Abstract
Similar to clinically used antidepressants, cannabinoids can also regulate anxiety and depressive symptoms. Although the mechanisms of these effects are not completely understood, recent evidence suggests that changes in endocannabinoid system could be involved in some actions of antidepressants. Chronic antidepressant treatment modifies the expression of CB1 receptors and endocannabinoid (EC) content in brain regions related to mood and anxiety control. Moreover, both antidepressant and cannabinoids activate mitogen-activated protein (MAP) kinase and phosphoinositide 3-kinase(PI3-K)/Akt or PKB signaling, intracellular pathways that regulate cell proliferation and neural cell survival. Facilitation of hippocampal neurogenesis is proposed as a common effect of chronic antidepressant treatment. Genetic or pharmacological manipulations of cannabinoid receptors (CB1 and CB2) or enzymes responsible for endocannabinoid-metabolism have also been shown to control proliferation and neurogenesis in the hippocampus. In the present paper we reviewed the studies that have investigated the potential contribution of cannabinoids and neurogenesisto antidepressant effects. Considering the widespread brain distribution of the EC system, a better understanding of this possible interaction could contribute to the development of therapeutic alternatives to mood and anxiety disorders.
Keywords: Neurogenesis, antidepressant drugs, cannabinoids.
1. ADULT NEUROGENESIS
Until the early 60´s, a central dogma of neuroscience had been that no new neurons are added to the adult mammalian brain. For more than 100 years it has been assumed that neurogenesis, or the production of new neurons, occurs only during development and stops before puberty. Although the very first reports about neurogenesis came from Dr Rita Levi-Montalcini’s work with Nerve Growth Factor, it was Joseph Altmanin the early 60´s that published a series of papers presenting evidence that new neurons are added in specific regions of the young and adult rat brain, including the neocortex, hippocampal formation and olfactory bulb [1-3]. Subsequently, Eriksson and colleagues (1998) confirmed that new neurons are indeed generated in the hippocampus of adult humans [4] and established one of the most stimulating recent fields in neuroscience: neurogenesis in the adult brain.
Although a low proliferative activity has been reported in several brain regions such as the hypothalamus and the cell layers surrounding the third ventricle [5], a body of evidence supports the idea that in the adult mammalian brain only two regions show neurogenesis under physiological conditions: the subventricular zone (SVZ) of the lateral walls of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus of the hippocampal formation [6, 7].
Adult neurogenesis is a complex process that involves the initial division of a precursor cells and lasts until the existence of a new functionally new neuron. In the words of Dr. G. Kempermann: “neurogenesis is a process, not an event”. It can be more precisely defined as an in vivo process that involves division, survival (not all dividing cells will survive), migration and differentiation [7, 8].
The physiological impact of adult neurogenesis is not yet completely understood. And importantly its relevance and existence in humans is matter of debate. SVZ neurogenesis seems to be regulated by the olfactory experience of animals [9, 10]. Odor exposure can increase the survival of newborn neurons and improve memory in a learned odor discrimination task [11], suggesting that in this region neurogenesis plays a role in learning and memory processes related to olfactory stimulation [11]. In the hippocampus SGZ, another major site of adult neurogenesis [12, 13], an association between this process and learning and memory has been found in rodents and humans [14-17]. Moreover, stimuli known to improve learning and memory processes, such as voluntary running and exposure to enriched environments [16, 18], increase SGZ cell proliferation and the survival of new neurons generated in this region [19, 20]. As a consequence, hippocampal neurogenesis has been suggested to be important for at least some forms of learning and memory [14-17]. Despite these pieces of evidence, adult neurogenesis is not necessarily always good to brain function. For example, increased neurogenesis after hippocampus injury could be involved in the development of temporal seizures [7].
The hippocampal formation is not an homogenous structure, showing differential connectivity along its dorsal-ventral (septum-temporal) axis. It has been proposed that, while the dorsal portions of hippocampus have a preferential role in learning and memory, the ventral portions of the hippocampus are involved in affective behaviors [21]. Also, several lines of evidence suggest that, in addition to learning and memory process, adult hippocampal neurogenesis could play an important role in the genesis of psychiatric disorders such as anxiety, schizophrenia and mood disorders [22-25]. In this way, stressful experiences, that are closely related to the development of anxiety and mood disorders, down-regulate hippocampal neurogenesis [26]. More recently, Snyder and colleagues (2011) showed that DG, but not SVZ neurogenesis, impairs stress-induced depressive-like symptoms and facilitates the negative hippocampal influence on the hypothalamic-pituitary-adrenal (HPA) axis [27]. Interestingly, drugs used in the clinical practice to treat these psychiatry disorders, such as antidepressants or lithium, normalize or even increase hippocampal neurogenesis [24, 28-30]. Together these findings support the proposal that adult hippocampal neurogenesis, in addition to influencing learning and memory process, is also involved in the genesis of psychiatry disorders and could, therefore, be a therapeutic target in these disorders.
2. NEUROGENESIS AND ANTIDEPRESSANTS
The mechanism of action of antidepressants (AD) has been the focus of a large number of studies in the last 50 years. Most of these studies were based on the monoaminergic theory of depression [31-37]. However, in the last decade, a neurogenic mechanism of action for AD opened new venues of investigation, particularly because the latency for antidepressants clinical effects (2-4weeks) coincides with the minimum time course necessary for the maturation of new neurons in the dentate gyrus [38]. Initial studies have showed that subchronic and chronic, but not acute, treatment with different classes of AD, such as fluoxetine (selective serotonin reuptake inhibitor, SSRI), imipramine (tricyclic, TC), reboxetine (norepinephrine reuptake inhibitor, NRI), tranylcypromine (monoamine oxidase inhibitor, MAOI), venlafaxine (serotonin-norepinephrine reuptake inhibitor, SNRI) and others increase adult hippocampal neurogenesis (see Table 1) and, at the same time, cause antidepressive and anxiolytic effects and improvement of stress-disrupted responses [23, 28, 39].
Table 1.
Effect of Different Classes of Antidepressants on Adult Neurogenesis: in vitro and in vivo Studies
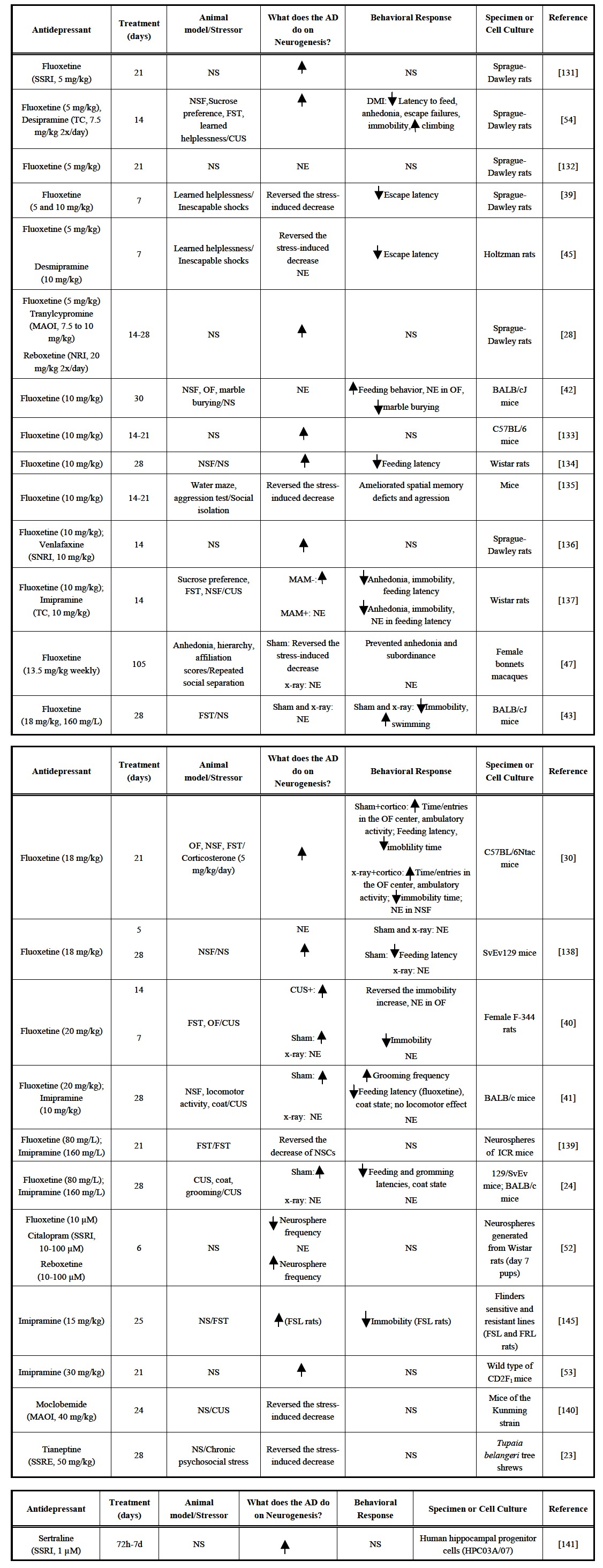 |
SSRI = selective serotonin reuptake inhibitor; TC = tricyclic; NRI = norepinephrine reuptake inhibitor; MAOI = monoamino oxidase inhibitor; SNRI = serotonin-norepinephrine reuptake inhibitor; SSRE = selective serotonin reubtake enhancer; NSF = novelty supressed feeding; FST = forced swimming test; OF = open field test; x-ray = x-irradiation of the subgranular zone of the dentate gyrus; sham = not irradiated animals; MAM = methylazoxymethanol; CUS = chronic unpredictable stress; NS = not studied; NE = no effect observed.
