LPS-activated human monocyte–derived macrophages was also observed, as well as in SGBS preadipocytes when cocultured with the U937 macrophage cell model (78, 83). Underlining the importance of this pathway, the IKKβ inhibitor sc-514 almost totally abrogated the ability of THP-1-MacCM to inhibit human adipocyte differentiation (82).
To examine the role of cell cycle signaling and the MCE in the context of MacCM and adipogenesis, mouse 3T3-L1 preadipocytes and mouse J774A.1 macrophages were studied. The effective time window for the antiadipogenic effect was early in the differentiation protocol, consistent with a perturbation of the MCE (84). During this time, retinoblastoma protein (Rb) phosphorylation is required for dissociation of E2F from Rb, allowing E2F to participate in the induction of PPARγ. J774A.1-MacCM inhibited Rb phosphorylation and PPARγ induction (84). It also blocked an upstream kinase of Rb, cyclin-dependent kinase 2, as well as the induction of cyclin A, which normally stimulates cyclin-dependent kinase 2 activity (85). In this experimental system, the antiadipogenic activity was located in a ≤3-kD fraction and was heat resistant. This is in contrast to a heat-labile antiadipogenic activity found in human macrophages that was associated with increased cyclin D1 and proliferation (78). As investigations continue in the future to further delineate factors that inhibit adipocyte differentiation, careful attention should be paid to catalogue the specific experimental models used to develop a comprehensive view of these interactions.
Concomitant with their inhibitory effect on adipogenesis, macrophage-secreted factors also exert a profibrotic action on human preadipocytes (86, 87). At present, it remains unknown whether the resulting higher levels of extracellular matrix proteins, known downstream targets of the ERK and NF-κB pathway, are causally linked to the blockade of differentiation induced by MacCM. Other studies directly manipulating ECM have shown that this can be sufficient to block adipogenesis (39, 88).
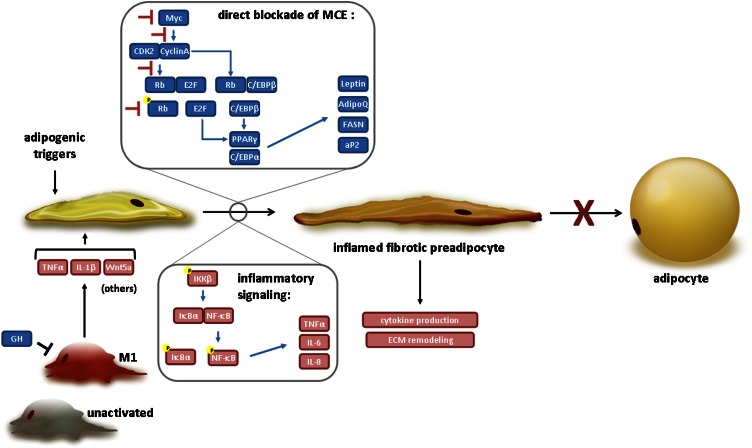
Figure 1 summarizes the main findings discussed previously with respect to how MacCM can inhibit adipogenic preadipocyte responses. Potential macrophage-secreted factors and their target pathways are illustrated. Macrophage-adipocyte interactions are beyond the focus of this review, but it should be mentioned that investigators are actively examining the influence of macrophage factors on adipocyte inflammation and insulin resistance. Furthermore, release of fatty acids from adipocytes triggers macrophage inflammation in a coculture system (89).
Figure 1.
Mechanisms by which macrophages block adipogenesis. Macrophage-derived factors can inhibit the adipogenic program by interfering with cell cycle proteins [Myc, cyclin A, cyclin-dependent kinase (CDK) 2, retinoblastoma protein (Rb)] that regulate mitotic clonal expansion (MCE) and expression of key adipogenic transcriptional factors (CCAAT/enhancer binding proteins, and peroxisome-proliferator activated receptor γ). They may also activate nuclear factor κB and the expression of inflammatory antiadipogenic cytokines (TNF-α, IL-6, and IL-8). The result is an inflamed and profibrotic preadipocyte phenotype. See text for details.
