Abstract
Ruminants are more vulnerable to copper deficiency than humans because rumen sulfide generation lowers copper availability from forage, increasing the risk of conditions such as swayback in lambs. Molybdenum-rich pastures promote thiomolybdate (TM) synthesis and formation of unabsorbable Cu-TM complexes, turning risk to clinical reality (hypocuprosis). Selection pressures created ruminant species with tolerance of deficiency but vulnerability to copper toxicity in alien environments, such as specific pathogen–free units. By contrast, cases of copper imbalance in humans seemed confined to rare genetic aberrations of copper metabolism. Recent descriptions of human swayback and the exploratory use of TM for the treatment of Wilson’s disease, tumor growth, inflammatory diseases, and Alzheimer’s disease have created unexpected common ground. The incidence of pre–hemolytic copper poisoning in specific pathogen–free lambs was reduced by an infection with Mycobacterium avium that left them more responsive to treatment with TM but vulnerable to long-term copper depletion. Copper requirements in ruminants and humans may need an extra allowance for the “copper cost” of immunity to infection. Residual cuproenzyme inhibition in TM-treated lambs and anomalies in plasma copper composition that appeared to depend on liver copper status raise this question “can chelating capacity be harnessed without inducing copper-deficiency in ruminants or humans?” A model of equilibria between exogenous (TM) and endogenous chelators (e.g., albumin, metallothionein) is used to predict risk of exposure and hypocuprosis; although risk of natural exposure in humans is remote, vulnerability to TM-induced copper deficiency may be high. Biomarkers of TM impact are needed, and copper chaperones for inhibited cuproenzymes are prime candidates.
Introduction
Until the turn of the millennium, those investigating the impact of copper deficiency in grazing livestock and humans could find little of mutual interest in their respective endeavors. Since then, the picture has changed, and an exchange of information now might benefit human and livestock health in unforeseen ways. Ruminants still provide the most spectacular example of a nutritional interaction that affects health, involving a 3-way interaction among copper, molybdenum, and sulfur (Cu × Mo × S). Where concentrations of the 2 antagonists in pastures are both higher than normal, copper-responsive disorders would still be endemic without appropriate intervention. Disorders took a variety of easily recognized forms, depending on the species involved and its physiological stage of development when exposed. Lambs exposed in utero were born with or soon developed ataxia (neonatal or delayed swayback), which was incurable. Acute exposure of cows caused diarrhea and loss of coat color, which were quickly cured by administering copper. Anemia was seen only after extreme, prolonged copper deprivation. Vulnerability was eventually linked to the rumen synthesis of thiomolybdates [TM2 (MoSnO4-n)], which formed physiologically unavailable complexes with copper. Use of new, slow-release copper supplements in affected herds and flocks appeared to bring clinical problems under contro1, research activity decreased, and little progress was made toward understanding the pathogenesis of copper-responsive disorders in the 19 y separating 2 reviews (1, 2). By contrast, there has been an upsurge of research interest in copper deficiency in humans since the year 2000, stimulated by reports of human swayback, a copper-responsive myelopathy associated with hypocupremia (3). In a review of 55 case reports, mostly in women aged 50 to 70 y, risk factors were identified and included bariatric surgery and hyperzincemia (4). Previously, cases of unequivocal clinical copper deficiency in humans were largely confined to children born with a rare genetic mutation, Menkes disease, resulting from defective copper absorption and manifested in early childhood in similar symptoms to those seen in ruminants (5).
Several areas of common ground have opened up. Crude attempts to use copper supplements as an antidote to allegedly endemic TM toxicity in cattle throughout the United Kingdom may have contributed to the appearance of a new health hazard, bovine copper toxicity (2). Fears of a “myelopathy epidemic” could entice an increasing number of people to unnecessarily self-medicate with copper supplements. There is a common need to define optimum levels of copper supplementation for preventing copper deficiency (2, 6). Copper toxicity has long been recognized as a health hazard for intensively reared sheep, and the only treatment relied on copper chelation with parenteral TM, given as tetrathiomolybdate (MoS4) (2). However, potentially harmful side effects in the form of cuproenzyme inhibition occur (7) that are of relevance to those using MoS4 to treat patients with Wilson’s disease who store copper excessively (8) and those considering TM as potential treatments for cancers (9), inflammatory diseases (10), and Alzheimer’s disease (11).
Current status of knowledge
Contrasting susceptibilities of ruminants and humans to copper imbalance
There are few similarities in the “macro metabolism” of copper ruminants and humans and their respective susceptibilities to copper deficiency (Table 1) . Fundamental differences in the anatomy of the gut between herbivores and omnivores leave the former reliant on roughages for nutrition, and when anaerobic digestion takes place in the herbivore forestomachs, as it does in ruminants, absorbability of dietary copper is reduced to a uniquely low level in the sulfide-rich rumen milieu. All species contrasts in Table 1 stem from this fundamental anatomic difference. Lethal consequences of low copper absorbability (abortion, swayback) probably created natural selection pressure for traits that favored survival: capacity to store copper in times of plenty, ability to tolerate hypocupremia in times of scarcity, breeds that absorbed copper well in environments providing scant dietary supplies of copper (2). Artificial selection for enhanced productivity traits in environments where copper deficiency limited livestock performance may have unintentionally complemented natural selection for efficient use of dietary copper. Susceptibilities to copper toxicity between ruminants and humans differ as markedly as those to deficiency and have similar origins: the strong capacity of ruminants to store copper leaves them vulnerable to toxicity when they are given diets low in roughage from which copper is well absorbed. Copper toxicity has rarely been reported in humans, with most cases occurring in individuals with Wilson's disease, caused by a rare genetic defect in copper metabolism.
Table 1.
Some contrasts in susceptibility of ruminants and humans to copper deficiency
| Copper deficiency | Ruminants | Humans |
| Natural occurrence | Would be endemic in grazing livestock in some regions without regular use of copper supplements | Exceedingly rare: largely confined to individuals with genetic disorders; may occur in malnourished children |
| Unnatural occurrence | Induced by industrial or agricultural exposure to antagonists (e.g., iron, zinc) via exploitation of underground resources of minerals and water | Bariatric surgery reduces capacity to absorb copper and may in time cause copper-responsive neuropathy and anemia |
| Antagonism from molybdenum | Most common cause of clinical symptoms (hypocuprosis): these include retarded growth, bone deformities, loss of hair/wool strength and color, infertility, diarrhea; neuropathy in lambs when exposure is in utero; anemia in severe cases | Unrecorded |
| Copper reserves | Abundant in liver, including neonate | Meagre |
| Genetic variation | Breeds vary in susceptibility: high heritabilities for plasma and liver copper | Single gene mutations (e.g., Menkes disease: symptoms similar to young molybdenum- exposed ruminants); polymorphisms? |
| Hypocupremia | Marked tolerance | Tolerance unknown but probably low |
| Biochemical markers | Liver and plasma copper commonly relied on but imprecise: oxidase activity of ceruloplasmin unreliable | Oxidase activity of ceruloplasmin widely used as surrogate for plasma copper: more sensitive alternatives sought |
Extrapolations between ruminants and humans are therefore error prone. Human swayback (3, 4) bears little resemblance to any of the 3 forms of swayback identified in lambs, in which the primary lesion shifts from the brainstem to the spinal cord to the cerebral cortex with age at onset (fetus, suckling, and periweanling) and is probably a consequence of anoxic dysfunction in developing nerve cells and a resultant myelin aplasia (2). Myelinopathy in adults cannot result from myelin aplasia and appears to involve nonspecific myelin degeneration, with similar clinical manifestations to the subacute combined degeneration of vitamin B-12 deficiency. Delayed swayback in lambs is a preventable but irreversible locomotor disorder, whereas human swayback is primarily a sensory ataxia that responds to copper supplementation (3, 4). Other phenotypic effects of copper deficiency such as anemia also vary in expression among species (1, 12). However, the earliest visible effect of copper deficiency in species as far apart as zebrafish (13) and mammals (2) is depigmentation of the integument caused by loss of tyrosinase activity.
