Abstract
Dietary nutrients are essential for gastrointestinal (GI) growth and function, and nutritional support of GI growth and development is a significant component of infant care. For healthy full-term neonates, nutritional provisions of the mother’s milk and/or formula will support normal maturation of structure and function of the GI tract in most infants. The composition of breast milk affects GI barrier function and development of a competent mucosal immune system. The functional nutrients and other bioactive components of milk support a microenvironment for gut protection and maturation. However, premature infants struggle with feeding tolerance impairing normal GI function, leading to intestinal dysfunction and even death. The high prevalence worldwide of enteric diseases and dysfunction in neonates has led to much interest in understanding the role of nutrients and food components in the establishment and maintenance of a functioning GI tract. Neonates who do not receive enteral feeding as either mother’s milk or formula are supported by total parental nutrition (TPN). The lack of enteral nutrition can compound intestinal dysfunction, leading to high morbidity and mortality in intestinally compromised infants. Reciprocally, enteral stimulation of an immature GI tract can also compound intestinal dysfunction. Therefore, further understanding of nutrient interactions with the mucosa is necessary to define nutritional requirements of the developing GI tract to minimize intestinal complications and infant morbidity. Piglet models of intestinal development and function are similar to humans, and this review summarizes recent findings regarding nutrient requirements for growth and maintenance of intestinal health. In particular, this article reviews the role of specific amino acids (arginine, glutamine, glutamate, and threonine), fatty acids (long chain polyunsaturated, medium chain, and short chain), various prebiotic carbohydrates (short-chain fructo-oligosaccharide, fructo--oligosaccharide, lacto-N-neotetraose, human milk oligosaccharide, polydextrose, and galacto-oligosaccharide), and probiotics that have been examined in the suckling piglet model of intestinal health.
Introduction
Gastrointestinal (GI)3 maladies rank among the highest causes of neonatal morbidity and mortality across most mammalian species, including domestic livestock species (1) and human infants (2). At birth, there is a significant and immediate demand on the GI tract to digest and absorb nutrients efficiently to maintain the high rate of growth in the neonate (3, 4). Intestinal epithelial cell integrity is of prime importance considering that this single layer of epithelium is simultaneously responsible for secretion of fluid and absorption of water, electrolytes, and nutrients, and, concurrently, this layer of cells must provide a selective barrier against the complex and potentially noxious environment of the gut lumen. The intestinal epithelium is constantly exposed to a wide array of antigens essential to the development of the innate immune system and programming of the adaptive immune system (5). The cell layer is continuously exposed to a variety of both commensal and pathogenic microbes and antigens. To contain this bacterial, viral, and antigenic load, features of the mucosal epithelium have evolved that allow it to function as an active participant in mucosal immune responses and a physical barrier to the uptake of noxious substances (6, 7). Therefore, the establishment and maintenance of homeostatic GI function are vitally important in reducing neonatal morbidity and mortality. Neonatal nutrition is a critical component in the establishment of normal GI function from digestion and absorption to barrier function and development of the mucosal immune system. The demands on the GI tract of full-term neonates are remarkable, but the challenge for a preterm neonatal immature gut to develop and maintain GI homeostasis is immense.
Adaptation to enteral nutrition (EN) is one of the first challenges faced by the neonate because, in utero, most nutrients are supplied via the umbilical circulation with swallowing of amniotic fluid in the last weeks before birth (3). Adapting to enteral nutrient delivery is well tolerated in most term neonates, and mother’s milk or formula provides the needed nutrients to establish and maintain GI homeostasis, allowing digestion, absorption, gut barrier function, and the development of immune tolerance (8). Conversely, many preterm neonates have feeding intolerance, challenging their very survival, given that dietary nutrients are the most effective stimuli for intestinal growth (9, 10). The inability to tolerate EN leads to further atrophy of the GI tract because of the absence of nutrient-stimulated local growth factor production such as glucagon-like peptide 2 (GLP-2), gastrin, cholecystokinin, peptide YY, and neurotensin (11, 12). The essential function of development of digestive and absorptive capacity is only compounded by a severely underdeveloped mucosal immune response and the inability to gain passive immunity from mother’s milk and stimulate the newborn’s own host defense (13). When a neonate is unable to be fed mother’s milk or formula, the microenvironment of the gut breaks down. The lack of metabolism of essential nutrients is impaired, but, additionally, the ability to tolerate colonization of the GI tract leads to intestinal dysfunction, breakdown of barrier function, and increased aberrant inflammation further exacerbating the intestinal stress and leading to an increased incidence of intestinal dysfunction, necrotizing enterocolitis (NEC), intestinal permeability, bacterial translocation, sepsis, stunted growth, and even death (14).
Although the pathophysiology of enteric diseases and neonatal intestinal dysfunction is understood on a global level, the molecular mechanisms associated with development of these diseases are not well established. Furthermore, the understanding of how dietary bioactive molecules affect prevention and/or treatment of intestinal dysfunctions is an important area of research. The influence of perinatal diet on the developing mucosal immune system and interaction with the intestinal microbiota is an area of much research emphasis to improve infant survival and protect against the long-term developmental intestinal disorders for the at-risk infant (15–17).
Neonatologists struggle to optimize nutritional support for preterm infants, who most often require total parental nutrition (TPN) or TPN with minimal EN (5, 14, 18). It is well documented that TPN, although necessary, can further impede the developing intestine because of the lack of luminal-nutrient stimulated trophic factors (5, 12). Additionally, long-term use of parenteral nutrition (PN) in infants can lead to metabolic liver dysfunction (19). The mucosal breakdown is linked to the lack of enteral nutrients and intestinal immaturity (14). More recently, Stoll et al. (20) demonstrated that the type of EN significantly affects the neonatal piglet’s metabolic dysfunction and trophic polypeptide production. These findings support the need for continued investigation of macronutrient composition and chemical form of the nutrients that directly and/or indirectly stimulate trophic and cytoprotective pathways involved in the developing intestine. A more comprehensive understanding of the individual nutrients, mode of nutrient supplementation, type of nutrient supplementation, and the cellular and immunological adaptations is needed to optimize infant nutrition for growth and development as well as long-term quality of life. This review examines the contributions of various nutrients in support of optimal intestinal growth, development, and health, with particular emphasis on work conducted in the suckling piglet model.
What dietary nutrients affect suckling neonatal intestinal health? (Table 1)
The impact of amino acids on neonatal intestinal health
Dietary proteins elicit an extensive array of nutritional and biological functions. Nutritionally, protein is composed of amino acids, which are classified as nutritionally essential or nonessential. However, the stage of developmental maturation and health status affects dietary essentiality of amino acids (21, 22). Dietary proteins and individual amino acids play critical physiological regulatory roles throughout the body, but especially in the intestine, beyond their traditional role in protein synthesis. Physicochemical properties, amino acid concentration, and bioactive peptides affect mechanical, hormonal and neuroendocrine functions of the GI tract (12, 23). In-depth reviews on this topic in pigs and other mammals were recently published by Wu (21, 22) and Jahan-Mihan et al. (24). In this review, we focus on the recent advances in parenteral and enteral amino acid influence on GI development in suckling piglet intestinal health models.
Table 1.
