Abstract
It is a common belief that voltage-gated calcium channels (VGCC) cannot carry toxic amounts of Ca2+ in neurons. Also, some of them as L-type channels are essential for Ca2+-dependent regulation of prosurvival gene-programs. However, a wealth of data show a beneficial effect of drugs acting on VGCCs in several neurodegenerative and neurovascular diseases. In the present review, we explore several mechanisms by which the “harmless” VGCCs may become “toxic” for neurons. These mechanisms could explain how, though usually required for neuronal survival, VGCCs may take part in neurodegeneration. We will present evidence showing that VGCCs can carry toxic Ca2+ when: a) their density or activity increases because of aging, chronic hypoxia or exposure to β-amyloid peptides or b) Ca2+-dependent action potentials carry high Ca2+ loads in pacemaker neurons. Besides, we will examine conditions in which VGCCs promote neuronal cell death without carrying excess Ca2+. This can happen, for instance, when they carry metal ions into the neuronal cytoplasm or when a pathological decrease in their activity weakens Ca2+-dependent prosurvival gene programs. Finally, we will explore the role of VGCCs in the control of nonneuronal cells that take part to neurodegeneration like those of the neurovascular unit or of microglia.
Keywords: Neurovascular unit, voltage-gated Ca2+ channels, neurodegeneration, beta-amyloid, Parkinson’s disease, Alzheimer’s disease, Multiple Sclerosis.
INTRODUCTION
A strong increase in [Ca2+]i may cause the saturation of mitochondrial buffering capacity, mitochondrial damage and, eventually, neuronal death [1, 2]. This is considered the main mechanism responsible for neuronal cell death in neurodegeneration. The first evidence that Ca2+ overload is a death-triggering signal came from experiments performed in nonneuronal cells like hepatocytes and skeletal muscle cells [3, 4]. In 1984, Roger Simon discovered that a massive Ca2+ build-up takes place in the hippocampus after an ischemic insult. This finding suggested that Ca2+ overload could be lethal also in neurons and marked the beginning of the Ca2+ theory of neurodegeneration [5]. Up to date, hundreds of papers have been published on this issue. Since the early days, it was proposed that the pharmacological blockade [Ca2+]i overload could rescue neurons from death. Therefore, there was (and there still is) a formidable interest in identifying excess Ca2+ sources in neurodegenerative diseases. At the time, the most obvious candidates were the varied families of ionotropic glutamate receptors and voltage-gated Ca2+ channels (VGCCs) (see Table 1 and references [6] and [7] for detailed reviews on these channels). They were, indeed, the best characterized Ca2+ influx pathways in neurons. Many papers showed benefits of a new class of drugs blocking L-type Ca2+ channels, the Ca2+ channel blockers (CCBs), in diverse preclinical models of neurodegenerative disorders. Soon, however, serious doubts arose about the importance of VGCCs in neurodegenerative cell death and other ion channels and transporters appeared way more important in this process. Among them, glutamate receptors were initially thought the most important. More recently, fancier channels and transporters like acid sensitive ion channels (ASICs), transient receptor potential cation channel, subfamily M, member 7 (TRPM7), or sodium calcium exchanger (NCX) [8, 9] emerged as major death effectors in neurons. The failure of some clinical trials with CCBs in neurodegenerative diseases like stroke [10] was an important reason to abandon the idea that VGCCs are relevant in neurodegeneration. Moreover, several studies in vitro suggested that these ion channels were not the right channels to carry a “toxic” Ca2+ influx into neurons. However, the old idea that VGCCs could be targeted to treat neurodegenerative and neurovascular diseases was never abandoned. Recent data provide new evidence in its support [11]. In the present review, we will go through these developments, and we will show how they are changing the old dogma that VGCCs simply act as a general and “nonspecific” source of excess Ca2+ in neurons. To be specific, we will review evidence suggesting that neuronal VGCCs activation, though usually ineffective in killing neurons, may become lethal in specific neuronal subpopulations or in specific disease states. We will also examine how these channels may become lethal for neurons even without carrying excess Ca2+. Finally, we will describe the role of VGCCs on nonneuronal cells that take part to neurodegeneration, like vascular smooth muscle cells, microglia or macrophages.
Table 1.
Molecular Diversity of VGCC
| Current | Pore Forming Subunit | Localization | Specific Antagonists | Cellular Functions |
|---|---|---|---|---|
| L | CaV1.1 | Skeletal muscle transverse tubles | Dihydropyridines, phenylalkylamines, benzothiazepines | Excitation-contraction coupling |
| CaV1.2 | Cardiac myocytes, endocrine cells, neuronal cell bodies and proximal dendrites | Dihydropyridines, phenylalkylamines, benzothiazepines | Excitation-contraction coupling, hormone release, regulation of transcription, synaptic integration | |
| CaV1.3 | endocrine cells, neuronal cell bodies and dendrites | Dihydropyridines, phenylalkylamines, benzothiazepines | Excitation-contraction coupling, hormone release, regulation of transcription, synaptic integration | |
| CaV1.4 | Retina | Not established | Neurotransmitter release from rods and bipolar cells | |
| P/Q | CaV2.1 | Nerve terminals and dendrites | ω-agatoxin-IVA | Neurotransmitter release, dendritic Ca2+ transients |
| N | CaV2.2 | Nerve terminals and dendrites | ω-CTx-GVIA | Neurotransmitter release, dendritic Ca2+ transients |
| R | CaV2.3 | Neuronal cell bodies and dendrites | SNX-482 | Repetitive firing |
| T | CaV3.1 | Neuronal cell bodies and dendrites | NNC 55-0396, R-efonidipine | Pacemaking, Repetitive firing |
| CaV3.2 | Neuronal cell bodies and dendrites | NNC 55-0396, R-efonidipine | Pacemaking, Repetitive firing | |
| CaV3.3 | Neuronal cell bodies and dendrites | NNC 55-0396, R-efonidipine | Pacemaking, Repetitive firing |
The table reports the current classification of the different VGCCs according to the type of Ca2+ current carried and of the gene encoding the pore forming subunit. For each class the main pharmacological properties and physiological roles are reported. Reproduced under permission with slight modifications from Catteral, W.A., Striessnig, J., Snutch, T.P., Perez-Reyes, E. International Union of Pharmacology. XL. Compendium of Voltage-Gated Ion Channels: calcium Channels. Pharmacol. Rev., 2003, 55(4), 579-581.
1. Conditions that Confer to VGCCs the Ability to Carry Toxic Ca2+ Loads
As originally intended, the Ca2+ neurotoxicity, or Ca2+ load, theory assumed that neurons die whenever their [Ca2+]i becomes higher than a critical threshold [12]. According to this theory, every Ca2+ source, also including VGCCs, may induce cell death if it carries large Ca2+ loads into neurons. The important experimental work of Michael Tymianski and his group seriously questioned this idea and led to the influential Ca2+ source specificity theory [13]. Briefly, this theory assumes that Ca2+ is toxic for neurons if it may activate specific death triggering systems. This may happen when Ca2+ enters the neurons through specific pathways that are close to these death effectors. According to the Ca2+ source specificity theory and in contrast with the Ca2+ load theory, not all the Ca2+ sources are capable of triggering cell death. Importantly, evidence suggested that VGCCs are not the right channels to kill neurons. Specifically, only 20% of cultured spinal [13] and cortical [14] neurons died when these channels were activated with 50 mM K+ given alone or with the L-type channel activator Bay-K 8644. On the contrary, NMDA agonists killed neurons in a dose-dependent manner. Cell death was also proportional to the increase in [Ca2+]i caused by these compounds [14]. These data suggested that whereas NMDA channels may trigger neuronal cell death VGCCs may not. Interestingly, NMDA agonists caused more cell death than high K+ even in cultures that showed a similar increase in [Ca2+]i in response to these treatments [14]. This suggested that the Ca2+ carried by VGCCs is not the “right” Ca2+ to kill neurons. It was suggested, indeed, that Ca2+ entering through these channels has different physiological roles than triggering cell death. Specifically, it activates gene programs, like that involving CREB, that promote cell survival [15-18]. Several explanations have been proposed to explain why, differently from VGCCs, NMDA receptors have a privileged role in neuronal death. The most popular of these hypotheses is that they are close to neuronal nitric oxide synthase (nNOS) in the postsynaptic density [19, 20]. So, Ca2+ entering through NMDA receptors activates nNOS leading to the production of toxic peroxynitrites [20]. These toxic compounds could further increase Ca2+ overload by activating TRPM7 channels [20]. In conclusion, the source specificity theory assumes that VGCCs cannot cause death in neurons because they do not couple with death effectors like nNOS. Recent data questioned these conclusions. They showed, indeed, great differences in the [Ca2+]i responses and in the cell damage caused by depolarization in different single cortical and hippocampal neurons [21] (Fig. 1A-D). In most of the neurons, membrane depolarization with high K+ caused an [Ca2+]i response lower than after NMDA receptor stimulation and no cell damage [21]. On the contrary, in a small group of neurons, it caused a steady increase in [Ca2+]i up to values close to those induced by NMDA agonists and signs of impending cell death [21]. These included a massive build-up of Ca2+ ions into the mitochondria, mitochondrial depolarization, and relevant structural changes in these organelles [21] (Fig. 1C). Thus, it appears likely that in a subset of neurons, membrane depolarization may cause a marked increase in [Ca2+]i and cell death. In keeping with this hypothesis, neuronal aging in vitro causes a parallel increase in the percentages of dying neurons, of neurons showing high L-type VGCC density and of neurons showing high [Ca2+]i responses to depolarization (Fig. 1A and B). Based on these data, Stanika et al. [21] proposed that VGCCs do not cause neuronal death because in the majority of neurons they do not carry enough Ca2+. Indeed, in cells where their activation causes large Ca2+ loads, membrane depolarization triggers a mitochondrial-dependent cell death [21]. These data suggest that VGCCs could promote neurodegeneration if their density or activity is increased over the average. This raises the question of whether this phenomenon happens in specific neurodegenerative diseases. VGCC channelopathies are a good answer to this question. Indeed, in these diseases, mutations in VGCC genes cause changes in the activity of these channels and death of selected neuronal populations. These rare disorders are a paradigmatic example of the involvement of VGCCs in neurodegeneration. However, we will not discuss about them because they have been the object of excellent reviews where the interested reader can find an in-depth analysis of their pathophysiology [22-24]. Instead, we will focus, on common neurodegenerative disorders not caused by mutations in VGCC genes, like Alzheimer’s disease (AD) or Parkinson’s disease (PD). We will distinguish two generally different situations leading to VGCC-dependent cell death in these diseases. First, we will examine conditions where specific pathological stimuli increase the expression or activity of VGCCs, thus killing neurons otherwise refractory to depolarization-induced death. Second, we will explore conditions in which depolarization-induced cell death occurs in neurons that are intrinsically more susceptible to VGCC-dependent cell death. In these cases, factors increasing VGCC activity or density are not required to induce cell death. Specifically, this happens in neurons relying on these channels for action potentials generation or propagation.
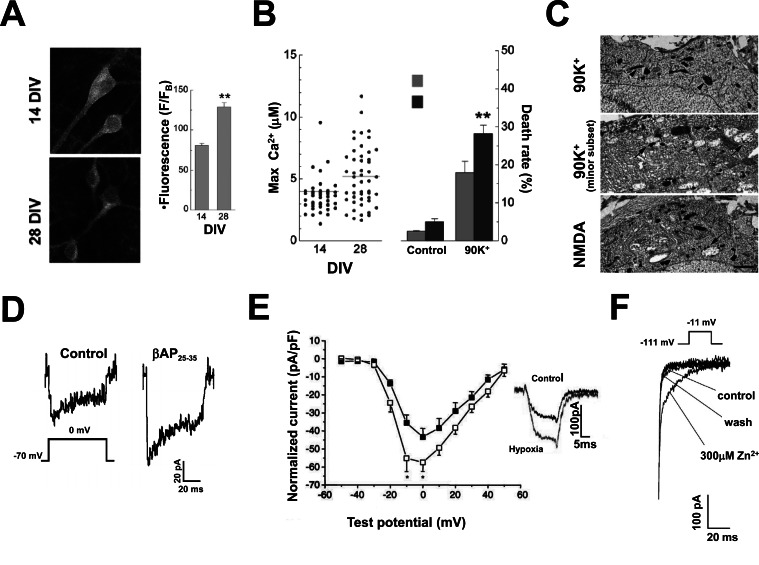
Fig. (1).
Factors causing a pathological VGCC current increase in neurodegenerative diseases. A, B and C, neurons aged in vitro carry larger VGCC currents than young neurons and are more vulnerable to death. A, confocal fluorescence images of hippocampal neurons incubated with anti CaV1.2 primary antibodies and FITC-conjugated secondary antibodies. Note that CaV1.2 immunoreactivity is higher in 28 than in 14 DIV cultured neurons. The bar graph in the inset reports the mean±SEM of the fluorescence intensities measured in 28 DIV (n=24) and 14 DIV (n=46) neurons. B, the scatter plot on the left reports the values of maximal [Ca2+]i increase elicited by a 90 mM K+- containing extracellular solution in single 14 and 28 DIV neurons. The bar graph on the right shows the fractional cell death in the same conditions. Note that cell death is higher in 28 DIV than in 14 DIV neurons. C, electron micrographs obtained in 28 DIV hippocampal neurons freeze-dried and sectioned immediately after being exposed to either 90 mM K+ or 100 µM NMDA. Note the presence of mitochondrial swelling (arrows) both in NMDA and in 90 mM K+ treated cultures. Arrowheads indicate normal mitochondria for comparison (A, B and C reproduced with permission from Stanika et al., 2012 [21]). D, βAPs increase VGCC currents in pyramidal hippocampal neurons. Cultured hippocampal neurons were exposed for 16 hours to 10 µM βAP25-35 before patch-clamp recording (reproduced with permission from Ueda et al., 1997 [44]). E, chronic hypoxia causes an increase in VGCC current amplitude. The graph shows voltage to current plots obtained in rat cerebellar neurons cultured under normoxic (■) and hypoxic conditions (□). Chronic hypoxia was obtained by incubating the neurons for 24 h before patching in a 2.5% O2, 5%CO2 and 92.5% N2 atmosphere. The holding potential was set at -90 mV and currents were elicited by voltage steps up to 0 mV. The inset shows superimposed the means of all the traces recorded in control and hypoxic neurons as indicated (reproduced with permission from Webster et al., 2006 [52]). F, Zn2+ slows down current deactivation kinetics in recombinant CaV3.3 T-type channels. The panel shows the tail currents recorded upon membrane repolarization in control condition, in the presence of 300µM Zn2+ and after washing out this transition metal in a CaV3.3 stably expressing HEK-293 cell held at -111mV and stepped up to -11mV (voltages corrected for junction potential). Note that in the presence of Zn2+ current deactivation is greatly slowed down (reproduced with permission from Cataldi et al., 2007 [87]).
1a. Conditions Leading to a Pathological Increase in the Expression or Activity of VGCCs
Aging increases the risk of Alzheimer’s disease, and vascular dementia [25-27]. These diseases often coexist in the same patient giving rise to a mixed Alzheimer’s disease and vascular dementia syndrome [28]. Furthermore, neurovascular disorders are an important risk factor for different forms of dementia [29-31]. So, the incidence of this disease is doubled in subjects aged less than 85 years that previously suffered from brain ischemic episodes [29-31]. Several findings suggest that a reason for linking these conditions the one to the other and all to aging could be a pathological increase in L-type VGCC currents.
The idea that normal aging does increase the activity of L-type Ca2+ channels was proposed more than 20 years ago. It is supported by a wealth of experimental data. For instance, in aged animals, neuronal whole-cell L-type currents are larger than in younger animals [32]. Besides, Ca2+-dependent action potentials or Ca2+-dependent after-hyperpolarization, that depend both on these currents, increase as well [32]. Importantly, the increase in L-type currents caused by aging also leads to a potentiation of Ca2+-dependent synaptic depression [34]. This could have a role in cognitive impairment of the elderly [33]. Cultured aged neurons are more vulnerable to death than younger neurons and their higher vulnerability is at least in part L-type channel dependent [34]. In keeping with this evidence, a maximal depolarizing challenge evokes [Ca2+]i responses larger than 5 µM in a higher percentage of 28 DIV “aged” than of 14 DIV “young” neurons [21]. This finding suggests that L-type dependent Ca2+ influx could be responsible for the high vulnerability of aged neurons [21]. Our knowledge of the mechanism responsible for the increase in L-type currents in aged neurons is still limited. We know that this process does not depend on changes in L-type channel expression. Indeed, the expression of the major L-type isoform, CaV1.2, strongly decreases in aging whereas the less represented isoform CaV1.3 only marginally increases [35-36]. Instead, the available evidence points to an increase in L-type channel activity whose origin remains, however, obscure. Davare and Hell reported evidence that an increase in protein kinase A (PKA)-mediated channel phosphorylation could be involved [37]. According to recent evidence, the imunophilin FKBP1b could also have a role in aging-induced L-type channel potentiation [38]. The levels of this protein, that binds to ryandodine receptor type 2 in the brain and keeps it closed [39, 40], decrease in normal aging [41]. Aging-induced changes in FKBP12.6 were reproduced by RNA interference both in vitro, in cultured neurons, and in vivo, in young rats [38]. Intriguingly, in these conditions, both L-type currents and Ca2+-dependent slow after-hyperpolarization greatly increased [38].