Copper transport
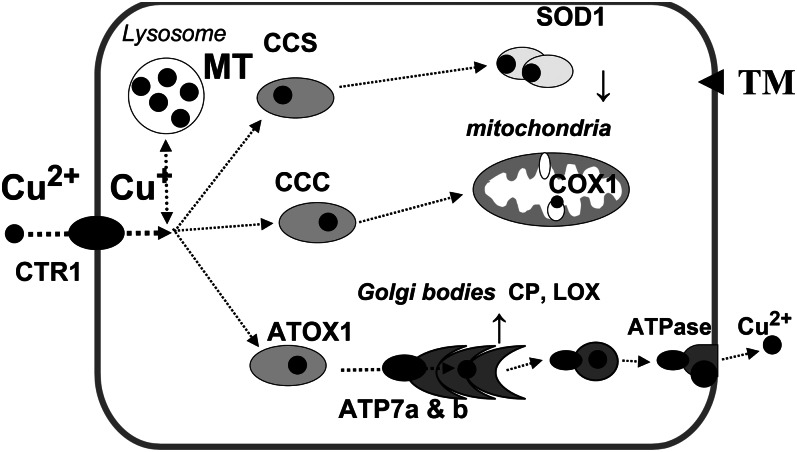
Common ground is found in mechanisms of intracellular copper transport (i.e., at the micrometabolism level) that were first elucidated in cultures of single-celled species, confirmed in laboratory animals (see References 12–15 for reviews) and are summarized in Figure 1. Copper chelation, along with gene deletion, has revealed the relative physiological importance of each step in the pathways illustrated. Susceptibility of cuproenzymes to inhibition by copper chelators over a wide range of concentrations allows both the chelator and target to be ranked for potency. In the malarial parasite Plasmodium falciparae, an intracellular chelator, neocuproine, inhibited superoxide dismutase (SOD) more than an extracellular chelator (16). Gene deletion studies in rodents showed that SOD was not essential for survival, but deletion of the copper transporter 1 gene (Ctr1) caused developmental abnormalities in mice (12). Ctr 1 was “switched on” by reductions in dietary copper supply in mice (12) and cattle (17). In both species, copper chaperones (CC) for SOD (CCS) were upregulated at several sites after copper depletion (18, 19). CC may be the Achilles heel of the copper transport system (Fig. 1). Menkes disease in humans is caused by a mutation affecting ATP7A, a CC that normally facilitates the export of absorbed copper from enterocytes and the incorporation into cuproenzymes in the tissues. Wilson’s disease is caused by a lack of ATP7B, a CC that incorporates copper into the major plasma protein ceruloplasmin (CP) in hepatocytes. Chelators such as MoS4 have an affinity for CC, forming metal clusters with antioxidant 1 in yeast cells exposed in vitro and in kidneys of rats exposed in vivo (20). The potential of MoS4 for the control of human diseases may depend on its ability to disrupt chaperone function and thus inhibit activity of cuproenzymes such as SOD1 (13, 14).
Figure 1.
Mechanisms of copper transport in mammalian cells. A copper-transporter (CTR1) takes Cu2+ across the cell membrane as Cu+ (assuming help from a copper reductase). Specific intracellular copper chaperones with repeating SH domains bind and convey Cu+ to sites of cuproenzyme synthesis, such as superoxide dismutase (SOD) in the cytosol (CCS) and cytochrome oxidase (CCC) in mitochondria. A second group of transport proteins, including antioxidant 1(Atox 1) and ATP7, carry Cu+ to the Golgi apparatus, where cuproproteins such as ceruloplasmin and those with lysyl oxidase (LOX) activity are synthesized. A third group of ATPases, together with CTR 2, export excess copper from cells when the storage capacity [bound to metallothionein (MT) in lysosomes] approaches its limit. Thiomolybdates (TM) cross the cell membrane by unknown pathways with the potential to perturb copper transport by forming metal clusters with copper chaperones and MT, while sequestering copper from SOD within erythrocytes. Adapted from Reference 14 with permission.
New information
Infection and copper imbalance.
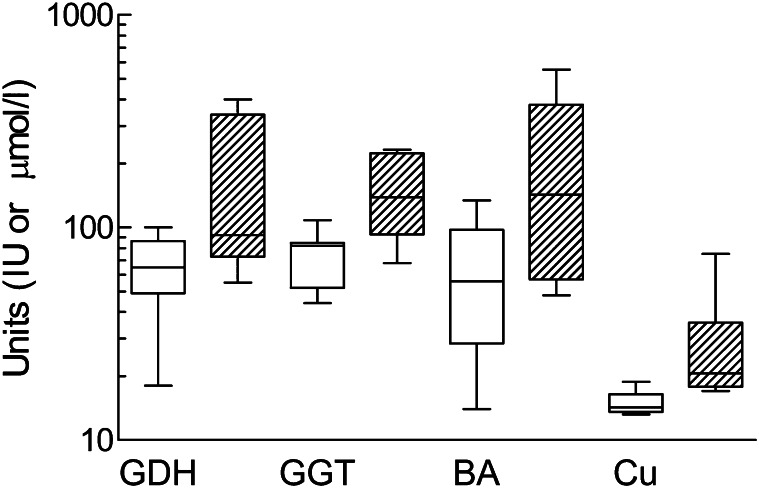
The primary focus in studies of interactions between infection and copper status has been on effects of copper deficiency on components of the immune response, with claims that immunity was one of the first functions to become compromised (21). However, resistance to infection in lambs was no more compromised by copper deficiency than other copper-dependent functions, such as anemia and growth retardation (22).There are now many examples of copper deficiency, some only marginal, affecting one or other cell type and cytokine of the inflammatory cascade (23) but only one more of an effect on the outcome of an infection. Increased virulence of the coxsackievirus in copper-deficient mice has been reported, but the deficiency was extreme: pregnant mice were given a diet containing only 0.5 mg Cu/kg dry matter (DM), and offspring had grossly enlarged hearts when challenged at just 1 wk old (24). The effect of infections on copper requirement may prove to have a greater impact on health in ruminants and humans. Infections have manifold effects on copper metabolism (23), and the first consequence of pathogenic significance has just been reported (7). An outbreak of pre-hemolytic copper poisoning (HCP) occurred in specific pathogen–free (SPF) lambs during a controlled infection experiment and was less severe in 2 groups infected as neonates with Mycobacterium avium subspecies than in an uninfected control group (Fig. 2). Infection with the gut pathogen had in some way reduced the copper burden being carried by 1-y-old lambs. It was speculated that SPF lambs had unusually low copper requirements because they were spared the copper cost of the development of immunity to microbes from a normal environment, both pathogenic and commensal. Should the copper requirement for all species include a component for immunity?