Survival
As noted earlier, 3T3-L1 preadipocytes undergo apoptosis when deprived of growth factors (serum withdrawal). Molgat et al. (90) observed a survival effect of MacCM on serum-deprived 3T3-L1 mouse preadipocytes. This was demonstrated with several mouse macrophage models: J774A.1, RAW264.7, and mouse bone marrow–derived macrophages. Cell death and the protective effect of MacCM were assessed by enumeration of viable cells, by nuclear staining, and by annexin V staining with flow cytometry.
Further molecular studies with the J774A.1 model identified PDGF as a key component accounting for much of the preadipocyte survival activity (90). Signaling studies indicated that macrophage-secreted factors activate Akt and ERK1/2 as well as generate ROS. Inhibitor studies demonstrated that the survival activity induced by macrophage-conditioned medium depends in part on the PI3K-Akt pathway, the MEK-ERK1/2 pathway, and ROS generation (91).
Questions remain about the origin and action of ROS in this context. For example, serum deprivation itself also increased ROS levels with associated cell death. Furthermore, reduction of ROS led to improved survival in the serum-free conditions, pointing to ROS as damaging agents. This contrasts with the prosurvival effect of ROS associated with MacCM. It is possible that ROS generated by growth factor withdrawal are of mitochondrial origin and detrimental to cell survival, whereas ROS related to MacCM and survival are produced at the cell surface by NAD(P)H oxidase associated with growth factor receptors such as PDGF receptors (91). More work is needed in this area.
The state of macrophage activation and its effect on preadipocyte survival has been investigated (92). LPS stimulation of J774A.1 macrophages inhibits the ability of MacCM to suppress apoptosis of 3T3-L1 preadipocytes. It does not alter MacCM-induced preadipocyte PDGF signaling, but does induce macrophage TNF-α expression. The importance of the TNF-α with regard to the reduction in the prosurvival activity of the MacCM was demonstrated by the observation that immunoneutralization of TNF-α in the MacCM restored the full prosurvival activity.
Studies on apoptosis using human macrophage models and human preadipocyte models have also been reported (92). As in the mouse models, secreted factors from THP-1 human macrophages or from human monocyte–derived macrophages inhibit human preadipocyte apoptosis triggered by TNF-α and cycloheximide in serum-free medium. This prosurvival effect was also lost when macrophages were activated by proinflammatory LPS, whereas it was preserved with anti-inflammatory IL-4 treatment of the macrophages. The identity of the factors from human macrophages mediating human preadipocyte survival is not yet known.
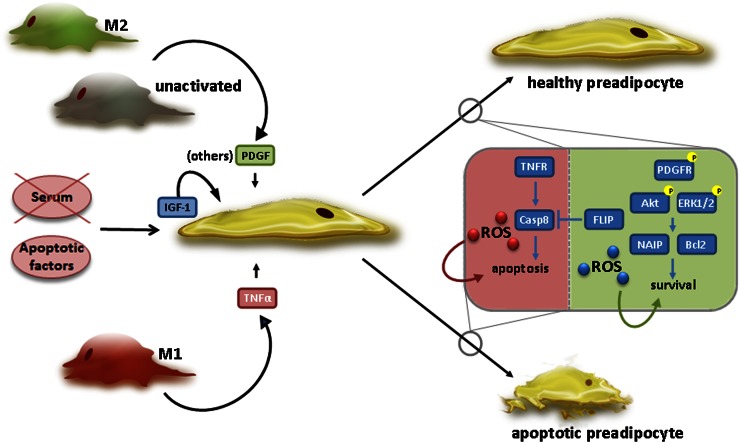
Figure 2 summarizes the main findings discussed previously with respect to macrophages and their influence on preadipocyte survival. It depicts the potential factors that are secreted from macrophages and shows how they alter pathways in the preadipocyte that may lead to survival or death.