β-Amyloid Peptides (βAPs) accumulate in Alzheimer’s disease resulting in amyloid plaques. These peptides are considered responsible for neuronal cell death in this disease. Importantly, βAPs markedly increase VGCC currents. This was first demonstrated in differentiated mouse N1E-115 neuroblastoma cells [42]. Later, this effect of βAPs was observed also in hippocampal and cortical neurons and in cerebellar granules [43-45] (Fig. 1D). Early studies reported a significant potentiation not only of L-, but also of N- and P-type currents upon βAP exposure [46]. More recently, data have been reported suggesting that βAPs do inhibit P-type currents and that this effect could be relevant in causing the cognitive disorders typical of Alzheimer’s disease [47]. βAP also inhibits recombinant P/Q type channels expressed in Xenopus oocytes [48]. The reason of the discrepancy among these studies is presently unknown. Aggregation status influences the ability of βAP to modify VGCC currents. The “toxic” forms of these peptides, the βAP oligomers, activate VGCCs much more than the aggregated forms that are not toxic [46]. The mechanism by which βAPs increase VGCC currents remains elusive. However, L-type channel activation could depend on βAP-induced free radical generation [44] or channel phosphorylation by MAP-kinases [49]. Recent work on recombinant L-type channels showed that βAP25-35 affects channel trafficking to the plasma membrane by binding to the accessory β3 subunits [50].
L-type currents increase also in the presence of chronic hypoxia that occurs, for instance, in conditions like atherosclerotic dementia. The first evidence of the increase in L-type currents was obtained in PC12 cells [51] and in cerebellar neurons [52] cultured in a low-oxygen atmosphere (Fig. 1E). Some evidence suggests that βAPs are responsible for the increase in L-type channel activity caused by hypoxia. This could explain why AD and cerebrovascular disorders often occur together in the same patients. Brain ischemia increases the expression of amyloid precursor protein (APP) mRNAs and proteins [53, 54]. Specifically, mRNAs for APP isoforms with a Kunitz-type serine protease inhibitor domain (KPI) build-up in ischemia [53]. Also the activity of β- and γ-secretase is enhanced in this condition [55]. The following evidence supports the hypothesis that βAP is responsible for the increase in L-type channel activity caused by hypoxia. First, the pharmacological blockade of β- or γ-secretase [56], or the immunoneutralization of βAP with monoclonal antibodies prevent the effect of hypoxia on VGCCs [51]. Second, the exposure to βAP25-35, βAP1-40 or βAP1-42 replicate this effect [51, 52]. L-type channels carry most of the inward currents induced by hypoxia. On the contrary, the contribution of other HVA subtypes like N-type channels is negligible [51, 52]. Nonspecific ion channels made by βAPs dissolved into the plasma-membrane carry only a minor, Cd2+-resistant, component of hypoxia-induced currents [57]. Hypoxia does not induce changes in the gating properties of L-type channels. Western blot and immunocytochemistry data show that, instead, it increases channel density in the plasma-membrane [56]. This effect depends on an enhanced translocation of L-type channels to the plasma membrane [56]. It is prevented, indeed, by drugs that disrupt vesicle recycling [56]. Antioxidant drugs prevent the effect of hypoxia on L-type channels [58]. Furthermore, hypoxia does not affect L-type currents in cell lines that have been depleted of mitochondria with ethidium bromide [58]. These findings suggest that radical oxygen species (ROS) have a role in L-type current potentiation by hypoxia. Importantly, the levels of these compounds are high in hypoxia because mitochondrial respiration is impaired [58]. The increase in L-type channel activity induced by βAP1-40 is not affected when ROS levels are decreased. These data suggest that hypoxia promotes, by a ROS-dependent mechanism, the generation of βAPs that, then, modulate L-type channels [56]. Recent evidence shows that, indeed, hypoxia increases the transcription of β-secretase 1 (BACE1) via ROS and the oxygen-sensitive transcription factor hypoxia-inducible factor 1 (HIF-1) [59]. Coimmunoprecipitation experiments suggest that βAPs could affect L-type channels binding to the channel proteins [56]. In keeping with this hypothesis, in heterologous expression systems βAPs affect L-type channel trafficking by interacting with their β3 subunits [50].
Do all these data support the use of CCBs in Alzheimer’s disease or in dementia syndromes? The hypothesis that CCBs could be helpful in these conditions was proposed already in the early days of the Ca2+ neurotoxicity theory. At the time several studies showed the protective effects of CCBs on βAP-induced cell death in neurons cultured in vitro [60, 61]. Other studies, however, did not confirm these findings [62]. Several epidemiological studies evaluated whether patients treated with CCBs for cardiovascular disorders are protected from AD and dementia. They yielded, however, contradictory results. Some studies but not others showed, indeed, that CCBs improve AD symptoms in the short term and prevent or delay, disease progression [63]. These inconsistencies could be due to differences among the different CCBs. For instance, some CCBs but not others efficiently cross the blood-brain barrier. Also, some CCBs may affect βAP toxicity by mechanisms unrelated to VGCC blockade. For instance, nimodipine promotes βAP1-42 secretion in a L-type channel-independent way [64]. Also, bepridil affects βAP processing by interfering with endosome pH [65]. Finally, some CCBs like verapamil reduce the clearance of βAP because they block P-glycoprotein, the pump responsible for its removal from the brain [66-68]. Nivaldipine [63, 69] and isradipine [70] are the most promising CCBs for the treatment of AD and other forms of dementia. Specifically, nivaldipine retarded the progression of cognitive decline in patients with mild cognitive impairment [71, 72]. Besides, it stabilized cognition and improved executive function in AD patients. This effect was higher in patients not showing the APOE ε4 phenotype [69]. Isradipine is neuroprotective for MC65 cells [73, 74]. This cell line represents a model that reproduces in vitro βAP neurotoxicity more faithfully than cultured neuronal cells exposed to exogenous βAP [73, 74]. It is a neuroblastoma cell line stably transfected with amyloid precursor protein (APP)-C99 under control of a tetracycline (Tet)-repressor cassette. Therefore, it releases βAPs and undergoes βAP-dependent cell death when tet is removed from the culture medium [73]. Isradipine prevented βAP neurotoxicity also in several AD models in vivo including drosophila, the moth Manduca sexta and 3xTgAD mice, which harbor the presenilin-1 (PS1) (M146V), APPswe and tau (P301L) transgenes [74]. Despite this encouraging evidence, the blood pressure lowering effect of CCBs may be of concern for it lowers brain perfusion. We will discuss further the neurovascular effects of CCBs in AD in section 3a. A more rational approach than blocking L-type channels would be to correct the molecular mechanisms responsible for the increase in their activity or expression. Unfortunately, although some information on this issue begins to be available, no drug therapy specifically directed against these targets has been developed yet.
Membrane potential becomes depolarized in neurons after hypoxia in vitro or ischemic stroke in vivo. Therefore, VGCCs are expected to be strongly activated in these conditions (see section 3a for further details) [75]. This suggested that VGCC blocking drugs could be neuroprotective in stroke. It was also reported that these compounds may counteract Ca2+-dependent hypoxic vasoconstriction and improve the perfusion of ischemic brain [76]. A wealth of preclinical data confirmed the neuroprotective properties of L-, N- and P-type channels blockers [77]. However, CCBs failed in several clinical trials on stroke in humans [10]. Nowadays, it is clear that they are not effective in this disease. On the contrary, the information available is still insufficient to establish the role of N- or P-type channel blockade in brain ischemia [77, 78]. A likely reason of the failure of L-type channel blockers in stroke is that, in the ischemic core, Ca2+ enters the neurons through many other ion channels and transporters [8,9]. In this part of the brain, cerebral blood flow (CBF) dramatically decreases after stroke and neurons die almost immediately after the ischemic insult [8, 9]. Around the ischemic core there is a region, the penumbra, where CBF, though lower than normal, still preserves ionic homeostasis. Neurons in the penumbra die, indeed, only hours after the initial ischemic event. Therefore, there is enough time to rescue neurons this region of through pharmacological interventions. Surprisingly, L-type currents in the neurons of the penumbra are not increased, as expected, but significantly decreased respect to control conditions [79]. The decrease in L-type currents occurs in CA1 hippocampal neurons, that are sensitive to ischemia, but not in CA3 neurons, that are more resistant to this insult [79]. Protein expression of L-type channels is comparable in the ischemic penumbra and in control tissue. On the contrary, L-type single channel open probability is significantly decreased [79]. This effect could depend on the oxidation of the channel [79], a common mechanism affecting ion channel activity in stroke [80]. What is the pathophysiological meaning of the decrease in L-type channel currents in the penumbra? The authors of the cited paper speculated that it contributes to delayed neuronal cell death [79]. A decrease in Ca2+ influx through L-type channels could, indeed, blunt neuroprotective gene programs like that mediated by CREB [15-18]. Consistent, the L-type channel agonist Bay-K 8644 protects neurons in models in vitro and in vivo of brain ischemia [79]. Recently, the group of Ricardo Dolmetsch in Stanford reported that glutamate exposure in vitro causes a decrease in the density of L-type channels in the plasma membrane [80]. This decrease occurred because of the internalization and the lysosomal degradation of the CaV1.2 pore-forming subunits [80]. This process involves the following consecutive steps. First, CaV1.2 binds to Pikfyve, an enzyme that generates phosphatidylinositol (3,5)-bisphosphate (PtdIns(3,5)P2). Second, CaV1.2-containing endosomes become enriched with PtdIns(3,5)P2. Finally, PtdIns(3,5)P2-enriched vesicles are targeted to lysosomes [80]. Because they are degraded in lysosomes, CaV1.2 containing channels cannot be recycled to the plasma-membrane. This causes a persistent decrease in L-type currents. Therefore, glutamate targets CaV1.2 channels to lysosomal degradation. On the contrary, after depolarization, CaV1.2 channels are internalized but not targeted to lysosomes. Therefore, they may rapidly return to plasma membrane [81]. Differently from what reported by Li [79], glutamate-induced CaV1.2 degradation seems to protect neurons from Ca2+ overload and cell death. Pikfyve silencing in vitro causes, indeed, an increase in glutamate-induced cell death presumably because more Ca2+ can enter the neuron through L-type channels [80]. In keeping with this hypothesis, old data show, indeed, that L-type channels become activated after NMDA receptor stimulation and contribute to glutamate-induced Ca2+ overload and cell death [14]. Also, glutamate-induced cell death in Pikfyve silenced neurons can be prevented by L-type channel blockade with nimodipine [80]. Pikfyve-dependent decrease in L-type channel density could be a key neuroprotective mechanism in glutamate-dependent forms of neurodegeneration such as in stroke or in cell death after seizures. This intriguing hypothesis remains, however, to be directly explored in vivo.
T-type or low voltage-activated (LVA) channels are another class of VGCC that could have a role in ischemic neuronal cell death. These channels, differ from HVA Ca2+ channels under many respects including permeation and gating properties [82, 83]. Importantly, in LVA but not in HVA channels, the voltage dependence of activation and the voltage dependence of inactivation overlap at voltages around -50 mV [82]. This implies that when membrane resting potential assumes values inside this window of overlap, a definite fraction of T-type channels stays stably open and can carry excess Ca2+ inside the neuron. This could happen in the early phases of ischemia when neurons start to depolarize and their resting membrane potential move into the T-type channel window [84]. Therefore, it has been suggested that T-type channels could be an important Ca2+ influx path in ischemic neurons. The only evidence that T-type channels take part to brain ischemia comes from experiments performed with the unselective T-type channel blockers mibefradil, pimozide and nickel [84, 85]. These drugs decreased ischemic neuronal cell death in brain slices undergoing oxygen-glucose deprivation [85]. Also, they lessened cell loss in the hippocampus of rats subjected to global ischemia [86]. We still ignore which of the three T-type channel isoforms, CaV3.1, CaV3.2 and CaV3.3 [82], takes part to ischemic death in neurons. Further experiments in knock-out animals or in vitro silencing studies will be necessary to clarify this point.
Transition metals cause an increase in the activity of T-type channels that could be relevant in brain ischemia. In particular, we showed that Zn2+ affects the gating properties of the CaV3.3 isoform of T-type channels [87]. To be specific, it markedly slows down their deactivation [87]. This is expected to increase the amount of Ca2+ entering the neurons on membrane repolarization. The importance of this mechanism in neurodegeneration remains to be proved. However, it is worth to remind that Zn2+ ions have a well established role in neurodegeneration. These ions, indeed, are coreleased with glutamate and cooperate with this neurotransmitter in causing neuronal cell death in conditions like brain ischemia and AD [88, 89].
1b. VGCC “Toxicity” in Neurons Showing VGCC-Dependent Action Potentials
In the previous sections we showed that specific factors may cause neuronal cell death by increasing VGCC density or activity. Now we will review evidence that selected neuronal populations are intrinsically susceptible to Ca2+ overload. This may happen in neurons that show a Ca2+-dependent pacemaker activity. These cells undergo large oscillations in [Ca2+]i. Therefore, even in physiological conditions, they have average [Ca2+]i significantly higher than other neuronal populations. Pacemaking is common in neurons but it is Ca2+-dependent only in a minority of cases. Indeed, in most CNS neurons Ih channels or TTX-sensitive persistent Na+ currents are responsible for pacemaking [90]. In few neuronal populations [90] repetitive action potential generation relies on VGCCs. This resembles the pacemaking of nonneuronal cells like sinoatrial cardiac cells [91] and pituitary cells [92, 93]. CaV3 low-voltage activated T-type channels or CaV1.3- containing L-type channels are the VGCCs involved in this process [82, 94]. These channels open at subthreshold voltages and, therefore, may trigger action potentials [82, 95]. Thalamic neurons are typical examples of T-type dependent pacemaker cells [82]. Instead, CaV1.3 channels are responsible for spontaneous firing in substantia nigra pars compacta (SNc), in midspiny striatal neurons, in adrenal chromaffin cells and in cochlear immature inner hair cells [94] (Fig. 2A). In pacemaker cells, high Ca2+ amounts enter the cytoplasm at each spike. Therefore, these neurons are exposed to higher Ca2+ loads than non-oscillating cells or cells with nonVGCC-dependent pacemaking. Also, in Ca2+-dependent pacemaker cells, Ca2+ influx through L-type channels causes NO generation and cGMP increase [96] (Fig. 2B). These data suggest that neurons with Ca2+-dependent pacemaker cells could be especially susceptible to cell death. The group of D.J. Surmeier showed that this occurs in SNc neurons [97]. SNc neurons have a special relevance in neurodegeneration because they are the first to degenerate in PD. SNc neurons fire spontaneous Ca2+-dependent action potentials at a frequency of 2-4 Hz [98]. Classical studies showed that in these neurons action potential generation depends on L-type channel opening. Dihydropyridines like nifedipine, nimodipine or isradipine make, indeed, it stop [97-100] (Fig. 2A).
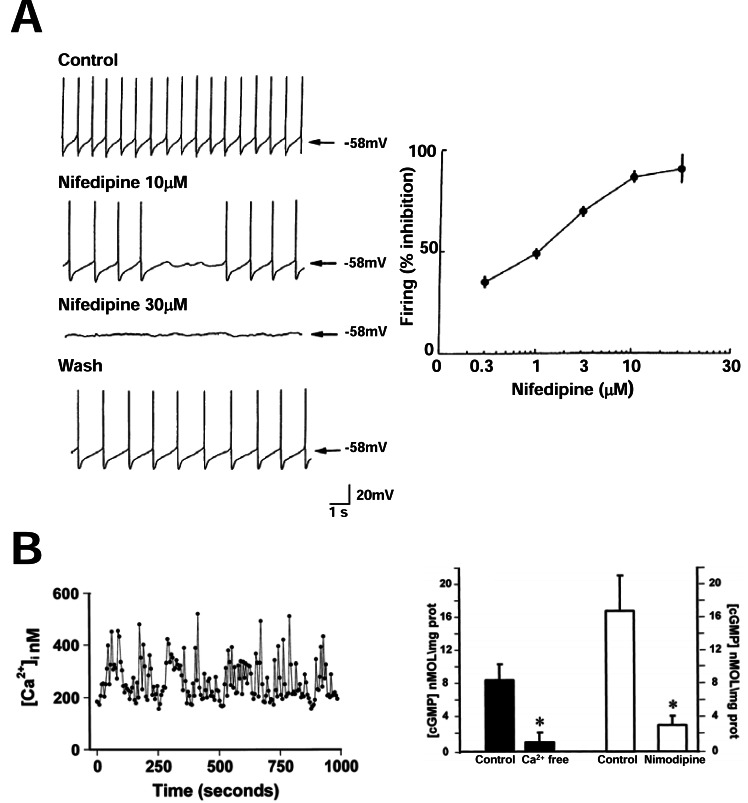
Fig. (2).
High Ca2+ loads in cells showing Ca2+-dependent oscillations. A, SNC neurons fire spontaneous Ca2+-dependent action potentials. The panel shows representative current clamp recordings obtained in a SNC neuron recorded from an acute rat brain slice in basal condition, in the presence of two different concentrations of nifedipine and after washing out this CCB. Note that in the presence of nifedipine spontaneous action potential generation is prevented. The plot on the right of the panel shows the dose-dependence of the suppression of SNC neuron firing by nifedipine (reproduced with permission from Mercuri et al., 1994 [99]). B, Ca2+-dependent oscillations in pituitary GH3 cells are coupled with the activation of the NO-cGMP cascade. The trace on the left of the panel shows typical [Ca2+]i oscillations in a single fura2-loaded GH3 cell. The bar graph on the right shows the effect on intracellular cGMP concentrations of the removal of extracellular Ca2+ or of the incubation with the CCB nimodipine, two conditions that abrogate [Ca2+]i oscillations in GH3 cells; *= p<0.05 (reproduced from Cataldi et al., 1998 [96] with permission).