In 2003 Santarelli and colleagues published a landmark study showing that some behavioral effects of AD depend on neurogenesis in the subgranular zone of the dentate gyrus [24]. Chronic treatment with fluoxetine and imipramine induced anxiolytic-like effects in the novelty suppressed feeding test in control mice but not in animals that were submitted to x-ray-irradiation of the SGZ (SGZ-x-irradiation), a procedure that blunts neurogenesis by killing cells undergoing proliferation. Since then, other studies using different animal models have corroborated these results [40-41]. However, it is unlikely that neurogenesis facilitation explains all the behavioral effects of AD. For example, chronic treatment with fluoxetine induces anxiolytic responses in BALBc/J mice without interfering in neurogenesis [42]. Moreover, mice submitted to the SGZ-x-irradiation or methylazoxymethanol, a cytostatic agent used to arrest neurogenesis, showed similar antidepressive responses to fluoxetine than control animals [43]. It is probable, therefore, that depending on the animal model and species used, multiple mechanisms are responsible for the effects of AD.
Whereas most experimental data so far has suggested that a decrease of adult hippocampal neurogenesis is not directly responsible for depressive disorders [24, 40, 44] exposure to chronic stressors such as inescapable shocks, unpredictable stress, forced swim, social isolation and psychosocial conflict, decreases neuroproliferative processes in this brain region. Chronic AD treatment prevents this effect in different species such as rats, mice and primates [39, 40, 45-47]. In non-human primates, repeated social isolation, in addition to inducing depressive-like behaviors (anhedonia and subordinance), is also able to decrease cell proliferation and granule cell layer volume. Treatment with fluoxetine (15 weeks) prevented these effects in control animals but had no effect in SGZ-x-irradiated macaques, indicating neurogenesis-dependent action [47].
A question that remains open is how AD modulate neurogenesis. Most AD act by blocking monoamine uptake, and both serotonin and norepinephrine have been implicated in the increase of neuronal proliferation. A pioneer study showed that dl-fenfluramine, a compound that facilitates the release of 5-HT, promoted cellular division in the dentate gyrus, an effect that was blocked by the 5HT1A receptor antagonist, WAY100,635 [48]. Also, administration of different 5HT1A antagonists decreased the number of BrdU-immunoreactive cells in the dentate gyrus [49]. In 5HT1A receptors knockout animals treated chronically with fluoxetine, both hippocampal neurogenesis and anxiolytic-like responses were abolished [24]. The deletion of 5HT1A and 5HT1B receptors decreased the expression of genes involved in long-term potentiation and adult neurogenesis and reduced hippocampal neurons survival [50]. Norepinephrine also stimulates cell division. It increases the proliferation of neural precursor derived cells, an effect that is blocked by selective β2-receptor antagonists [51]. Moreover, AD selectively increase nor epinephrine activated adult hippocampal precursors via β3-adrenergic receptors and β-adrenergic agonists enhanced nestin-GFAP positive neurons [52]. Finally, activation of 5-HT and β-adrenergic receptors influences the expression of important factors that modulate neuronal synaptic remodeling, proliferation, maturation and survival, including the brain derived neurotrophic factor (BDNF, [53]), the vascular endothelial growth factor (VEGF, [54]), proteins belonging to the cAMP-CREB cascade [54, 55], the Wnt3a signaling [56] and the p21 protein [57].
3. CANNABINOIDS AND NEUROGENESIS
3.1. Cannabinoids and the Endocannabinoid System
Cannabinoids were first extracted from the plant Cannabis sativa, which has at least 60 components that belong to this class of substances [58-63]. The observation that the activity of psychotropic cannabinoids was intrinsically related to its chemical structure [62, 63] led to the hypothesis that cannabinoid receptors exist in the organism. Subsequently, the cloning of CB1 and CB2 receptors confirmed their presence in rats, mice and humans (Howlett et al., 2002) and their activation inhibit the enzyme adenylatecyclase through a Gi/o protein [64-66].
CB1 receptors are now considered the most abundant metabotropic receptor in the mammalian brain and are also present in peripheral tissues [67]. Immunohistochemical evidence indicates that CB1 are located in several different adult brain regions, including those related to emotion and responses to aversive stimuli. They include the hippocampus [68, 69] striatum, substantia nigra, periaqueductal grey (PAG), amygdala, nucleus accumbens [69] and the cortex, especially the prefrontal cortex and cingulate [70, 71]. On the other hand, CB2 receptors are found mainly in cells of hematopoietic and immune system but are also present in the brain [72, 73].
Following the identification of these receptors various endogenous neuromodulators, named endocannabinoids (ECs), were discovered. Nowadays, the endocannabinoid (EC) system is proposed to comprise the CB1 and CB2 receptors, endogenous agonists derived from the arachidonic acid such as (N-arachidonoylethanolamide, AEA) and 2-arachidonoylglycerol (2-AG), and the proteins responsible for the synthesis and degradation of these molecules [74].
Although marijuana is considered a drug of abuse, some of its beneficial effects, including anticonvulsant, antipsychotic, antidepressant and anxiolytic actions, are due to its ability to regulate the endocannabinoid system [75-80]. Cannabinoids are able to alter brain activity by inhibiting calcium and activating potassium channels, resulting in inhibition of neurotransmitter release [81]. They can also promote neuronal plasticity, affecting short-term neuronal excitability by depolarization-induced suppression of inhibition (DSI), mainly in GABAergic synapses, and depolarization-induced suppression of excitation (DSE) in synapses governing the release of glutamate and the neuropeptide cholecystokinin [82-85]. Moreover, cannabinoids display neuroprotective actions, being involved in the control of glutamate-induced excitotoxicity [86-88]. In the last decade, other important mechanism of action of cannabinoids has been related to the improvement of emotional states: its regulatory role of adult hippocampal neurogenesis (see Table 2).
Table 2.