Figure 2.
A rare, if not unique, example of an infection changing the clinical outcome of dietary copper imbalance: lambs, reared in separate pens in a specific pathogen-free unit after cesarean delivery, had either been orally dosed with Mycobacterium avium (open columns) or left uninfected (hatched columns); a case of hemolytic copper poisoning (HCP) occurred in an uninfected lamb 1 y later, and blood tests on cohorts for 3 plasma markers of liver disorder [glutamate dehydrogenase (GDH) and γ-glutamyl transpeptidase (GGT)] activities and bile acid concentrations [BA] were higher and more variable in uninfected lambs, some of which had plasma copper (Cu) concentrations above the normal range (>19 μmol/L) that correlated with indices of liver disorder. Infection protected lambs from pre-HCP.
Responses of sheep to parenteral TM
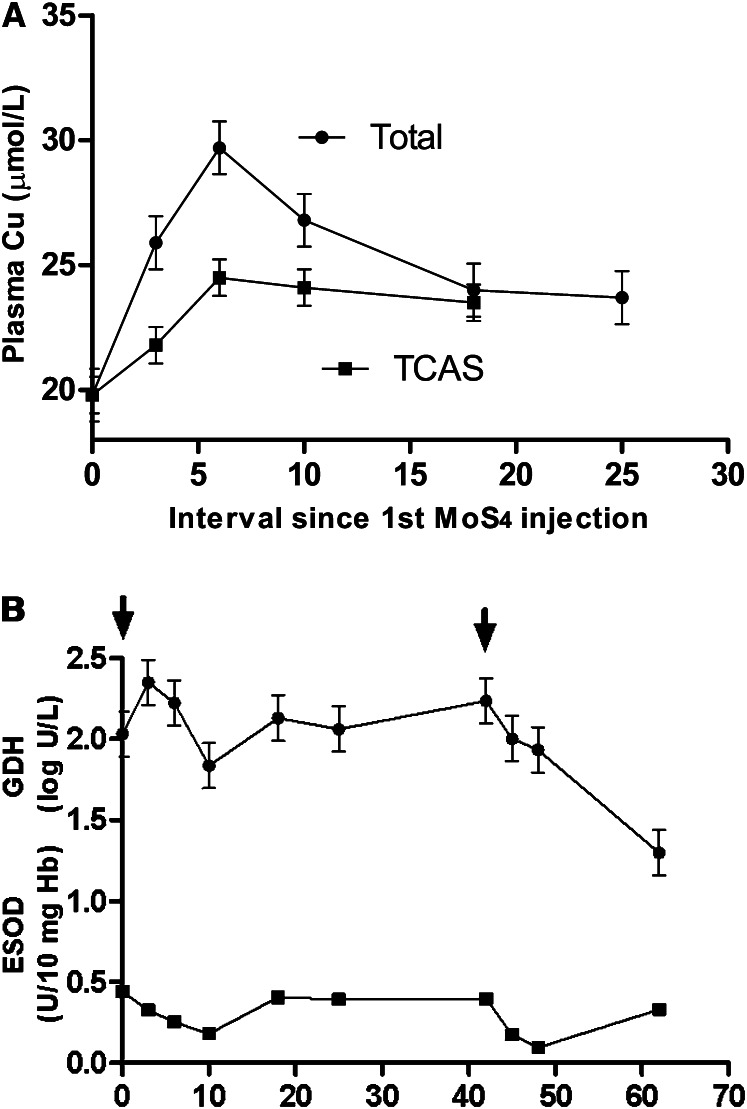
All SPF lambs in the above experiment were treated with subcutaneously administered MoS4, and their responses reveal the macrometabolic effect of the chelator, yielding information that cannot be obtained from cultured cells. As in previous studies (2), there was a rapid increase in plasma copper, but it was relatively small and only partly due to accumulation of trichloroacetic acid-insoluble (TCAI) copper (Fig. 3A), indicating the formation of a complex with albumin (Cu-TM-albumin) (2). In vitro, UV/visible spectrophotometric studies suggest that Cu+1 becomes bound to the terminal amino-N group of the albumin molecule via 2 S atoms in MoS4 (25). There were also residual effects of MoS4 at 2 other locations, hepatocytes and erythrocytes (Fig. 3B). It was hypothesized that MoS4 or its derivatives had crossed cell membranes of both cell types, chelating copper that was causing intracellular hepatocyte dysfunction and sequestering copper from and thus inactivating SOD 1 in the erythrocyte. There was no depletion of erythrocyte copper, and it was assumed that copper became temporarily trapped in unexportable complexes in the erythrocyte (7). Subsequent, coincident recovery of erythrocyte SOD (ESOD) activity and resurgence of hepatotoxicity (Fig. 3B) suggested that simultaneous breakdown of copper complexes in erythrocytes, hepatocytes, and possibly elsewhere had allowed free copper concentrations to return to toxic levels within hepatocytes. When the course of MoS4 injections was repeated after 42 d, hepatoxicity and ESOD activity again showed coincident reductions, but control of hepatoxicity was sustained and inhibition of ESOD 1 was more severe, although still temporary (7). Sufficient copper had apparently been removed from the body by retreatment and ongoing dietary copper depletion to prevent recurrence of hepatotoxicity, but 2 fundamental questions were raised concerning the safety of chelation therapy with MoS4: Is short-term, multifocal disruption of copper-dependent functions inevitable and is it harmful?
Figure 3.
Changes in blood biochemistry of uninfected sheep from Figure 2 after administration of tetrathiomolybdate (MoS4). A: Total and trichloroacetic acid–soluble (TCAS) copper (Cu), the gap between the lines indicating the contribution of trichloroacetic acid–insoluble copper. B: Glutamate dehydrogenase activity (GDH) and erythrocyte superoxide dismutase (ESOD) 1 activity: starts of two 7-d courses of subcutaneous MoS4 are indicated by arrows. Values are pooled mean ± SE for 5 lambs after repeated-measures analysis to remove repeatable differences between sheep. Plasma copper was moderately increased by MoS4 with similar contributions from trichloroacetic acid-insoluble- and -soluble copper after the first MoS4 course, which temporarily reduced hepatoxicity (normal log GDH <1.08) and inhibited ESOD.