Elegant studies showed that Ca2+-dependent pacemaking is responsible for the high susceptibility of SNc neurons to neurodegeneration. Briefly, Chan and coworkers were interested in establish whether CaV1.3 channels are responsible for pacemaking in SNc neurons that strongly express these channels [97]. Therefore, they studied the spontaneous electrical activity of SNc neurons from CaV1.3 knock-out mice [97]. To their surprise they found that these cells were still discharging at the expected frequency [97]. However, differently from controls, in knock-out mice pacemaking depended on Na+ channels and Ih and not on L-type channels [97]. This form of pacemaking is typical of juvenile SNc neurons. This suggested that the developmental switch from Na+- to Ca2+-dependent pacemaking does not take place in CaV1.3 knock-out mice [97]. SNc neurons from CaV1.3 knock-out mice are more resistant to to neurotoxic compounds, like the parkinsonigen agent rotenone [101], than those from their littermates [97]. This suggested that Ca2+-dependent pacemaking has a role in SNc susceptibility to death. Consistent with this idea, isradipine, an L-type channel blocker with some selectivity for CaV1.3 channels [102], protect SNc neurons from parkinsonigen agents. Interestingly, isradipine forces SNc neurons from wild type mice to switch to a Na+-dependent juvenile pacemaking pattern [97]. Isradipine is also neuroprotective in models in vivo of the disease based on rotenone, MTPP or 6-OH dopamine toxicity [97-103]. Isradipine also prevents abnormal movements in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD diskinesia [104]. These findings suggest that CCBs could be helpful in PD. However, the available clinical evidence do not support this hypothesis [105]. For instance, a large retrospective analysis of Ontario’s health care databases did not show any evidence that long-term treatment with dihydropyridines for hypertension could lower the risk of PD [106]. This could be due to the fact that most of currently marketed CCBs only weakly block CaV1.3 channels [102]. The only exception could be isradipine [102] that, therefore, is currently evaluated for PD. The results of a pilot dose escalation study using this drug in early PD have been published already [107]. A randomized double blinded phase II study, the “Safety, Tolerability and Efficacy Assessment of Dynacirc CR in Parkinson’s Disease (STEADY-PD)” (http://www.clinicaltrials.gov/ct2/show/NCT00909545?term=STEADY-PD&rank=1) is underway and its results are eagerly awaited.
The evidence reviewed above suggested that aging SNc neurons degenerate and die much faster than other kinds of neurons because they are exposed to high Ca2+ loads during all life. In this scenario, PD is an inescapable result of extreme aging. PD may occur earlier in life in conditions that weaken the ability of mitochondria to cope with the high Ca2+ loads. This could happen because of mutations of key mitochondrial proteins or of the exposure to mitochondrial toxins [108]. Ca2+ oscillations may cause SNc damage also by another mechanism. L-type channels activation leads, indeed, to an increase in the synthesis of dopamine. The metabolism of this neurotransmitter with the generation of free radicals may further damage SNc neurons [109].
In neurodegenerative diseases Ca2+-dependent pacemaking may have roles independent from their involvement in cell death. For instance, T-type-dependent pacemaking in the subthalamic nucleus (STN) has a role in the genesis of the parkinsonian tremor [110]. STN is part of the indirect dopaminergic pathway. It has a role in the control of extrapyramidal movements. Therefore, it is often the target of the deep brain stimulation protocols used control PD symptoms [110]. Isolated STN neurons show repetitive single-spike firing. This spontaneous electrical activity depends on the interplay between voltage-gated Na+ channels and a Ca2+-dependent K+ conductance [110]. When they are hyperpolarized, STN neurons switch to burst firing [110]. Interestingly, the percentage of burst firing STN neurons significantly increases in experimental models of PD [111]. T-type Ca2+ channels maintain burst firing in STN neurons. Diverse T-type channel inhibitors including mibefradil, efonidipine or NNC 55-0396 block, indeed, STN burst firing in brain slices and in living rats undergoing single unit extracellular recordings [110]. In rats made parkinsonian by 6OH-DA these drugs normalize the percentage of burst-firing neurons [110]. Importantly, they also relieve the locomotor deficits observed in these animals [110]. These results suggest that the pharmacological blockade of T-type channels could decrease motor symptoms in PD. A drug acting on T-type channels, zonisamide (ZNS), was approved for PD even before the discovery of the role of these channels in STN. ZNS is an antiepileptic drug acting on multiple targets. It blocks voltage-gated Na+ channels, carbonic anydrase, MAO-B, and T-type channels [112]. In March 2009 ZNS was approved in Japan for PD. Drug approval was granted on the basis of the results of a multicenter, randomized, double-blind, parallel-treatment, placebo-controlled study showing the improvement in motor function upon ZNS add-on in patients not adequately responding to L-Dopa [113]. The effect of ZNS on PD motor symptoms was discovered by chance in a PD patient that was treated with this drug because of a concurrent seizure [114]. Since then, other clinical studies confirmed these initial findings. The mechanistic bases of the beneficial effects of ZNS in PD remain, however, obscure. The ability of the drug to reduce oxidative stress could have a role. ZNS, indeed, up- regulates manganese-superoxide dismutase (MnSOD) and inhibits monoamine oxidase B (MAO-B). These pharmacological effects contribute in protecting dopaminergic neurons from MTPT toxicity [115-118] and human neuroblastoma SHSY5Y cells from staurosporine-induced apoptosis [119]. Astrocytes could be responsible for ZNS neuroprotection. This drug increases, indeed, glutathione levels in these cells, but not in neurons [120]. Moreover, ZNS induces the expression of anti-oxidative and neurotrophic factors in astrocytes [121]. The new evidence that we reviewed above about the role of STN T-type channels in motor symptoms of PD strongly suggests that T-type channel blockade contributes to the favorable effect of the drug in PD. This hypothesis remains, however, to be proved. We would like to close this section with essential tremor (ET), another neurodegenerative disease whose symptoms depend on a T-type-dependent neuronal pacemaker. ET is a motor disorder characterized by tremor in the arms and hands during voluntary movements. In this disease, Lewy bodies occur in the brain stem, and cell loss or degenerative changes like torpedoes in cerebellar Purkinje cells [122, 123]. Therefore, ET is considered as a neurodegenerative disorder [122, 123]. The indole alkaloid harmaline produces a condition similar to ET in mice. In this experimental model, tremor is maintained by the synchronized oscillatory discharge of inferior olive (IO) neurons [124]. Pacemaking in these cells depends on CaV3.1 T-type channels [124]. In agreement with these findings, harmaline treatment does not induce tremor in CaV3.1 knock-out mice. This treatment induces 4-10-Hz oscillations in the IO in wild type but not in CaV3.1 knock-out mice [124]. The local infusion of lentivirus harboring CaV3.1-specific shRNA into the IO neurons also prevents harmaline-induced tremor in mice [124]. Intriguingly, the T-type channel blockers ethosuximide, zonisamide, KYS05064, and NNC 55-0396 and the neuroactive steroid (3β,5α,17β)-17-hydroxyestrane-3-carbonitrile (ECN), also ameliorate the neurological symptoms in experimental ET [125]. Several clinical studies evaluated ZNS in ET patients [126]. Authorities in the field feel, however, that the available evidence is inadequate to recommend ZNS in this disease [126].
2. VGCCs may be Toxic for Neurons Without Carrying Excess Ca2+
One of the most surprising findings emerging from recent work on VGCCs in neurodegeneration is that these ion channels may cause neuronal cell death even without transporting toxic Ca2+ loads. A decrease in the ability of VGCCs to carry Ca2+ ion may, indeed, kill neurons. Neurons may also die because VGCCs let toxic metal ion enter their cytoplasm.
2a. Paradoxical Decrease of VGCC-Dependent Ca2+ Load in Neurodegenerative Diseases: the Case of Prion Disease
We mentioned above that Ca2+ entry through L-type channels promotes neuron survival by activating neuroprotective Ca2+-dependent gene programs [15-18]. We also mentioned that a decrease in the activity of these channels could be responsible for delayed neuronal death in the ischemic penumbra [79]. Here we will focus on prion disease, another condition where VGCC currents strongly decrease. The earliest evidence that prion protein could suppress VGCC was reported many years ago by Florio and coworkers [127]. They noted that, in GH3 pituitary cells, a synthetic peptide homologous to residues 106–126 of PrP (PrP106–126) suppressed L-type currents and the [Ca2+]i response to high K+ solutions. These findings were later confirmed also in neurons [128]. Recently, Senatore et al. [129] suggested a molecular mechanism to explain prion effect on VGCCs. Briefly, they compared the [Ca2+]i response to high K+ depolarization and the activity of VGCC currents in cerebellar granule cells from wild type and Tg(P14) mice [129]. These mice express a misfolded form of prion protein and develop a progressive fatal neurological disorders with ataxia, kyphosis, and foot clasp reflexes [129]. Both the [Ca2+]i response to depolarization and VGCC current density were lower in transgenic than in control mice. These differences were already evident at the time of the first neurological symptoms, well before the histological evidence of neurodegeneration [129]. No change in VGCC gating properties was observed but the density of the channels in plasma membrane increased. The accessory α2δ subunits are crucial regulators of VGCC trafficking [130]. They are also part of the PrP interactome [131]. Therefore they are good candidates as effectors of prion modulation of VGCCs. Senatore et al. [129] showed that the α2δ-1 isoform physically interacts with mutant PrPs. Importantly, this causes VGCC retention in intracellular compartments and prevents their targeting to the plasma membrane [129]. The decrease in VGCC density at the plasma membrane causes a general decrease in synaptic transmission that could be responsible for the neurological symptoms observed in transgenic animals. The formal proof that the decrease in VGCC trafficking has also a role in causing neuronal cell death in prion disease is still missing. However, this seems a very likely possibility. Ca2+ influx through VGCCs is, indeed, crucial for neuronal survival [15-18] and neurons die of apoptotic cell death when exposed for many hours to CCBs [132]. Moreover, neuronal cell degeneration occurs in mutant mice showing spontaneous mutations or targeted deletions of the CACNA2D2 gene that encodes α2δ-2 subunits [133, 134]. Further studies are needed to establish whether drugs that increase VGCC currents could be effective in this devastating neurodegenerative disorder.
2b. Metal Ion Influx Through VGCCs
VGCCs have always been considered just Ca2+ influx systems. However, biophysical studies clearly show that other cations beside Ca2+ may permeate through these ion channels. The pore of L-type channels is large enough to let large organic cations permeate into the cell [135]. These cations may also permeate through T-type channels though their pore is smaller [83]. Importantly, also transition metals can flux trough VGCCs. This could be relevant in neurodegeneration because some of them including aluminum, manganese, iron and zinc have a role in the pathogenesis of major neurodegenerative disorders like AD, PD and multiple sclerosis (MS). These metals can cross the blood brain barrier through specific transporters and interact with neuronal VGCCs [136, 137]. Therefore, the hypothesis that VGCCs contribute to the toxicity of transition metals by carrying them into the neurons appears intriguing. Recent publications provide convincing experimental evidence that this could occur in the case of iron. This metal is essential for the normal functioning of the brain. It is necessary, indeed, for mitochondrial respiration and for the activity of many enzymes that synthesize or degrade the neurotransmitters [138]. The main among them are tyrosine hydroxylase, tryptophan hydroxylase, glutamate decarboxylase and glutamate transaminase, and the monoamine oxidases A and B [138]. However, when iron concentration increases too much, this metal becomes neurotoxic because it generates free radicals. The main mechanism by which this occurs is the conversion of Fe2+ into Fe3+ in the Fenton reaction. The free radicals so produced make the cells die by apoptosis [139]. Importantly, free radicals also cause the denaturation of specific proteins that precipitate and form protein aggregates. These are typical of many neurodegenerative diseases as in the case of PD in which Lewy bodies accumulate in neurons [140]. Protein aggregation and precipitation in intracellular compartments are an important cause of endoplasmic reticulum stress and, thus, of apoptotic cell death [140]. Recently, Dixon reported that iron causes a new form of non-apoptotic neuronal cell death known as ferroptosis in organotypic hippocampal slices challenged with glutamate [141]. Iron exerts its toxic effects after entering the neurons. In recent years, there was a considerable progress on how iron reaches these cells and enters their cytoplasm [142-144]. Briefly, iron, complexed with transferrin (Tf), crosses the blood brain barrier (BBB) upon binding to transferrin receptors (TfR) on endothelial cells. Once entered into the endothelial cell Fe2+ can be released into brain interstitial space in two different ways. First, it can exit the endothelial cells still bound to Tf. Second, it can leave their endosomes as Fe2+ going through the divalent metal transporter-1 (DMT-1). Two different iron pools do exist in the interstitial space in the brain, iron bound to transferrin (Tf-I) and iron not bound to TF (NTBI). In NTBI, iron is bound to lactoferrin or melanotransferrin or, very loosely, to organic anions like lactate. Because extracellular iron concentrations in the brain exceeds the binding capacity of Tf [145], NTBI significantly contributes to brain iron homeostasis. Its role becomes crucial when iron load is increased or, as in PD, the concentration of Fe-binding proteins like Tf or neuromelanin is lower than normal. Tf-I enters the neurons by receptor-mediated endocytosis. The mechanism of NTBI uptake is, instead, controversial. Until recently, the prevalent view was that it requires the activation of DMT-1 [146]. This transporter is activated by NMDA receptors through a complex signaling cascade that involves the following steps. First, Ca2+ enters the cells through these receptors and causes nNOS activation. Then, a specific brain member of the ras family known as DEXras is S-nitrosylated. This leads to DMT-I activation by the AMP-kinase binding protein Pap7 [146]. Recent data strongly questioned the idea that DMT-1 is the only influx system for NTBI. Evidence has been reported, indeed, that VGCCs also represent a route of iron entry into the cytoplasm. This was demonstrated for the first time in the heart. In iron-overload cardiomyopathy, L-type Ca2+ channels are, indeed, a major patway for iron entry into cardiomyocytes [147, 148]. Gaasch and coworkers [149] demonstrated that a similar mechanism exists also in neuronal cells. They showed that membrane depolarization promotes the uptake of radioactive 55Fe into murine N2α neuroblastoma and in rat pheochromocytoma PC12 cells [149]. This effect was abrogated by the L-type Ca2+ channel blocker nimodipine [149]. This evidence suggested that L-type channels could be a influx path for iron in neurons. More recently, the group of F. Grohovaz in Milan, Italy confirmed and extended this conclusion [150]. They measured Fe2+ influx into the cytoplasm of hippocampal and cortical neurons with calcein fluorescence or fura-2 quenching [150]. Using this approach, they showed that basal and depolarization-induced iron influx is attenuated but not abolished by L-type channel blockade with nimodipine [150]. Instead, it was totally inhibited by an inhibitor cocktail contining nimodipine plus the T-type blocker NNC 55-0396 and ω-conotoxin MVIIC that blocks N-, P-, and Q-type channels [150] (Fig. 3A). Therefore, not only L-type channels but also other subtypes of VGCCs may carry Fe2+ in neurons. Specifically, T-type channels could be involved. Intriguingly, these channels also contribute to cardiac iron overload in thalassemic mice [151]. Whatever the influx pathway involved, excess Fe2+ into the cytoplasm causes ROS generation, mitochondrial depolarization and cell death [150] (Fig. 3B). This could explain why membrane depolarization with high K+, though usually ineffective in causing cell death in cultured neurons in vitro, becomes toxic in the presence of Fe2+ in the extracellular medium [150]. Therefore, the presence of high extracellular Fe2+ concentration should be added to the list of conditions that make potentially lethal VGCC activation in neurons. A key point that remains to be clarified concerns the role of extracellular Ca2+ in VGCC-dependent iron toxicity. Fe2+ ions are believed, indeed, to compete with Ca2+ for permeation. In keeping with this idea, the amount of Fe2+ ions entering the cell through these channels decreases as the extracellular Ca2+ concentration increases [149, 150] and it is maximal when extracellular Ca2+ concentration is well below its physiological value (Fig. 3C). This raises the question of whether or not Fe2+ influx through VGCCs could be relevant in neurodegeneration in vivo. However, it should be pointed out that extracellular Ca2+ concentrations may decrease well below the “normal” values because of the sink activity of neurons both in conditions of intense synaptic stimulation as in epilepsy [152] or when damaged neurons become abnormally permeable to Ca2+ as in most of the neurodegenerative conditions [153]. Interestingly, evidence has been reported that L-type channel blockade with nifedipine could be effective in preventing iron accumulation in dopaminergic neurons also in vivo in rats treated with iron-dextran, an experimental model of iron overload [154].
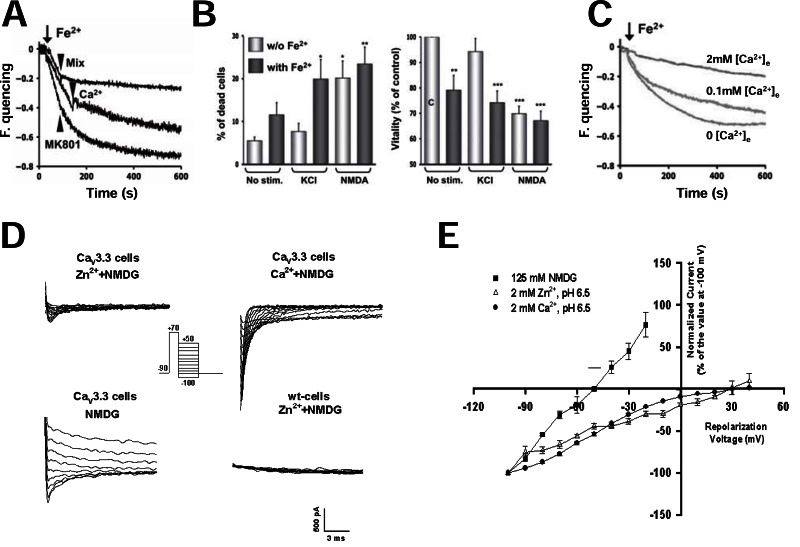
Fig. (3).