Figure 2.
Mechanisms by which macrophages regulate preadipocyte survival. In the presence of apoptotic inducers, either intrinsic, e.g., serum (growth factor) withdrawal, or extrinsic, e.g., TNF-α, anti-inflammatory M2 or unactivated macrophages promote preadipocyte survival by releasing platelet-derived growth factor (PDGF) (and other factors). Prosurvival intracellular pathways are then activated, involving Akt and ERK1/2, as well as reactive oxygen species (ROS) production, likely from NAD(P)H oxidases. This may result in the increased expression of antiapoptotic proteins, such as neuronal apoptosis inhibitor protein (NAIP) and Bcl-2. In contrast, proinflammatory M1 macrophages release TNF-α (and other factors) that cause preadipocyte death, possibly involving caspase 8 (Casp8) and/or mitochondria-derived ROS. TNFR, TNF receptor. See text for details.
Proliferation
Maumus et al. (93) reported that MacCM reduced adipose progenitor proliferation in culture, but evidence of increased proliferation in situ as a function of obesity was shown in human adipose tissue. The higher rates of in situ proliferation are consistent with observations in obese versus lean mice (94). Others also report a proliferative effect of macrophages on preadipocytes (86, 95).
Conclusions
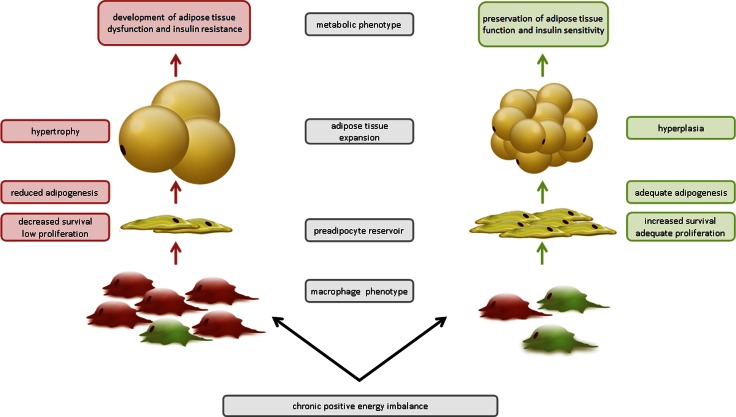
The developing concept of metabolic inflammation associated with obesity and insulin resistance views adipose tissue as a seething world of interconnected cell-specific subpopulations subjected to the pressure of nutrient excess. The effects of obesity on the overall extent of adipose tissue inflammation can be modulated by nutritional factors such as the type of dietary fat consumed, as has been reviewed (96). However, it is not yet known whether dietary factors can directly alter how macrophages interact with preadipocytes. Dynamic remodeling of the tissue architecture occurs with its expansion, as shown in Figure 3. The number and functionality of preadipocytes are crucial factors that influence how superfluous energy is to be stored and distributed. Adequate numbers of preadipocytes that are differentiation competent allow for hyperplastic growth with the effect being to preserve metabolic function in the face of obesity, depicted on the right side of Figure 3. Interactions between immune cells and adipose progenitor cells are important to consider because they may influence the number of preadipocytes and/or their differentiation capacity and induce adipose tissue dysfunction by inhibiting overall adipogenic capacity, depicted on the left side of Figure 3. If such intercellular communication proves to be meaningful in vivo, macrophage molecules acting on preadipocytes could be considered potential future targets for restoring healthy adipose tissue function in obese individuals, distinct from weight-reduction strategies.
Figure 3.