Nutritional factors influencing intestinal health of the suckling neonate1
| Nutrient | Effects | References |
| Arginine | ↑ cell migration by NO and FAK mechanism | (27, 28) |
| ↑ polyamine synthesis | (27, 28) | |
| ↓ NEC preterm piglets | (29) | |
| ↑ or no effect SMA blood flow depends on health status | (30, 31, 35) | |
| ↑ protein synthesis, mTOR, MAPK, ribosomal p70s6k | (28, 38–40) | |
| ↓ intestinal permeability | (39) | |
| ↑ intestinal structure and function in neonatal pig intestinal epithelial cells model | (41) | |
| No effect on diarrhea in infection models | (39, 43) | |
| ? possible immune regulation | (44) | |
| Glutamine/glutamate | ↑ energy supply to cells | (12, 23) |
| ↑ protein synthesis | (44) | |
| ↓ apoptosis in enterocytes and lymphocytes | (46) | |
| ↑ cellular antioxidant capacity | (47) | |
| No effect in rotavirus model | (48) | |
| ↑ glutathione, proline, arginine synthesis | (49) | |
| Threonine | Main substrate for mucin synthesis | (51, 53–59) |
| Medium-chain triglycerides | Quick absorption and oxidation | (61) |
| Potentially augment intestinal structure | (62) | |
| Antimicrobial | (64, 66–68) | |
| PUFA | Enrich enterocyte phospholipids | (72) |
| When fed to sow during gestation and suckling phase, enriches piglet tissues and ↑intestinal structure | (73–77) | |
| ↓ mast-cell degranulation | (73) | |
| ↑ glucose absorption, GLUT2, SGLT-1 | 75, 77) | |
| ↑ transepithelial resistance after ischemic injury | (84) | |
| ↓ villi denudation, mucosal to serosal flux | (84) | |
| SCFA | ↑ colonic mucosal proliferation | (88) |
| ↓ intestinal atrophy in TPN-fed pigs | (89) | |
| ↑ glucose, amino acid, and dipeptidase transports | (89–91) | |
| ↑ plasma GLP-2 | (92) | |
| Prebiotics | ↓ recovery time after Salmonella infection | (98) |
| ↓ luminal pH | (99, 100, 107) | |
| May reduce incidence of NEC | (104) | |
| Does not increase bacterial translocation | (105) | |
| ↑ intestinal structure | (106) | |
| Effects dependent on age, diet | (107) | |
| Probiotics | Effects dependent on microbial population, age, diet | (106) |
FAK, focal adhesion kinase; GLP-2, glucagon-like peptide-2; GLUT2, glucose transporter-2; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NEC, necrotizing enterocolitis; NO, nitric oxide; p70s6k, p70S6 kinase; SGLT-1, sodium-dependent glucose cotransporter 1; SMA, superior mesenteric artery; TPN, total parental nutrition.
Arginine.
Arginine (Arg) is an essential amino acid in neonates synthesized by enterocytes and required for protein synthesis and maximum growth (21, 22, 25). Injury to the intestinal mucosa increases amino acid requirements for growth and repair. The importance of Arg signaling in the intestine, as applied to all species and developmental ages, was reviewed in detail by Rhoads and Wu (23). However, supplemental Arg in neonatal piglets has long been known to be beneficial in intestinal integrity and function because it is an essential substrate for synthesis of nitric oxide (NO), a major nonadrenergic, noncholinergic vasodilator (26). More recently, supplemental Arg before a razor wound injury in neonatal piglet intestinal IPEC-J2 cells was shown to increase cell migration in a dose-dependent biphasic manner via NO and focal adhesion kinase-dependent mechanisms (27, 28). Additionally, it was shown that Arg-dependent migration required synthesis of polyamines and that Arg is a major amino acid precursor of polyamines essential in GI repair (22, 27, 28). The increased NO production and increased enterocyte migration are important mechanisms after mucosal injury for rapid restoration of epithelial continuity over the denuded villus surface area and may be mechanistically related to previous work demonstrating attenuation of NEC in newborn pigs pretreated with intravenous Arg (29).
Recent work in neonatal piglets has demonstrated that TPN adversely affects gut barrier function and reduces the intestinal blood flow and induces small intestine atrophy (30, 31). Research has also shown that Arg synthesis is decreased in TPN-fed piglets, and the piglets have a significant reduction in inducible NO synthase activity, a key enzyme in NO synthesis (31). The decrease in NO production contributes to the decrease in superior mesenteric blood flow in the piglets and induction of mucosal atrophy (31). Arg supplementation effectively improves intestinal barrier function and vascular development in adult pigs (32), and many rodent models have demonstrated increased intestinal recovery after ischemia-reperfusion injury when Arg is supplemented (33, 34). Puiman et al. (35) demonstrated that partial EN does increase blood flow compared with TPN-fed piglets, but enteral physiological or pharmacological doses of Arg do not increase superior mesenteric artery blood flow. Additionally, they found that only pharmacological doses of Arg modestly increased intestinal growth independent of NO, and there was no effect on protein synthesis, cell proliferation, or activation of mammalian target of rapamycin (mTOR) signaling (35). Although contradictory of some rodent data (33, 34) and data from ischemic-reperfusion models in older pigs (36), the authors hypothesize that the differences may be related to the immaturity of the piglet gut and the fact this was not an intestinal health challenge model. Under healthy development of an immature GI tract, enteral Arg may have a less potent affect than during immaturity compounded by intestinal dysfunction.
In weanling piglets, supplemental Arg decreases the mucosal injury caused by LPS endotoxemia by decreasing intestinal lesions and increasing cell proliferation (37). Rhoads et al. (28, 38) and Corl et al. (39) demonstrated that Arg activates mTOR, MAPK, and ribosomal p70S6 kinase (p70s6k) signaling in enterocytes, increasing jejunal protein synthesis, cell migration, and intestinal restitution in neonatal pigs. Corl et al. (39) demonstrated that oral Arg supplementation to rotavirus-infected piglets enhances intestinal protein synthesis in part by stimulating p70s6k and reduces intestinal permeability by increasing transepithelial resistance via an mTOR/p70S6k-independent mechanism (39). Bauchart-Thevret et al. (40) recently demonstrated similar results by showing that protein synthesis in IPEC-J2 cells (neonatal pig intestinal cell line) via activation of mTOR/p70s6k is increased when treated with Arg. Additionally, Wang et al. (41) demonstrated that supplemental Arg improves intestinal development in intra-uterine growth retardation (IUGR) piglets by decreasing enterocyte apoptotic index through an Akt (protein kinase B) dependent mechanism and increasing intestinal weight, structural soundness, and phosphorylated mTOR compared with unsupplemented IUGR littermates.
Determining the correct supplementation level of dietary Arg is a key component of neonatal nutrition because dosing may determine its effectiveness (42). For example, although dietary Arg increases protein synthesis in rotavirus-infected piglets, it has no effect on the severity of diarrhea (39). This is consistent with findings of Gookin et al. (43) demonstrating that Cryptosporidium parvum–infected suckling piglets fed enteral Arg had increased prostaglandin-dependent secretory diarrhea. The potential immunomodulatory roles of Arg in neonatal piglets need further investigation. However, Li et al. (44) comprehensively reviewed many studies in mammals and humans implicating Arg as an important component of intestinal immune response. In piglets, Arg is known to participate in B- and T-cell development (45), establishing a role for Arg in the innate and adaptive immune response.
Glutamine and glutamate.
The GI tract of the neonatal pig preferentially uses dietary glutamine (Gln) and glutamate (Glu) as its key respiratory fuels, with Glu being almost completely oxidized in the mucosal cells of the piglet intestine as an energy source for growth and function (12). Gln cell signaling was recently comprehensively reviewed by Rhoads and Wu (23). In addition to its function as a primary energy source for intestinal epithelial cells and leukocytes, Gln contributes to key metabolic processes from protein synthesis to the immune response and regulation of cellular redox state (44). The amide nitrogen of Gln also is required for the synthesis of purines and pyrimidines comprising RNA and DNA. Domeneghini et al. (46) demonstrated that supplementation of dietary Gln decreased apoptosis of enterocytes and lymphatic cells. Additionally, Wang et al. (47) reported that in the small intestine dietary Gln improved antioxidant capacity and cell proliferation. However, we were unable to detect beneficial effects of supplemental dietary Gln or alanine-Gln during recovery of piglets from rotaviral gastroenteritis (48). Glu is synthesized from Gln and is the precursor to glutathione, proline, and Arg and also is a neurotransmitter (49). Its importance in the GI tract seems to be essential in neonatal growth and development because it is almost 100% oxidized in the intestinal mucosa. The metabolic fate of Glu was extensively reviewed by Burrin and Stoll (50).