VGCCs act as influx paths for metal ions. A, B and C, Fe2+ ions enter the neuronal cytoplasm through VGCCs and cause cell death. A, the administration of 100µM Fe2+ to fura2-loaded hippocampal neurons incubated in a Ca2+ free extracellular solution causes fura2- fluorescence quencing. Fura2 quencing is unaffected by the NMDA blocker MK-801 (20µM), partially prevented by restoring physiological concentrations of extracellular Ca2+ (2mM) and virtually abolished by a mix of VGCC blockers including 10 µM nimodipine, 10 µM NNC 55-0396, and 1 µM ω-conotoxin MVIIC. B, fractional cell death increases and cell viability decreases in the presence of Fe2+ in the extracellular solution. Note that this detrimental effect of Fe2+ can be observed both in basal conditions and after cell depolarization with KCl. Interestingly, when hippocampal neurons are depolarized with KCl in the presence of extracellular Fe2+, fractional cell death increases up to values similar to those observed upon NMDA exposure. C, Fe2+-induced fura2 quencing is partially and dose-dependently reversed by increasing extracellular Ca2+ concentrations. (A, B and C reproduced with permission from Pelizzoni et al., 2011 [150]). D and E, Zn2+ permeates through CaV3.3 T-type channels. D, tail currents evoked by step repolarization of HEK-293 cells stably expressing CaV3.3 channels to progressively more positive potentials after depolarizing the cell to +70 mV. The panel shows representative traces of the currents recorded in cells perfused with extracellular solutions containing, as indicated, 140 mM N-methyl-D-glucamine (NMDG) (to replace extracellular Na+) with no Zn2+ and no Ca2+ or plus either 2mM Zn2+ and no Ca2+ or 2mM Ca2+ and no Zn2+. For comparison, the traces recorded with Zn2+ in a control, untransfected HEK cell are also shown. All the recordings were performed at pH 6.5. E, reports the mean voltage to current plots of the experiments depicted in D. (D and E, unpublished data by Cataldi et al. presented in abstract form in ref. 162).
Another neurotoxic metal that enters the neurons through VGCCs is Zn2+. In brain ischemia, it is coreleased with glutamate, enters the neurons and contributes to their death [88, 155, 156]. AMPA/kainate receptors are the main route of entry of Zn2+ in neurons [155]. However, VGCCs may also have a role [157]. Zn2+ influx in neurons is, indeed, promoted by membrane depolarization and blocked by CCBs and ω-conotoxin GVIA. In addition, in insect muscles fibers [158] and snail neurones [159], action potentials still occur when Zn2+ substitutes for Ca2+ in the extracellular solution. Also, patch clamp experiments showed Zn2+ currents in Helix neurones [160] and in mice cortical neurons [161]. This provided the direct demonstration that Zn2+ may permeate through HVA channels. Experiments performed with Zn2+-sensitive fluorimetric probes showed that VGCCs may carry this metal ion also in the presence of physiological Ca2+ concentrations [161]. L- and N-type channels are the VGCC subtypes involved in Zn2+ permeation. Depolarization-evoked Zn2+ influx in neurons was, indeed, blocked, by CCBs and ω-conotoxin GVIA. However, more recently, we showed that also recombinant CaV3.3 channels may carry Zn2+ when Ca2+ is omitted from the extracellular solution [162] (Fig. 3D and F). This suggests a role for T-type channels in Zn2+ permeation. Further studies are necessary to confirm this theory in neurons and prove its relevance in neurodegeneration.
3. VGCCs may Affect Neuronal Cell Survival Indirectly Through Actions Exerted on Nonneuronal Cells
The reason of the interest on VGCCs in neurodegeneration has been traditionally linked to the role that these channels may play directly in neurons. In the previous sections we examined the evidence indicating that VGCCs may act as a route of entry of toxic Ca2+ loads as it was supposed since the early days of the Ca2+ neurotoxicity hypothesis. In addition, we reviewed data showing that neuronal VGCCs may also take part to neurodegeneration without carrying excess Ca2+ either because they transport less Ca2+ than normal or because they carry metal ions inside the neuron. However, neuronal cell survival may also be indirectly affected by VGCCs through effects that these ion channels exert on non-neuronal cells. This represents an emerging and exciting field of investigation in neurodegeneration. Here we will go through some clear examples recently emerged in the literature that illustrate how VGCCs could be involved in the control of blood flow at the neurovascular unit and of immune responses by microglia.
3a. Critical Role of VGCCs at the Neurovascular Unit
Cerebral blood flow matches the metabolic needs of neurons. This neurovascular coupling occurs thanks to the interaction of neurons, astrocytes, pericytes and vascular cells. These cell types form a highly integrated system known as the neurovascular unit [163, 164]. The neurovascular unit has also other physiological roles besides controlling brain perfusion. For instance, it controls BBB permeability, neurotransmitter availability in the extracellular space and brain inflammatory responses [161, 162]. Recent evidence suggests that pathological changes in the neurovascular unit contribute to the origin neurovascular and neurodegenerative disorders [165]. In this section we will report data on the role of VGCCs in the dysfunctions of the neurovascular unit in some of these conditions.
Strong evidence suggests that a dysfunction of the neurovascular unit causes the secondary expansion of traumatic, hemorrhagic or ischemic focal brain lesions. In focal brain ischemia, the ischemic core immediately becomes hypoperfused and rapidly dies. On the contrary, neurons in the ischemic penumbra die hours later unless they are rescued by specific pharmacological interventions. Similarly, brain trauma and subarachnoid hemorrhage (SAH) cause a focal brain damage that gradually expands after the initial insult. In all these conditions, recurrent episodes of vasoconstriction occur for several days after the acute event [166, 167]. They are elicited by depolarization waves that originate in the core of the lesion and propagate into the neighboring healthy brain [168]. To be specific, immediately after a focal ischemic brain insult, a massive depolarization, the anoxic depolarization, arises in the ischemic core. The failure of the Na+/K+ ATPase causes its appearance. It induces, indeed, the accumulation of K+ ions in the extracellular space. This triggers the opening of multiple and still poorly defined cationic conductances [168]. In the core, anoxic depolarization causes cytotoxic edema. In addition, it forces the failing Na+/K+ ATPase to burn more ATP and further exhaust the cell of this nucleotide [168]. Anoxic depolarization does not remain secluded into the core [168]. Indeed, at the border between the ischemic core and the penumbra it elicits depolarization waves, known as peri-infact depolarizations (PIDs), that invade the penumbra at a speed of 2-5 mm/min [169]. PIDs propagate as a wave of negative deflection of extracellular direct current potential 10-20 mV wide [169]. Spreading depolarizations similar to PIDs propagate from the primary lesion into healthy brain after neurotrauma [170-173] or SAH [174]. The more generic term of cortical spreading depolarizations (CSD) is often used to refer to these depolarizing waves. Electrophysiologically, CSDs resembles the spreading depression that occurs during the aura of migraine attacks [175]. Indeed, they consist, of a large depolarization wave followed by a long lasting suppression of any spontaneous activity in the invaded network [168]. In stroke, CSDs recur for several days from the initial ischemic event and contribute to extend the ischemic damage [169]. This is suggested by the proportionality between the number and duration of PIDs and the growth of the ischemic lesion at the expenses of the penumbra [176-178]. Risher and coworkers used a two photon microscope to visualize the degenerative changes caused by PIDs in the cortical pyramidal neurons of a transgenic mice strain expressing GFP in these cells [179]. They observed, indeed, that rapid dendritic beading occurred after each PID episode [179]. Initially, neurons completely recovered at the end of each CSD [179]. However, after several CSDs dendritic damage became irreversible [179]. The mechanism by which CSDs cause brain damage is still a matter of debate. The massive neuronal depolarization caused by CSDs probably has a role. However, the prevalent view is that neuronal death depends on spreading ischemia. This term indicates waves of ischemia caused by the vasoconstriction elicited by PIDs. Shin and coworkers were the first to describe this process [166]. They simultaneously monitored cerebral blood flow with laser spekle flowmetry and extracellular potential with intracortical glass microelectrodes in mice undergoing middle cerebral artery occlusion (MCAO) [166]. Using this approach, they found that each PID causes a drop in brain perfusion. In addition, it increases by 140% the volume of the brain parenchyma in which blood flow is 20% or less than normal (Fig. 4A). Strong et al. [167] reported similar findings in anestetized cats undergoing MCAO. CSDs extend focal brain damage also in humans [180]. This was demonstrated for the first time in patients with subarachnoid hemorrage (SAH). In this clinical condition the rupture of an aneurysm in the circle of Willis is followed by a vasospasm and ischemia downstream [180]. In his famous commentary on these findings in Nature Medicine, Costantino Iadecola [181] introduced the term killer waves to describes CSDs. This designation emphasizes the deadly potential of CSDs. Later studies demonstrated that CSDs extend brain damage also in stroke [182] and in neurotrauma [183]. Nakamura and coworkers showed that after stroke CSDs cycle around the ischemic core for several days and progressively enlarge the area of dead brain tissue [182] (Fig. 4B). CSDs elicit different vasomotor responses in the healthy brain, as in migraineurs, and after focal brain damage. In the first case, they induce vasodilation, in the second, vasoconstriction. Therefore, the term of inverse hemodynamic response is often used to describe the vasomotor changes evoked by CSDs [168]. The reason of the different response of healthy and damaged brain to CSDs is unknown. It has been proposed, however, that it depends on the different levels of NO generated in these different conditions [184,185]. Because CSDs enlarge ischemic, traumatic or hemorrhagic focal brain lesions there is a major interest in identifying the factors responsible for their propagation or for the generation of the aberrant vasomotor responses that they elicit. Some data is emerging to suggest that VGCC could be involved.
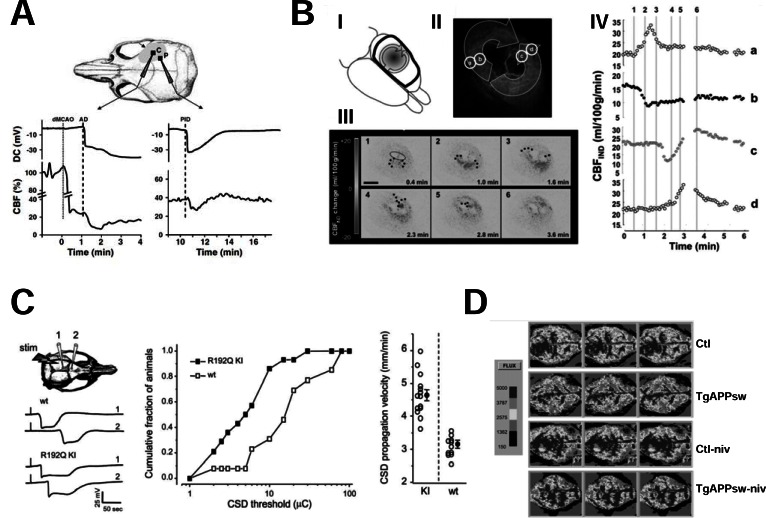
Fig. (4).
Changes in CBF in neurodegenerative and neurovascular disorders and their modulation by VGCCs. A, after MCAO, anoxic depolarization (AD) and postischemic depolarizations (PIDs) cause a marked drop in CBF. The two plots show the time course of DC currents and of CBF simultaneously recorded in the core (on the left) and in the penumbra (on the right) of the ischemic lesion caused by MCAO in a C57BL/6J mouse. The straight line in the left plot indicates the time of MCAO induction whereas the dotted lines correspond to the beginning of AD in the plot on the left, and to the beginning of PID in the plot on the right, respectively. The schematic draw on the top illustrates the position of the two extracellular recording electrodes. Note the sudden drop in CBF immediatly after AD in the core or PID, in the penumbra (reproduced with permission from Shin et al., 2006 [166]). B, waves of vasoconstriction cycle around the ischemic core and contribute to enlarging the ischemic lesion. The arrows in the schematic draw in I represent the front of propagation of the changes in indicative CBF (CBFIND) occurring after MCAO in adult male Wistar rats. In II a laser speckle image from a representative animal is shown. Note that CBFIND in the inner “core” region markedly decreases (black in II), that the region immediately surrounding the core (b and c in II) shows a hypoaemic response whereas a hyperemic response with an increase in CBFIND increases occurs in the outer border of the lesion (a and d in II). These changes in CBFIND propagate distally from the core in opposite directions thus originating two different fronts of propagation. As shown in III, these fronts of propagation (indicated by the black dots) cycle around the ischemic core and collide in a position opposite to their origin. The plot in IV reports the time course of CBFIND in the different regions of interest a, b, c and d identified in II. Note that whereas in the outer regions (a and d) CBFIND significantly increases (hyperaemic response), in the inner regions b and c a decrease in CBFIND (hypoaemic response) is observed followed (as in c) or not (as in b) by a secondary hyperaemia (reproduced with permission from Nakamura et al., 2010 [182]). C, CaV2.1-encoded P/Q channels have a role in the propagation of CSDs. The plot on the left of the panel shows that CSDs last longer and propagate faster in mice harboring the R192Q migraine mutation in the Ca2.1 gene. The graph in the middle shows that the distribution of CSD threshold is right-shifted in R192Q CaV2.1 transgenic mice as compared with controls whereas the scatter plot on the right reports the individual values of CSD propagation velocity in the different animals of the two groups (reproduced with permission from van den Maagdenberg et al., 2004 [187]). D, nivaldipine restores normal CBF in a transgenic mouse model of Alzheimer’s disease. The panel shows two-dimensional maps of the regional blood flow measured with laser Doppler flowmetry in the cortex of 13 month old control mice (ctl), transgenic mice harboring the APPK670N,M671L mutation (TgAPPsw), and control (ctl-niv) or TgAPPsw (TgAPPsw-niv) mice treated with nivaldipine (1 mg/kg of body weight daily for 15 days). Different CBF values correspond to different gray intensities as indicated in the gray scale reported on the left of the panel. Note that the very low CBF in TgAPPsw cortex is restored to values comparable to those of control mice upon treatment with nivaldipine. Conversely, this CCB does not modify CBF in control mice (reproduced with permission from Paris et al., 2004 [223]).
Specifically, recent evidence clearly point to P/Q VGCCs as important mediators of the propagation of CSDs into the penumbra. Considerable work performed on spreading depression in migraine provided clear evidence that its propagation involves P/Q channel activation. In particular, useful information came from the analysis of leaner and tottering mice. These two strains of mice carry mutations in the CaV2.1 gene that hinder the activity of P/Q type channels. Both leaner and tottering mice have a high threshold for the induction of cortical spreading depression by KCl application on the pial surface or by electrical stimulation of the cortex [186]. On the contrary, mutant mice bearing migraine mutations that increase P/Q type channel activity have a high susceptibility to the same stimuli [187-189] (Fig. 4C). Further arguments supporting the involvement of P/Q channels in spreading depression come from pharmacological experiments in vitro. Nonspecific VGCC blockers like Ni2+ and Cd2+ and selective P/Q type channel blocker ω-agatoxin-IVA blocked spreading depression elicited by electrical stimulation in a Na-acetate buffer in hyppocampal organotypic slices [190]. Instead, the L-type channel blocker nifedipine and the N-type channel blocker ω-conotoxin GVIA were ineffective [190]. P/Q channels also have a major role of ischemic CSDs. This was demonstrated by studies performed in transgenic mice with familial hemiplegic migraine type 1 (FHM1) mutations of the CACNA1A gene encoding for CaV2.1. FHM is an autosomal dominant inherited form of migraine whose aura is characterized by episodes of hemiparesis [191]. As in other forms of migraine, FHM1 patients have a higher risk of stroke than age-matched controls and usually develop more serious forms of the disease [192, 193]. To explore the role the P/Q channels in ischemic CSDs, Eikermann-Haerter and coworkers [194] performed transient MCAO experiments in transgenic mice with the human R192Q or S218L FHM1 mutations of the CACNA1A gene [187, 188] and in their wild type littermates. Transgenic mice showed a higher frequency of PIDs recorded in vivo with intracortical microelectrodes than controls. These electrical events often occurred in clusters suggesting that, as in humans after stroke, their were cycling around the ischemic core [182]. Ischemic brain damage and mortality were also higher in mutant mice than in controls [194]. Importantly, the worse clinical course of stroke in transgenic mice respect to controls was related to a more rapid growth of hyperacute ischemic core [194]. These results suggest that stroke is more severe in mice with CACNA1A mutations because postischemic CSDs are enhanced. Another conclusion emerging from the cited study is that P/Q-type channels take part to the generation and/or propagation of postischemic CSDs. The mechanism involved remains, however, obscure. Given the role of this class of VGCCs in neurotransmitter release at the presynaptic terminal, the most likely explanation is that P/Q-type channel opening cause glutamate release in the ischemic brain. The activation of NMDA receptors is required, indeed, for PID propagation [195]. Consistent with this hypothesis, in FHM1 mice, excitatory neurotransmission is enhanced because of a higher probability of glutamate release at pyramidal cell synapses [196]. A potentiation in glutamate release could also explain why the CBF threshold for ischemic damage is lower in FHM1 mice than in controls [194]. Alernatively, P/Q type channels could affect post/ischemic CSDs by directly controlling the tone of brain microvessels. P/Q type channel are expressed, indeed, in brain vessels [197]. The role of P/Q type channels in the control of vascular tone was demonstrated in afferent glomerular arteries in the kidney but not yet in brain vessels [198, 199].
In Subarachnoid hemorrage (SAH), the rupture of a subarachnoid aneurysm is followed by vasospasm in blood vessels of the circle of Willis. This causes brain ischemia in the region perfused by the constricted vessels. Vasoconstriction in SAH depends on Ca2+ influx into vascular smooth cells through VGCCs. L-type channels were originally identified as the class of VGCCs involved. Therefore, the use of CCBs was proposed to relieve vasospasm and prevent secondary cerebral ischemia in this disease. The current clinical evidence is solid enough to recommend the use of nimodipine in SAH [200]. However, the clinical response to this drug is often suboptimal. This led to the widespread perception that other mechanisms could be involved. Indeed, recent studies showed that other VGCC subtypes besides L-type channels are expressed in vascular smooth muscle cells [201, 202]. They contribute to a nimodipine-resistant component of vascular tone that becomes larger after SAH [202]. Our knowledge of the molecular choreography of ion channels in vascular smooth cells and, specifically, of VGCCs is still only partial. However, it is increasingly clear that a significant heterogeneity does exist among different vascular beds. Not only different ion channel subtypes do exist in different vascular districts but also different splicing variants of the same channels (e.g. L-type channels) could be expressed in different regions [102, 203]. L-type channels predominate in larger caliber proximal vessels [197, 202]. Instead, in smaller diameter resistance vessels, T-and R-type channels are also significantly expressed [197, 202, 204]. Intriguingly, SAH causes an increase in the expression in the basilar artery of the pore forming subunits of R-type channels, CaV2.3, and of T-type channels, CaV3.1 and CaV3.3. On the contrary, the protein expression of the L-type channel subunits CaV1.2 and CaV1.3 decreases in the same condition [205]. The molecular mechanism responsible for the changes in T- and R-type channel expression in SAH is still unknown. However, the release of oxyhemoglobin from extravasated erythrocytes could have a role in this process. This protein promotes, indeed, the expression of R-type channels in isolated rat basilar arteries [206]. Recent evidence highlights the role in SAH of the parenchimal small resistance arteries that express R- and T-type channels. Specifically, cerebral ischemia and CSDs were observed in SAH patients also after the surgical placement of nicardipine prolonged-release implants in the subarachnoid space [207, 208]. In these subjects, there was no angiographic evidence of proximal vasospasm [207, 208].