Effect of Cannabinoids Compounds on Adult Neurogenesis: in vitro and in vivo Studies
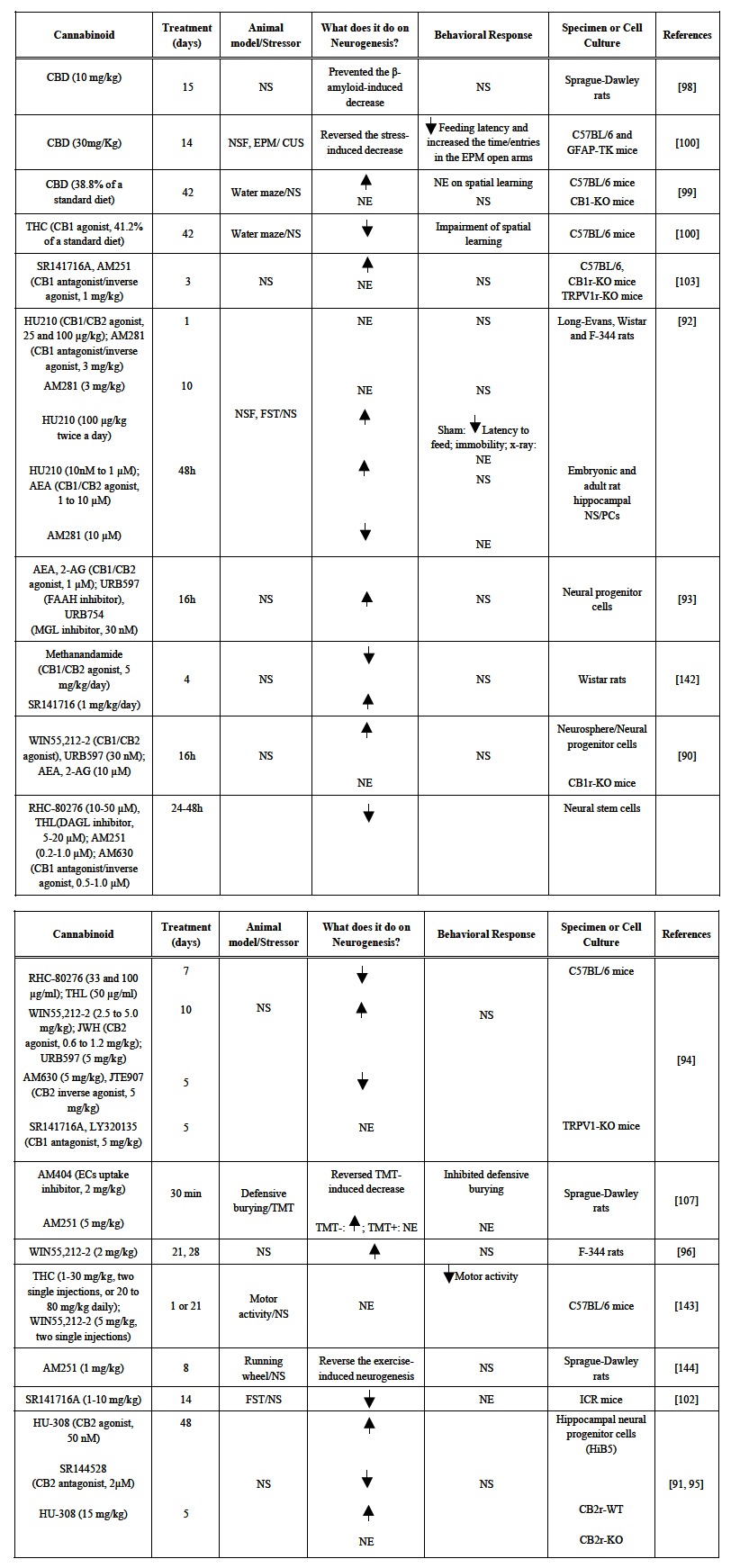 |
CBD = cannabidiol; AEA = anandamide; NSF = novelty supressed feeding; EPM= elevated plus maze FST = forced swimming test; x-ray = x-irradiation of the subgranular zone of the dentate gyrus; sham = not irradiated animals; TMT = trimethylthiazoline; NS = not studied; NE = no effect observed.
3.2. Evidence from in vitro Studies
The EC system is present in the central nervous system since early stages of embryonic development and is involved in neuronal migration, survival and differentiation [89]. Embryonic neural progenitor cells (NPs) in culture express CB1, CB2 receptors and FAAH. This is observed in cells that express nestin and incorporate BrdU, indicating that dividing cells express components of the EC system. Moreover, NPs can produce AEA and 2-AG, which are involved in the modulation of neuronal fate [90, 91]. Similar to the findings obtained in embryonic tissue, the EC system remains expressed and functional in adult stem/progenitor cells, inducing cell proliferation after cannabinoid challenge [92, 93].
NPs incubation with non-selective cannabinoid agonists such as AEA, 2-AG, HU210 and WIN55,212-2, as well as the enhancement of EC signaling with drugs that blocks ECs degradation (URB597 and URB574), increase cell proliferation [90, 92] whereas treatment with WIN55,212-2 and URB597 in CB1 knockout NPs failed to alter neurogenesis, indicating the requirement of CB1 receptors in cannabinoids induced NPs cell division [93]. Moreover, FAAH knockout mice, which present increased ECs levels, displayed a larger number of hippocampal BrdU+ cells [90]. On the same direction, studies in vitro showed that cannabinoid antagonists such as AM251, AM281, AM630, and the diacylglycerol lipase (DAGL) inhibitors RHC-80276 and THL, which decrease ECs biosynthesis, blocked the effect of cannabinoid agonists or decreased cell proliferation by themselves [92, 94].
Similar to CB1, CB2 receptors also seem to be involved in the modulation of adult hippocampal neurogenesis. Hippocampal NPs treated with the CB2 selective agonist HU-308 present increased cell proliferation whereas the CB2 antagonist SR144528 reduced neurogenesis [91, 95]. Interestingly, regulation of neurogenesis by DAGL-derived 2-AG has been shown to involve, at least in part, CB2 receptors [94].
3.3. Evidence from in vivo Studies
In accordance to these in vitro results, studies in vivo have also demonstrated the importance of the EC system to modulate cell proliferation, differentiation, maturation and survival. Moreover, there is a positive association between cannabinoid-induced neurogenesis and the behavioral improvement observed in animal models of anxiety, psychosis and depression. Chronic (10 days), but not acute, administration of HU210 induced anxiolytic- and antidepressive-like effects by increasing neurogenesis, once animals that were submitted to SGZ-x-ray did not show any behavioral response. Repeated administration of WIN55,212-2 was also able to promote cell division in mice and rats [92, 94, 96].
In addition to injections of exogenous agonists, the participation of ECs in the modulation of neurogenesis has also been investigated. Chronic treatment with URB597 (10 days) increased cell proliferation, while the ECs uptake inhibitor, AM404, reversed the trimethylthiazoline(TMT)-induced decrease of neurogenesis and inhibited defensive burying [94, 97].
Akin to the results observed with synthetic cannabinoids and ECs, two major constituents of the plant Cannabis sativa, the psychoactive compound delta-9-tetrahydrocannabinol (THC) and the non-psychoactive cannabidiol (CBD), may also affect adult hippocampal neurogenesis. Repeated treatment with CBD for 15-days prevented β-amyloid-induced neurotoxicity via activation of the proliferator-activated receptor-γ (PAAR-γ), suggesting a mechanism for CBD neuroprotective effects [98]. Also, CBD (42 days), despite decreasing cell proliferation, stimulated cell survival without promoting amelioration on spatial learning [99]. These responses were mediated by CB1 receptors, since CBD effects were absent in CB1-KO mice. More recently, a studied conducted with transgenic mice (GFAP-TK mice) showed that the anxiolytic effect of chronic CBD administration (14 days) in stressed mice depends on its proneurogenic action in the adult hippocampus by facilitating endocannabinoid-mediated signaling [100]. However it is important to stress that THC, a CB1 receptor partial agonist, can decrease cell proliferation and impair spatial memory [101]. In addition, Zhang and colleagues [101] have recently shown that mice lacking CB1 only on astrocytes were protected from memory impairments induced by high doses of THC, suggesting a THC mechanism independent of neuronal located CB1 receptors [101].