Threonine.
Almost 15 years ago, the essential amino acid threonine was found to have particular importance in mucin synthesis and maintenance of gut barrier function in neonatal pigs (51). Formula-fed piglets use and retain as much as 60% of dietary threonine in the GI tract (52). Bertolo et al. (51) confirmed the high GI tract threonine retention demonstrating TPN-fed piglets use ∼40% of the total body requirement compared with enterally fed piglets. High GI tract threonine requirements are related to use in the synthesis of threonine-rich mucins produced in intestinal goblet cells (51). Mucins are large gel-forming glycoproteins synthesized and secreted by the goblet cells, and mucin 2 (MUC2) is the predominant secretory mucin in the human intestinal tract (53, 54). The peptide component of MUC2 is predominantly composed of the essential amino acid threonine, which constitutes ∼30% of the total amino acids in this protein (55, 56). Availability of threonine affects mucosal protein synthesis and mucin synthesis in pigs (57, 58). In fact, Puiman et al. (59) demonstrated in preterm formula-fed piglets that there is decreased mucosal growth with an associated decrease in first-pass splanchnic threonine use, protein synthesis, and MUC2 synthesis in the distal small intestine compared with colostrum-fed piglets. Mucin production is important to gut barrier function because the mucus layer that overlies the gut epithelium provides protection against luminal pathogens and toxic substances. Disruption of the mucin layer decreases barrier function and may facilitate bacterial translocation. This type of intestinal insult combined with a neonatal immature immune system leaves the neonate at greater risk of the development of intestinal inflammation, sepsis, and further intestinal dysfunction.
Fatty acids and associated metabolites
Fatty acids are a major energy source, important components of the cell membrane, metabolic substrates in many biochemical pathways, cell-signaling molecules, and play a role as immunomodulators (60). These pathways are significantly affected by diet. Dietary manipulation is a major advantage in the ability of a nutritionist to have a considerable impact on GI health, possibly minimizing use of pharmacological treatments in the future. Medium-chain triglycerides (MCT), long-chain PUFA (LC-PUFA), and SCFA are essential components in providing energy and maintaining GI growth and development for neonates.
Medium-chain triglycerides.
Medium-chain fatty acids (MCFA) are saturated 6–12 carbon fatty acids, which occur naturally as MCT in milk fat and other dietary nutritional supplements. MCFA and MCT have specialized nutritional and metabolic effects, including being readily digestible, passive absorption, and obligatory oxidation, making them of particular interest for neonatal nutrition (61). Exploiting the biochemical and physiological properties of MCFA and MCT in neonatal nutrition due to the small amounts in EN consumption and the development of feeding intolerance in preterm neonates is a way to increase energy intake needed to meet the high growth rate demand. Additionally, MCFA length and emulsification may augment intestinal structure and morphology after a period of limited to no feed intake (62), as is seen in preterm neonates. In fact, augmentation of the small intestine mucosa has been reported in a rat model of feed restriction (63). Similarly, MCFA-fed weanling piglets have been shown to have increased villus length, decreased crypt depth, and decreased intraepithelial lymphocytes compared with unsupplemented piglets (64). Villus height and crypt depth are used as indicators of mucosal turnover, and one could speculate that a decrease in intraepithelial lymphocytes may be related to a reduced apoptotic index in the intestine or adapted immune surveillance. However, the impact of MCFA and MCT on gut-associated immune function has not been thoroughly investigated, therefore, further research is needed to make a conclusive decision on their impact on nutritional immunomodulation. Although Wang et al. (65) have identified MCFA as ligands for the orphan receptor G-coupled protein receptor 84, a cell-signaling protein highly expressed in monocytes and macrophages, upon LPS activation.
As early as the 1960s, high dietary supplementation of MCFA was linked to potential antimicrobial regulation of gastric microbes (66). More recently, milk lipids, in particular MCFA and long-chain unsaturated fatty acids, in their FFA and monoglyceride forms, have been associated with antimicrobial and antiviral activity in gastric lining and proximal small intestine of mammalian neonates (66). Based on this ideology, weanling piglets were supplemented with high dietary MCT, which were found to modulate microbial gastric and intestinal populations in vitro and in vivo (64, 67, 68). In addition, Zentek et al. (66) showed that low dietary MCFA supplementation affected gastric microbial ecology and altered concentrations of SCFA in the small intestine.
PUFA.
LC-PUFA contribute to normal perinatal growth and development. In the past 10 y, there has been great interest in how LC-PUFA affect neonatal nutrition. In particular, the focus has been on the balance between (n-3) and (n-6) LC-PUFA and how the dietary ratio affects perinatal brain and retinal development as well as immune function involved in overall neonatal health (69). Modification of dietary LC-PUFA intake greatly affects membrane structure through incorporation into cellular membrane phospholipids in many tissues, including the brain, retina, and intestine (70–72). Membrane LC-PUFA are synthesized from dietary essential fatty acids and then can be metabolized to produce prostanoids, which are essential in many cellular functions. Approximately 30–35% of fatty acids comprising small intestine membranes are dietary essential fatty acids and cannot be synthesized de novo. The majority of these essential fatty acids are of the (n-6) series, including linoleic acid and arachidonic acid (ARA). ARA (n-6) is a natural component of breast milk, and in 2002, on FDA approval, began being added to infant formulas along with DHA (n-3). Defining supplemental doses of ARA and DHA similar to breast milk can be challenging in the case of DHA because of the variability found in breast milk, which is directly dependent on the mother’s diet (69).
Modification of (n-3) LC-PUFA concentrations in mother’s milk has been demonstrated in pig studies in which the sows were supplemented with (n-3) LC-PUFA at the beginning of gestation or in late gestation and continued supplementation through a minimum of 14 d of piglet suckling (73–76). In addition to changes in sow's milk composition in these studies, Boudry et al. (73) demonstrated higher (n-3) LC-PUFA levels in maternal and piglet red blood cells at birth and ileum fatty acid composition 7 d after birth. Moreover, (n-3) LC-PUFA supplementation decreased villi height and crypt cell depth and mast cell degranulation compared with lard-fed animals (73). Nutritional immunomodulation was also seen in sows fed supplemental (n-3) LC-PUFA, increasing suckling piglets (n-3) LC-PUFA concentrations in mesenteric lymph nodes and spleen, with marginal changes in CD4-CD8+ lymphocyte subpopulations (76). In addition to modulation of immune function, in utero and postnatal suckling exposure to (n-3) LC-PUFA also enhances intestinal glucose absorption in the weaned piglets via increased jejunal glucose transporter 2 membrane protein and increased expression of sodium-dependent glucose cotransporter 1 with mechanisms related to the cellular energy–sensing kinase, AMP-activated protein kinase at piglet weaning (75, 77).