A dysfunction of the neurovascular unit also occurs in neurodegenerative diseases not vascular in origin [209]. This happens, for instance, in Alzheimer’s disease (AD) that is now considered a progressive disorder of both neurons and brain vasculature [210, 211]. A severe decrease of regional brain perfusion occurs in transgenic mice models of the disease [212, 213] and in human patients [214]. This decrease in brain perfusion is a further argument to suggest that AD and cerebrovascular ischemic disorders are closely related (see section 1a). A weakening of cerebrovascular coupling (the ability to increase CBF in response to neuronal activity), cerebrovascular autoregulation (the ability to adjust vascular tone to compensate for changes in systemic blood pressure), and vasoreactivity to CO2 also occur in AD [215-217]. Vascular dysfunction in AD depends on amyloid deposition in blood vessel wall. This is part of cerebral amyloid angiopathy, a specific form of vascular pathology occurring in AD [218, 219]. Other features of this condition are small cortical infarcts and microhemorrages [218, 219]. βAP induces free radical generation, causes endothelial dysfunction and hinders the activity of endothelial NOS (eNOS) [220, 221]. This leads to an increase vascular tone [220,221]. Similar events also occur in vivo, in experimental animal models of AD [222]. The final vasoconstrictive response to βAPs requires the opening of L-type channels. Nivaldipine, indeed, prevents βAP1-40-induced vasoconstriction in isolated rat aortas [223]. It also normalizes CBF in the transgenic AD mouse model Tg APPsw [223] (Fig. 4D). The beneficial effect of nivaldipine on regional blood flow has been demonstrated also in human AD patients [72]. Therefore, CCBs may be helpful in AD not only because of their direct neuroprotective effects but also because they prevent the effect of βAP on CBF. Nivaldipine has also other pharmacological properties that contribute to its neuroprotective effect. Indeed, it also decreases the synthesis of βAPs and increases their removal from the brain that is often low in AD patients [224, 225].
3b. N-Type Channels Control Chemokine Release from Microglia
In central nervous system, microglia can both cause tissue damage and promote healing [226-228]. In multiple sclerosis (MS), it has a role in the demyelination process [229]. It is well known that glial cells like astrocytes and oligodendrocytes express VGCCs [230-232]. On the contrary, their presence in microglia has been a matter of debate [233, 234]. Until recently, the only evidence of a role of VGCCs in microglial physiology was from pharmacological studies in vitro. Specifically, VGCCs take part in the [Ca2+]i response to βAP25-35, PrP106-126, chemokines or HIV proteins in cultured microglia [235, 236]. Recently, evidence was reported of the relevance of these channels in microglial activation in living animals. Specifically, microglial N-type channels have been implicated in demyelination in experimental allergic encephalomyelitis (EAE), a model of MS. In animals with this disease, N-type channels accumulate in the plasma membrane of degenerating axons [237, 238]. Also, the N-type channel blocker ω-conotoxin GVIA protects Norway rats from EAE-induced optic neuritis [238]. Tokuhara and coworkers compared the clinical and pathological evolution of EAE in knockout mice for N-type channel pore forming subunit CaV2.2 (α1B) and in their wild type littermates [239]. As expected, the neurological symptoms and the spinal cord lesions were less severe in knockout than in wild type mice [239]. Also perilesional inflammation was less severe in knockout mice. Fewer macrophages and microglial cells amassed, indeed, around the demyelinating lesions in knockout than in wild-type mice [239]. In these lesions, T-lymphocytes and microglial cells produce and release, respectively, monocyte chemotactic protein-1 (MCP-1) and macrophage inflammatory protein-1γ (MIP-1γ) [239]. These two chemokines further worsen tissue damage by recruiting inflammatory and immune cells to the demyelinating foci [239]. Importantly, less MCP-1 and MIP-1γ were released in knockout mice than in wild-type controls [239]. CaV2.2 channels control MCP-1 release form microglia [239]. The N-type channel blocker ω-conotoxin GVIA suppresses, indeed, the release of this chemokine from microglial cells in vitro. Besides, MCP-1 synthesis and release is lower in cultured microglia from CaV2.2 knockout than from control mice [239]. As a whole, these results suggest that N-type channels take part to microglia-dependent spinal cord damage in MS. Therefore, N-type channels should be added to the list of ion channels and transporters whose pharmacological modulation could be helpful in MS [240, 241].
CONCLUSIONS
In conclusion, in neurodegenerative and neurovascular disorders, VGCCs influence cell survival in different ways (Table 2). This may happen when their activity increases above average as in aging, in the presence of βAP or during chronic hypoxia. In addition, neurons showing Ca2+ dependent pacemaking are exposed to higher than average Ca2+ loads. Therefore, they are exquisitely vulnerable to neurotoxic insults. Also a decrease in VGCC activity below the normal may be detrimental for neurons. These ion channels, indeed, promote the influx of the Ca2+ ions that activate neuronal cell survival programs. VGCCs may kill neurons by letting toxic metal ions enter the cytoplasm. Finally, these channels may indirectly affect neuronal survival. In fact, they control vascular tone in the brain and the release of cytokines and chemochines by microglia. These data suggest that VGCCs could be a useful pharmacological target in neurodegenerative and neurovascular disorders. However, a major obstacle to developing effective VGCC-based therapies in humans is the lack of drugs that specifically act on dysfunctional brain VGCCs. This limit could be overcome by targeting the molecular mechanisms responsible for VGCC dysfunction in neurodegenerative diseases. This will represent a major challenge and, we hope, an active area of investigation in the years to come.
Table 2.
Mechanisms of VGCC-Dependent Neurotoxicity
| Cell Compartment Involved | Pathogenetic Mechanism | Diseases |
|---|---|---|
|
| ||
| Neurons | ||
| with Ca2+ overload | Enhanced expression/activity of L-type Ca2+ channels | Ageing |
| Chronic Hypoxia | ||
| Alzheimer’s disease | ||
| without Ca2+overload | Ca2+-dependent pacemaker activity | Parkinson’s disease |
| Essential tremor | ||
| Pathological decrease of Ca2+influx through VGCC | Prion disease (?) | |
| Metal ion influx | Iron neurotoxicity | |
| Zinc neurotoxicity | ||
| Neurovascular unit | Enhanced generation/propagation of spreading depression | Ischemic Stroke, Neurotrauma, Subarachnoid hemorrage |
| Enhanced vasomotor tone of small caliber brain blood vessels | Subarachnoid hemorrage | |
| Microglia | Enhanced chemokine release | Multiple Sclerosis |
ACKNOWLEDGEMENTS
Declared none.
ABBREVIATIONS
- AD
= Alzheimer disease
- APP
= amyloid precursor protein
- ASICs
= acid sensitive ion channels
- β-AP
= β-amyloid peptide
- BBB
= blood brain barrier
- CBF
= cerebral blood flow
- CCBs
= calcium channel blockers
- cGMP
= cyclic GMP
- CSD
= cortical spreading depolarizations
- DMT-1
= divalent metal transporter-1
- DIV
= days in vitro
- EAE
= experimental autoimmune encephalomyelitis
- eNOS
= endothelial nitric oxide synthase
- ET
= essential tremor
- FHM1
= familial hemiplegic migraine type 1
- GSH
= glutathione
- HIF-1
= hypoxia-inducible factor 1
- HVA
= high voltage activated
- IO
= inferior olive
- LVA
= low voltage activated
- MnSOD
= manganese-superoxide dismutase
- MAO-B
= mono-aminooxidase B
- MCAO
= middle cerebral artery occlusion
- MCP-1
= monocyte chemotactic protein-1
- MIP-1γ
= macrophage inflammatory protein-1γ
- MPTP
= 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NCX
= sodium calcium exchanger
- MS
= multiple sclerosis
- NO
= nitric oxide
- nNOS
= neuronal nitric oxid synthase
- NTBI
= non/transferrin bound iron
- PD
= Parkinson’s disease
- PtdIns(3,5)P2
= phosphatidylinositol (3,5)-bisphosphate
- PIDs
= peri-infact depolarizations
- PS1
= presenilin-1
- PKA
= protein kinase A
- ROS
= radical oxygen substance
- SNc
= substantia nigra pars compacta
- STN
= subthalamic nucleus
- 6OH-DA
= 6-hydroxy-dopamine
- Tet
= tetracycline
- SAH
= subarachnoid hemmorage
- Tf
= transferrin
- Tf-I
= iron bound to transferring
- TfR
= transferrin receptors
- TRPM7
= transient receptor potential cation channel, subfamily M, member
- VGCC
= voltage-gated calcium channels
- ZNS
= zonisamide
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6(3):337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 2.Zündorf G, Reiser G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal. 2011;14(7):1275–1288. doi: 10.1089/ars.2010.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schanne FAX, Kane A B, Young EE, Farber JL. Calcium dependence of toxic cell death a final common pathway. Science. 1979;206:700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- 4.Leonard JP, Salpeter MM. Agonist-induced myopathy at the neuromuscular junction is mediated by calcium. J. Cell. Biol. 1979;82:811–819. doi: 10.1083/jcb.82.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon R P, Griffiths T, Evans MC, Swan JH, Meldrum BS. Calcium overload in selectively vulnerable neurons of the hippocampus during and after ischemia: An electron microscopy study in the rat. J. Cereb. Blood. Flow. Metab. 1984;4:350–361. doi: 10.1038/jcbfm.1984.52. [DOI] [PubMed] [Google Scholar]
- 6.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell. Dev. Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 7.Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr. Opin. Neurobiol. 2009;19(3):237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Annunziato L, Pignataro G, Boscia F, Sirabella R, Formisano L, Saggese M, Cuomo O, Gala R, Secondo A, Viggiano D, Molinaro P, Valsecchi V, Tortiglione A, Adornetto A, Scorziello A, Cataldi M, Di Renzo GF. ncx1, ncx2, and ncx3 gene product expression and function in neuronal anoxia and brain ischemia. Ann. N. Y. Acad. Sci. 2007;1099:413–426. doi: 10.1196/annals.1387.050. [DOI] [PubMed] [Google Scholar]
- 9.Annunziato L, Cataldi M, Pignataro G, Secondo A, Molinaro P. Glutamate-independent calcium toxicity: introduction. Stroke. 2007;38(2 Suppl):661–664. doi: 10.1161/01.STR.0000247942.42349.37. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Yang J, Zhang C, Jiang X, Zhou H, Liu M. Calcium antagonists for acute ischemic stroke. Cochrane Database of Systematic Reviews. 2012;5:CD001928. doi: 10.1002/14651858.CD001928.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Yagami T, Kohma H, Yamamoto Y. L-type voltage-dependent calcium channels as therapeutic targets for neurodegenerative diseases. Curr. Med. Chem. 2012;19(28):4816–4827. doi: 10.2174/092986712803341430. [DOI] [PubMed] [Google Scholar]
- 12.Hartley DM, Kurth MC, Bjerkness L, Weiss JH, Choi DW. Glutamate receptor-induced 45Ca2+ accumulation in cortical cell culture correlates with subsequent neuronal degeneration. J. Neurosci. 1993;13:1993–2000. doi: 10.1523/JNEUROSCI.13-05-01993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tymianski M, Charlton MP, Carlen PL, Tator CH. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J. Neurosci. 1993;13:2085–2104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sattler R, Charlton MP, Hafner M, Tymianski M. Distinct influx pathways, not calcium load, determine neuronal vulnerability to calcium neurotoxicity. J. Neurochem. 1998;71:2349–2364. doi: 10.1046/j.1471-4159.1998.71062349.x. [DOI] [PubMed] [Google Scholar]
- 15.West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc. Natl. Acad. Sci. U.S.A. 2001;98(20):11024 –11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294(5541):333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 17.Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260(5105):181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- 18.Bengtson CP, Bading H. Nuclear calcium signaling. Adv. Exp. Med. Biol. 2012;970:377–405. doi: 10.1007/978-3-7091-0932-8_17. [DOI] [PubMed] [Google Scholar]
- 19.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 20.Forder JP, Tymianski M. Postsynaptic mechanisms of excitotoxicity: Involvement of postsynaptic density proteins, radicals, and oxidant molecules. Neuroscience. 2009;12, 158(1):293–300. doi: 10.1016/j.neuroscience.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Stanika RI, Villanueva I, Kazanina G, Andrews SB, Pivovarova NB. Comparative impact of voltage-gated calcium channels and NMDA receptors on mitochondria-mediated neuronal injury. J. Neurosci. 2012;32(19):6642–6650. doi: 10.1523/JNEUROSCI.6008-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heintz N, Zoghbi HY. Insights from mouse models into the molecular basis of neurodegeneration. Annu. Rev. Physiol. 2000;62:779–802. doi: 10.1146/annurev.physiol.62.1.779. [DOI] [PubMed] [Google Scholar]
- 23.Pietrobon D. Function and dysfunction of synaptic calcium channels: insights from mouse models. Curr. Opin. Neurobiol. 2005;15:257–265. doi: 10.1016/j.conb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Pietrobon D. CaV2.1 channelopathies. Pflugers Arch. 2010;460(2):375–393. doi: 10.1007/s00424-010-0802-8. [DOI] [PubMed] [Google Scholar]
- 25.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 901 study. Ann. Neurol. 2010;67:114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 2009;11(2):111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1-2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selnes OA, Vinters HV. Vascular cognitive impairment. Nat. Clin. Pract. Neurol. 2006;2(10):538–547. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- 29.Savva GM, Stephan BC Alzheimer’s Society Vascular Dementia Systematic Review Group. Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41(1):e41–e46. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- 30.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 31.Reitz C, Bos MJ, Hofman A, Koudstaal PJ, Breteler MM. Prestroke cognitive performance, incident stroke, and risk of dementia: the Rotterdam Study. Stroke. 2008;39(1):36–41. doi: 10.1161/STROKEAHA.107.490334. [DOI] [PubMed] [Google Scholar]
- 32.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell. 2007;6(3):307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J. Neurosci. 2001;21(24):9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, Garcia-Ramos G, Kraner S, Landfield PW, Porter NM. Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res. 2007;1151:20–31. doi: 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Land?eld PW. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J. Neurosci. 2007;27(12):3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herman JP, Chen KC, Booze R, Landfield PW. Up-regulation of alpha1D Ca2+ channel subunit mRNA expression in the hippocampus of aged F344 rats. Neurobiol. Aging. 1998;19:581–587. doi: 10.1016/s0197-4580(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 37.Davare MA, Hell JW. Increased phosphorylation of the neuronal L-type Ca(2+) channel Ca(v)1,2 during aging. Proc. Natl. Acad. Sci U.S.A. 2003;100:16018–16023. doi: 10.1073/pnas.2236970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gant JC, Chen KC, Norris CM, Kadish I, Thibault O, Blalock EM, Porter NM, Landfield PW. Disrupting function of FK506-binding protein 1b/12.6 induces the Ca²+-dysregulation aging phenotype in hippocampal neurons. J. Neurosci. 2011;31(5):1693–1703. doi: 10.1523/JNEUROSCI.4805-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehnart SE, Huang F, Marx SO, Marks AR. Immunophilins and coupled gating of ryanodine receptors. Curr. Top. Med. Chem. 2003;3:1383–1391. doi: 10.2174/1568026033451907. [DOI] [PubMed] [Google Scholar]
- 40.Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu. Rev. Biochem. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- 41.Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J. Neurosci. 2009;29:1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson RM, Shajenko L, Donta TS. Amyloid beta-peptide (A beta P) potentiates a nimodipine-sensitive L-type barium conductance in N1E-115 neuroblastoma cells. Brain Res. 1994;643(1-2):324–327. doi: 10.1016/0006-8993(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 43.MacManus A, Ramsden M, Murray M, Henderson Z, Pearson HA, Campbell VA. Enhancement of 45Ca2+ influx and voltage-dependent Ca2+ channel activity by beta-amyloid-(1-40) in rat cortical synaptosomes and cultured cortical neurons. Modulation by the proinflammatory cytokine interleukin-1beta. J. Biol. Chem. 2000;275(7):4713–4718. doi: 10.1074/jbc.275.7.4713. [DOI] [PubMed] [Google Scholar]