Early studies used a similar dose of MoS4 given intravenously to sheep with incipient HCP and reported much larger (2- to 3-fold) increases in plasma copper due entirely to increases in TCAI copper (1). When MoS4 was first given subcutaneously to sheep with pre-HCP and lower presumed liver copper concentrations, the relatively small increase in plasma copper (∼20%) was made up by equal contributions of TCAI and TCAS copper. but there was no evidence that TCAS copper was induced when the second course was given and pre-HCP was finally being controlled (Fig. 3A). In sheep without pre-HCP, MoS4 did not induce TCAS copper (26). Why should effects of MoS4 on plasma copper distribution appear to be so profoundly affected by the severity of HCP? Responses to MoS4 in the Long-Evans Cinnamon (LEC) rat, which has a genetic abnormality causing excessive copper accumulation in the liver, may provide an answer.
Comparative responses of rats and ruminants to TM
Ruminant livers are normally rich in metallothionein (MT), a cysteine-rich protein with an abundance of SH ligands that can bind heavy metals, including copper. Exposure of ruminants to excess copper induces MT in hepatic lysosomes (Fig. 1) (2). In LEC rats that carry a genetic mutation causing excessive hepatic copper storage, intraperitoneally administered MoS4 binds to copper in MT, is removed from the liver as a complex and partitioned between the biliary secretion and the bloodstream in a 70:30 ratio: in the bloodstream, copper MT forms a soluble complex with albumin (27, 28). The large increases in TCAI copper induced by intravenously administered MoS4 in sheep with incipient HCP and very high plasma copper concentrations probably resulted from the immediate formation of Cu-TM-albumin complexes with free copper. In sheep with pre-HCP and little or no hypercupremia, more parenteral MoS4 probably reached the liver: there, MoS4 may have complexed with MT copper and displaced some to the bloodstream, where it was detected as TCAS copper (Fig. 3A). In sheep without pre-HCP and lower presumed liver copper stores (26) or given a second course of MoS4 (Fig. 3A), less MoS4 might be trapped in the liver as Cu-MT complexes and more remains in the bloodstream to inhibit ESOD 1. Other studies with LEC rats showed that extremely high MoS4 doses induced insoluble Cu-MT complexes in the liver (29), whereas lower doses had the same effect if the liver had been depleted of glutathione (30).
Reappraisal of parenteral TM as copper-depleting agent
The propensity of ruminants to store copper in the liver (Table 1) means that the range of liver copper concentrations in any population gathered together for an experiment can be wide. Calves due to be placed on a low copper diet to compare 2 injectable copper supplements had initial liver copper concentrations ranging 5-fold and reaching near-toxic levels. Those with the highest values were therefore given subcutaneous MoS4 [∼3 mg/kg body weight (BW) in total], 10–14 d before copper injection to narrow the range, but the depletion of liver copper was only marginally accelerated (31). Furthermore, the subsequent depletion rate in calves not injected with copper decreased by half, rather than increased, in those given MoS4. Plasma copper was not increased by injecting copper but remained higher in MoS4-treated than untreated calves throughout the experiment, a characteristic response to MoS4. It was concluded that parenteral MoS4 had lengthy residual copper-sparing rather than -depleting effects in calves but how might this happen? If free copper concentrations in hepatocytes were decreased by retained TM, fundamental relationships between hepatocyte concentration and both biliary and plasma copper concentrations might have been perturbed.
Reappraisal of the literature on the use of parenteral MoS4 in sheep indicated that depleting capacity had been exaggerated by failure to discount concurrent accretion of copper from the diet during concurrent oral dosing with copper; short (6-d) periods of assessment, which may have missed the recycling copper to the liver and failure to realize that the spectacular short-term (6 h) increases in biliary copper concentration induced by MoS4 removed only small amounts of copper from the liver (7). The relatively low copper-depleting capacity of MoS4 in ruminants compared with rats (27, 28) could be attributable to a greater capacity to retain copper by minimizing biliary copper secretion (2), but the lower MoS4 dose rate used in ruminants may also have been influential. Nevertheless, parenteral MoS4 removed relatively little copper from the livers of sheep or cattle in the short term and subsequently drew copper into a complex recycling process that delayed further copper excretion. The dependence of that process on liver copper status has implications for the tolerance of humans to TM and is discussed later.
Future therapeutic use of TM
Priorities for future research.
A deeper understanding of TM metabolism is required on 3 fronts if therapeutic potential is to be realized. First, the mechanisms by which TM gain entry to cells must be established. The readiness with which TM form complexes with albumin suggests that TM may not circulate in free form. MoS4 may be less harmful in vivo than the less thiolated TM, MoS3O and MoS2O2: in vitro studies showed that the latter formed less complex polymers of higher solubility (25). Can MoS2O2 and MoS3O be carried by cell-penetrating peptides, conjugated through a disulfide bridge or cross react with cell surface thiols (32)? Is this how they accomplish the unexpected feat of being absorbed from the rumen (33)?
Second, the full physiological impact of TM on copper transport and cuproenzyme activity must be established. Similarities between the molecular structure of CCS and other CC could indicate that other cuproenzymes might be inhibitable by MoS4. Most of the evidence obtained to date comes from in vitro studies. Reduced angiogenesis in cultured tumor cells after the addition of MoS4 has been linked to inhibition of lysyl oxidase and related proteins (13). When follicular granuloma cells were cultured in serum-free media containing 10 mg MoS4/L, estradiol production was reduced and attributed, without direct evidence, to reduced expression of lysyl oxidase (34). Under similar conditions, differentiation of bovine theca cells was inhibited by >1 mg MoS4/L and attributed to impaired steroidogenesis (35). However, the “in vitro bark” of TM may be worse than in its “in vivo bite.” The concentration of MoS4 required for 50% inhibition of bovine SOD 1 in vitro was increased 7-fold by the presence of human plasma (36). Lysyl oxidase (and other cuproenzymes) may be less readily inhibited in vivo, where there is competition for chelator from MT in the liver, albumin in plasma, and CCS in erythrocytes and hepatocytes, to name but a few. However, protection afforded by formation of complexes with MoS4 in vivo does not preclude subsequent intracellular interactions of less thiolated breakdown products with MT or CC.