Some early studies of dietary PUFA supplementation in suckling neonatal pigs examined its impact on recovery after an intestinal insult. Malnutrition results in shortened villi, mucosa atrophy, and decreases in digestive function (78). Malnourished piglets fed a recovery diet supplemented with PUFA (including ARA) recovered small intestine digestive activity and morphology more rapidly than piglets fed an unsupplemented diet supplying only linoleic and linolenic acids (79). Cell culture studies showed that ARA significantly enhanced cellular migration of the rat intestinal epithelial cell line 6 after razor wounding, a model of intestinal restitution (78). Further examination revealed greater prostaglandin E2 production in ARA-supplemented cultures, with ARA-stimulated restitution being attenuated by cyclooxygenase 2 inhibitors (80), demonstrating that enrichment of intestinal cells with ARA increases prostaglandin E2 production and stimulates restitution. These observations indicate the benefits of PUFA for robust development and function of the small intestine and clearly indicate a functional role of ARA in facilitating recovery of damaged intestinal mucosa. Previous studies in our laboratory showed that prostanoids stimulate rapid recovery of the gut barrier function, as measured by transepithelial resistance, and restore baseline levels of permeability after ischemic injury (81). Blikslager et al. (82, 83) have accumulated extensive evidence indicating that prostanoids orchestrate recovery of paracellular resistance within restituting epithelium (82, 83). Recently, we showed that ARA (n-6), when included as 5% of the diet (percentage of total fatty acids), reduces histological lesions, increases transepithelial resistance recovery, and inhibits mucosal-to-serosal flux after ileal ischemia (84). The beneficial effects of (n-6) LC-PUFA go against the central dogma in the literature that ascribes (n-6) PUFA as proinflammatory and “bad” and (n-3) PUFA as anti-inflammatory and “good.” One could argue that inflammation is not bad, but the inability of a neonate to resolve inflammation that causes aberrant intestinal problems. In fact, given the immense and constant antigenic load of the intestine, the ability of the mucosal immune system to monitor the luminal microenvironment, mount a proinflammatory response, and resolve the response is the appropriate goal. As with any nutrient, defining the ratio of (n-6)/(n-3) dietary LC-PUFA and understanding the changes in the ratio under differing physiological health status will be the long-term goal.
SCFA, prebiotics, and probiotics.
SCFA are a group of intestine-specific fuels produced by microbial fermentation of diet-resistant carbohydrates and fiber (85). Acetate, propionate, and butyrate are the major SCFA accounting for ∼85% of luminal SCFA production at a molar ratio of 60:25:15 (86). Among their many properties, SCFA are quickly absorbed by the colonic mucosa, relatively high in energy content, readily metabolized by the intestinal epithelium and liver, trophic to intestinal mucosa even when administered by PN, and a potent stimulus of Na and H2O absorption in the colon (12). The mechanisms supporting these SCFA properties all participate in improving neonatal intestinal health and promoting growth and development of the neonate. The effects of SCFA on colonic electrolyte uptake are thought to modulate diarrheal episodes associated with starvation and refeeding in a rat model (87). It has been known for some time that intraluminal infusion of SCFA stimulates colonic mucosal proliferation in multiple species in a dose-dependent manner, with varying potency among the SCFA (butyrate > propionate > acetate) (88). More recently, Bartholome et al. (89) demonstrated that butyrate-supplemented TPN given to neonatal piglets after intestinal bowel resection surgery prevented TPN-associated small intestine mucosal atrophy and augmented structural indices of GI adaptation. Specifically, SCFA increase epithelial surface area, increase enterocyte proliferation, inhibit apoptosis, and concomitantly increase mucosal glucose, amino acid, and dipeptidase transporters (89–91). Tappenden et al. (92) reported that the epithelial structure and function changes are linked to increased plasma GLP-2 in response to systemic butyrate. Increased production of GLP-2 has significant trophic implications for the intestine. Sangild et al. (93) found that cesarean-delivered neonatal piglets receiving TPN + GLP-2 had larger small intestines with greater sodium-dependent glucose cotransporter 1 mRNA expression and higher capacity for absorbance. Moreover, the intestines of GLP-2 piglets were comparable in size and absorptive capacities with those of sow-reared piglets. Taken together, supplementing enteral butyrate to promote infant intestinal adaptation is an attractive idea, except that SCFA are extremely volatile and have a rancid smell. Therefore, alternative methods for shifting luminal SCFA profiles are needed.
One alternative is the use of supplemental dietary prebiotics and/or probiotics. Prebiotics are selectively fermented nondigestible ingredients that allow specific changes in both the luminal microbial population and their activity to promote intestinal health by being fermented to SCFA (94). Prebiotics are classified not only by their selective stimulation of bacterial growth but also by their fermentation properties that have equally important roles in GI homeostasis (95). In addition to changes in the microbial profiles, the rate of gas production and SCFA profiles are also considered. These responses to fermentation are related to an individual’s GI microbial profile but also to the prebiotic’s chemical structure (95). Prebiotics are glycoside-linked monosaccharides (glucose, fructose, galactose, and xylose) with varying degrees of polymerization. Studies suggest the longer chain length prebiotics have slower fermentation rates and are less bifidogenic than shorter chain prebiotics (95). Some of the common prebiotics being characterized are short-chain fructo-oligosaccharides (scFOS), fructo-oligosaccharide (FOS), galacto-oligosaccharide (GOS), polydextrose (PDX), lacto-N-neotetraose, and human milk oligosaccharide (HMO). Human milk has been shown to be the gold standard in infant nutrition, providing nutrients and bioactive factors involved in immunomodulation (13, 96). Human milk is unique compared with other species when considering the oligosaccharide complexity; HMO concentration ranges from 10- to 100-fold higher than other species (96). Understanding prebiotic structures, fermentation patterns, and facilitation of bacterial selection can be further used to facilitate the bacterial selection by using prebiotic and probiotic combinations in formula. Probiotics are living microorganisms that, when ingested at a significant dose, can exert health benefits in addition to basic nutrition (97). Prebiotics, probiotics, and, perhaps more importantly, their symbiotic relationship in changing the microenvironment of the intestinal lumen provide great potential for developing infant supplemental nutrition protocols allowing optimal growth and development under less than ideal situations.
Recent work in supplemental pre- and probiotics in the neonatal piglet shows promising results from these functional nutrients. In 2002, Correa-Matos et al. (98) demonstrated the addition of fermentable substrates to formula-fed piglets reduced recovery time and improved infection-associated symptoms in piglets after Salmonella typhimurium infections by reducing diarrhea and increasing ileal enzyme activities, transepithelial resistance, glutamine transport, and colonic SCFA concentrations. Additionally, studies from our lab have shown that PDX supplementation in formula-fed piglets can change the cecal and colonic environment by increasing Lactobacilli and decreasing luminal pH in a dose-dependent manner (99, 100), the latter resulting from linear increases in propionate and lactic acid concentrations in the cecum. Lactobacillus strains from weanling pigs were previously shown to produce high levels of lactic acid compared with other organic acids in the absence of excess fermentable carbohydrates (101). Although most research suggests that the beneficial effects of prebiotics are weighted heavily in the changes of SCFA concentrations on intestinal adaptation, there is research suggesting that lactic acid can help maintain intestinal health (102–104). In fact, in an NEC model, piglets fed lactose had significantly higher colonic lactic acid concentrations, lower butyrate concentrations, and fewer incidences of NEC (104). In addition to lactic acid production, there are other potential benefits of PDX. Monaco et al. (105) demonstrated that PDX, GOS, or PDX+GOS formula-fed piglets do not have an increased risk of bacterial translocation compared with piglets fed formula only.
Understanding the interaction between luminal bacteria or supplemented probiotics and the fermentable carbohydrate sources represents a critical crossroads for the clinical use of prebiotic and/or probiotics in cases of intestinal dysfunction. Barnes et al. (106) used a piglet model of bowel resection to evaluate the effect of the prebiotic, scFOS, the probiotic Lactobacillus rhamnosus GG (LGG), and the symbiotic scFOS + LGG to evaluate a method to possibly administer butyrate to augment intestinal adaptation. These piglets received 80% nutritional supplementation from PN and 20% from EN. The study showed that scFOS and symbiotic supplementation enhanced structure and function of the intestine, but LGG alone or in the symbiotic treatment did not produce additive effects (106). This study reinforces the beneficial use of prebiotics in the delivery of SCFA for intestinal adaptation, but additionally emphasizes the need for greater understanding of prebiotic fermentation patterns with specific interest in luminal microbial populations. Li et al. (107) addressed part of this equation by demonstrating via in vitro fermentation that the composition of GI microbiota is influenced by piglet diet and age, which led to differing carbohydrate fermentation patterns for HMO, lacto-N-neotetraose, PDX/GOS, and scFOS. More studies elucidating the fermentation patterns and bacterial selections will be valuable in determining nutritional support combinations for individualized dietary treatment in GI compromised infants.