- 44.Ueda K, Shinohara S, Yagami T, Asakura K, Kawasaki K. Amyloid beta protein potentiates Ca2+ influx through L-type voltage-sensitive Ca2+ channels: a possible involvement of free radicals. J. Neurochem. 1997;68(1):265–271. doi: 10.1046/j.1471-4159.1997.68010265.x. [DOI] [PubMed] [Google Scholar]
- 45.Price SA, Held B, Pearson HA. Amyloid beta protein increases Ca2+ currents in rat cerebellar granule neurons. Neuroreport. 1998;9:539–545. [PubMed] [Google Scholar]
- 46.Ramsden M, Henderson Z, Pearson HA. Modulation of Ca2+ channel currents in primary cultures of rat cortical neurones by amyloid β protein (1-40) is dependent on solubility status. Brain Res. 2002;956:254–261. doi: 10.1016/s0006-8993(02)03547-3. [DOI] [PubMed] [Google Scholar]
- 47.Nimmrich V, Grimm C, Draguhn A, Barghorn S, Lehmann A, Schoemaker H, Hillen H, Gross G, Ebert U, Bruehl C. Amyloid beta oligomers (A beta(1-42) globulomer) suppress spontaneous synaptic activity by inhibition of P/Q-type calcium currents. J. Neurosci. 2008;28:788–797. doi: 10.1523/JNEUROSCI.4771-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mezler M, Barghorn S, Schoemaker H, Gross G, Nimmrich V. A β-amyloid oligomer directly modulates P/Q-type calcium currents in Xenopus oocytes. Br. J. Pharmacol. 2012;165(5):1572–1583. doi: 10.1111/j.1476-5381.2011.01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ekinci FJ, Malik KU, Shea TB. Activation of the L Voltage-sensitive calcium channel by Mitogen-activated Protein (MAP) Kinase following Exposure of Neuronal cells to βAmyloid. MAP kinase mediates β-amyloid-induced neurodegeneration. J. Biol. Chem. 1999;274(42):30322–30327. doi: 10.1074/jbc.274.42.30322. [DOI] [PubMed] [Google Scholar]
- 50.Kim S, Rhim H. Effects of amyloid-β peptides on voltage-gated L-type Ca(V)1.2 and Ca(V)1.3 Ca(2+) channels. Mol. Cells. 2011;32(3):289–294. doi: 10.1007/s10059-011-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green KN, Peers C. Amyloid beta peptides mediate hypoxic augmentation of Ca(2+) channels. J. Neurochem. 2001;77(3):953–956. doi: 10.1046/j.1471-4159.2001.00338.x. [DOI] [PubMed] [Google Scholar]
- 52.Webster NJ, Ramsden M, Boyle JP, Pearson HA, Peers C. Amyloid peptides mediate hypoxic increase of L-type Ca2+ channels in central neurones. Neurobiol Aging. 2006;27(3):439–445. doi: 10.1016/j.neurobiolaging.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Koistinaho J, Pyykonen I, Keinanen R, Hokfelt T. Expression of beta-amyloid precursor protein mRNAs following transient focal ischaemia. Neuroreport. 1996;7:2727–2731. doi: 10.1097/00001756-199611040-00064. [DOI] [PubMed] [Google Scholar]
- 54.Pluta R, Kida E, Lossinsky AS, Golabek AA, Mossakowski MJ, Wisniewski HM. Complete cerebral ischemia with short-term survival in rats induced by cardiac arrest. I. Extracellular accumulation of Alzheimer's beta-amyloid protein precursor in the brain. Brain Res. 1994;649(1-2):323–328. doi: 10.1016/0006-8993(94)91081-2. [DOI] [PubMed] [Google Scholar]
- 55.Pluta R, Furmaga-Jabłońska, W, Maciejewski R, Ułamek-Kozioł M, Jabłoński M. Brain Ischemia Activates β- and γ-Secretase Cleavage of Amyloid Precursor Protein: Significance in Sporadic Alzheimer's Disease. Mol. Neurobiol. 2013;47(1):425–434. doi: 10.1007/s12035-012-8360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scragg JL, Fearon IM, Boyle JP, Ball SG, Varadi G, Peers C. Alzheimer’s amyloid peptides mediate hypoxic up-regulation of L-type Ca2+ channels. FASEB J. 2005;19(1):150–152. doi: 10.1096/fj.04-2659fje. [DOI] [PubMed] [Google Scholar]
- 57.Lin H, Zhu Y, Ratneshwar L. Amyloid β Protein (1-40) forms calcium-permeable, Zn2+-sensitive channel in reconstituted lipid vesicles. Biochemistry. 1999;38:11189–11196. doi: 10.1021/bi982997c. [DOI] [PubMed] [Google Scholar]
- 58.Brown ST, Scragg JL, Boyle JP, Hudasek K, Peers C, Fearon IM. Hypoxic augmentation of Ca2+ channel currents requires a functional electron transport chain. J. Biol. Chem. 2005;280(23):21706–21712. doi: 10.1074/jbc.M503144200. [DOI] [PubMed] [Google Scholar]
- 59.Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, Colombatto S, Danni O, Parola M, Smith MA, Perry G, Tamagno E, Tabaton M. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1alpha. J. Neurochem. 2009;108(4):1045–1056. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- 60.Weiss JH, Pike CJ, Cotman CW. Ca2+ channel blockers attenuate beta-amyloid peptide toxicity to cortical neurons in culture. J. Neurochem. 1994;62(1):372–375. doi: 10.1046/j.1471-4159.1994.62010372.x. [DOI] [PubMed] [Google Scholar]
- 61.Yagami T, Ueda K, Sakaeda T, Itoh N, Sakaguchi G, Okamura N, Hori Y, Fujimoto M. Protective effects of a selective L-type voltage-sensitive calcium channel blocker, S-312-d, on neuronal cell death. Biochem. Pharmacol. 2004;67(6):1153–1165. doi: 10.1016/j.bcp.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Whitson JS, Appel SH. Neurotoxicity of A beta amyloid protein in vitro is not altered by calcium channel blockade. Neurobiol. Aging. 1995;16(1):5–10. doi: 10.1016/0197-4580(95)80002-9. [DOI] [PubMed] [Google Scholar]
- 63.Kennelly SP, Abdullah L, Paris D, Parish J, Mathura V, Mullan M, Crawford F, Lawlor BA, Kenny RA. Demonstration of safety in Alzheimer’s patients for intervention with an anti-hypertensive drug Nilvadipine: results from a 6-week open label study. Int. J. Geriatr. Psychiatry. 2011;26(10):1038–1045. doi: 10.1002/gps.2638. [DOI] [PubMed] [Google Scholar]
- 64.Facchinetti F, Fasolato C, Del Giudice E, Burgo A, Furegato S, Fusco M, Basso E, Seraglia R, D’Arrigo A, Leon A. Nimodipine selectively stimulates beta-amyloid 1-42 secretion by a mechanism independent of calcium influx blockage. Neurobiol Aging. 2006;27(2):218–227. doi: 10.1016/j.neurobiolaging.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Mitterreiter S, Page RM, Kamp F, Hopson J, Winkler E, Ha HR, Hamid R, Herms J, Mayer TU, Nelson DJ, Steiner H, Stahl T, Zeitschel U, Rossner S, Haass C, Lichtenthaler SF. Bepridil and amiodarone simultaneously target the Alzheimer’s disease beta- and gamma-secretase via distinct mechanisms. J. Neurosci. 2010;30(26):8974–8983. doi: 10.1523/JNEUROSCI.1199-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J. Clin. Invest. 2005;115(11):3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abuznait AH, Cain C, Ingram D, Burk D, Kaddoumi A. Up-regulation of P-glycoprotein reduces intracellular accumulation of beta amyloid: investigation of P-glycoprotein as a novel therapeutic target for Alzheimer’s disease. J. Pharm. Pharmacol. 2011;63(8):1111–1118. doi: 10.1111/j.2042-7158.2011.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Assema DM, Lubberink M, Bauer M, van der Flier WM, Schuit RC, Windhorst AD, Comans EF, Hoetjes NJ, Tolboom N, Langer O, Müller M, Scheltens P, Lammertsma AA, van Berckel BN. Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain. 2012;135(Pt 1):181–189. doi: 10.1093/brain/awr298. [DOI] [PubMed] [Google Scholar]
- 69.Kennelly S, Abdullah L, Kenny RA, Mathura V, Luis CA, Mouzon B, Crawford F, Mullan M, Lawlor B. Apolipoprotein E genotype-specific short-term cognitive benefits of treatment with the antihypertensive nilvadipine in Alzheimer’s patients—an open-label trial. Int J Geriatr Psychiatry. 2012;7(4):415–422. doi: 10.1002/gps.2735. [DOI] [PubMed] [Google Scholar]
- 70.Anekonda TS, Quinn JF. Calcium channel blocking as a therapeutic strategy for Alzheimer’s disease: the case for isradipine. Biochim. Biophys. Acta. 2011;1812(12):1584–1590. doi: 10.1016/j.bbadis.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hanyu H, Hirao K, Shimizu S, Sato T, Kiuchi A, Iwamoto T. Nilvadipine prevents cognitive decline of patients with mild cognitive impairment. Int. J. Geriatr. Psychiatry. 2007;22(12):1264–1266. doi: 10.1002/gps.1851. [DOI] [PubMed] [Google Scholar]
- 72.Hanyu H, Hirao K, Shimizu S, Iwamoto T, Koizumi K, Abe K. Favourable effects of nilvadipine on cognitive function and regional cerebral blood flow on SPECT in hypertensive patients with mild cognitive impairment. Nucl. Med. Commun. 2007;28(4):281–287. doi: 10.1097/MNM.0b013e32804c58aa. [DOI] [PubMed] [Google Scholar]
- 73.Sopher BL, Fukuchi K, Smith AC, Leppig KA, Furlong CE, Martin GM. Cytotoxicity mediated by conditional expression of a carboxylterminal derivative of the beta-amyloid precursor protein. Brain Res. Mol. Brain Res. 1994;26:207–217. doi: 10.1016/0169-328x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 74.Anekonda TS, Quinn JF, Harris C, Frahler K, Wadsworth TL, Woltjer RL. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer’s disease. Neurobiol Dis. 2011;41(1):62–70. doi: 10.1016/j.nbd.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanaka E, Yamamoto S, Kudo Y, Mihara S, Higashi H. Mechanisms underlying rapid depolarization produced by deprivation of oxygen and glucose in rat hippocampal CA1 neurons in vitro. J. Neurophysiol. 1997;78:891–902. doi: 10.1152/jn.1997.78.2.891. [DOI] [PubMed] [Google Scholar]
- 76.Steen PA, Newberg LA, Milde JH, Michenfelder JD. Nimodipine improves cerebral blood flow and neurologic recovery after complete cerebral ischemia in the dog. J. Cereb. Blood Flow Metab. 1983;3(1):38–43. doi: 10.1038/jcbfm.1983.4. [DOI] [PubMed] [Google Scholar]
- 77.Belrose JC, Caetano FA, Yang K, Lockhart BMW, Jackson MF, MacDonald JF. Mechanism of calcium influx following stroke. In: Li YV, Zhang JH, editors. Metal ion in stroke. New York: Springer; 2012. pp. 15–39. [Google Scholar]
- 78.Lee ST, Jeon D, Chu K. Subtypes of voltage-gated Ca2+ channels and ischemic brain injury. In: Annunziato L, editor. New strategies in stroke intervention. New York: Humana Press; 2010. pp. 189–209. [Google Scholar]
- 79.Li XM, Yang JM, Hu DH, Hou FQ, Zhao M, Zhu XH, Wang Y, Li JG, Hu P, Chen L, Qin LN, Gao TM. Contribution of downregulation of L-type calcium currents to delayed neuronal death in rat hippocampus after global cerebral ischemia and reperfusion. J. Neurosci. 2007;27(19):5249–5259. doi: 10.1523/JNEUROSCI.0802-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsuruta F, Green EM, Rousset M, Dolmetsch RE. PIKfyve regulates CaV1.2 degradation and prevents excitotoxic cell death. J. Cell Biol. 2009;187(2):279–294. doi: 10.1083/jcb.200903028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Green EM, Barrett CF, Bultynck G, Shamah SM, Dolmetsch RE. The tumor suppressor eIF3e mediates calcium-dependent internalization of the L-type calcium channel CaV1.2. Neuron. 2007;55:615–632. doi: 10.1016/j.neuron.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol. Rev. 2003;83(1):117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 83.Cataldi M, Perez-Reyes E, Tsien RW. Differences in apparent pore sizes of low and high voltage-activated Ca2+ channels. J. Biol. Chem. 2002;277(48):45969–45976. doi: 10.1074/jbc.M203922200. [DOI] [PubMed] [Google Scholar]
- 84.Martin RL, Lloyd HG, Cowan AI. The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci. 1994;17(6):251–257. doi: 10.1016/0166-2236(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 85.Nikonenko I, Bancila M, Bloc A, Muller D, Bijlenga P. Inhibition of T-type calcium channels protects neurons from delayed ischemia-induced damage. Mol. Pharmacol. 2005;68(1):84–89. doi: 10.1124/mol.104.010066. [DOI] [PubMed] [Google Scholar]
- 86.Bancila M, Copin JC, Daali Y, Schatlo B, Gasche Y, Bijlenga P. Two structurally different T-type Ca2+ channel inhibitors, mibefradil and pimozide, protect CA1 neurons from delayed death after global ischemia in rats. Fundam. Clin. Pharmacol. 2011;25(4):469–478. doi: 10.1111/j.1472-8206.2010.00879.x. [DOI] [PubMed] [Google Scholar]
- 87.Cataldi M, Lariccia V, Marzaioli V, Cavaccini A, Curia G, Viggiano D, Canzoniero LM, di Renzo G, Avoli M, Annunziato L. Zn2+ slows down CaV3.3 gating kinetics: implications for thalamocortical activity. J. Neurophysiol. 2007;98(4):2274–2284. doi: 10.1152/jn.00889.2006. [DOI] [PubMed] [Google Scholar]
- 88.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005;6(6):449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 89.Shuttleworth CW, Weiss JH. Zinc: new clues to diverse roles in brain ischemia. Trends Pharmacol. Sci. 2011;32(8):480–486. doi: 10.1016/j.tips.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bean BP. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007;8(6):451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 91.Verheijck EE, van Ginneken AC, Wilders R, Bouman LN. Contribution of L-type Ca2+ current to electrical activity in sinoatrial nodal myocytes of rabbits. Am. J. Physiol. 1999;276(3 Pt 2):H1064–H1077. doi: 10.1152/ajpheart.1999.276.3.H1064. [DOI] [PubMed] [Google Scholar]
- 92.Stojilkovic SS, Catt KJ. Calcium oscillations in anterior pituitary cells. Endocr. Rev. 1992;13:256–280. doi: 10.1210/edrv-13-2-256. [DOI] [PubMed] [Google Scholar]
- 93.Cataldi M, Taglialatela M, Guerriero S, Amoroso S, Lombardi G, di Renzo G, Annunziato L. Protein-tyrosine kinases activate while protein-tyrosine phosphatases inhibit L-type calcium channel activity in pituitary GH3 cells. J. Biol. Chem. 1996;271(16):9441–9446. doi: 10.1074/jbc.271.16.9441. [DOI] [PubMed] [Google Scholar]
- 94.Vandael DH, Marcantoni A, Mahapatra S, Caro A, Ruth P, Zuccotti A, Knipper M, Carbone E. Ca(v)1.3 and BK channels for timing and regulating cell firing. Mol. Neurobiol. 2010;42(3):185–198. doi: 10.1007/s12035-010-8151-3. [DOI] [PubMed] [Google Scholar]
- 95.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. alpha 1D (CaV1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J. Biol. Chem. 2001;276(25):22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- 96.Cataldi M, Secondo A, D’Alessio A, Sarnacchiaro F, Colao AM, Amoroso S, Di Renzo GF, Annunziato L. Involvement of phosphodiesterase-cGMP-PKG pathway in intracellular Ca2+ oscillations in pituitary GH3 cells. Biochim. Biophys. Acta. 1999;1449(2):186–193. doi: 10.1016/s0167-4889(99)00013-0. [DOI] [PubMed] [Google Scholar]
- 97.Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447(7148):1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 98.Hainsworth AH, Röper J, Kapoor R, Ashcroft FM. Identification and electrophysiology of isolated pars compacta neurons from guinea-pig substantia nigra. Neuroscience. 1991;43(1):81–93. doi: 10.1016/0306-4522(91)90419-o. [DOI] [PubMed] [Google Scholar]
- 99.Mercuri NB, Bonci A, Calabresi P, Stratta F, Stefani A, Bernardi G. Effects of dihydropyridine calcium antagonists on rat midbrain dopaminergic neurones. Br. J. Pharmacol. 1994;113(3):831–838. doi: 10.1111/j.1476-5381.1994.tb17068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Puopolo M, Raviola E, Bean BP. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J. Neurosci. 2007;27:645–656. doi: 10.1523/JNEUROSCI.4341-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bové J, Perier C. Neurotoxin-based models of Parkinson’s disease. Neuroscience. 2012;211:51–76. doi: 10.1016/j.neuroscience.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 102.Cataldi M, Bruno F. 1,4-Dihydropyridines: the multiple personalities of a blockbuster drug family. Translational Medicine. 2012;4(2):10–24. [PMC free article] [PubMed] [Google Scholar]
- 103.Ilijic E, Guzman JN, Surmeier DJ. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol Dis. 2011;43(2):364–371. doi: 10.1016/j.nbd.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schuster S, Doudnikoff E, Rylander D, Berthet A, Aubert I, Ittrich C, Bloch B, Cenci MA, Surmeier DJ, Hengerer B, Bezard E. Antagonizing L-type Ca2+ channel reduces development of abnormal involuntary movement in the rat model of L-3,4-dihydroxyphenylalanine-induced dyskinesia. Biol. Psychiatry. 2009;65(6):518–526. doi: 10.1016/j.biopsych.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 105.Rees K, Stowe R, Patel S, Ives N, Breen K, Ben-Shlomo Y, Clarke CE. Anti-hypertensive drugs as disease-modifying agents for Parkinson’s disease: evidence from observational studies and clinical trials. Cochrane Database Syst. Rev. 2011;9(11):CD008535. doi: 10.1002/14651858.CD008535.pub2. [DOI] [PubMed] [Google Scholar]