Third, it is essential to know whether impairment of cuproenzyme activity results in dysfunction. There was no evidence of erythrocyte instability in the form of hemolysis during periods of severe ESOD 1 inhibition, such as those shown in Figure 3B, raising the possibility that, like glutathione peroxidase in the erythrocyte (2), ESOD 1 functions largely as an expendable store. Nevertheless, inhibition of SOD 1 by MoS4 in other cell types in culture was considered to be the most likely cause of inhibited angiogenesis (9). Phenotpyic abnormalities have been induced by TM in various species. Regular parenteral doses of MoS4 were investigated as possible defleecing agents and reduced both pigmentation and crimp in the wool fibers of Merino sheep (37), but no loss of crimp was visible in Texel sheep (26) (N. Suttle, unpublished observations). Serial subcutaneous doses of MoS4 in monkeys caused alopecia (36), and copper dependent dysfunction may have been happening beneath the surface.
Optimal use of thiomolybdate in ovine copper toxicity outbreaks
Once a case of post-HCP has been diagnosed, veterinary practitioners must decide how to bring the inevitable pre-HCP in some cohorts under control. There is normally a wide range of pre-HCP (Fig. 2), with some individuals showing no evidence of hepatotoxicity. If all sheep are given the same dose of MoS4, extrahepatic side effects may be most marked in those at lowest risk. The veterinarian cannot be sure that fertility of a ram or ewe with little or no liver damage will not be harmed by the side effects of MoS4 on reproductive hormones. Repeated large parenteral doses of MoS4 have caused pituitary endocrinopathy in sheep (38). Until all side effects are clarified, it is recommended that a single intravenous dose of 1 mg MoS/kg BW be given to sheep presenting biochemical evidence of severe hepatotoxicity and that reliance is placed on dietary copper depletion of the whole flock, using sulfur and zinc as antagonists (39). Sheep should only be re-treated with MoS4 if there is evidence of resurgent hepatotoxicity.
Optimizing TM therapy in humans
The efficacy of oral MoS4 in Wilson’s disease patients has been attributed to concurrent reduction in copper absorption and systemic sequestration of cytotoxic copper. Although the dose rate is relatively low (∼1 mg MoS4/kg BW orally), systemic side effects have been reported, including liver damage (8). Liver damage has also been noted in MoS4-treated rats, but dose rates were considerably higher (at least 10 mg/kg BW, delivered parenterally) and sufficient to grossly deplete the liver of SOD 1 and plasma of CP (27, 28). Administration of sulfide and di-TM, possible metabolites of MoS4, also increased liver enzyme activity in normal rats, but dose rates were equally high (27). Parenteral administration of 1.7–3.4 mg/kg BW in sheep without pre-HCP (26) did not increase liver enzyme activity (N. Suttle, unpublished data). With a paradigm shift away from chelation to zinc therapy for Wilson’s disease being advocated in some quarters because of iatrogenic effects (40) and a similar move advocated in sheep (39, 41), changes in the use of MoS4 in Wilson’s disease that minimize the risk of cuproenzyme inhibition may merit consideration. Two complementary possibilities are a priming parenteral MoS4 dose to lower free copper concentrations in blood and tissues and oral MoS4 that is gradually replaced by zinc as copper status declines.
The use of TM to control other human diseases may require specific location of the chelator as well as specificity of chelation if cuproenzyme inhibition is shown to be a common and multifocal iatrogenic effect of MoS4 therapy. No unequivocal explanation has been given for the apparent increased incidence of copper deficiency in humans, although copper intakes from staple foods such as wheat may have decreased (42). The closer copper intakes are to the marginal, the greater will be the need for caution in using copper chelation therapies.
Nutritional implications of TM exposure
Simulating natural thiomolybdate exposure
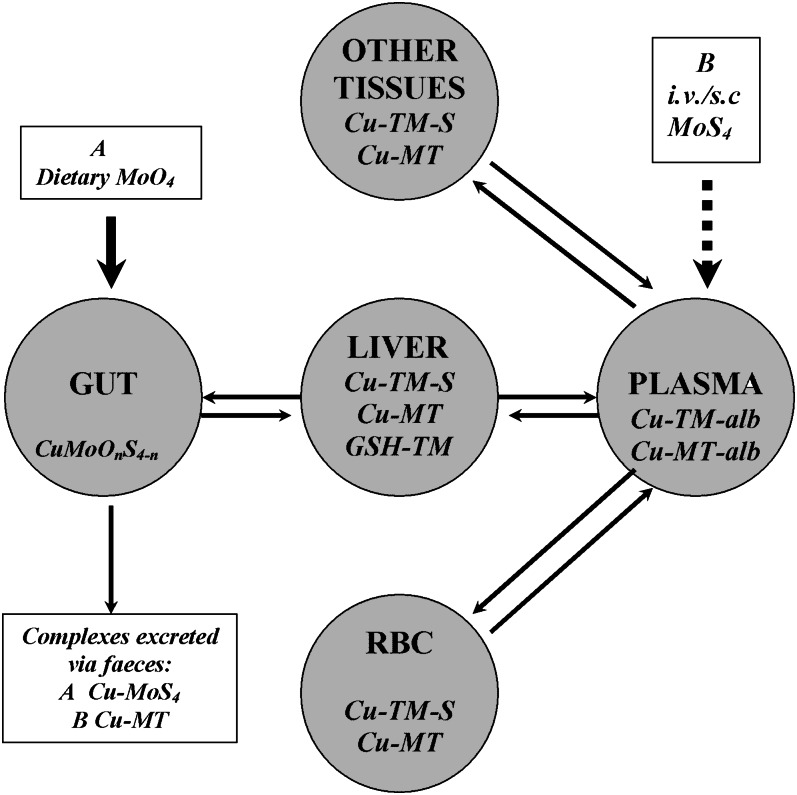
The aforementioned influences of dose, size and cellular distribution of the liver copper store, and species on responses to parenteral TM allow a simple model for TM metabolism to be constructed (Fig. 4). This model can predict the experimental conditions that are most likely to yield nutritionally significant information. Close simulation of natural TM absorption in vivo requires continuous infusions of TM at relatively low rates via the hepatic portal vein. Jugular infusion of 1 mg MoS4/h has inhibited oxidase activity of ceruloplasmin (CPO) in sheep plasma (33), but the route is unphysiological and the rate exceeds that experienced by most adult sheep. The ingestion rate would be ∼0.2 mg Mo/h from pasture containing a high level of 5 mg Mo/kg DM: even if conversion to MoS4 was complete, much would form unabsorbable complexes with dietary copper, i.e., have no systemic potency. When given subcutaneously, MoS4 slowly enters the venous system in albumin-free lymph, and CPO showed no inhibition 24 h after successive doses (26). The capacity of plasma and liver to bind MoS4 may have to be saturated before absorbed MoS4 can begin to inhibit cuproenzymes at other sites. Liver perfusion studies with and without TM in artificial serum might elucidate the dynamic role played by that organ in determining responses to TM arriving from the gut. Effects of MoS4 in cell culture will have greater physiological and nutritional significance to mammals if culture media contain albumin at concentrations characteristic of adult plasma (i.e., not fetal calf serum) and exposure rates that translate to realistic levels of in vivo exposure
Figure 4.