Conclusions and future directions
An understanding of the dependent relationships (Fig. 1) among nutrients, mode of delivery, microbes, and the specific physiological state of the intestinal epithelium are what must be dissected to optimize nutritional support protocols and provide optimal outcomes for infants with compromised intestinal integrity. Functional nutrients are essential parts of this equation, and defining each component’s role in GI growth and development is critical for this optimization (3, 5, 12). Human milk seems to be the standard to strive for in infant nutrition because of its abundant functional nutrients and bioactive components involved in developing the infant’s mucosal immune system and barrier function (13, 96). Although not addressed in detail in this review, nutrient stimulation of systemic growth factors and cytokines will also provide critical information in determining optimal nutritional support. An ever-changing target of individual genetics, age, and daily-changing status of the infant’s GI health makes the need for continuing research, both clinical and basic, necessary to answer these challenging questions. Vigilance will be necessary to design successful EN and PN protocols that use the knowledge of functional macro- and micronutrients with the emergence of pre- and probiotic supplements that seem to have great promise in furthering the ability of neonatologist to provide successful outcomes for GI-compromised infants.
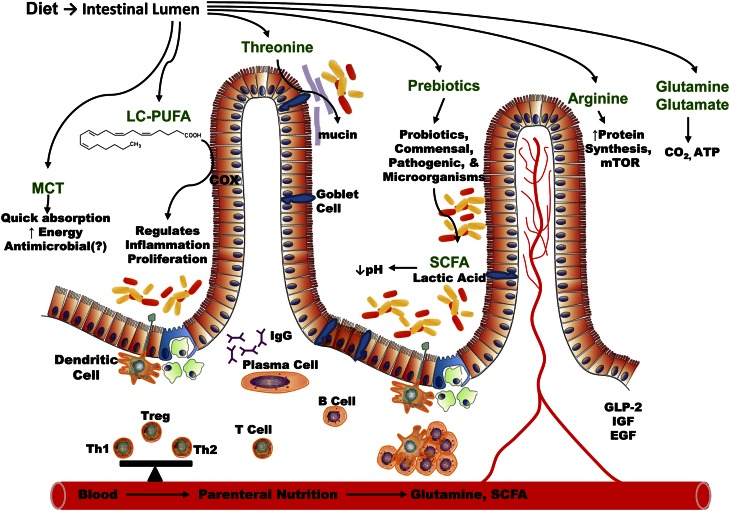
Figure 1.
Macronutrients, prebiotic and probiotic interactions in the lumen affecting nutrient digestion, gut microbiota, and mucosal immunology during growth and development. COX, cyclooxygenase; EGF, epidermal growth factor; GLP-2, glucagon-like peptide 2; IGF, insulin-like growth factor; LC-PUFA, long-chain PUFA; MCT, medium-chain triglycerides; mTOR, mammalian target of rapamycin; Th1, T helper type 1 cells; Th2, T helper type 2 cells; Treg, regulatory T cells. Adapted from Reference (12) with permission.
Acknowledgments
Both authors have read and approved the final manuscript.
Footnotes
Supported in part by Cooperative State Research, Education and Extension Service, USDA-National Research Initiative grant no. 2005-35200-16174 and by the North Carolina Agriculture Research Service.
Author disclosures: S. K. Jacobi and J. Odle, no conflicts of interest.
Abbreviations used: ARA, arachidonic acid; Arg, arginine; EN, enteral nutrition; GI, gastrointestinal; Gln, glutamine; GLP-2, glucagon-like peptide-2; Glu, glutamate; GOS, galacto-oligosaccharide; HMO, human milk oligosaccharide; neonatal pig intestinal epithelial cells; LC-PUFA, long-chain PUFA; LGG, Lactobacillus rhamnosus GG; MCFA, medium-chain fatty acids; MCT, medium-chain triglyceride; mTOR, mammalian target of rapamycin; MUC2, mucin 2; NEC, necrotizing enterocolitis; NO, nitric oxide; PDX, polydextrose; PN, parenteral nutrition; p70s6k, p70S6 kinase; scFOS, short-chain fructo-oligosaccharide; TPN, total parental nutrition.
Literature Cited
- 1. USDA. Part III: Reference of swine health and environmental management in the United States, 2006. 2008 Mar 1.
- 2.AGA American Gastroenterological Association Medical Position Statement: guidelines on intestinal ischemia. Gastroent. 2000;118:953. [DOI] [PubMed] [Google Scholar]
- 3.Buddington RK, Sangild PT. Companion Animals Symposium: development of the mammalian gastrointestinal tract, the resident microbiota, and the role of diet in early Life. J Anim Sci. 2011;89:1506–19 [DOI] [PubMed] [Google Scholar]
- 4.Buddington RK. Bacterial influences on mammalian gut development. J Dairy Sci. 2010;93:263 [Google Scholar]
- 5.Neu J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am J Clin Nutr. 2007;85:629S–34S [DOI] [PubMed] [Google Scholar]
- 6.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–20 [DOI] [PubMed] [Google Scholar]
- 7.Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr Res. 2011;69:465–72 [DOI] [PubMed] [Google Scholar]
- 8.Klein N, Schwertmann A, Peters M, Kunz C, Strobel S. Immunomodulatory effects of breast milk oligosaccharides.. Adv Exp Med Biol. 2000;478:251–9 [DOI] [PubMed] [Google Scholar]
- 9.Burrin DG, Stoll B, Jiang RH, Chang XY, Hartmann B, Holst JJ, Greeley GH, Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr. 2000;71:1603–10 [DOI] [PubMed] [Google Scholar]
- 10.Bjornvad CR, Thymann T, Deutz NE, Burrin DG, Jensen SK, Jensen BB, Molbak L, Boye M, Larsson LI, Schmidt M, et al. Enteral feeding induces diet-dependent mucosal dysfunction, bacterial proliferation, and necrotizing enterocolitis in preterm pigs on parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1092–103 [DOI] [PubMed] [Google Scholar]
- 11.Odle J, Zijlstra RT, Donovan SM. Intestinal effects of milkborne growth factors in neonates of agricultural importance. J Anim Sci. 1996;74:2509–22 [DOI] [PubMed] [Google Scholar]
- 12.Burrin DG, Stoll B. Key nutrients and growth factors for the neonatal gastrointestinal tract. Clin Perinatol. 2002;29:65. [DOI] [PubMed] [Google Scholar]
- 13.Walker A. Breast milk as the gold standard for protective nutrients. J Pediatr. 2010;156:S3–7 [DOI] [PubMed] [Google Scholar]
- 14.Sangild PT. Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med (Maywood). 2006;231:1695–711 [DOI] [PubMed] [Google Scholar]
- 15.Sangild PT, Petersen YM, Schmidt M, Elnif J, Petersen TK, Buddington RK, Greisen G, Michaelsen KF, Burrin DG. Preterm birth affects the intestinal response to parenteral and enteral nutrition in newborn pigs. J Nutr. 2002;132:2673–81 [DOI] [PubMed] [Google Scholar]
- 16.Tappenden KA. Quest for excellence: inspiration from the ileum. JPEN J Parenter Enteral Nutr. 2010;34:716–22 [DOI] [PubMed] [Google Scholar]
- 17.Burrin DG, Stoll B, Jiang R, Petersen YM, Buddington RK, Schmidt M, Holst JJ, Hartmann B, Sangild PT. Glucagon-like peptide-2 stimulates intestinal growth by decreasing proteolysis and apoptosis in TPN-fed preterm piglets. Gastroent. 2000;118:A546. [DOI] [PubMed] [Google Scholar]
- 18.Tappenden KA. Mechanisms of enteral nutrient-enhanced intestinal adaptation. Gastroenterology. 2006;130 (2 Suppl 1):S93–9 [DOI] [PubMed] [Google Scholar]