- 106.Marras C, Gruneir A, Rochon P, Wang X, Anderson G, Brotchie J, Bell CM, Fox S, Austin PC. Dihydropyridine calcium channel blockers and the progression of parkinsonism. Ann. Neurol. 2012;71(3):362–369. doi: 10.1002/ana.22616. [DOI] [PubMed] [Google Scholar]
- 107.Simuni T, Borushko E, Avram MJ, Miskevics S, Martel A, Zadikoff C, Videnovic A, Weaver FM, Williams K, Surmeier DJ. Tolerability of isradipine in early Parkinson’s disease: a pilot dose escalation study. Mov. Disord. 2010;25(16):2863–2866. doi: 10.1002/mds.23308. [DOI] [PubMed] [Google Scholar]
- 108.Chan CS, Gertler TS, Surmeier DJ. Calcium homeostasis, selective vulnerability and Parkinson’s disease. Trends Neurosci. 2009;32(5):249–256. doi: 10.1016/j.tins.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62(2):218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tai CH, Yang YC, Pan MK, Huang CS, Kuo CC. Modulation of subthalamic T-type Ca(2+) channels remedies locomotor deficits in a rat model of Parkinson disease. J. Clin. Invest. 2011;121(8):3289–3305. doi: 10.1172/JCI46482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J. Neurophysiol. 1994;72(2):507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 112.Biton V. Clinical pharmacology and mechanism of action of zonisamide. Clin. Neuropharmacol. 2007;30(4):230–240. doi: 10.1097/wnf.0b013e3180413d7d. [DOI] [PubMed] [Google Scholar]
- 113.Murata M. Zonisamide: a new drug for Parkinson’s disease. Drugs Today (Barc) 2010;46(4):251–258. doi: 10.1358/dot.2010.46.4.1490077. [DOI] [PubMed] [Google Scholar]
- 114.Murata M, Horiuchi E, Kanazawa I. Zonisamide has beneficial effects on Parkinson’s disease patients. Neurosci. Res. 2001;41(4):397–399. doi: 10.1016/s0168-0102(01)00298-x. [DOI] [PubMed] [Google Scholar]
- 115.Choudhury ME, Moritoyo T, Yabe H, Nishikawa N, Nagai M, Kubo M, Matsuda S, Nomoto M. Zonisamide attenuates MPTP neurotoxicity in marmosets. J. Pharmacol. Sci. 2010;114(3):298–303. doi: 10.1254/jphs.10120fp. [DOI] [PubMed] [Google Scholar]
- 116.Choudhury ME, Moritoyo T, Kubo M, Kyaw WT, Yabe H, Nishikawa N, Nagai M, Matsuda S, Nomoto M. Zonisamide-induced long-lasting recovery of dopaminergic neurons from MPTP-toxicity. Brain Res. 2011;1384:170–178. doi: 10.1016/j.brainres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 117.Yano R, Yokoyama H, Kuroiwa H, Kato H, Araki T. A novel anti-Parkinsonian agent, zonisamide, attenuates MPTP-induced neurotoxicity in mice. J. Mol. Neurosci. 2009;39(1-2):211–219. doi: 10.1007/s12031-009-9181-z. [DOI] [PubMed] [Google Scholar]
- 118.Yokoyama H, Yano R, Kuroiwa H, Tsukada T, Uchida H, Kato H, Kasahara J, Araki T. Therapeutic effect of a novel anti-parkinsonian agent zonisamide against MPTP (1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine) neurotoxicity in mice. Metab. Brain Dis. 2010;25(3):305–313. doi: 10.1007/s11011-010-9212-z. [DOI] [PubMed] [Google Scholar]
- 119.Kawajiri S, Machida Y, Saiki S, Sato S, Hattori N. Zonisamide reduces cell death in SH-SY5Y cells via an anti-apoptotic effect and by upregulating MnSOD. Neurosci. Lett. 2010;481(2):88–91. doi: 10.1016/j.neulet.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 120.Asanuma M, Miyazaki I, Diaz-Corrales FJ, Kimoto N, Kikkawa Y, Takeshima M, Miyoshi K, Murata M. Neuroprotective effects of zonisamide target astrocyte. Ann. Neurol. 2010;67(2):239–249. doi: 10.1002/ana.21885. [DOI] [PubMed] [Google Scholar]
- 121.Choudhury M, Sugimoto K, Kubo M, Iwaki H, Tsujii T, Kyaw W, Nishikawa N, Nagai M, Tanaka J, Nomoto M. Zonisamide up-regulated the mRNAs encoding astrocytic anti-oxidative and neurotrophic factors. Eur J Pharmacol. 2012;689(1-3):72–80. doi: 10.1016/j.ejphar.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 122.Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, Rajput A, Pahwa R, Lyons KE, Ross GW, Borden S, Moskowitz CB, Lawton A, Hernandez N. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(Pt 12):3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 123.Louis ED. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurol. 2010;9(6):613–622. doi: 10.1016/S1474-4422(10)70090-9. [DOI] [PubMed] [Google Scholar]
- 124.Park YG, Park HY, Lee CJ, Choi S, Jo S, Choi H, Kim YH, Shin HS, Llinas RR, Kim D. Ca(V)3.1 is a tremor rhythm pacemaker in the inferior olive. Proc. Natl. Acad. Sci. U S A. 2010;107(23):10731–1076. doi: 10.1073/pnas.1002995107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Handforth A, Homanics GE, Covey DF, Krishnan K, Lee JY, Sakimura K, Martin FC, Quesada A. T-type calcium channel antagonists suppress tremor in two mouse models of essential tremor. Neuropharmacology. 2010;59(6):380–387. doi: 10.1016/j.neuropharm.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zesiewicz TA, Elble RJ, Louis ED, Gronseth GS, Ondo WG, Dewey RB, Jr, Okun MS, Sullivan KL, Weiner WJ. Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology. 2011;77(19):1752–1755. doi: 10.1212/WNL.0b013e318236f0fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Florio T, Thellung S, Amico C, Robello M, Salmona M, Bugiani O, Tagliavini F, Forloni G, Schettini G. Prion protein fragment 106-126 induces apoptotic cell death and impairment of L-type voltage-sensitive calcium channel activity in the GH3 cell line. J. Neurosci. Res. 1998;54:341–352. doi: 10.1002/(SICI)1097-4547(19981101)54:3<341::AID-JNR5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 128.Korte S, Vassallo N, Kramer ML, Kretzschmar HA, Herms J. Modulation of L-type voltage-gated calcium channels by recombinant prion protein. J. Neurochem. 2003;87:1037–1042. doi: 10.1046/j.1471-4159.2003.02080.x. [DOI] [PubMed] [Google Scholar]
- 129.Senatore A, Colleoni S, Verderio C, Restelli E, Morini R, Condliffe SB, Bertani I, Mantovani S, Canovi M, Micotti E, Forloni G, Dolphin AC, Matteoli M, Gobbi M, Chiesa R. Mutant PrP suppresses glutamatergic neurotransmission in cerebellar granule neurons by impairing membrane delivery of VGCC α(2)δ-1 subunit. Neuron. 2012;74(2):300–313. doi: 10.1016/j.neuron.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr. Opin. Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 131.Rutishauser D, Mertz KD, Moos R, Brunner E, Rülicke T, Calella AM, Aguzzi A. The comprehensive native interactome of a fully functional tagged prion protein. PLoS One. 2009;4(2):e4446. doi: 10.1371/journal.pone.0004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koh JY, Cotman CW. Programmed cell death: its possible contribution to neurotoxicity mediated by calcium channel antagonists. Brain Res. 1992;587(2):233–240. doi: 10.1016/0006-8993(92)91002-v. [DOI] [PubMed] [Google Scholar]
- 133.Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, Perez-Reyes E, Lander ES, Frankel WN, Gardiner RM, Dolphin AC, Rees M. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J. Neurosci. 2001;21(16):6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ivanov SV, Ward JM, Tessarollo L, McAreavey D, Sachdev V, Fananapazir L, Banks MK, Morris N, Djurickovic D, Devor-Henneman DE, Wei MH, Alvord GW, Gao B, Richardson JA, Minna JD, Rogawski MA, Lerman MI. Cerebellar ataxia, seizures, premature death, and cardiac abnormalities in mice with targeted disruption of the Cacna2d2 gene. Am. J. Pathol. 2004;165(3):1007–1018. doi: 10.1016/S0002-9440(10)63362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McCleskey EW, Almers W. The Ca channel in skeletal muscle is a large pore. Proc. Natl. Acad. Sci. U. S. A. 1985;82(20):7149–7153. doi: 10.1073/pnas.82.20.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li YV, Zhang J H, editors. Metal ion in stroke. New York: Springer; 2012. [Google Scholar]
- 137.Yokel RA. Blood-Brain Barrier Flux of Aluminum, Manganese, Iron and Other Metals Suspected to Contribute to Metal-Induced Neurodegeneration. J. Alzheimers Dis. 2006;10(2-3):223–253. doi: 10.3233/jad-2006-102-309. [DOI] [PubMed] [Google Scholar]
- 138.Hidalgo C, Nuñez MT. Calcium, Iron and Neuronal Function. IUBMB Life. 2007;59(4-5):280–285. doi: 10.1080/15216540701222906. [DOI] [PubMed] [Google Scholar]
- 139.Nuñez MT, Urrutia P, Mena N, Aguirre P, Tapia V, Salazar J. Iron toxicity in neurodegeneration. Biometals. 2012;25:761–776. doi: 10.1007/s10534-012-9523-0. [DOI] [PubMed] [Google Scholar]
- 140.Liu Y, Connor JR. Iron and ER stress in neurodegenerative disease. Biometals. 2012;25(4):837–845. doi: 10.1007/s10534-012-9544-8. [DOI] [PubMed] [Google Scholar]
- 141.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Crichton RR, Dexter DT, Ward RJ. Brain iron metabolism and its perturbation in neurological diseases. J. Neural Transm. 2011;118(3):301–314. doi: 10.1007/s00702-010-0470-z. [DOI] [PubMed] [Google Scholar]
- 143.Moos T, Morgan EH. The metabolism of neuronal iron and its pathogenic role in neurological disease: review. Ann. N. Y. Acad. Sci. 2004;1012:14–26. doi: 10.1196/annals.1306.002. [DOI] [PubMed] [Google Scholar]
- 144.Moos T, Rosengren Nielsen T, Skjørringe T, Morgan EH. Iron trafficking inside the brain. J. Neurochem. 2007;103(5):1730–1740. doi: 10.1111/j.1471-4159.2007.04976.x. [DOI] [PubMed] [Google Scholar]
- 145.Moos T, Morgan EH. Evidence for low molecular weight, non-transferrin-bound iron in rat brain and cerebrospinal fluid. J. Neurosci. Res. 1998;54(4):486–489. doi: 10.1002/(SICI)1097-4547(19981115)54:4<486::AID-JNR6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 146.Cheah JH, Kim SF, Hester LD, Clancy KW, Patterson SE, 3rd, Papadopoulos V, Snyder SH. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron. 2006;51(4):431–440. doi: 10.1016/j.neuron.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tsushima RG, Wickenden AD, Bouchard RA, Oudit GY, Liu PP, Backx PH. Modulation of iron uptake in heart by L-type Ca2+ channel modifiers: possible implications in iron overload. Circ. Res. 1999;84(11):1302–1309. doi: 10.1161/01.res.84.11.1302. [DOI] [PubMed] [Google Scholar]
- 148.Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C, Yazdanpanah M, Wilson GJ, Schwartz A, Liu PP, Backx PH. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat. Med. 2003;9(9):1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 149.Gaasch JA, Geldenhuys WJ, Lockman PR, Allen DD, Van der Schyf CJ. Voltage-gated calcium channels provide an alternate route for iron uptake in neuronal cell cultures. Neurochem. Res. 2007;32(10):1686–1693. doi: 10.1007/s11064-007-9313-1. [DOI] [PubMed] [Google Scholar]
- 150.Pelizzoni I, Macco R, Morini MF, Zacchetti D, Grohovaz F, Codazzi F. Iron handling in hippocampal neurons: activity-dependent iron entry and mitochondria-mediated neurotoxicity. Aging Cell. 2011;10(1):172–183. doi: 10.1111/j.1474-9726.2010.00652.x. [DOI] [PubMed] [Google Scholar]
- 151.Kumfu S, Chattipakorn S, Srichairatanakool S, Settakorn J, Fucharoen S, Chattipakorn N. T-type calcium channel as a portal of iron uptake into cardiomyocytes of beta-thalassemic mice. Eur. J. Haematol. 2011;86(2):156–166. doi: 10.1111/j.1600-0609.2010.01549.x. [DOI] [PubMed] [Google Scholar]
- 152.Heinemann U, Louvel J. Changes in [Ca2+]o and [K+]o during repetitive electrical stimulation and during pentetrazol induced seizure activity in the sensorimotor cortex of cats. Pflugers Arch. 1983;398(4):310–317. doi: 10.1007/BF00657240. [DOI] [PubMed] [Google Scholar]
- 153.Harris RJ, Symon L, Branston NM, Bayhan M. Changes in extracellular calcium activity in cerebral ischemia. J. Cereb. Blood Flow Metab. 1981;1(2):203–209. doi: 10.1038/jcbfm.1981.21. [DOI] [PubMed] [Google Scholar]
- 154.Ma Z, Zhou Y, Xie J. Nifedipine Prevents Iron Accumulation and Reverses Iron-Overload-Induced Dopamine Neuron Degeneration in the Substantia Nigra of Rats. Neurotox. Res. 2012 doi: 10.1007/s12640-012-9309-8. in press, DOI: 10.1007/s12640-012-9309-8. [DOI] [PubMed] [Google Scholar]
- 155.Sensi SL, Paoletti P, Koh JY, Aizenman E, Bush AI, Hershfinkel M. The neurophysiology and pathology of brain zinc. J. Neurosci. 2011;31(45):16076–16085. doi: 10.1523/JNEUROSCI.3454-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stork CJ, Li YV. Rising zinc: a significant cause of ischemic neuronal death in the CA1 region of rat hippocampus. J. Cereb. Blood Flow Metab. 2009;29(8):1399–1408. doi: 10.1038/jcbfm.2009.64. [DOI] [PubMed] [Google Scholar]
- 157.Sensi SL, Canzoniero LM, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J. Neurosci. 1997;17(24):9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fukuda J, Kawa K. Permeation of manganese, cadmium, zinc, and beryllium through calcium channels of an insect muscle membrane. Science. 1977;196(4287):309–311. doi: 10.1126/science.847472. [DOI] [PubMed] [Google Scholar]
- 159.Kawa K. Zinc-dependent action potentials in giant neurons of the snail, Euhadra quaestia. J. Membr. Biol. 1979;49(4):325–344. doi: 10.1007/BF01868990. [DOI] [PubMed] [Google Scholar]
- 160.Oyama Y, Nishi K, Yatani A, Akaike N. Zinc current in Helix soma membrane. Comp. Biochem. Physiol. C. 1982;72(2):403–410. doi: 10.1016/0306-4492(82)90111-3. [DOI] [PubMed] [Google Scholar]
- 161.Kerchner GA, Canzoniero LM, Yu SP, Ling C, Choi DW. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J. Physiol. 2000;528(1):39–52. doi: 10.1111/j.1469-7793.2000.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cataldi M, Lariccia V, Canzoniero MLT, Di Renzo GF, Annunziato L. Zinc permeates T-type calcium channels and affects their gating. Conference Proceedings of the National Congress of the Italian Society for Neuroscience and joint Italian-Swedish Neuroscience Meeting; 1-4 october 2005; Lacco Ameno Ischia (Napoli). [Google Scholar]
- 163.Lo EH, Rosenberg GA. The neurovascular unit in health and disease: introduction. Stroke. 2009;40(3 Suppl):S2–S3. doi: 10.1161/STROKEAHA.108.534404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, Lo EH. Cell-cell signaling in the neurovascular unit. Neurochem. Res. 2007;32(12):2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 165.Guo S, Lo EH. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke. 2009;40(3 Suppl):S4–S7. doi: 10.1161/STROKEAHA.108.534388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J. Cereb. Blood Flow Metab. 2006;26(8):1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- 167.Strong AJ, Anderson PJ, Watts HR, Virley DJ, Lloyd A, Irving EA, Nagafuji T, Ninomiya M, Nakamura H, Dunn AK, Graf R. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain. 2007;130(Pt 4):995–1008. doi: 10.1093/brain/awl392. [DOI] [PubMed] [Google Scholar]
- 168.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nature Med. 2011;17(4):439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 169.Hossmann KA. Periinfarct depolarizations. Cerebrovasc. Brain Metab. Rev. 1996;8:195–208. [PubMed] [Google Scholar]
- 170.Mayevsky A, Doron A, Manor T, Meilin S, Zarchin N, Ouaknine GE. Cortical spreading depression recorded from the human brain using a multiparametric monitoring system. Brain Res. 1996;740(1-2):268–274. doi: 10.1016/s0006-8993(96)00874-8. [DOI] [PubMed] [Google Scholar]
- 171.Fabricius M, Fuhr S, Willumsen L, Dreier JP, Bhatia R, Boutelle MG, Hartings JA, Bullock R, Strong AJ, Lauritzen M. Association of seizures with cortical spreading depression and peri-infarct depolarisations in the acutely injured human brain. Clin. Neurophysiol. 2008;119(9):1973–1984. doi: 10.1016/j.clinph.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hartings JA, Watanabe T, Bullock MR, Okonkwo DO, Fabricius M, Woitzik J, Dreier JP, Puccio A, Shutter LA, Pahl C, Strong AJ. The Co-Operative Study on Brain Injury Depolarizations. Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain. 2011;134(Pt 5):1529–1540. doi: 10.1093/brain/awr048. [DOI] [PubMed] [Google Scholar]
- 173.Hartings JA, Strong AJ, Fabricius M, Manning A, Bhatia R, Dreier JP, Mazzeo AT, Tortella FC, Bullock MR. Co-Operative Study of Brain Injury Depolarizations. Spreading depolarizations and late secondary insults after traumatic brain injury. J. Neurotrauma. 2009;26(11):1857–1866. doi: 10.1089/neu.2009.0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann TN, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- 175.Lauritzen M. Pathophysiology of the migraine aura: the spreading depression theory. Brain. 1994;117(1):199–210. doi: 10.1093/brain/117.1.199. [DOI] [PubMed] [Google Scholar]