Four compartment model for possible flows of dietary molybdate (A) and parenteral tetrathiomolybdate (MoS4) (B) through the body of sheep, based on a sequence of assumptions. A: MoS4 formed in the rumen will be excreted in feces as copper complexes; at high MoO4 intakes, absorbable di- and tri-thiomolybdates (MoS2O2 and MoS3O), as well as MoS4, may continuously enter the liver via the portal bloodstream and form metal clusters with copper in metallothionein (Cu-MT) and copper-transporting proteins [copper-thiomolybdates (TM)-S]; Cu-MT may be lost from liver and body via biliary secretion; once hepatic binding sites are saturated, excess TM enter the peripheral bloodstream and complex with plasma albumin (Cu-alb); as albumin becomes saturated, TM enters erythrocytes (RBC) and forms complexes with copper from superoxide dismutase 1 (and MT?) that cannot be exported, leaving the enzyme temporarily inhibited. As binding sites in RBC become saturated, TM may flow from plasma into extrahepatic tissues, where cuproenzyme activity is inhibited; within and between each compartment, copper may be exchanged between complexes as they degrade. B: Intravenously injected MoS4 follows a different sequence. First, complex formation in plasma and RBC; second, complex formation in soft tissues, extrahepatic tissues increasing in importance as sites of copper fixation relative to liver if little copper is stored there. With subcutaneous (s.c.) and continuous intravenous (i.v.) administration of MoS4, the sequence will move closer to but not accurately simulate that of exposure via the diet; in vitro exposure will generally exaggerate the consequences of physiological exposure to TM.
TM and molybdenum toxicity in ruminants
Inactivation of CC by MoS4 has recently been invoked to explain the problem that first aroused interest in the Cu × Mo × S interaction (13, 20): how small increments in pasture Mo could cause copper-responsive disorders such as swayback in young lambs, when copper intakes appeared adequate (1). A recent review of historical data on the potential for TM synthesis in ruminants and the consequences of simulated TM absorption appeared to sustain the TM toxicity hypothesis (33). However, 3 important questions must be answered before that hypothesis becomes credible: How much TM is absorbed by grazing ruminants? How much is needed to inhibit cuproenzyme activity? How much inhibition can be tolerated before dysfunction occurs?
Crystallographic studies of metal clusters produced from the admixture of solutions of chaperone (antioxidant 1) and MoS4 (3 mmol/L and 1.2 mmol/L, respectively (20) cannot provide unequivocal answers. However, the metal clusters produced in vitro bear similarities to ferridoxin/molybenopterin complexes found in nitrogen-fixing bacteria. A quest for similar clusters in continuous, molybdenum-enriched cultures of rumen microorganisms might be rewarding, particularly if allied to spectroscopic analysis of TM in the culture fluid (43). Synthesis of TM in the rumen must be sufficient to saturate copper binding in the gut before systemic effects can occur, and saturation cannot be achieved without inducing a state of copper deficiency, the clinical consequences of which are indistinguishable from those of molybdenum or thiomolybdate toxicity (2). It is conceivable that depletion of liver copper stores in molybdenum-induced copper deficiency leaves ruminants vulnerable to short periods of systemic TM exposure: this would explain why iron-induced hypocupremia in cattle was asymptomatic, whereas hypocupremia of equal severity induced by molybdenum- caused hypocuprosis (44).
TM and molybdenum toxicity in nonruminants
Humans may be more vulnerable to absorbed TM than ruminants for 3 reasons: less TM would be needed to saturate binding capacity in digesta; less TM would be needed to saturate binding capacity of much smaller liver copper stores; in the absence of selection pressure, humans may not have developed tolerance of subnormal copper status. Published evidence of natural synthesis of TM in monogastric species is dated and scant. Abnormally bound plasma copper was found in guinea pigs given 26 mg of Mo as MoO4/kg diet DM (1), and much higher levels (500 mg/kg DM) caused similar anomalies in rats (45). In studies of the nematodicidal properties of molybdate in post-weanling rats given the equivalent of only 10 mg Mo/kg diet via the drinking water, plasma copper increased almost 2-fold (E. Ortolani, D. Knox, and N. F. Suttle, unpublished data) and at 70 mg of Mo/kg DM, ESOD 1 activity was concomitantly reduced. Femur enlargement after 6–7 wk of exposure at the higher level (N. Sangwan, N. F. Suttle, and D. Knox, unpublished data) was reminiscent of gross bone abnormalities induced in weanling rats by low dietary exposure to MoS4 (6 mg/kg DM) (46). In dogs, adding MoS4 to the diet reduced cross-linking in proteoglycans from the epiphyseal growth plate (47). Bone development may have been impaired through the inhibition of lysyl oxidase by TM and resultant impairment of angiogenesis in the remodeling zone, proximal to the growth plate. These characteristic responses to MoS4 induced by MoO4 exposure suggest that TM can be synthesized without the rumen, probably in the cecum where sulfide can be generated from the breakdown of slowly degradable protein. TM generated in the cecum would not impair copper absorption but would probably be more absorbable than those generated by rumen synthesis. Premature publicity is given to unpublished data here because of the risk that adolescents could be harmed by therapeutic exposure to TM. Furthermore, it is conceivable that humans absorb TM after ingesting a molybdenum-rich seafood meal.
Detection of TM in blood and tissues
The pathogenic significance of dietary and therapeutic exposure to TM in grazing ruminants and in humans will not be clarified until the dietary circumstances that permit TM absorption are defined and the metabolic fate of retained TM is unraveled. Clarification will require methods for detecting TM in the bloodstream and tissues. In ruminants, CPO is an unreliable indicator of plasma copper status, partly because oxidase activity can come from sources other than CP during parturition, infection, and vaccination (2). Measurement of non-CP copper rather than CPO, a commonly used surrogate for plasma copper in the human context, has much to offer (Fig. 3A). However, the presence of TM in the circulation may not indicate adverse effects of TM on copper-dependent processes, health, and production. It has even been suggested that by slowing the turnover of copper in the body, Cu-TM complexes can act as a slow-release source of copper (2). Reductions in free plasma copper concentration during oral MoS4 treatment of Wilson’s disease have recently been reported (48), indirectly determined by deducting TM-bound and CPO- copper from total plasma copper and therefore with large cumulative error. It took five weeks for the effect to become significant, although there was steady neurological improvement in patients given MoS4. Free copper concentrations in plasma may be inversely correlated with systemic TM exposure but better measured directly (49) than by difference.
In species in which ESOD is susceptible to TM inhibition (e.g., mice and sheep), a low ratio of ESOD activity to milligrams of ESOD protein may indirectly indicate TM absorption. More promising still is the recent suggestion that induction CCS in erythrocytes provides the earliest and most sensitive indicator yet of marginal copper deficiency in rats (18). It seems likely that CCS would be induced by systemic exposure to MoS4.The specific activity of other cuproenzymes (per milligram of copper or enzyme protein), or the abundance of their chaperones may also indicate the presence of inhibitory TM. For example, anomalies in tyrosinase or thiol oxidase activity in hair or wool follicles from the dermis may indicate subclinical dysfunction before depigmentation becomes visible. In monkeys, TM exposure causes a reversible decrease in the number of endothelial progenitor cells (36).