- 19.Stoll B, Horst DA, Cui LW, Chang XY, Ellis KJ, Hadsell DL, Suryawan A, Kurundkar A, Maheshwari A, Davis TA, et al. chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J Nutr. 2010;140:2193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoll B, Puiman PJ, Cui L, Chang X, Benight NM, Bauchart-Thevret C, Hartmann B, Holst JJ, Burrin DG. Continuous parenteral and enteral nutrition induces metabolic dysfunction in neonatal pigs. JPEN J Parenter Enteral Nutr. Epub 2012. May 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu G. Functional amino acids in growth, reproduction, and health. Adv Nutr. 2010;1:31–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17 [DOI] [PubMed] [Google Scholar]
- 23.Marc Rhoads J, Wu GY. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids. 2009;37:111–22 [DOI] [PubMed] [Google Scholar]
- 24.Jahan-Mihan A, Luhovyy BL, El Khoury D, Anderson GH. Dietary proteins as determinants of metabolic and physiologic functions of the gastrointestinal tract nutrients. 2011;3:574–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu G, Bazer FW, Davis TA, Kim SW, Li P, Rhoads JM, Satterfield MC, Smith SB, Spencer TE, Yin YL. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu G, Morris SM. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhoads JM, Chen W, Gookin J, Wu GY, Fu Q, Blikslager AT, Rippe RA, Argenzio RA, Cance WG, Weaver EM, et al. Arginine stimulates intestinal cell migration through a focal adhesion kinase dependent mechanism. Gut. 2004;53:514–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhoads M, Fu Q, Rippe R, Odle J, Graves LM. Focal adhesion kinase (FAK) and p70 s6 kinase are critical for arginine stimulated intestinal cell migration. J Investig Med. 2004;52:S291–2 [Google Scholar]
- 29.Di Lorenzo M, Bass J, Krantis A. Use of L-arginine in the treatment of experimental necrotizing enterocolitis. J Pediatr Surg. 1995;30:235–40; discussion 240–1 [DOI] [PubMed] [Google Scholar]
- 30.Kansagra K, Stoll B, Rognerud C, Niinikoski H, Ou CN, Harvey R, Burrin D. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1162–70 [DOI] [PubMed] [Google Scholar]
- 31.Niinikoski H, Stoll B, Guan XF, Kansagra K, Lambert BD, Stephens J, Hartmann B, Holst JJ, Burrin DG. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-Fed neonatal piglets. J Nutr. 2004;134:1467–74 [DOI] [PubMed] [Google Scholar]
- 32.Albin DM, Wubben JE, Rowlett JM, Tappenden KA, Nowak RA. Changes in small intestinal nutrient transport and barrier function after lipopolysaccharide exposure in two pig breeds. J Anim Sci. 2007;85:2517–23 [DOI] [PubMed] [Google Scholar]
- 33.Sayan H, Ozacmak VH, Altaner S, Aktas RG, Arslan SO. Protective effects of L-arginine on rat terminal ileum subjected to ischemia/reperfusion. J Pediatr Gastroenterol Nutr. 2008;46:29–35 [DOI] [PubMed] [Google Scholar]
- 34.Fukatsu K, Ueno C, Maeshima Y, Hara E, Nagayoshi H, Omata J, Mochizuki H, Hiraide H. Effects of L-arginine infusion during ischemia on gut blood perfusion, oxygen tension, and circulating myeloid cell activation in a murine gut ischemia/reperfusion model. JPEN J Parenter Enteral Nutr. 2004;28:224–30 [DOI] [PubMed] [Google Scholar]
- 35.Puiman PJ, Stoll B, van Goudoever JB, Burrin DG. Enteral arginine does not increase superior mesenteric arterial blood flow but induces mucosal growth in neonatal pigs. J Nutr. 2011;141:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spanos CP, Papaconstantinou P, Spanos P, Karamouzis M, Lekkas G, Papaconstantinou C. The effect of L-arginine and aprotinin on intestinal ischemia-reperfusion injury. J Gastrointest Surg. 2007;11:247–55 [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Huang JJ, Hou YQ, Zhu HL, Zhao SJ, Ding BY, Yin YL, Yi GF, Shi JX, Fan W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr. 2008;100:552–60 [DOI] [PubMed] [Google Scholar]
- 38.Rhoads JM, Corl BA, Harrell R, Niu XM, Gatlin L, Phillips O, Blikslager A, Moeser A, Wu GY, Odle J. Intestinal ribosomal p70(S6K) signaling is increased in piglet rotavirus enteritis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G913–22 [DOI] [PubMed] [Google Scholar]
- 39.Corl BA, Odle J, Niu XM, Moeser AJ, Gatlin LA, Phillips OT, Blikslager AT, Rhoads JM. Arginine activates intestinal p70(S6k) and protein synthesis in piglet rotavivrus enteritis. J Nutr. 2008;138:24–9 [DOI] [PubMed] [Google Scholar]
- 40.Bauchart-Thevret C, Cui LW, Wu GY, Burrin DG. Arginine-induced stimulation of protein synthesis and survival in IPEC-J2 cells is mediated by mTOR but not nitric oxide. Am J Physiol Endocrinol Metab. 2010;299:E899–909 [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Zhang L, Zhou G, Liao Z, Ahmad H, Liu W, Wang T. Dietary l-arginine supplementation improves the intestinal development through increasing mucosal Akt and mammalian target of rapamycin signals in intra-uterine growth retarded piglets. Br J Nutr. 2012;5:1–11 [DOI] [PubMed] [Google Scholar]
- 42.Kim SW, Wu GY. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr. 2004;134:625–30 [DOI] [PubMed] [Google Scholar]
- 43.Gookin JL, Foster DM, Coccaro MR, Stauffer SH. Oral delivery of L-arginine stimulates prostaglandin-dependent secretory diarrhea in Cryptosporidium parvum-infected neonatal piglets. J Pediatr Gastroenterol Nutr. 2008;46:139–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li P, Yin YL, Li D, Kim SW, Wu GY. Amino acids and immune function. Br J Nutr. 2007;98:237–52 [DOI] [PubMed] [Google Scholar]
- 45.Bailey M, Haverson K, Inman C, Harris C, Jones P, Corfield G, Miller B, Stokes C. The development of the mucosal immune system pre- and post-weaning: balancing regulatory and effector function. Proc Nutr Soc. 2005;64:451–7 [DOI] [PubMed] [Google Scholar]
- 46.Domeneghini C, Di Giancamillo A, Arrighi S, Bosi G. Gut-trophic feed additives and their effects upon the gut structure and intestinal metabolism. State of the art in the pig, and perspectives towards humans. Histol Histopathol. 2006;21:273–83 [DOI] [PubMed] [Google Scholar]
- 47.Wang WW, Qiao SY, Li DF. Amino acids and gut function. Amino Acids. 2009;37:105–10 [DOI] [PubMed] [Google Scholar]
- 48.Mareskes C. Therapeutic effects of oral rehydration solution (ORS) and L-glutamine (GLN) on porcine rotaviral enteritis. Thesis, North Carolina State University. 1997. p. A401.