- 176.Mies G, Iijima T, Hossmann KA. Correlation between peri-infarct DC shifts and ischaemic neuronal damage in rat. Neuroreport. 1993;4:709–711. doi: 10.1097/00001756-199306000-00027. [DOI] [PubMed] [Google Scholar]
- 177.Dijkhuizen RM, Beekwilder JP, van der Worp HB, Berkelbach van der Sprenkel JW, Tulleken KA, Nicolay K. Correlation between tissue depolarizations and damage in focal ischemic rat brain. Brain Res. 1999;840:194–205. doi: 10.1016/s0006-8993(99)01769-2. [DOI] [PubMed] [Google Scholar]
- 178.Busch E, Gyngell ML, Eis M, Hoehn-Berlage M, Hossmann KA. Potassium-induced cortical spreading depressions during focal cerebral ischemia in rats: contribution to lesion growth assessed by diffusion-weighted NMR and biochemical imaging. J. Cereb. Blood Flow Metab. 1996;16:1090–1099. doi: 10.1097/00004647-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 179.Risher WC, Ard D, Yuan J, Kirov SA. Recurrent spontaneous spreading depolarizations facilitate acute dendritic injury in the ischemic penumbra. J. Neurosci. 2010;30(29):9859–9868. doi: 10.1523/JNEUROSCI.1917-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ COSBID study group. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132(Pt 7):1866–1881. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Iadecola C. Bleeding in the brain: killer waves of depolarization in subarachnoid bleed. Nat. Med. 2009;15:1131–1132. doi: 10.1038/nm1009-1131. [DOI] [PubMed] [Google Scholar]
- 182.Nakamura H, Strong AJ, Dohmen C, Sakowitz OW, Vollmar S, Sué M, Kracht L, Hashemi P, Bhatia R, Yoshimine T, Dreier JP, Dunn AK, Graf R. Spreading depolarizations cycle around and enlarge focal ischaemic brain lesions. Brain. 2010;133(Pt 7):1994–2006. doi: 10.1093/brain/awq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hartings JA, Watanabe T, Bullock MR, Okonkwo DO, Fabricius M, Woitzik J, Dreier JP, Puccio A, Shutter LA, Pahl C, Strong AJ Co-Operative Study on Brain Injury Depolarizations. Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain. 2011;134(Pt 5):1529–1540. doi: 10.1093/brain/awr048. [DOI] [PubMed] [Google Scholar]
- 184.Dreier JP, Körner K, Ebert N, Görner A, Rubin I, Back T, Lindauer U, Wolf T, Villringer A, Einhäupl KM, Lauritzen M, Dirnagl U. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J. Cereb. Blood Flow Metab. 1998;18(9):978–990. doi: 10.1097/00004647-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 185.Windmüller O, Lindauer U, Foddis M, Einhäupl KM, Dirnagl U, Heinemann U, Dreier JP. Ion changes in spreading ischaemia induce rat middle cerebral artery constriction in the absence of NO. Brain. 2005;128(Pt 9):2042–2051. doi: 10.1093/brain/awh545. [DOI] [PubMed] [Google Scholar]
- 186.Ayata C, Shimizu-Sasamata M, Lo EH, Noebels JL, Moskowitz MA. Impaired neurotransmitter release and elevated threshold for cortical spreading depression in mice with mutations in the α1A subunit of P/Q type calcium channels. Neuroscience. 2000;95:639–645. doi: 10.1016/s0306-4522(99)00446-7. [DOI] [PubMed] [Google Scholar]
- 187.van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, Cesetti T, van de Ven RC, Tottene A, van der Kaa J, Plomp JJ, Frants RR, Ferrari MD. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41(5):701–710. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 188.van den Maagdenberg AM, Pizzorusso T, Kaja S, Terpolilli N, Shapovalova M, Hoebeek FE, Barrett CF, Gherardini L, van de Ven RC, Todorov B, Broos LA, Tottene A, Gao Z, Fodor M, De Zeeuw CI, Frants RR, Plesnila N, Plomp JJ, Pietrobon D, Ferrari MD. High cortical spreading depression susceptibility and migraine-associated symptoms in Ca(v)2.1 S218L mice. Ann. Neurol. 2010;67:85–98. doi: 10.1002/ana.21815. [DOI] [PubMed] [Google Scholar]
- 189.Eikermann-Haerter K, Dilekoz E, Kudo C, Savitz SI, Waeber C, Baum MJ, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, Ayata C. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J. Clin. Invest. 2009;119:99–109. doi: 10.1172/JCI36059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kunkler PE, Kraig RP. P/Q Ca2+ Channel blockade stops spreading depression and related pyramidal neuronal Ca2+ rise in hippocampal organ culture. Hippocampus. 2004;14:356–367. doi: 10.1002/hipo.10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.van den Maagdenberg AM, Haan J, Terwindt GM, Ferrari MD. Migraine: gene mutations and functional consequences. Curr. Opin. Neurol. 2007;20(3):299–305. doi: 10.1097/WCO.0b013e3281338d1f. [DOI] [PubMed] [Google Scholar]
- 192.Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63. doi: 10.1136/bmj.38302.504063.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. B.M.J. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Eikermann-Haerter K, Lee JH, Yuzawa I, Liu CH, Zhou Z, Shin HK, Zheng Y, Qin T, Kurth T, Waeber C, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, Ayata C. Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations. Circulation. 2012;125(2):335–345. doi: 10.1161/CIRCULATIONAHA.111.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lauritzen M, Hansen AJ. The effect of glutamate receptor blockade on anoxic depolarization and cortical spreading depression. J. Cereb. Blood Flow Metab. 1992;12:223–229. doi: 10.1038/jcbfm.1992.32. [DOI] [PubMed] [Google Scholar]
- 196.Tottene A, Conti R, Fabbro A, Vecchia D, Shapovalova M, Santello M, van den Maagdenberg AM, Ferrari MD, Pietrobon D. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca (v) 2.1 knockin migraine mice. Neuron. 2009;61:762–773. doi: 10.1016/j.neuron.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 197.Kuo IY, Ellis A, Seymour VA, Sandow SL, Hill CE. Dihydropyridine-insensitive calcium currents contribute to function of small cerebral arteries. J. Cereb. Blood Flow Metab. 2010;30(6):1226–1239. doi: 10.1038/jcbfm.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hansen PB, Jensen BL, Andreasen D, Friis UG, Skøtt O. Vascular smooth muscle cells express the alpha(1A) subunit of a P-/Q-type voltage-dependent Ca2+ channel, and It is functionally important in renal afferent arterioles. Circ Res. 2000;87(10):896–902. doi: 10.1161/01.res.87.10.896. [DOI] [PubMed] [Google Scholar]
- 199.Andreasen D, Friis UG, Uhrenholt TR, Jensen BL, Skøtt O, Hansen PB. Coexpression of voltage-dependent calcium channels Cav1.2, 2.1a, and 2.1b in vascular myocytes. Hypertension. 2006;47(4):735–741. doi: 10.1161/01.HYP.0000203160.80972.47. [DOI] [PubMed] [Google Scholar]
- 200.Dorhout Mees SM, Rinkel GJ, Feigin VL, Algra A, van den Bergh WM, Vermeulen M, van Gijn J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev. 2007;18(3):CD000277. doi: 10.1002/14651858.CD000277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Simard JM. Calcium channel currents in isolated smooth muscle cells from the basilar artery of the guinea pig. Pflugers Arch. 1991;417(5):528–536. doi: 10.1007/BF00370950. [DOI] [PubMed] [Google Scholar]
- 202.Ishiguro M, Wellman TL, Honda A, Russell SR, Tranmer BI, Wellman GC. Emergence of a R-Type Ca2 Channel (CaV 2.3) Contributes to Cerebral Artery Constriction After Subarachnoid Hemorrhage. Circ. Res. 2005;96:419–426. doi: 10.1161/01.RES.0000157670.49936.da. [DOI] [PubMed] [Google Scholar]
- 203.Fisher SA. Vascular smooth muscle phenotypic diversity and function. Physiol. Genomics. 2010;42A(3):169–187. doi: 10.1152/physiolgenomics.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Navarro-Gonzalez MF, Grayson TH, Meaney KR, Cribbs LL, Hill CE. Non-L-type voltage-dependent calcium channels control vascular tone of the rat basilar artery. Clin. Exp. Pharmacol. Physiol. 2009;36(1):55–66. doi: 10.1111/j.1440-1681.2008.05035.x. [DOI] [PubMed] [Google Scholar]
- 205.Nikitina E, Kawashima A, Takahashi M, Zhang ZD, Shang X, Ai J, Macdonald RL. Alteration in voltage-dependent calcium channels in dog basilar artery after subarachnoid hemorrhage. Laboratory investigation. J. Neurosurg. 2010;113(4):870–880. doi: 10.3171/2010.2.JNS091038. [DOI] [PubMed] [Google Scholar]
- 206.Link TE, Murakami K, Beem-Miller M, Tranmer BI, Wellman GC. Oxyhemoglobin-induced expression of R-type Ca2+ channels in cerebral arteries. Stroke. 2008;39(7):2122–2128. doi: 10.1161/STROKEAHA.107.508754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Woitzik J, Dreier JP, Hecht N, Fiss I, Sandow N, Major S, Winkler M, Dahlem YA, Manville J, Diepers M, Muench E, Kasuya H, Schmiedek P, Vajkoczy P COSBID study group. Delayed cerebral ischemia and spreading depolarization in absence of angiographic vasospasm after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2012;32(2):203–212. doi: 10.1038/jcbfm.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Strong AJ, Macdonald RL. Cortical spreading ischemia in the absence of proximal vasospasm after aneurysmal subarachnoid hemorrhage: evidence for a dual mechanism of delayed cerebral ischemia. J. Cereb Blood Flow Metab. 2012;32(2):201–202. doi: 10.1038/jcbfm.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sagare AP, Bell RD, Zlokovic BV. Neurovascular Dysfunction and Faulty Amyloid β-Peptide Clearance in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012;2(10):1–17. doi: 10.1101/cshperspect.a011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nicolakakis N, Hamel E. Neurovascular function in Alzheimer’s disease patients and experimental models. J. Cereb. Blood Flow Metab. 2011;31(6):1354–1370. doi: 10.1038/jcbfm.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol. Dis. 2002;9:61–68. doi: 10.1006/nbdi.2001.0460. [DOI] [PubMed] [Google Scholar]
- 213.Kara F, Dongen ES, Schliebs R, Buchem MA, Groot HJ, Alia A. Monitoring blood flow alterations in the Tg2576 mouse model of Alzheimer’s disease by in vivo magnetic resonance angiography at 17.6 T. Neuroimage. 2012;60(2):958–966. doi: 10.1016/j.neuroimage.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 214.Sabayan B, Jansen S, Oleksik AM, van Osch MJ, van Buchem MA, van Vliet P, de Craen AJ, Westendorp RG. Cerebrovascular hemodynamics in Alzheimer’s disease and vascular dementia: a meta-analysis of transcranial Doppler studies. Ageing Res. Rev. 2012;11(2):271–277. doi: 10.1016/j.arr.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 215.Claassen JA, Zhang R. Cerebral autoregulation in Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2011;31(7):1572–1577. doi: 10.1038/jcbfm.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cantin S, Villien M, Moreaud O, Tropres I, Keignart S, Chipon E, Le Bas JF, Warnking J, Krainik A. Impaired cerebral vasoreactivity to CO2 in Alzheimer’s disease using BOLD fMRI. Neuroimage. 2011;58(2):579–587. doi: 10.1016/j.neuroimage.2011.06.070. [DOI] [PubMed] [Google Scholar]
- 217.Niwa K, Younkin L, Ebeling C, Turner S K, Westaway D, Younkin S, Ashe K H, Carlson G A, Iadecola C. Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yamada M, Naiki H. Cerebral amyloid angiopathy. Prog. Mol. Biol. Transl. Sci. 2012;107:41–78. doi: 10.1016/B978-0-12-385883-2.00006-0. [DOI] [PubMed] [Google Scholar]
- 219.Yamada M. Cerebral amyloid angiopathy: an overview. Neuropathology. 2000;20(1):8–22. doi: 10.1046/j.1440-1789.2000.00268.x. [DOI] [PubMed] [Google Scholar]
- 220.Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. β-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 221.Gentile MT, Vecchione C, Maffei A, Aretini A, Marino G, Poulet R, Capobianco L, Selvetella G, Lembo G. Mechanisms of soluble beta-amyloid impairment of endothelial function. J. Biol Chem. 2004;279(46):48135–48142. doi: 10.1074/jbc.M407358200. [DOI] [PubMed] [Google Scholar]
- 222.Niwa K, Porter VA, Kazama K, Cornfield D, Carlson GA, Iadecola C. A beta-peptides enhance vasoconstriction in cerebral circulation. Am. J. Physiol. Heart Circ. Physiol. 2001;281(6):H2417–H2424. doi: 10.1152/ajpheart.2001.281.6.H2417. [DOI] [PubMed] [Google Scholar]
- 223.Paris D, Quadros A, Humphrey J, Patel N, Crescentini R, Crawford F, Mullan M. Nilvadipine antagonizes both Abeta vasoactivity in isolated arteries, and the reduced cerebral blood flow in APPsw transgenic mice. Brain Res. 2004;999(1):53–61. doi: 10.1016/j.brainres.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 224.Paris D, Bachmeier C, Patel N, Quadros A, Volmar CH, Laporte V, Ganey J, Beaulieu-Abdelahad D, Ait-Ghezala G, Crawford F, Mullan MJ. Selective antihypertensive dihydropyridines lower Aβ accumulation by targeting both the production and the clearance of Aβ across the blood-brain barrier. Mol. Med. 2011;17(3-4):149–162. doi: 10.2119/molmed.2010.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.van Assema DM, Lubberink M, Bauer M, van der Flier WM, Schuit RC, Windhorst AD, Comans EF, Hoetjes NJ, Tolboom N, Langer O, Müller M, Scheltens P, Lammertsma AA, van Berckel BN. Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain. 2012;135(Pt 1):181–189. doi: 10.1093/brain/awr298. [DOI] [PubMed] [Google Scholar]
- 226.Czeh M, Gressens P, Kaindl AM. The yin and yang of microglia. Dev. Neurosci. 2011;33(3-4):199–209. doi: 10.1159/000328989. [DOI] [PubMed] [Google Scholar]
- 227.Wee Yong V. Inflammation in neurological disorders: a help or a hindrance? Neuroscientist. 2010;16(4):408–420. doi: 10.1177/1073858410371379. [DOI] [PubMed] [Google Scholar]
- 228.Polazzi E, Monti B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog. Neurobiol. 2010;92(3):293–315. doi: 10.1016/j.pneurobio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 229.Howell OW, Rundle JL, Garg A, Komada M, Brophy PJ, Reynolds R. Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2010;69(10):1017–1033. doi: 10.1097/NEN.0b013e3181f3a5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Verkhratsky A, Steinhäuser C. Ion channels in glial cells. Brain Res. Brain Res. Rev. 2000;32(2-3):380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 231.Sontheimer H. Voltage-dependent ion channels in glial cells. Glia. 1994;11(2):156–172. doi: 10.1002/glia.440110210. [DOI] [PubMed] [Google Scholar]
- 232.Latour I, Hamid J, Beedle AM, Zamponi GW, Macvicar BA. Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia. 2003;41(4):347–353. doi: 10.1002/glia.10162. [DOI] [PubMed] [Google Scholar]
- 233.Moller T. Calcium signaling in microglial cells. Glia. 2002;40(2):184–194. doi: 10.1002/glia.10152. [DOI] [PubMed] [Google Scholar]
- 234.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 235.Silei V, Fabrizi C, Venturini G, Salmona M, Bugiani O, Tagliavini F, Lauro GM. Activation of microglial cells by PrP and beta-amyloid fragments raises intracellular calcium through L-type voltage sensitive calcium channels. Brain Res. 1999;818(1):168–170. doi: 10.1016/s0006-8993(98)01272-4. [DOI] [PubMed] [Google Scholar]
- 236.Hegg CC, Hu S, Peterson PK, Thayer SA. Beta-chemokines and human immunodeficiency virus type-1 proteins evoke intracellular calcium increases in human microglia. Neuroscience. 2000;98(1):191–199. doi: 10.1016/s0306-4522(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 237.Kornek B, Storch MK, Bauer J, Djamshidian A, Weissert R, Wallstroem E, Stefferl A, Zimprich F, Olsson T, Linington C, Schmidbauer M, Lassmann H. Distribution of a calcium channel subunit in dystrophic axons in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain. 2001;124(Pt 6):1114–1124. doi: 10.1093/brain/124.6.1114. [DOI] [PubMed] [Google Scholar]
- 238.Gadjanski I, Boretius S, Williams SK, Lingor P, Knöferle J, Sättler MB, Fairless R, Hochmeister S, Sühs KW, Michaelis T, Frahm J, Storch MK, Bähr M, Diem R. Role of N-type voltage-dependent calcium channels in autoimmune optic neuritis. Ann. Neurol. 2009;66(1):81–93. doi: 10.1002/ana.21668. [DOI] [PubMed] [Google Scholar]
- 239.Tokuhara N, Namiki K, Uesugi M, Miyamoto C, Ohgoh M, Ido K, Yoshinaga T, Yamauchi T, Kuromitsu J, Kimura S, Miyamoto N, Kasuya Y. N-type calcium channel in the pathogenesis of experimental autoimmune encephalomyelitis. J. Biol. Chem. 2010;285(43):33294–33306. doi: 10.1074/jbc.M109.089805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Skaper SD. Ion channels on microglia: therapeutic targets for neuroprotection. CNS Neurol. Disord. Drug. Targets. 2011;10(1):44–56. doi: 10.2174/187152711794488638. [DOI] [PubMed] [Google Scholar]
- 241.Boscia F, Gala R, Pannaccione A, Secondo A, Scorziello A, Di Renzo G, Annunziato L. NCX1 expression and functional activity increase in microglia invading the infarct core. Stroke. 2009;40(11):3608–3617. doi: 10.1161/STROKEAHA.109.557439. [DOI] [PubMed] [Google Scholar]