Abstract
Objective
To estimate if live birth in single blastocyst transfers is correlated with the number of sibling supernumerary vitrified blastocysts (embryos not transferred) generated from that same cycle.
Design
Retrospective cohort study.
Setting
A large academic ART clinic.
Patients
All single blastocyst transfers in 2010 of a SART grade ‘good’ embryo.
Interventions
None.
Main Outcome Measures
Implantation and live birth.
Results
655 single blastocyst transfers met inclusion criteria. Implantation occurred in 65% and live birth in 54% of cycles. In chi-square analysis, patients with supernumerary vitrified blastocysts had statistically higher implantation rate (65% versus 50%) and live birth rate (56% versus 41%) when compared to patients without supernumerary blastocysts. Univariate logistic regression demonstrated an increase in implantation (OR 1.09, 95%CI 1.03–1.15) and live birth (OR 1.06, 95%CI 1.02–1.09) with increasing number of supernumerary blastocysts. Multivariate logistic regression analysis demonstrated patient age and the number of supernumerary blastocysts to be significantly associated with implantation and live birth.
Conclusion
The number of supernumerary vitrified blastocyst correlated positively with the odds of implantation and live birth in good quality single blastocyst transfers. Patients with supernumerary blastocysts are good candidates for single embryo transfer.
Keywords: supernumerary embryos, vitrified, blastocyst, live birth, implantation
Introduction
The ability to predict embryo implantation is clinically useful for counseling patients and in determining the ideal number of embryos to transfer. Cycles with the highest implantation potential are ideal cycles for single embryo transfer to minimize the risks of multi-fetal gestation while maximizing the likelihood of pregnancy (1). It is well established that embryo quality correlates with implantation potential (1–5). Supernumerary embryos are excess embryos obtained during assisted reproductive technologies (ART) that are not transferred in the fresh cycle in which they are generated. The presence of supernumerary embryos for cryopreservation has been used as a marker of embryo quality and is an independent predictor of implantation (2, 6–8). In cleavage-stage embryo transfers, supernumerary embryo development to the blastocyst stage was more likely to occur in cycles where the transferred embryos implanted (9). Cleavage-stage embryo transfers with additional cryopreserved embryos were more likely to implant than similar grade embryo transfer without supernumerary embryos available for cryopreservation (2).
There are gaps in the current knowledge about supernumerary embryos. The current literature comes primarily from cleavage stage transfers. It is possible that the importance of supernumerary embryos in predicting outcomes may not apply to blastocysts, which benefit from a higher implantation rate as a result of improved embryo selection. The statistical analyses in the current studies rely primarily on Chi-square statistics. While this has demonstrated that the presence of supernumerary embryos is superior to the absence, this statistical test does not allow to predict if the number of supernumerary embryos correlates with ART outcomes or to control for important potential confounders in the analysis. Finally, the current studies have been performed in multiple embryo transfer cycles. A single embryo transfer study design would control for the actual number of embryos transferred.
While the absence or presence of supernumerary embryos is correlated to implantation, we are not aware of any data examining the correlation of the number of supernumerary embryos on implantation in single blastocyst transfers. The objective of this study was to estimate if live birth in single blastocyst transfers was correlated with the number of sibling supernumerary vitrified blastocysts generated from that same cycle. We hypothesized that the number of supernumerary blastocysts available for vitrification would positively correlate with implantation and live birth in single blastocyst transfers.
Materials and Methods
Study Design
This was a retrospective cohort analysis of 720 fresh, autologous single blastocyst embryo transfer cycles during 2010. Since the quality of the actual embryo being transferred would be a significant confounder in the implantation and live birth of each cycle, we limited to the analysis to only embryos transferred which were scored as SART grade good in order to control for embryo quality. These are embryos with an inner cell mass grade A and a trophectoderm grade A or B. Of 720 single blastocyst transfers, 655 were transfers of a SART grade ‘good’ embryo. Supernumerary blastocysts were defined as additional blastocyst generated in that same fresh ART cycle that were not transferred and were of a quality to be cryopreserved. Vitrification was performed in inner cell mass/trophectoderm grade BB or better embryos. The study was performed at Shady Grove Fertility Reproductive Science Center in Rockville, MD and was IRB approved.
Patients
All patients who underwent a fresh autologous embryo transfer of a SART grade ‘good’ embryo during 2010 were included in that analysis. Additional subgroup analyses were done on embryos that were expanded or hatched blastocysts with a grade A trophectoderm and grade A inner cell mass, representing the highest quality embryos for transfer. The study included elective single blastocyst transfers as well as patients with only a single blastocyst available for transfer. Exclusion criteria included cycles with multiple embryos transferred, donor-recipient cycles, frozen-thaw transfers, cleavage stage transfers, morula stage transfers, and transfers of SART grade fair or poor blastocysts.