Conclusions
A copper cost of immunity has been inferred from controlled infection studies with sheep and may add to the human requirement for copper. A systemic role for TM inhibition of CC in the pathogenesis of hypocuprosis in grazing ruminants has yet to be established and would probably be confined to short periods of acute molybdate exposure, when TM were generated in the cecum as well as the rumen, and while new equilibria in copper transport were being established within cells and between organs. New knowledge of MoS4 as copper chelator in ruminants suggests that residual inhibition of cuproenzyme activity may restrict opportunities for TM therapy in the treatment of cancers, inflammatory diseases, and Alzheimer’s disease. Realization of the therapeutic potential for TM has 2 primary requisites: first, biomarkers for the systemic impact of TM and second, realistic exposure rates used in experimental models that closely simulate the in vivo environment.
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Author disclosure: N. F. Suttle, no conflicts of interest.
Abbreviations used: BW, body weight; CC, copper chaperone; CCS, copper chaperone for superoxide dismutase; CP, ceruloplasmin; CPO, oxidase activity of ceruloplasmin; DM, dry matter; ESOD, erythrocyte superoxide dismutase; HCP, hemolytic copper poisoning; LEC, Long-Evans Cinnamon; MoS4, tetrathiomolybdate; MT, metallothionein; SOD, superoxide dismutase; SPF, specific pathogen–free; TCAI, trichloroacetic acid-insoluble; TCAS, trichloroacetic acid-soluble; TM, thiomolybdate.
Literature Cited
- 1.Suttle NF. The role of thiomolybdates in the nutritional interactions of copper, molybdenum and sulfur: fact or fantasy. Ann N Y Acad Sci. 1980;355:195–207 [DOI] [PubMed] [Google Scholar]
- 2.Suttle NF. Copper. In Mineral nutrition of livestock. 4th ed. Wallingford, UK: CABI International; 2010. p. 255–305. [Google Scholar]
- 3.Kumar N. Copper deficiency myelopathy (human swayback). Mayo Clin Proc. 2006;81:1371–84 [DOI] [PubMed] [Google Scholar]
- 4.Jaiser SR, Winston GP. Copper deficiency myelopathy. J Neurol. 2010;257:869–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danks DM. Copper deficiency in humans. Annu Rev Nutr. 1988;8:235–57 [DOI] [PubMed] [Google Scholar]
- 6.Harvey LJ, Ashton K, Hooper L, Casgrain A, Fairwether-Tait SJ. Methods of assessment of copper status in humans: a systematic review. Am J Clin Nutr. 2009;89:2025S–39S [DOI] [PubMed] [Google Scholar]
- 7.Suttle NF. Residual effects of Mycobacterium avium infection on the susceptibility of sheep to copper toxicity and the efficacy of conservative treatment with tetrathiomolybdate. Vet Rec. 2012; doi:10.1136/vr.100716 [DOI] [PubMed] [Google Scholar]
- 8.Medici V, Rossaro L, Sturniolo GC. Wilson’s disease - A practical approach to diagnosis, treatment and follow-up. Dig Liver Dis. 2007;39:601–9 [DOI] [PubMed] [Google Scholar]
- 9.Juarez JC, Betancourt O, Pirie-Shepherd SR, Guan X, Price ML, Shaw DE, Mazar AP, Donate F. Copper binding by tetrathiomolybdate attenuates angiogenesis and tumor cell proliferation through inhibition of superoxide dismutase. Clin Cancer Res. 2006;12:4974–82 [DOI] [PubMed] [Google Scholar]
- 10.Wei H, Frei B, Beckman JS, Zhang W-J. Copper chelation by tetrathiomolybdate inhibits lipospolysaccharide-induced inflammatory responses in vivo. Am J Physiol Heart Circ Physiol. 2011;301:H712–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinn JF, Harris CJ, Cobb KE, Domes C, Ralle M, Brewer G, Wadsworth TL. A copper-lowering strategy attenuates amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;21:903–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prohaska JR. Impact of copper limitation on expression and function of multicopper oxidases (ferroxidases). Adv Nutr. 2011;2:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turski ML, Thiele DR. New roles for copper metabolism in cell proliferation, signalling and disease. J Biol Chem. 2009;284:717–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogra Y. Research tools and techniques for copper metabolism in mammals. J Health Sci. 2011;57:385–96 [Google Scholar]
- 15.Prohaska JR. Copper. In: Bowman BA, Russell RM, editors. Present knowledge in nutrition. 7th ed. Washington, DC: International Life Science Institute–Nutrition Foundation; 2006. p. 458–70. [Google Scholar]
- 16.Rasoloson D, Shi L, Chong CR, Kafsack BF, Sullivan DJ. Copper pathways in Plasmodium falciparum-infected erythrocytes indicate an efflux role for the copper P-ATPase. Biochem J. 2004;381:803–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen SL, Ashwell MS, Legleiter LR, Fry RS, Lloyd KE, Spears JW. The addition of high manganese to a copper-deficient diet further depresses copper status and growth of cattle. Br J Nutr. 2009;101:1068–78 [DOI] [PubMed] [Google Scholar]
- 18.Hepburn JJ, Arthington JD, Hansen SL, Spears JW, Knutson MD. Technical note: copper chaperones for copper, zinc superoxide dismutase: a potential marker for copper status in cattle. J Anim Sci. 2009;87:4641–6 [DOI] [PubMed] [Google Scholar]
- 19.Lassi KC, Prohaska JR. Erythrocyte chaperone for superoxide dismutase is increased following marginal copper deficiency in adult and post-weanling mice. J Nutr. 2012;142:292–7 [DOI] [PubMed] [Google Scholar]
- 20.Alvarez HM, Xue Y, Robinson CD. Tetrathiomolybdate inhibits copper trafficking proteins through metal cluster formation. Science. 2010;327:331–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonham M, O'Connor JM, Hannigan BM, Strain JJ. The immune system as a physiological indicator of marginal copper status? Br J Nutr. 2002;87:393–403 [DOI] [PubMed] [Google Scholar]
- 22.Jones DG, Suttle NF. Copper and disease resistance. In: Hemphill D, editor. Proceedings of the 21st Annual Conference on Trace Substances in Environmental Health. University of Missouri, Columbia, MO: 1987. p514–25.