Similar to in vitro studies, CB2 was also shown to influence neurogenesis. Repeated administration of HU-308 during 5 days increased cell proliferation [91, 95], whereas the CB2 inverse agonist JTE907 or the antagonist SR144528 caused opposite results [94]. The involvement of CB2 receptors in these results was confirmed by the failure of the CB2 agonist to induce any change in CB2 deficient mice [95].
Although in vitro studies with NPs exposed to CB1 and CB2 antagonists/inverse agonists usually demonstrate unidirectional effect on neurogenesis, the use of these compounds in vivo shows contradictory results. While repeated administration of SR141716A and AM630 decreased neurogenesis in some studies [94, 102], Jin et al. [103] found that AM251 and SR141716A increased it, an effect present even in CB1-KO mice but absent in TRPV1-KO mice, suggesting the participation of the vanilloid system in the modulation of neurogenesis [94, 102]. These discrepancies may involve the animal species or gender used, the drug and BrdU treatment schedule, the drug dose and, importantly, the time-point where these measurements are performed, which may induce confusing interpretations. For example, Wolf et al. [99] found increased cell proliferation 1 and 24h after treatment with AM251, but a decrease in cell maturation 48h and 7 days later [99]. These results suggest that the role of cannabinoids on neurogenesis is complex and requires additional investigation.
4. ANTIDEPRESSANT TREATMENT MODULATES THE ENDOCANNABINOID SYSTEM
The putative role of cannabinoid in the control of mood and anxiety disorders has been describe by numerous authors [103, 104]. In addition, it has been suggested that the majority of the available treatments for depression modulates sendo-cannabinoid signaling. For instance, sleep deprivation, which can induce antidepressant effects, increases circulating levels of AEA in humans [103] and elevates 2-AG levels in the hippocampus [105]. A similar picture was found in the amygdala [106]. However, a decrease in CB1 receptor binding and in the amount of AEA in the prefrontal cortex was described by the same group [107]. Several studies have also provided evidence that chronic treatment with anti-depressant drugs such as SSRIs and tricyclic might modify the endocannabinoid system. For example, the tricyclic antidepressant desipramine, a noradrenergic uptake inhibitor, increases cannabinoid CB1 receptor density without changing endocannabinoid levels in the hypothalamus and hippocampus [107]. In addition, imipramine chronic treatment increases CB1 receptor binding in amygdaloid complex, but reduces CB1 receptor binding in the hypothalamus and striatum [108]. The SSRI fluoxetine increases the expression and promotes a facilitation of CB1 receptor mediated signaling in limbic areas such as the prefrontal cortex [109-111]. Conversely, in the study of Hesketh and colleagues [112], citalopram reduced CB1 mediated neurotransmission in the hippocampus and hypothalamus [112]. More recently however, it was shown that acute stimulation of CB1 receptors modulates the effect of citalopram on serotonin levels in the medial prefrontal cortex [113]. Regarding the monoamine oxidase (MAO) inhibitors, tranylcypromine reduced AEA content and increased CB1 receptor binding in the hippocampus and prefrontal cortex [110]. Even if there are contradictory results, in overall these findings support the hypothesis that the recruitment of the endocannabinoid system could be involved in the long lasting neuroplastic events (neurogenesis) promoted by AD chronic treatment.
Cannabinoids can also modulate serotonergic neurotransmission and serotonin subtypes 1A and 2A/2C receptor expression in the brain [114, 115]. Genetic deletion of the eCB degradation enzyme FAAH increases the firing of serotonergic neurons located in dorsal raphe nucleus. As a consequence, serotonin release is increased in limbic areas such as the prefrontal cortex [116]. Moreover, CB1 knockout mice displayed functional impairment of 5-HT1A and 5-HT2A/C receptor-mediated neurotransmission in the hippocampus [117] while a loss of antidepressants behavioral effects was described after genetic blocked of CB1 receptors [118].
Several studies point to an important bi-directional influence between the EC system and AD effects. For example, previous treatment with a CB1 receptor antagonist prevented the effects of imipramine on stress-induced activation of the hypothalamus-pituitary-adrenal axis [107]. Furthermore, treatment with the SSRI fluoxetine failed to facilitate serotonergic neurotransmission in the prefrontal cortex of CB1 knockout mice [119]. Likewise, long-term fluoxetine treatment up-regulated CB1 receptor signaling at the G protein transduction level in the prefrontal cortex [111].
However, even considering the possible role of neurogenesis facilitation by AD in their therapeutic effects [24, 28, 44, 120], no study, to our knowledge, has yet directly investigated if the disruption of the endocannabinoids system signaling could influence the pro-neurogenic effects of AD. Since facilitation of hippocampal endocannabinoid signaling (via CB1/CB2 receptor) is known to promote cell proliferation and neurogenesis [90, 91, 92, 94], and based on the evidence that AD treatment promotes changes in endocannabinoid signaling, it is possible that antidepressant chronic treatment modulates hippocampal neurogenesis via endocannabinoid system.
The results reviewed in the present paper so far suggest a common link between neurogenesis, antidepressant and endocannabinoids. Moreover, part of the positive effects of AD has also been related to changes in signaling pathways that regulate cellular plasticity and survival. Interestingly, a significant number of these intracellular pathways are also modulated by cannabinoid signaling. Long-term treatment with ADs up-regulates the cAMP-protein kinase A (PKA) and extracellular signal-regulated kinase (ERK) signaling pathway [117, 118]. Similarly, CB1 receptors are also coupled to ERK cascades and the proneurogenic action of cannabinoids seems to be related to facilitation of ERK signaling [91, 122, 123]. Also, brain derived neurotrophic factor (BDNF), a neurotrophin that is found reduced in depressed patients, and that is up regulated after AD or cannabinoids treatment could be involved [121, 124-127]. This neurotrophic factor has been implicated in adult hippocampal neurogenesis [128]. Activation of the BDNF receptor, TrkB, induces phosphorylation of ERK1/2 and Akt [129]. The Akt-mediated pathway is up regulated by dual reuptake inhibitor (SNRI) venlafaxine, which also facilitates hippocampal neurogenesis [130]. In a similar way, cannabinoids can increase in vitro neuroprogenitor cell proliferation by increasing the activation of the phosphatidylinositol 3-kinase/Akt signaling [93]. Therefore, additive or synergic effects on signaling pathways related to neurogenesis, cellular plasticity and survival mechanisms could be relevant for the endocannabinoids facilitatory effects on the therapeutic responses of ADs (Fig. 1).
Fig. (1).
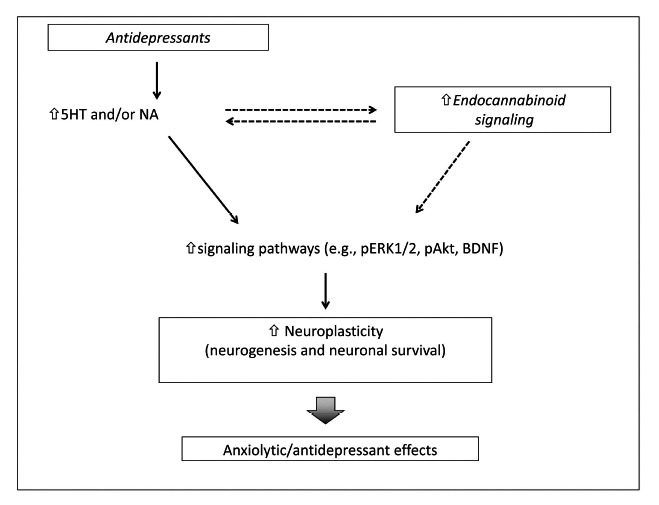
Interaction between antidepressants and the endocannabinoid system. Continuous arrows show proposed mechanisms of the neuroplastic hypothesis of antidepressant actions. Dashed arrows indicate possible interaction sites between antidepressant and endocannabinoids effects. 5HT: serotonin, NE: norepinephrine.