Model of how macrophages regulate adipose tissue remodeling during chronic positive energy imbalance. Chronic positive energy imbalance leads to obesity, and macrophage phenotype may influence the mechanism by which adipose tissue expands. When proinflammatory M1 macrophages (red) dominate, an inadequate preadipocyte reservoir may exist due to reduced preadipocyte survival, proliferation, and/or adipogenic capacity. Energy storage will occur via exaggerated adipocyte hypertrophy, resulting in dysfunctional adipose tissue and contributing to an inflamed, insulin-resistant state. In contrast, when anti-inflammatory M2 macrophages are dominant (green), this will favor a functional pool of preadipocytes capable of differentiating into new adipocytes will allow for adequate adipose tissue hyperplasia, with the maintenance of normal cellular functionality and insulin sensitivity. See text for details.
Acknowledgments
All authors have read and approved the final manuscript.
Footnotes
Abbreviations used: ECM, extracellular matrix; IGF, insulin-like growth factor; MacCM, macrophage-conditioned medium; MCE, mitotic clonal expansion; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; PI3K, phosphoinositide 3-kinase; Rb, retinoblastoma protein; ROS, reactive oxygen species; SGBS, Simpson-Golabi-Behmel syndrome.
Literature Cited
- 1.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–74 [DOI] [PubMed] [Google Scholar]
- 2.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch J, Fried SK, Edens NK, Leibel RL. The fat cell. Med Clin North Am. 1989;73:83–96 [DOI] [PubMed] [Google Scholar]
- 5.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–7 [DOI] [PubMed] [Google Scholar]
- 6.Strawford A, Antelo F, Christiansen M, Hellerstein MK. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am J Physiol Endocrinol Metab. 2004;286:E577–88 [DOI] [PubMed] [Google Scholar]
- 7.Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107:18226–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arner E, Westermark PO, Spalding KL, Britton T, Rydén M, Frisén J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oñate B, Vilahur G, Ferrer-Lorente R, Ybarra J, Díez-Caballero A, Ballesta-López C, Moscatiello F, Herrero J, Badimon L. The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB J. 2012;26:4327–36 [DOI] [PubMed] [Google Scholar]
- 10.Tchoukalova Y, Koutsari C, Jensen M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia. 2007;50:151–7 [DOI] [PubMed] [Google Scholar]
- 11.Permana PA, Nair S, Lee Y-H, Luczy-Bachman G, Vozarova de Courten B, Tataranni PA. Subcutaneous abdominal preadipocyte differentiation in vitro inversely correlates with central obesity. Am J Physiol Endocrinol Metab. 2004;286:E958–62 [DOI] [PubMed] [Google Scholar]
- 12.Kursawe R, Eszlinger M, Narayan D, Liu T, Bazuine M, Cali AM, D'Adamo E, Shaw M, Pierpont B, Shulman GI, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes. 2010;59:2288–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. 2011;57:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samocha-Bonet D, Chisholm DJ, Tonks K, Campbell LV, Greenfield JR. Insulin-sensitive obesity in humans - a 'favorable fat’ phenotype? Trends Endocrinol Metab. 2012;23:116–24 [DOI] [PubMed] [Google Scholar]
- 15.Danforth E., Jr Failure of adipocyte differentiation causes type 2 diabetes mellitus? Nat Genet. 2000;26:13. [DOI] [PubMed] [Google Scholar]
- 16.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type 2 diabetes mellitus. Int J Obes. 2004;28:S12–21 [DOI] [PubMed] [Google Scholar]