- 49.Burrin DG, Janeczko MJ, Stoll B. Emerging aspects of dietary glutamate metabolism in the developing gut. Asia Pac J Clin Nutr. 2008;17:368–71 [PubMed] [Google Scholar]
- 50.Burrin DG, Stoll B. Metabolic fate and function of dietary glutamate in the gut. Am J Clin Nutr. 2009;90:850S–6S [DOI] [PubMed] [Google Scholar]
- 51.Bertolo RFP, Chen CZL, Law G, Pencharz PB, Ball RO. Threonine requirement of neonatal piglets receiving total parenteral nutrition is considerably lower than that of piglets receiving an identical diet intragastrically. J Nutr. 1998;128:1752–9 [DOI] [PubMed] [Google Scholar]
- 52.Stoll B, Burrin DG, Henry J, Yu H, Jahoor F, Reeds PJ. Substrate oxidation by the portal drained viscera of fed piglets. J Physiol. 1999;277:E168–75 [DOI] [PubMed] [Google Scholar]
- 53.Van Klinken BJW, Tytgat KMAJ, Buller HA, Einerhand AWC, Dekker J. Biosynthesis of intestinal mucins: MUC1, MUC2, MUC3 and more. Biochem Soc Trans. 1995;23:814–8 [DOI] [PubMed] [Google Scholar]
- 54.Van Klinken BJW, Dekker J, Buller HA, Einerhand AWC. Mucin gene structure and expression - protection vs adhesion. Am J Physiol. 1995;269:G613–27 [DOI] [PubMed] [Google Scholar]
- 55.Gum JR. Mucin genes and the proteins they encode - structure, diversity, and regulation. Am J Respir Cell Mol Biol. 1992;7:557–64 [DOI] [PubMed] [Google Scholar]
- 56.Gum JR, Jr, Hicks JW, Toribara NW, Rothe EM, Lagace RE, Kim YS. The human MUC2 intestinal mucin has cysteine-rich subdomains located both upstream and downstream of its central repetitive region. J Biol Chem. 1992. Oct 25;267(30):21375–83 [PubMed] [Google Scholar]
- 57.Law G, Chen CZL, Bertolo RFP, Ball RO, Pencharz PB. Threonine requirement is lower in neonatal piglets receiving intravenous (IV) (total parenteral nutrition, TPN) compared to intragastric (IG) feeding. FASEB J. 1998;12:A858 [Google Scholar]
- 58.Nichols NL, Bertolo RF. Luminal threonine concentration acutely affects intestinal mucosal protein and mucin synthesis in piglets. J Nutr. 2008;138:1298–303 [DOI] [PubMed] [Google Scholar]
- 59.Puiman PJ, Jensen M, Stoll B, Renes IB, de Bruijn ACJM, Dorst K, Schierbeek H, Schmidt M, Boehm G, Burrin DG, et al. Intestinal threonine utilization for protein and mucin synthesis is decreased in formula-fed preterm pigs. J Nutr. 2011;141:1306–11 [DOI] [PubMed] [Google Scholar]
- 60.Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73 [DOI] [PubMed] [Google Scholar]
- 61.Odle J. New insights into the utilization of medium-chain triglycerides by the neonate: Observations from a piglet model. J Nutr. 1997;127:1061–7 [DOI] [PubMed] [Google Scholar]
- 62.Price KL. Improving fat utilization by the weaned pig: effect of diet physical form, fatty acid chain length, and emulsification [Thesis]. Raleigh (NC): North Carolina State University; 2007 [Google Scholar]
- 63.Takase S, Goda T. Effects of medium-chain triglycerides on brush border membrane-bound enzymes activity in rat small intestine. J Nutr. 1990;120:969–76 [DOI] [PubMed] [Google Scholar]
- 64.Dierick N, Michiels J, Van Nevel C. Effect of medium chain fatty acids and benzoic acid, as alternatives for antibiotics, on growth and some gut parameters in piglets. Commun Agric Appl Biol Sci. 2004;69:187–90 [PubMed] [Google Scholar]
- 65.Wang J, Wu X, Simonavicius N, Tian H, Ling L. Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J Biol Chem. 2006;281:34457–64 [DOI] [PubMed] [Google Scholar]
- 66.Zentek J, Buchheit-Renko S, Manner K, Pieper R, Vahjen W. Intestinal concentrations of free and encapsulated dietary medium-chain fatty acids and effects on gastric microbial ecology and bacterial metabolic products in the digestive tract of piglets. Arch Anim Nutr. 2012;66:14–26 [DOI] [PubMed] [Google Scholar]
- 67.Dierick NA, Decuypere JA, Molly K, Van Beek E, Vanderbeke E. The combined use of triacylglycerols (TAGs) containing medium chain fatty acids (MCFAs) and exogenous lipolytic enzymes as an alternative to nutritional antibiotics in piglet nutrition - II. In vivo release of MCFAs in gastric cannulated and slaughtered piglets by endogenous and exogenous lipases; effects on the luminal gut flora and growth performance. Livest Prod Sci. 2002;76:1–16 [Google Scholar]
- 68.Dierick NA, Decuypere JA, Molly K, Van Beek E, Vanderbeke E. The combined use of triacylglycerols containing medium-chain fatty acids (MCFAs) and exogenous lipolytic enzymes as an alternative for nutritional antibiotics in piglet nutrition I. In vitro screening of the release of MCFAs from selected fat sources by selected exogenous lipolytic enzymes under simulated pig gastric conditions and their effects on the gut flora of piglets. Livest Prod Sci. 2002;75:129–42 [Google Scholar]
- 69.Fleith M, Clandinin MT. Dietary PUFA for preterm and term infants: review of clinical studies. Crit Rev Food Sci Nutr. 2005;45:205–29 [DOI] [PubMed] [Google Scholar]
- 70.Clandinin MT. Brain development and assessing the supply of polyunsaturated fatty acid. Lipids. 1999;34:131–7 [DOI] [PubMed] [Google Scholar]
- 71.Suh M, Wierzbicki AA, Lien EL, Clandinin MT. Dietary 20:4n-6 and 22:6n-3 modulates the profile of long- and very-long-chain fatty acids, rhodopsin content, and kinetics in developing photoreceptor cells. Pediatr Res. 2000;48:524–30 [DOI] [PubMed] [Google Scholar]
- 72.Hess HA, Corl BA, Lin X, Jacobi SK, Harrell RJ, Blikslager AT, Odle J. Enrichment of intestinal mucosal phospholipids with arachidonic and eicosapentaenoic acids fed to suckling piglets is dose and time dependent. J Nutr. 2008;138:2164–71 [DOI] [PubMed] [Google Scholar]
- 73.Boudry G, Douard V, Mourot J, Lalles JP, Huerou-Luron I. Linseed oil in the maternal diet during gestation and lactation modifies fatty acid composition, mucosal architecture, and mast cell regulation of the ileal barrier in piglets. J Nutr. 2009;139:1110–7 [DOI] [PubMed] [Google Scholar]
- 74.Leonard SG, Sweeney T, Bahar B, Lynch BP, O'Doherty JV. Effect of maternal fish oil and seaweed extract supplementation on colostrum and milk composition, humoral immune response, and performance of suckled piglets. J Anim Sci. 2010;88:2988–97 [DOI] [PubMed] [Google Scholar]
- 75.Gabler NK, Spencer JD, Webel DM, Spurlock ME. In utero and postnatal exposure to long chain (n-3) PUFA enhances intestinal glucose absorption and energy stores in weanling pigs. J Nutr. 2007;137:2351–8 [DOI] [PubMed] [Google Scholar]
- 76.Binter C, Khol-Parisini A, Gerner W, Schafer K, Hulan HW, Saalmuller A, Zentek J. Effect of maternally supplied n-3 and n-6 oils on the fatty acid composition and mononuclear immune cell distribution of lymphatic tissue from the gastrointestinal tract of suckling piglets. Arch Anim Nutr. 2011;65:341–53 [DOI] [PubMed] [Google Scholar]
- 77.Gabler NK, Radcliffe JS, Spencer JD, Webel DM, Spurlock ME. Feeding long-chain n-3 polyunsaturated fatty acids during gestation increases intestinal glucose absorption potentially via the acute activation of AMPK. J Nutr Biochem. 2009;20:17–25 [DOI] [PubMed] [Google Scholar]