5. PERSPECTIVES AND CONCLUSIONS
The present paper reviewed the possible role of hippocampal neurogenesis on the behavioral effects of AD and cannabinoids. Several pieces of evidence support the proposal that the endocannabinoid signaling pathway could participate in behavioral actions of AD that may depend on hippocampal neurogenesis (Fig. 1). In addition, disruption of endocannabinoid signaling by stressful situations could be involved in the stress-induced reduction of hippocampal neurogenesis. Additional studies, designed to test these possibilities, are needed to elucidate the role of the endocannabinoid system on the behavioral and pro-neurogenic effects of AD.
ACKNOWLEDGEMENTS
This work was supported by grants from FAPESP, CNPq, NAPNA-USP. Research in IG-R.lab was financially supported by Ministerio de Ciencia e Innovación (PLE2009-0117), Comunidad de Madrid-Universidad Complutense de Madrid (S2011/BMD-2336) and CAPES (to F.S.G. and I.G.-R, DGU 217/2010). MVF is a FAPESP fellowship recipient. ACC is a CNPq fellowship recipient.
CONFLICT OF INTEREST STATEMENT
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Aloe L. Rita Levi-Montalcini: the discovery of nerve growth factor and modern neurobiology. Trends Cell Biol . 2004;14(7 ):395–9. doi: 10.1016/j.tcb.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Altman J. Autoradiographic study of degenerative and regenerative proliferation of neuroglia cells with tritiated thymidine. Exp. Neurol . 1962;5:302–18. doi: 10.1016/0014-4886(62)90040-7. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol . 1965;124(3 ):319–35. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4(11 ):1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 5.Yuan TF, Arias-Carrion O. Adult neurogenesis in the hypothalamus: evidence functions, and implications. CNS Neurol. Disord Drug Targets. 2011;10(4 ):433–9. doi: 10.2174/187152711795563985. [DOI] [PubMed] [Google Scholar]
- 6.Lois C, Alvarez-Buylla A. Long distance neuronal migration in the adult mammalian brain. Science . 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 7.Kempermn G. Cold Spring Harbor. N.Y: Cold Spring Harbor Laboratory Press; c2008. Adult Neurogenesis: Stem Cells and Neuronal Development in the Adult Brain. [Google Scholar]
- 8.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell . 2008;132(4 ):645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci . 2006;7(3 ):179–93. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 10.Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28(5 ):248–54. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Alonso M, Viollet C, Gabellec MM, Meas-Yedid V, Olivo-Marin JC, Lledo PM. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J. Neurosci . 2006;26(41 ):10508–13. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol . 2004;14(2 ):186–91. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol. Rev . 2005;85(2 ):523–69. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 14.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 1999;2(3 ):260–5. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 15.Kempermann G, Gage FH. Genetic influence on phenotypic differentiation in adult hippocampal neurogenesis. Brain Res. Dev. Brain Res . 2002;134(1-2 ):1–12. doi: 10.1016/s0165-3806(01)00224-3. [DOI] [PubMed] [Google Scholar]
- 16.Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur. J. Neurosci . 2005;21(2 ):513–21. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 17.Coras R, Siebzehnrubl FA, Pauli E, Huttner HB, Njunting M, Kobow K, Villmann C, Hahnen E, Neuhuber W, Weigel D, Buchfelder M, Stefan H, Beck H, Steindler DA, Blumcke I. Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain . 2010;133(11 ):3359–72. doi: 10.1093/brain/awq215. [DOI] [PubMed] [Google Scholar]
- 18.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci . 1999;2(3 ):266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 19.Kee NJ, Preston E, Wojtowicz JM. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp. Brain Res . 2001;136(3 ):313–20. doi: 10.1007/s002210000591. [DOI] [PubMed] [Google Scholar]
- 20.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J. Neurosci . 2007;27(12 ):3252–9. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, hang WN, othuizen HH, eldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci. Biobehav. Rev . 2004;28(3 ):273–83. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Tanapat P, alea LA, ould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int J DevNeurosci. 1998;16(3-4 ):235–9. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- 23.Czéh B, ichaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. U. S. A . 2001;98(22 ):12796–801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science . 2003;301(5634 ):805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 25.Petrik D, Lagace DC, isch AJ. The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology . 2012;62(1 ):21–34. doi: 10.1016/j.neuropharm.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16(3 ):233–8. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 27.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361 ):458–61. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malberg JE, Eisch AJ, Nestler EJ, uman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci . 2000;20(24 ):9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JS, Chang MY, Yu IT, Kim JH, Lee SH, ee YS, on H. Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J. Neurochem . 2004;89(2 ):324–36. doi: 10.1046/j.1471-4159.2004.02329.x. [DOI] [PubMed] [Google Scholar]
- 30.David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, erald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron . 2009;62(4 ):479–93. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Axelrod J. Biogenic amines and their impact in psychiatry. Semin. Psychiatry . 1972;4(3 ):199–210. [PubMed] [Google Scholar]
- 32.Carroll BJ. Monoamine precursors in the treatment of depression. Clin. Pharmacol. Ther. 1971;12(5 ):743–61. doi: 10.1002/cpt1971125743. [DOI] [PubMed] [Google Scholar]
- 33.Colonna L, Petit M, Lépine JP, Boismare F. Depressive states: clinical symptoms and monoaminergic hypothesis. Encephale. 978;4(1 ):5–17. [PubMed] [Google Scholar]
- 34.Charney DS, enkes DB, eninger GR. Receptor sensitivity and the mechanism of action of antidepressant treatment. Implications for the etiology and therapy of depression. Arch. Gen. Psychiatry . 1981;38(10 ):1160–80. doi: 10.1001/archpsyc.1981.01780350094011. [DOI] [PubMed] [Google Scholar]
- 35.Checkley S. Monoamines, depression and antidepressant drugs. Pharmacopsychiatry . 1988;21(1 ):6–8. doi: 10.1055/s-2007-1014637. [DOI] [PubMed] [Google Scholar]
- 36.Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch. Gen. Psychiatry . 1990;47(5 ):411–8. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- 37.Briley M, Moret C. 5-HT and antidepressants: in vitro and in vivo release studies. Trends Pharmacol. Sci. 1993;14(11 ):396–7. doi: 10.1016/0165-6147(93)90058-R. [DOI] [PubMed] [Google Scholar]
- 38.Ngwenya LB, Peters A, Rosene DL. Maturational sequence of newly generated neurons in the dentate gyrus of the young adult rhesus monkey. J. Comp. Neurol. 2006;498:204–216. doi: 10.1002/cne.21045. [DOI] [PubMed] [Google Scholar]
- 39.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28(9 ):1562–71. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 40.Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science . 2007;317(5839 ):819–23. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 41.Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry . 2008;64(4 ):293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Huang GJ, Bannerman D, Flint J. Chronic fluoxetine treatment alters behavior, but not adult hippocampal neurogenesis in BALB/cJmice. Mol. Psychiatry. 2008;13(2 ):119–21. doi: 10.1038/sj.mp.4002104. [DOI] [PubMed] [Google Scholar]