- 17.Dalmas E, Clément K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32:307–14 [DOI] [PubMed] [Google Scholar]
- 18.Van Harmelen V, Skurk T, Hauner H. Primary culture and differentiation of human adipocyte precursor cells. Methods Mol Med. 2005;107:125–35 [DOI] [PubMed] [Google Scholar]
- 19.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–56 [DOI] [PubMed] [Google Scholar]
- 20.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–9 [DOI] [PubMed] [Google Scholar]
- 22.Maumus M, Peyrafitte JA, D'Angelo R, Fournier-Wirth C, Bouloumié A, Casteilla L, Sengenès C, Bourin P. Native human adipose stromal cells: localization, morphology and phenotype. Int J Obes (Lond). 2011;35:1141–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Zimmerlin L, Marra KG, Donnenberg VS, Donnenberg AD, Rubin JP. Adipogenic potential of adipose stem cell subpopulations. Plast Reconstr Surg. 2011;128:663–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaiba S, França LP, França JP, Ferreira LM. Characterization of human adipose-derived stem cells. Acta Cir Bras. 2012;27:471–6 [DOI] [PubMed] [Google Scholar]
- 25.Majka SM, Barak Y, Klemm DJ. Concise review: adipocyte origins: weighing the possibilities. Stem Cells. 2011;29:1034–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafontan M. Historical perspectives in fat cell biology: the fat cell as a model for the investigation of hormonal and metabolic pathways. Am J Physiol Cell Physiol. 2012;302:C327–59 [DOI] [PubMed] [Google Scholar]
- 27.Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2004;101:9607–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2009;106:12670–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–33 [DOI] [PubMed] [Google Scholar]
- 30.Mandrup S, Loftus TM, MacDougald OA, Kuhajda FP, Lane MD. Obese gene expression at in vitro levels by fat pads derived from s.c. implanted 3T3–F442A preadipocytes. Proc Natl Acad Sci U S A. 1997;94:4300–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–3 [DOI] [PubMed] [Google Scholar]
- 32.Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci U S A. 2003;100:44–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skurk T, Hauner H. Primary culture of human adipocyte precursor cells: expansion and differentiation. Methods Mol Biol. 2012;806:215–26 [DOI] [PubMed] [Google Scholar]
- 34.Entenmann G, Hauner H. Relationship between replication and differentiation in cultured human adipocyte precursor cells. Am J Physiol. 1996;270:C1011–6 [DOI] [PubMed] [Google Scholar]
- 35.Wabitsch M, Brenner RE, Melzner I, Braun M, Möller P, Heinze E, Debatin KM, Hauner H. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int J Obes Relat Metab Disord. 2001;25:8–15 [DOI] [PubMed] [Google Scholar]
- 36.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Molec Biol. 2011;12:722–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smas CM, Sul HS. Control of adipocyte differentiation. Biochem J. 1995;309:697–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–91 [DOI] [PubMed] [Google Scholar]
- 41.Jonker JW, Suh JM, Atkins AR, Ahmadian M, Li P, Whyte J, He M, Juguilon H, Yin YQ, Phillips CT, et al. PPARγ-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laustsen PG, Michael MD, Crute BE, Cohen SE, Ueki K, Kulkarni RN, Keller SR, Lienhard GE, Kahn CR. Lipoatrophic diabetes in Irs1(−/−)/Irs3(−/−) double knockout mice. Genes Dev. 2002;16:3213–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magun R, Burgering BMT, Coffer PJ, Pardasni D, Lin Y, Chabot J, Sorisky A. Expression of a constitutively activated form of protein kinase B (c-Akt) in 3T3–L1 preadipose cells causes spontaneous differentiation. Endocrinology. 1996;137:3590–3 [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Liao K. Protein kinase B/AKT 1 plays a pivotal role in insulin-like growth factor-1 receptor signaling induced 3T3–L1 adipocyte differentiation. J Biol Chem. 2004;279:35914–22 [DOI] [PubMed] [Google Scholar]