- 23.Hodgkinson V, Petris MJ. Copper homeostasis at the host-pathogen interface. J Biol Chem. 2012;287:13549–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith AD, Botero S, Levander OA. Copper deficiency increases the virulence of amyocarditic and myocarditic strains of coxsackievirus B3 in mice. J Nutr. 2008;138:849–55 [DOI] [PubMed] [Google Scholar]
- 25.Quagraine EK, Read RS. UV/visible spectrophotometric studies of the interactions of thiomolybdates, copper (II) and other ligands. J Inorg Biochem. 2001;85:53–60 [DOI] [PubMed] [Google Scholar]
- 26.Suttle NF. Lack of effect of parenteral thiomolybdate on ovine caeruloplasmin activity: diagnostic implications. Vet Rec. 2008;162:593–4 [DOI] [PubMed] [Google Scholar]
- 27.Ogra Y, Komada Y, Suzuki KT. Comparative mechanism and toxicity of tetra- and di-thiomolybdates in the removal of copper. J Inorg Biochem. 1999;75:199–204 [DOI] [PubMed] [Google Scholar]
- 28.Komatsu Y, Sadakata I, Ogra Y, Suzuki KT. Excretion of copper complexed with thiomolybdate into the bile and blood in LEC rats. Chem Biol Interact. 2000;124:217–31 [DOI] [PubMed] [Google Scholar]
- 29.Ogra Y, Chikusa H, Suzuki KT. Metabolic fate of the insoluble copper/ tetrathiomolybdate complex found in the liver of LEC rats with excess tetrathiomolybdate. J Inorg Biochem. 2000;78:123–8 [DOI] [PubMed] [Google Scholar]
- 30.Ogra Y, Miyayama T, Anan Y. Effect of glutathione depletion on removal of copper from LEC rats by tetrathiomolybdate. J Inorg Biochem. 2010;104:858–62 [DOI] [PubMed] [Google Scholar]
- 31.Suttle NF. The use of fractional change in liver copper concentration to assess the efficacy of parenteral copper supplements in cattle given a copper-deficient diet, with or without pre-treatment with tetrathiomolybdate. N Z Vet J. 2012 [DOI] [PubMed] [Google Scholar]
- 32.Aubry S, Burlina F, Dupont D, Joliot S, Chaissaing G, Sagan S. Cell-surface thiols affect entry of di-sulphide-conjugated peptides. FASEB J. 2009;23:2956–67 [DOI] [PubMed] [Google Scholar]
- 33.Gould L. Kendall, NR. Role of the rumen in copper and thiomolybdate absorption. Nutr Res Rev. 2011;24:176–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendall NR, Marsters P, Scaramuzzi RJ, Campbell BK. Expression of lysyl oxidase and effect of copper chloride and ammonium tetrathiomolybdate on bovine ovarian follicle granulose cells cultured in serum-free media. Reproduction. 2003;125:657–65 [PubMed] [Google Scholar]
- 35.Kendall NR, Marsters P, Guo RJ, Scaramuzzi RJ, Campbell BK. Effect of copper and thiomolybdates on bovine theca cell differentiation in vitro. J Endocrinol. 2006;189:455–63 [DOI] [PubMed] [Google Scholar]
- 36.Doñate F, Juarez JC, Burnett ME, Manuia MM, Guan X, Shaw DE, Smith ELP, Timucin C, Braunstein MJ, Batuman OA, et al. Identification of biomarkers for the antiangiogenic and antitumour activity of the superoxide dismutase 1 (SOD 1) inhibitor tetrathiomolybdate (ATN-224). Br J Cancer. 2008;98:776–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon AJ, Hill JL. The effects of injecting sheep with thiomolybdate. In: Howell JMcC, Gawthorne JM, White CL, editors. Trace element metabolism in man and animals. Proceedings of the 4th International Symposium; 1981. Canberra: Australian Academy of Science: p 557–9.
- 38.Haywood S, Dincer Z, Jasani B, Loughram MJ. Molybdenum-associated pituitary endocrinopathy in sheep treated with ammonium tetrathiomolybdate. J Comp Pathol. 2004;130:21–31 [DOI] [PubMed] [Google Scholar]
- 39.Suttle NF. Responsiveness of pre-hemolytic copper poisoning in sheep from a specific pathogen-free environment to recommended doses of tetrathiomolybdate. Vet Rec. 2012; doi: 10.1136/vr 100722 [DOI] [PubMed] [Google Scholar]
- 40.Hoogenraad TU. Paradigm shift in treatment of Wilson’s disease: zinc therapy now the treatment of choice. Brain Dev. 2006;28:141–6 [DOI] [PubMed] [Google Scholar]
- 41.Suttle NF. Control of hepatic copper retention in Texel ram lambs by dietary supplementation with copper antagonists followed by a copper depletion regimen. Anim Feed Sci Technol. 2012;173:194–200 [Google Scholar]
- 42.Fan MS, Zhao FJ, Fairweather-Tait SJ, Poulton PR, Dunhan SJ, McGrath SP. Evidence of decreasing mineral density in wheat grain over the last 160 years. J Trace Elem Med Biol. 2008;22:315–24 [DOI] [PubMed] [Google Scholar]
- 43.Rhett C. Kinetics of thiomolybdate and copper-thiomolybdate interconversion processes. University of Saskatchewan [PhD Thesis], NR 62620; 2009.
- 44.Phillippo M, Humphries WR, Atkinson T, Henderson GD, Garthwaite PH. The effect of dietary molybdenum and iron on copper status, puberty, fertility and oestrus cycles in cattle. J Agric Sci Camb. 1987;109:321–36 [Google Scholar]
- 45.Nederbraght H. Changes in the distribution of copper and molybdenum after Mo administration and subsequent oral or intraperitoneal Cu administration in rats. Br J Nutr. 1982;48:353–64 [DOI] [PubMed] [Google Scholar]
- 46.Spence JA, Suttle NF, Wenham G, El-Gallad T, Bremner I. A sequential study of the skeletal abnormalities which develop in rats given a small dietary supplement of ammonium tetrathiomolybdate. J Comp Pathol. 1980;90:139–53 [DOI] [PubMed] [Google Scholar]
- 47.Read R, Sutherland J, Ghosh P. The matrix components of the epiphyseal growth plate and articular cartilage from dogs treated with ammonium tetrathiomolybdate, a copper antagonist. Aust J Exp Biol Med Sci. 1986;64:545–62 [DOI] [PubMed] [Google Scholar]
- 48.Brewer GJ, Askari F, Dick RB, Sitterly J, Fink JK, Carlson M, Kluin KJ, Lorincz MT. Treatment of Wilson’s disease with tetrathiomolybdate: V. control of free copper by tetrathiomolybdate and a comparison with trientine. Transl Res. 2009;154:70–7 [DOI] [PubMed] [Google Scholar]
- 49.Beattie JH, Reid MD, Fairweather-Tait S, Dainty JR, Majsak-Newman G, Harvey L. Selective extraction of blood plasma exchangeable copper for isotope studies of copper absorption. Analyst. 2001;126:2225–9 [DOI] [PubMed] [Google Scholar]