- 43.Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology . 2008;33(2 ):406–17. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- 44.Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc. Natl. Acad. Sci. U. S. A . 2007;104(11 ):4642–6. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H, Pandey GN, Dwivedi Y. Hippocampal cell proliferation regulation by repeated stress and antidepressants. Neuroreport . 2006;17(9 ):863–7. doi: 10.1097/01.wnr.0000221827.03222.70. [DOI] [PubMed] [Google Scholar]
- 46.Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, Kamei H, Nagai T, Yoneda Y, Nabeshima T, Yamada K. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J. Neurochem. 2008;105(3 ):921–32. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 47.Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci . 2007;27(18 ):4894–901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs BL, Tanapat P, Reeves AJ, Gould E. Serotonin stimulates the production of new hippocampal granule neurons via the 5-HT1A receptor in the adult rat. Soc. Neurosci. Abstr. 1998;24:1992. [Google Scholar]
- 49.Radley JJ, Jacobs BL. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 2002;955(1-2 ):264–7. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- 50.Xia L, Deloménie C, David I, Rainer Q, Marouard M, Delacroix H, David DJ, Gardier AM, Guilloux JP. Ventral hippocampal molecular pathways and impaired neurogenesis associated with 5-HT(1A) and 5-HT(1B) receptors disruption in mice. Neurosci. Lett . 2012;521(1 ):20–5. doi: 10.1016/j.neulet.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 51.Masuda T, Nakagawa S, Boku S, Nishikawa H, Takamura N, Kato A, Inoue T, oyama T. Noradrenaline increases neural precursor cells derived from adult rat dentate gyrus through beta2 receptor. Prog. Neuropsychopharmacol. Biol. Psychiatry . 2012;36(1 ):44–5. doi: 10.1016/j.pnpbp.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 52.Jhaveri DJ, Mackay EW, Hamlin AS, Marathe SV, Nandam LS, Vaidya VA, Bartlett PF. Norepinephrinedirectlyactivatesa- dulthippocampalprecursors via beta3-adrenergic receptors. J. Neurosci. 2010;30(7 ):2795–806. doi: 10.1523/JNEUROSCI.3780-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 2005;25(5 ):1089–94. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc. Natl. Acad. Sci. U. S. A. 2007;104(11 ):4647–52. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology . 2001;25(6 ):836–44. doi: 10.1016/S0893-133X(01)00358-X. [DOI] [PubMed] [Google Scholar]
- 56.Pinnock SB, Blake AM, Platt NJ, Herbert J. The roles of BDNF, pCREB and Wnt3a in the latent period preceding activation of progenitor cell mitosis in the adult dentate gyrus by fluoxetine. PLoS One . 2010;5(10 ):e13652. doi: 10.1371/journal.pone.0013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pechnick RN, Zonis S, Wawrowsky K, Cosgayon R, Farrokhi C, Lacayo L, Chesnokova V. Antidepressants stimulate hippocampal neurogenesis by inhibiting p21 expression in the subgranular zone of the hipppocampus. PLoS One . 2011;6(11 ):e27290. doi: 10.1371/journal.pone.0027290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J. Am. Chem. Soc . 1971;93(1 ):217–24. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- 59.Mechoulam R, Braun P, aoni Y. Syntheses of 1 -tetrahydrocannabinol and related cannabinoids. J. Am. Chem. Soc . 1972;94(17 ):6159–65. doi: 10.1021/ja00772a038. [DOI] [PubMed] [Google Scholar]
- 60.Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br. J.Pharmacol . 2006;147 (Suppl 1 ):S163–71. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol. Rev. 2010;62(4 ):588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mechoulam R, Lander N, Varkony TH, Kimmel I, Becker O, Ben-Zvi Z, Edery H, Porath G. Stereochemical requirements for cannabinoid activity . Med. Chem . 1980;23(10 ):1068–72. doi: 10.1021/jm00184a002. [DOI] [PubMed] [Google Scholar]
- 63.Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J. Pharmacol. Exp. Ther. 1998;247(3 ):1046–1051. [PubMed] [Google Scholar]
- 64.Howlett AC, Qualy JM, hachatrian LL. Involvement of Gi in the inhibition of adenylatecyclase by cannabimimetic drugs. Mol.Pharmacol . 1986;29(3 ):307–313. [PubMed] [Google Scholar]
- 65.Glass M, Northup JK. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol.Pharmacol . 1999;56(6 ):1362–2369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- 66.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev . 2002;54(2 ):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 67.Gómez-Ruiz M, Hernández M, de Miguel R, Ramos JA. An overview on the biochemistry of the cannabinoid system. Mol. Neurobiol . 2007;36:3–14. doi: 10.1007/s12035-007-0015-0. [DOI] [PubMed] [Google Scholar]
- 68.Brusco A, Tagliaferro P, Saez T, Onaivi ES. Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse . 2008;62(12 ):944–9. doi: 10.1002/syn.20569. [DOI] [PubMed] [Google Scholar]
- 69.Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides . 2000;21(11 ):1735–42. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- 70.Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb. Cortex. 2007;17(1 ):175–91. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- 71.Micale V, Cristino L, Tamburella A, Petrosino S, Leggio GM, Drago F, Di Marzo V. Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-1 channels. Neuropsychopharmacology . 2009;4(3 ):593–606. doi: 10.1038/npp.2008.98. [DOI] [PubMed] [Google Scholar]
- 72.Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071(1 ):10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 73.Onaivi ES. Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology. 2006;54(4 ):231–246. doi: 10.1159/000100778. [DOI] [PubMed] [Google Scholar]
- 74.Piomelli D. The endocannabinoid system: a drug discovery perspective. Curr. Opin. Investig. Drugs . 2005;6(7 ):672–9. [PubMed] [Google Scholar]
- 75.Zuardi AW, Rodrigues JA, Cunha JM. Effects of cannabidiol in animal models predictive of antipsychotic activity. Psychopharmacology (Berl) 1991;104(2 ):260–4. doi: 10.1007/BF02244189. [DOI] [PubMed] [Google Scholar]
- 76.Guimarães FS, Chiaretti TM, Graeff FG, Zuardi AW. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl) 1990;100(4 ):558–9. doi: 10.1007/BF02244012. [DOI] [PubMed] [Google Scholar]
- 77.Moreira FA, Aguiar DC, Guimarães FS. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30(8 ):1466–71. doi: 10.1016/j.pnpbp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 78.Moreira FA, Aguiar DC, Guimarães FS. Anxiolytic-like effect of cannabinoids injected into the rat dorsolateral periaquidutal gray. Neuropharmacology . 2007;52:958–965. doi: 10.1016/j.neuropharm.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 79.Bergamaschi MM, Queiroz RH, Chagas MH, deOliveira DC, DeMartinis BS, Kapczinski F, Quevedo J, Roeslerm R, Schröder N, Nardi AE, Martín-Santosm R, Hallak JE, Zuardi AW, Crippa JA. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology . 2011;36:1219–26. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. Handb. Exp. Pharmacol. 2005;168:327–165. doi: 10.1007/3-540-26573-2_11. [DOI] [PubMed] [Google Scholar]
- 81.Diana MA, Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE) Br. J. Pharmacol . 2004;142(1 ):9–19. doi: 10.1038/sj.bjp.0705726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M. The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells. J.Neurosci . 2002;22(5 ):1690–7. doi: 10.1523/JNEUROSCI.22-05-01690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ohno-Shosaku T, subokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J. Neurosci. 2002;22(10 ):3864–72. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Narushima M, Uchigashima M, Hashimoto K, Watanabe M, Kano M. Depolarization-induced suppression of inhibition mediated by endocannabinoids at synapses from fast-spiking interneurons to medium spiny neurons in the striatum. Eur. J. Neurosci . 2006;24(8 ):2246–52. doi: 10.1111/j.1460-9568.2006.05119.x. [DOI] [PubMed] [Google Scholar]