- 46.Fischer-Posovszky P, Tews D, Horenburg S, Debatin KM, Wabitsch M. Differential function of Akt1 and Akt2 in human adipocytes. Mol Cell Endocrinol. 2012;358:135–43 [DOI] [PubMed] [Google Scholar]
- 47.El-Chaar D, Gagnon A, Sorisky A. Inhibition of insulin signaling and adipogenesis by rapamycin: effect on phophorylation of p70 S6 kinase versus eIF4E–BP1. Int J Obes Relat Metab Disord. 2004;28:191–8 [DOI] [PubMed] [Google Scholar]
- 48.Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsuma A, Elchebly M, Spiegelman BM, et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E–BP1. Nat Med. 2001;7:1128–32 [DOI] [PubMed] [Google Scholar]
- 49.Nakae J, Kitamura T, Kitamura Y, Biggs WH, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–29 [DOI] [PubMed] [Google Scholar]
- 50.George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magun R, Boone DL, Tsang BK, Sorisky A. The effect of adipocyte differentiation on the capacity of 3T3–L1 cells to undergo apoptosis in response to growth factor deprivation. Int J Obes Relat Metab Disord. 1998;22:567–71 [DOI] [PubMed] [Google Scholar]
- 52.Magun R, Gagnon AM, Yaraghi Z, Sorisky A. Expression and regulation of neuronal apoptosis inhibitory protein during adipocyte differentiation. Diabetes. 1998;47:1948–52 [DOI] [PubMed] [Google Scholar]
- 53.Feng D, Tang Y, Kwon H, Zong H, Hawkins M, Kitsis RN, Pessin JE. High-fat diet-induced adipocyte cell death occurs through a cyclophilin D intrinsic signaling pathway independent of adipose tissue inflammation. Diabetes. 2011;60:2134–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gagnon A, Dods P, Roustan-Delatour N, Chen C-S, Sorisky A. Phosphatidylinositol-3,4,5-trisphosphate is required for IGF-1-mediated survival of 3T3–L1 preadipocytes. Endocrinology. 2001;142:205–12 [DOI] [PubMed] [Google Scholar]
- 55.Niesler CU, Urso B, Prins JB, Siddle K. IGF-1 inhibits apoptosis induced by serum withdrawal, but potentiates TNF-α-induced apoptosis, in 3T3–L1 preadipocytes. J Endocrinol. 2000;167:165–74 [DOI] [PubMed] [Google Scholar]
- 56.Prins JB, Niesler CU, Winterford CM, Bright NA, Siddle K, O'Rahilly S, Walker NI, Cameron DP. Tumor necrosis factor-α induces apoptosis of human adipose cells. Diabetes. 1997;46:1939–44 [DOI] [PubMed] [Google Scholar]
- 57.Papineau D, Gagnon A, Sorisky A. Apoptosis of human abdominal preadipocytes before and after differentiation into adipocytes in culture. Metabolism. 2003;52:987–92 [DOI] [PubMed] [Google Scholar]
- 58.Fischer-Posovszky P, Keuper M, Nagel S, Hesse D, Schurmann A, Debatin KM, Strauss G, Wabitsch M. Downregulation of FLIP by cycloheximide sensitizes human fat cells to CD95-induced apoptosis. Exp Cell Res. 2011;317:2200–9 [DOI] [PubMed] [Google Scholar]
- 59.Niesler CU, Siddle K, Prins JB. Human preadipocytes display a depot-specific susceptibility to apoptosis. Diabetes. 1998;47:1365–8 [DOI] [PubMed] [Google Scholar]
- 60.Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, Digby JE, Sewter CP, Lazar MA, Chatterjee KK, et al. Activators of peroxisome proliferator-activated receptor γ have depot-specific effects on human preadipocyte differentiation. J Clin Invest. 1997;100:3149–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nio Y, Zighelboim J, Berek J, Bonavida B. Cycloheximide-induced modulation of TNF-mediated cytotoxicity in sensitive and resistant ovarian tumor cells. Cancer Chemother Pharmacol. 1990;26:1–8 [DOI] [PubMed] [Google Scholar]