- 78.Lopez-Pedrosa JM, Torres MI, Fernandez MI, Rios A, Gil A. Severe malnutrition alters lipid composition and fatty acid profile of small intestine in newborn piglets. J Nutr. 1998;128:224–33 [DOI] [PubMed] [Google Scholar]
- 79.López-Pedrosa JM, Ramirez M, Torres MI, Gil A. Dietary phospholipids rich in long-chain polyunsaturated fatty acids improve the repair of small intestine in previously malnourished piglets. J Nutr. 1999;129:1149–55 [DOI] [PubMed] [Google Scholar]
- 80.Ruthig DJ, Meckling-Gill KA. N-3 and n-6 fatty acids stimulate restitution by independent mechanisms in the IEC-6 model of intestinal wound healing. J Nutr Biochem. 2002;13:27–35 [DOI] [PubMed] [Google Scholar]
- 81.Blikslager AT, Roberts MC, Rhoads JM, Argenzio RA. Prostaglandins I-2 and E-2 have a synergistic role in rescuing epithelial barrier function in porcine ileum. J Clin Invest. 1997;100:1928–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blikslager AT, Roberts MC, Argenzio RA. Prostaglandin-induced recovery of barrier function in porcine ileum is triggered by chloride secretion. Am J Physiol. 1999;276:G28–36 [DOI] [PubMed] [Google Scholar]
- 83.Blikslager AT, Pell SM, Young KM. Synergistic signaling via cAMP and Ca2+ (I)-coupled EP receptors is required for PGE(2)-triggered recovery of intestinal barrier function. Gastroenterol. 2000;118:A821 [Google Scholar]
- 84.Jacobi SK, Moeser AJ, Corl BA, Harrell RJ, Blikslager AT, Odle J. Dietary long-chain PUFA enhance acute repair of ischemic-injured intestine of suckling pigs. J Nutr. 2012; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bugaut M, Bentejac M. Biological effects of short-chain fatty-acids in nonruminant mammals. Annu Rev Nutr. 1993;13:217–41 [DOI] [PubMed] [Google Scholar]
- 86.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–64 [DOI] [PubMed] [Google Scholar]
- 87.Binder HJ, Mehta P. Short-chain fatty-acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology. 1989;96:989–96 [DOI] [PubMed] [Google Scholar]
- 88.Sakata T. Stimulatory effect of short-chain fatty-acids on epithelial-cell proliferation in the rat intestine - A possible explanation for trophic effects of fermentable fiber, gut microbes and luminal trophic factors. Br J Nutr. 1987;58:95–103 [DOI] [PubMed] [Google Scholar]
- 89.Bartholome AL, Albin DM, Baker DH, Holst JJ, Tappenden KA. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. JPEN J Parenter Enteral Nutr. 2004;28:210–22 [DOI] [PubMed] [Google Scholar]
- 90.Albin DM, Bartholome AL, Tappenden KA. Glucose transport is enhanced by short-chain fatty acid supplemented-total parenteral nutrition in a piglet model of intestinal adaptation. Proceedings of the 9th International Symposium on Digestive Physiology in Pigs. 2003 p. 220–2.
- 91.Albin DM, Bartholome AL, Tappenden KA. Amino acid and dipetide transport are enhanced by short-chain fatty acid supplemented total parenteral nutrition in a piglet model of intestinal adaptation. Proceedings of the 9th International Symposium on Digestive Physiology in Pigs. 2003 p. 241–3.
- 92.Tappenden KA, Albin DM, Bartholome AL, Mangian HF. Glucagon-like peptide-2 and short-chain fatty acids: A new twist to an old story. J Nutr. 2003;133:3717–20 [DOI] [PubMed] [Google Scholar]
- 93.Sangild PT, Tappenden KA, Malo C, Petersen YM, Elnif J, Bartholome AL, Buddington RK. Glucagon-like peptide 2 stimulates intestinal nutrient absorption in parenterally fed newborn pigs. J Pediatr Gastroenterol Nutr. 2006;43:160–7 [DOI] [PubMed] [Google Scholar]
- 94.Roberfroid M. Prebiotics: The concept revisited. J Nutr. 2007;137:830S–7S [DOI] [PubMed] [Google Scholar]
- 95.Hernot DC, Boileau TW, Bauer LL, Middelbos IS, Murphy MR, Swanson KS, Fahey GC. In vitro fermentation profiles, gas production rates, and microbiota modulation as affected by certain fructans, galactooligosaccharides, and polydextrose. J Agric Food Chem. 2009;57:1354–61 [DOI] [PubMed] [Google Scholar]
- 96.Donovan SM, Wang M, Li M, Friedberg I, Schwartz S, Chapkin R. Host-microbe interactions in the neonatal intestine: role of human milk oligosaccharides. Adv Nutr. 2012;3:450S–5S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weng MQ, Walker WA. Bacterial colonization, probiotics, and clinical disease. J Pediatr. 2006;149:S107–14 [Google Scholar]
- 98.Correa-Matos NJ, Donovan SM, Isaacson RE, Gaskins HR, White BA, Tappenden KA. Fermentable fiber reduces recovery time and improves intestinal function in piglets following Salmonella typhimurium infection. J Nutr. 2003;133:1845–52 [DOI] [PubMed] [Google Scholar]
- 99.Herfel TM, Jacobi SK, Lin X, Fellner V, Walker DC, Jouni ZE, Odle J. Polydextrose enrichment of infant formula demonstrates prebiotic characteristics by altering intestinal microbiota, organic acid concentrations, and cytokine expression in suckling piglets. J Nutr. 2011;141:2139–45 [DOI] [PubMed] [Google Scholar]
- 100.Herfel TM, Jacobi SK, Lin X, Walker DC, Jouni ZE, Odle J. Safety evaluation of polydextrose in infant formula using a suckling piglet model. Food Chem Toxicol. 2009;47:1530–7 [DOI] [PubMed] [Google Scholar]
- 101.Hacin B, Rogelj I, Matijasic BB. Lactobacillus isolates from weaned piglets’ mucosa with inhibitory activity against common porcine pathogens. Folia Microbiol (Praha). 2008;53:569–76 [DOI] [PubMed] [Google Scholar]
- 102.Ogawa K, Ben RA, Pons S, Depaolo MIL, Fernandez LB. Volatile fatty-acids, lactic-acid, and pH in the stools of breast-fed and bottle-fed infants. J Pediatr Gastroenterol Nutr. 1992;15:248–52 [DOI] [PubMed] [Google Scholar]
- 103.Lin J, Nafday SM, Chauvin SN, Magid MS, Pabbatireddy S, Holzman IR, Babyatsky MW. Variable effects of short chain fatty acids and lactic acid in inducing intestinal mucosal injury in newborn rats. J Pediatr Gastroenterol Nutr. 2002;35:545–50 [DOI] [PubMed] [Google Scholar]
- 104.Thymann T, Møller HK, Stoll B, Stoy ACF, Buddington RK, Bering SB, Jensen BB, Olutoye OO, Siggers RH, Mølbak L, et al. Carbohydrate maldigestion induces necrotizing enterocolitis in preterm pigs. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1115–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Monaco MH, Kashtanov DO, Wang M, Walker DC, Rai D, Jouni ZE, Miller MJ, Donovan SM. Addition of polydextrose and galactooligosaccharide to formula does not affect bacterial translocation in the neonatal piglet. J Pediatr Gastroenterol Nutr. 2011;52:210–6 [DOI] [PubMed] [Google Scholar]
- 106.Barnes JL, Hartmann B, Holst JJ, Tappenden KA. Intestinal adaptation is stimulated by partial enteral nutrition supplemented with the prebiotic short-chain fructooligosaccharide in a neonatal intestinal failure piglet model. J Parenter Enteral Nutr. Epub. 10 Apr 2012 [DOI] [PubMed] [Google Scholar]
- 107.Li M, Bauer LL, Chen X, Wang M, Kuhlenschmidt TB, Kuhlenschmidt MS, Fahey GC, Donovan SM. Microbial composition and in vitro fermentation patterns of human milk oligosaccharides and prebiotics differ between formula-fed and sow-reared piglets. J Nutr. 2012;142:681–9 [DOI] [PMC free article] [PubMed] [Google Scholar]