- 85.Zoppi S, Pérez Nievas BG, Madrigal JL, anzanares J, eza JC, García-Bueno B. Regulatory role of cannabinoid receptor 1 in stress-induced excitotoxicity and neuroinflammation. Neuropsychopharmacology . 2011;36(4 ):805–18. doi: 10.1038/npp.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, van der Stelt M, ópez-Rodriguez ML, Casanova E, Schütz G, ieglgänsberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302(5642 ):84–8. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 87.Shen M, Thayer SA. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol. Pharmacol . 1998;54(3 ):459–62. doi: 10.1124/mol.54.3.459. [DOI] [PubMed] [Google Scholar]
- 88.Harkany T, Guzmán M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 2007;28(2 ):83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Aguado T, Monory K, Palazuelos J, Stella N, ravatt B, Lutz B, Marsicano G, Kokaia Z, Guzmán M, Galve-Roperh I. The endocannabinoid system drives neural progenitor proliferation. FASEB J . 2005;19(12 ):1704–6. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- 90.Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzman M, Galve-Roperh I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20(13 ):2405–7. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- 91.Jiang S, Fu Y, Williams J, Wood J, Pandarinathan L, Avraham S, Makriyannis A, Avraham S, Avraham HK. Expression and function of cannabinoid receptors CB1 and CB2 and their cognate cannabinoid ligands in murine embryonic stem cells. PloSOne . 2007;2(7 ):e641. doi: 10.1371/journal.pone.0000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aguado T, Romero E, Monory K, Palazuelos J, Sendtner M, Marsicano G, Lutz B, Guzmán M, Galve-Roperh I. The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. J. Biol. Chem. 2007;282(33 ):23892–8. doi: 10.1074/jbc.M700678200. [DOI] [PubMed] [Google Scholar]
- 93.Goncalves MB, Suetterlin P, Yip P, Molina-Holgado F, Walker DJ, udin MJ, Zentar MP, Pollard S, Yáñez-Muñoz RJ, Williams G, Walsh FS, Pangalos MN, Doherty P. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol. Cell. Neurosci. 2008;38(4 ):526–36. doi: 10.1016/j.mcn.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 94.Palazuelos J, Ortega Z, Díaz-Alonso J, Guzmán M, Galve-Roperh I. CB2 cannabinoid receptors promote neural progenitor cell proliferation via mTORC1 signaling . Biol. Chem . 2012;287(2 ):1198–209. doi: 10.1074/jbc.M111.291294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marchalant Y, Brothers HM, Wenk GL. Cannabinoid agonist WIN-55, 212-2 partially restores neurogenesis in the aged rat brain. Mol. Psychiatry . 2009;14(12 ):1068–9. doi: 10.1038/mp.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hill MN, Kambo JS, Sun JC, Gorzalka BB, Galea LA. Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviours. Eur. J. Neurosci. 2006;24(7 ):1845–9. doi: 10.1111/j.1460-9568.2006.05061.x. [DOI] [PubMed] [Google Scholar]
- 97.Esposito G, Scuderi C, Valenza M, Togna GI, Latina V, De Filippis D, Cipriano M, Carratù MR, Iuvone T, Steardo L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One . 2011;6(12 ):e28668. doi: 10.1371/journal.pone.0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolf SA, Bick-Sander A, Fabel K, Leal-Galicia P, Tauber S, Ramirez-Rodriguez G, Müller A, Melnik A, Waltinger TP, Ullrich O, Kempermann G. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell. Commun. Signal . 2010;8:12. doi: 10.1186/1478-811X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Campos AC, Ortega Z, Palazuelos J, Fogaca MV, Aguiar DC, Díaz-Alonso J, Ortega-Gutierrez S, Vazquez-Villa H, Moreira FA, Guzman M, Galve-Roperh I, Guimarães F S. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. Intl. J. Neuropsychopharmacol . 2013 doi: 10.1017/S1461145712001502. (In press) [DOI] [PubMed] [Google Scholar]
- 100.Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, Koehl M, Abrous D N, Mendizabal-Zubiaga J, Grandes P, Liu Q, Bai G, Wang W, Xiong L, Ren W, Marsicano G, Zhang X. Acute cannabinoids impair working memory through astroglial CB1 receptor mod ulation of hippocampal LTD. Cell. 2012;148(5 ):1039–50. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 101.Lee S, Kim DH, Yoon SH, Ryu JH. Sub-chronic administration of rimonabant causes loss of antidepressive activity and decreases doublecortinimmunoreactivity in the mouse hippocampus. Neurosci. Lett. 2009;467(2 ):111–6. doi: 10.1016/j.neulet.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 102.Jin K, Xie L, Kim SH, Parmentier-Batteur S, Sun Y, Mao XO, Childs J, Greenberg DA. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol. Pharmacol. 2004;66(2 ):204–8. doi: 10.1124/mol.66.2.204. [DOI] [PubMed] [Google Scholar]
- 103.Vaughn LK, Denning G, Stuhr KL, de Wit H, Hill MN, Hillard CJ. Endocannabinoidsignalling: has it got rhythm? Br. J. Pharmacol . 2010;160(3 ):530–43. doi: 10.1111/j.1476-5381.2010.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen C, Bazan NG. Endogenous PGE2 regulates membrane excitability and synaptic transmission in hippocampal CA1 pyramidal neurons. J. Neurophysiol . 2005;93(2 ):929–41. doi: 10.1152/jn.00696.2004. [DOI] [PubMed] [Google Scholar]
- 105.Hill MN, Barr AM, Ho WS, Carrier EJ, Gorzalka BB, Hillard CJ. Electroconvulsive shock treatment differentially modulates cortical and subcortical endocannabinoid activity. J. Neurochem . 2007;103(1 ):47–56. doi: 10.1111/j.1471-4159.2007.04688.x. [DOI] [PubMed] [Google Scholar]
- 106.Hill MN, Ho WS, Sinopoli KJ, Viau V, Hillard CJ, Gorzalka BB. Involvement of the endocannabinoid system in the ability of long-term tricyclic antidepressant treatment to suppress stress-induced activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology . 2006;31(12 ):2591–9. doi: 10.1038/sj.npp.1301092. [DOI] [PubMed] [Google Scholar]
- 107.Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, Gorzalka BB. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J. Neurochem . 2008;106(6 ):2322–36. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30(3 ):508–15. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- 109.Hill MN, Ho WS, Hillard CJ, Gorzalka BB. Differential effects of the antidepressants tranylcypromine and fluoxetine on limbic cannabinoid receptor binding and endocannabinoid contents. J. Neural. Transm. 2008;115(12 ):1673–9. doi: 10.1007/s00702-008-0131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mato S, Vidal R, Castro E, Diaz A, Pazos A, Valdizan EM. Long-term fluoxetine treatment modulates cannabinoid type 1 receptor-mediated inhibition of adenylyl cyclase in the rat prefrontal cortex through 5-hydroxytryptamine 1A receptor-dependent mechanisms. Mol. Pharmacol . 2010;77(3 ):424–34. doi: 10.1124/mol.109.060079. [DOI] [PubMed] [Google Scholar]
- 111.Hesketh SA, Brennan AK, Jessop DS, Finn DP. Effects of chronic treatment with citalopram on cannabinoid and opioid receptor-mediated G-protein coupling in discrete rat brain regions. Psychopharmacology (Berl) 2008;198(1 ):29–36. doi: 10.1007/s00213-007-1033-3. [DOI] [PubMed] [Google Scholar]