- 63.Fischer-Posovszky P, Tornqvist H, Debatin K-M, Wabitsch M. Inhibition of death-receptor mediated apoptosis in human adipocytes by the insulin-like growth factor I (IGF-I)/IGF-I receptor autocrine circuit. Endocrinology. 2004;145:1849–59 [DOI] [PubMed] [Google Scholar]
- 64.Gupta S. A decision between life and death during TNF-alpha-induced signaling. J Clin Immunol. 2002;22:185–94 [DOI] [PubMed] [Google Scholar]
- 65.Richon VM, Lyle RE, McGehee RE., Jr Regulation and expression of retinoblastoma proteins p107 and p130 during 3T3–L1 adipocyte differentiation. J Biol Chem. 1997;272:10117–24 [DOI] [PubMed] [Google Scholar]
- 66.Bouloumié A, Planat VC, Devedjian JC, Valet P, Saulnier-Blache JS, Record M, Lafontan M. α2-adrenergic stimulation promotes preadipocyte proliferation. Involvement of mitogen-activated protein kinases. J Biol Chem. 1994;269:30254–9 [PubMed] [Google Scholar]
- 67.Valet P, Pages C, Jeanneton O, Daviaud D, Barbe P, Record M, Saulnier-Blache JS, Lafontan M. α2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. J Clin Invest. 1998;101:1431–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haraguchi K, Shimura H, Kawaguchi A, Ikeda M, Endo T, Onaya T. Effects of thyrotropin on the proliferation and differentiation of cultured rat preadipocytes. Thyroid. 1999;9:613–9 [DOI] [PubMed] [Google Scholar]
- 69.Hutley LJ, Herington AC, Shurety W, Cheung C, Vesey DA, Cameron DP, Prins JB. Human adipose tissue endothelial cells promote preadipocyte proliferation. Am J Physiol Endocrinol Metab. 2001;281:E1037–44 [DOI] [PubMed] [Google Scholar]
- 70.Van Harmelen V, Röhrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism. 2004;53:632–7 [DOI] [PubMed] [Google Scholar]
- 71.Considine RV, Nyce MR, Morales LM, Magosin SA, Sinha MK, Bauer TL, Rosato EL, Colberg J, Caro JF. Paracrine stimulation of preadipocyte-enriched cell cultures by mature adipocytes. Am J Physiol. 1996;270:E895–9 [DOI] [PubMed] [Google Scholar]
- 72.Hua K, Deng J, Harp JB. Interleukin-4 inhibits platelet-derived growth factor-induced preadipocyte proliferation. Cytokine. 2004;25:61–7 [DOI] [PubMed] [Google Scholar]
- 73.Artemenko Y, Gagnon A, Sorisky A. Catalytically inactive SHIP2 inhibits proliferation by attenuating PDGF signaling in 3T3–L1 preadipocytes. J Cell Physiol. 2009;218:228–36 [DOI] [PubMed] [Google Scholar]
- 74.Miller CW, Casimir DA, Ntambi JM. The mechanism of inhibition of 3T3–L1 preadipocyte differentiation by prostaglandin F2α. Endocrinology. 1996;137:5641–50 [DOI] [PubMed] [Google Scholar]
- 75.Carrière A, Fernandez Y, Rigoulet M, Pénicaud L, Casteilla L. Inhibition of preadipocyte proliferation by mitochondrial reactive oxygen species. FEBS Lett. 2003;550:163–7 [DOI] [PubMed] [Google Scholar]
- 76.Blüher M. The distinction of metabolically “healthy” from “unhealthy” obese individuals. Curr Opin Lipidol. 2010;21:38–43 [DOI] [PubMed] [Google Scholar]
- 77.Constant VA, Gagnon A, Landry A, Sorisky A. Macrophage-conditioned medium inhibits the differentiation of 3T3–L1 and human abdominal preadipocytes. Diabetologia. 2006;49:1402–11 [DOI] [PubMed] [Google Scholar]
- 78.Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology. 2007;148:868–77 [DOI] [PubMed] [Google Scholar]
- 79.Lu C, Kumar PA, Fan Y, Sperling MA, Menon RK. A novel effect of growth hormone on macrophage modulates macrophage-dependent adipocyte differentiation. Endocrinology. 2010;151:2189–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bilkovski R, Schulte DM, Oberhauser F, Mauer J, Hampel B, Gutschow C, Krone W, Laudes M. Adipose tissue macrophages inhibit adipogenesis of mesenchymal precursor cells via wnt-5a in humans. Int J Obes (Lond). 2011;35:1450–4 [DOI] [PubMed] [Google Scholar]
- 81.Constant VA, Gagnon A, Yarmo M, Sorisky A. The anti-adipogenic effect of macrophage-conditioned medium depends on ERK1/2 activation. Metabolism. 2008;57:465–72 [DOI] [PubMed] [Google Scholar]
- 82.Yarmo MN, Gagnon A, Sorisky A. The anti-adipogenic effect of macrophage-conditioned medium requires the IKKβ/NF-κB pathway. Horm Metab Res. 2010;42:831–6 [DOI] [PubMed] [Google Scholar]
- 83.O'Hara A, Lim FL, Mazzatti DJ, Trayhurn P. Stimulation of inflammatory gene expression in human preadipocytes by macrophage-conditioned medium: upregulation of IL-6 production by macrophage-derived IL-1β. Mol Cell Endocrinol. 2012;349:239–47 [DOI] [PubMed] [Google Scholar]
- 84.Yarmo MN, Landry A, Molgat AS, Gagnon A, Sorisky A. Macrophage-conditioned medium inhibits differentiation-induced Rb phosphorylation in 3T3–L1 preadipocytes. Exp Cell Res. 2009;315:411–8 [DOI] [PubMed] [Google Scholar]
- 85.Ide J, Gagnon A, Molgat AS, Landry A, Foster C, Sorisky A. Macrophage-conditioned medium inhibits the activation of cyclin-dependent kinase 2 by adipogenic inducers in 3T3–L1 preadipocytes. J Cell Physiol. 2011;226:2297–306 [DOI] [PubMed] [Google Scholar]
- 86.Keophiphath M, Achard V, Henegar C, Rouault C, Clément K, Lacasa D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol. 2009;23:11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gagnon A, Yarmo MN, Landry A, Sorisky A. Macrophages alter the differentiation-dependent decreases in fibronectin and collagen I/III protein levels in human preadipocytes. Lipids. 2012;47:873–80 [DOI] [PubMed] [Google Scholar]
- 88.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983;35:657–66 [DOI] [PubMed] [Google Scholar]
- 89.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes. Arterioscler Thromb Vasc Biol. 2005;25:2062–8 [DOI] [PubMed] [Google Scholar]
- 90.Molgat AS, Gagnon A, Sorisky A. Preadipocyte apoptosis is prevented by macrophage-conditioned medium in a PDGF-dependent manner. Am J Physiol Cell Physiol. 2009;296:C757–65 [DOI] [PubMed] [Google Scholar]
- 91.Molgat AS, Gagnon A, Sorisky A. Macrophage-induced preadipocyte survival depends on signaling through Akt, ERK1/2, and reactive oxygen species. Exp Cell Res. 2011;317:521–30 [DOI] [PubMed] [Google Scholar]
- 92.Molgat AS, Gagnon A, Foster C, Sorisky A. The activation state of macrophages alters their ability to suppress preadipocyte apoptosis. J Endocrinol. 2012;214:21–9 [DOI] [PubMed] [Google Scholar]
- 93.Maumus M, Sengenès C, Decaunes P, Zakaroff-Girard A, Bourlier V, Lafontan M, Galitzky J, Bouloumié A. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab. 2008;93:4098–106 [DOI] [PubMed] [Google Scholar]
- 94.Neese RA, Misell LM, Turner S, Chu A, Kim J, Cesar D, Hoh R, Antelo F, Strawford A, McCune JM, et al. Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci U S A. 2002;99:15345–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zaragosi LE, Wdziekonski B, Villageois P, Keophiphath M, Maumus M, Tchkonia T, Bourlier V, Mohsen-Kanson T, Ladoux A, Elabd C, et al. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes. 2010;59:2513–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Mol Aspects Med. 2012;33:26–34 [DOI] [PubMed] [Google Scholar]