- 112.Kleijn J, Cremers TI, Hofland CM, Westerink BH. CB-1 receptors modulate the effect of the selective serotonin reuptake inhibitor citalopram on extracellular serotonin levels in the rat prefrontal cortex. Neurosci. Res . 2011;70(3 ):334–7. doi: 10.1016/j.neures.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 113.Bambico FR, Nguyen NT, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol. Dis. 2010;37(3 ):641–55. doi: 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 114.Cassano T, Gaetani S, Macheda T, Laconca L, Romano A, Morgese MG, Cimmino CS, Chiarotti F, Bambico FR, Gobbi G, Cuomo V, Piomelli D. Evaluation of the emotional phenotype and serotonergic neurotransmission of fatty acid amide hydrolase-deficient mice. Psychopharmacology (Berl) 2011;214(2 ):465–76. doi: 10.1007/s00213-010-2051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bambico FR, Cassano T, Dominguez-Lopez S, Katz N, Walker CD, Piomelli D, Gobbi G. Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology . 2010;35(10 ):2083–100. doi: 10.1038/npp.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mato S, Aso E, Castro E, Martin M, Valverde O, Maldonado R, Pazos A. CB1 knockout mice display impaired functionality of 5-HT1A and 5-HT2A/C receptors. J. Neurochem . 2007;103(5 ):2111–20. doi: 10.1111/j.1471-4159.2007.04961.x. [DOI] [PubMed] [Google Scholar]
- 117.Steiner MA, Marsicano G, Nestler EJ, Holsboer F, Lutz B, Wotjak CT. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology . 2008;33(1 ):54–67. doi: 10.1016/j.psyneuen.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aso E, Renoir T, Mengod G, Ledent C, Hamon M, Maldonado R, Lanfumey L, Valverde O. Lack of CB1 receptor activity impairs serotonergic negative feedback. J. Neurochem . 2009;109(3 ):935–44. doi: 10.1111/j.1471-4159.2009.06025.x. [DOI] [PubMed] [Google Scholar]
- 119.Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John MJ, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsycho pharmacology. 2009;34(11 ):2376–89. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Malberg JE, Blendy JA. Antidepressant action: to the nucleus and beyond. Trends Pharmacol. Sci . 2005;26(12 ):631–8. doi: 10.1016/j.tips.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 121.Musazzi L, Mallei A, Tardito D, Gruber SH, El Khoury A, Racagni G, Mathe AA, Popoli M. Early-life stress and antidepressant treatment involve synaptic signaling and Erk kinases in a gene-environment model of depression. J. Psychiatr. Res. 2010;44(8 ):511–20. doi: 10.1016/j.jpsychires.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 122.Galve-Roperh I, Rueda D, Gomez del Pulgar T, Velasco G, Guzman M. Mechanism of extracellular signal-regulated kinase activation by the CB(1) cannabinoid receptor. Mol. Pharmacol . 2002;62(6 ):1385–92. doi: 10.1124/mol.62.6.1385. [DOI] [PubMed] [Google Scholar]
- 123.Aydemir O, Deveci A, Taskin OE, Taneli F, Esen-Danaci A. Serum brain-derived neurotrophic factor level in dysthymia: a comparative study with major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry . 2007;31(5 ):1023–6. doi: 10.1016/j.pnpbp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 124.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry . 2001;50(4 ):260–5. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 125.Blazquez C, Chiarlone A, Sagredo O, Aguado T, Pazos MR, Resel E, Palazuelos J, Julien B, Salazar M, Borner C, Benito C, Carrasco C, Diez-Zaera M, Paoletti P, Diaz-Hernandez M, Ruiz C, Sendtner M, Lucas JJ, de Yebenes JG, Marsicano G, Monory K, Lutz B, Romero J, Alberch J, Gines S, Kraus J, Fernandez-Ruiz J, Galve-Roperh I, Guzman M. Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington's disease. Brain . 2011;134(Pt 1 ):119–36. doi: 10.1093/brain/awq278. [DOI] [PubMed] [Google Scholar]
- 126.Elbatsh MM, Moklas MA, Marsden CA, Kendall DA. Antidepressant-like effects of Delta(9)-tetrahydrocannabinol and rimonabant in the olfactory bulbectomised rat model of depression. Pharmacol. Biochem. Behav . 2012;102(2 ):357–65. doi: 10.1016/j.pbb.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 127.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav.Pharmacol . 2007;18(5-6 ):391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 128.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem . 2003;72:609–42. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 129.Mostany R, Valdizan EM, Pazos A. A role for nuclear beta-catenin in SNRI antidepressant-induced hippocampal cell pr oliferation. Neuropharmacology . 2008;55(1 ):18–26. doi: 10.1016/j.neuropharm.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 130.Kodama M, Fujioka T, Duman R S. Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol. Psychiatry . 2004;56(8 ):570–80. doi: 10.1016/j.biopsych.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 131.Su XW, Li X Y, Banasr M, Duman R S. Eszopiclone and fluoxetine enhance the survival of newborn neurons in the adult rat hippocampus. Int. J. Neuropsychopharmacol . 2009;12(10 ):1421–8. doi: 10.1017/S1461145709990629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu X, Castrén E. Co-treatment with diazepam prevents the effects of fluoxetine on the proliferation and survival of hippocampal dentate granule cells. Biol. Psychiatry. 2009;66(1 ): 5–8. doi: 10.1016/j.biopsych.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 133.Marcussen A B, Flagstad P, Kristjansen P E, Johansen F F, Englund U. Increase in neurogenesis and behavioural benefit after chronic fluoxetine treatment in Wistar rats. Acta Neurol.Scand . 2008;117(2 ):94–100. doi: 10.1111/j.1600-0404.2007.00910.x. [DOI] [PubMed] [Google Scholar]
- 134.Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, Kamei H, Nagai T, Yoneda Y, Nabeshima T, Yamada K. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J. Neurochem . 2008;105(3 ):921–32. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 135.Khawaja X, Xu J, Liang J J, Barrett J E. Proteomic analysis of protein changes developing in rat hippocampus after chronic antidepressant treatment: Implications for depressive disorders and future therapies. J. Neurosci. Res . 2004;75(4 ):451–60. doi: 10.1002/jnr.10869. [DOI] [PubMed] [Google Scholar]
- 136.Bessa J M, Ferreira D, Melo I, Marques F, Cerqueira J J, Palha J A, Almeida O F, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol. Psychiatry. 2009;14(8 ):764–73. 739. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 137.Wang J W, David D J, Monckton J E, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J. Neurosci . 2008;28(6 ):1374–84. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hitoshi S, Maruta N, Higashi M, Kumar A, Kato N, Ikenaka K. Antidepressant drugs reverse the loss of adult neural stem cells following chronic stress. J. Neurosci. Res . 2007;85(16 ):3574–85. doi: 10.1002/jnr.21455. [DOI] [PubMed] [Google Scholar]
- 139.Li Y F, Zhang Y Z, Liu Y Q, Wang H L, Yuan L, Luo Z P. Moclobemide up-regulates proliferation of hippocampal progenitor cells in chronically stressed mice. Acta Pharmacol Sin . 2004;25(11 ):1408–12. [PubMed] [Google Scholar]
- 140.Anacker C, Zunszain P A, Cattaneo A, Carvalho L A, Garabedian M J, Thuret S, Price J, Pariante C M. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol. Psychiatry. 2011;16(7 ):738–50. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rueda D, Navarro B, Martinez-Serrano A, Guzman M, Galve-Roperh I. The endocannabinoidanandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J. Biol. Chem. 2002;29, 277(48 ):46645–50. doi: 10.1074/jbc.M206590200. [DOI] [PubMed] [Google Scholar]
- 142.Kochman L J, dos Santos A A, Fornal C A, Jacobs B L. Despite strong behavioral disruption, Delta9-tetrahydrocannabinol does not affect cell proliferation in the adult mouse dentate gyrus. Brain Res . 2006;1113(1 ):86–93. doi: 10.1016/j.brainres.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 143.Hill M N, Titterness A K, Morrish A C, Carrier E J, Lee T T, Gil-Mohapel J, Gorzalka B B, Hillard CJ, Christie B R. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus . 2010;20(4 ):513–23. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen F, Madsen T M, Wegener G, Nyengaard J R. Imipramine treatment increases the number of hippocampal synapses and neurons in a genetic animal model of depression. Hippocampus . 2010;20(12 ):1376–84. doi: 10.1002/hipo.20718. [DOI] [PubMed] [Google Scholar]