Stimulation protocol
Ovarian stimulation was accomplished with mixed FSH/LH protocols under GnRH antagonist or GnRH agonist pituitary suppression as previously described (10). For most patients, oral contraceptive treatment was initiated 19 days prior to stimulation. For GnRH antagonist cycles, the antagonist (Ganirelix) was initiated when the lead follicle was 14mm in size. During the last 3 days of oral contraceptives, 20 units of leuprolide acetate (Lupron) was initiated in GnRH agonist cycles. The leuprolide acetate dose was decreased to 5 units when ovarian suppression was confirmed. Ovarian stimulation was typically achieved with a mixed protocol employing recombinant FSH and hMG. When the lead follicle was ≥18mm, final oocyte maturation was triggered with 10,000 units of hCG or with 40 units of GnRH agonist in some of the GnRH antagonist cycles. Oocyte retrieval occurred 36 hours later and insemination was achieved with conventional IVF or ICSI as indicated. Ultrasound guided embryo transfer was performed on day 5 or day 6. Serum hCG levels were assessed two weeks after trigger injection and ultrasonographic confirmation of pregnancy was obtained in all pregnant patients.
Embryo grading
All blastocysts were evaluated by an embryologist using a modification of the grading system of Gardner and Schoolcraft (11, 12). The trophectoderm was assigned one of the following grades: A- many cells organized in epithelium, B- several cells organized in loose epithelium, C- few large cells. The inner cell mass was assigned one of the following grades: A- numerous, tightly packed cells, B- several and loosely packed cells, C- very few cells. Blastocyst expansion was assigned one of the following descriptors: Early- blastocele less than half the blastocyst, Expanded- blastocele fills the blastocyst with thin zona, Hatched- blastocyst has hatched out of the zona. Morula transfers were excluded from the analysis. SART grade ‘good’ embryos were defined as AA or AB graded embryos (inner cell mass/trophectoderm). Blastocysts were vitrified if the inner cell mass and trophectoderm were grade B or better. In cycles where patients declined blastocyst vitrification for financial, ethical, or religious reasons, the number of blastocysts meeting the quality to have otherwise been vitrified was recorded.
Statistics
Chi-square analysis was used to compare implantation and live birth rates in patients who had supernumerary vitrified blastocyst versus patients without supernumerary vitrified blastocyst. Continuous parameters were compared using a Students T test or Mann-Whitney rank sum test as appropriate. Univariate regression analysis was used to correlate the number of supernumerary blastocysts with implantation and live birth in the sibling single blastocyst transfer cycle. To control for other factors related to implantation, multivariate regression analysis was performed to include age, body mass index, the number of supernumerary vitrified blastocyst, and the stage, trophectoderm grade, and inner cell mass grade of the single blastocyst that was transferred. The quality of the inner cell mass was controlled for in the inclusion criteria, since SART grade good embryos are inner cell mass grade A. Statistical analysis was performed using SPSS version 17 (IBM, Armonk, NY) and Vassar Statistics (vassarstats.net). A p-value of <0.05 was considered statistically significant.
Results
Out of 720 single blastocyst transfers performed in 2010, there were 655 single blastocyst transfers of a SART grade good embryo. The mean age was 32.0 ± 3.5 years. The implantation rate for the cohort was 64% and the live birth rate was 54%. There were five twin gestation live births (0.7%) and no high order multiple gestation births. The average number of supernumerary vitrified blastocysts was 3.8 ± 2.9. There were no supernumerary embryos available in 98 cycles (15%) and were therefore non-elective single blastocyst transfers.
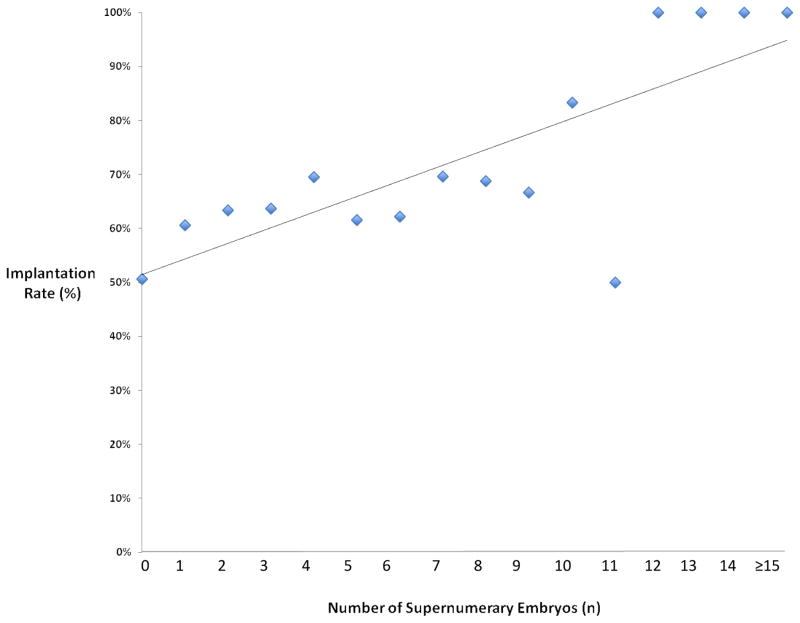
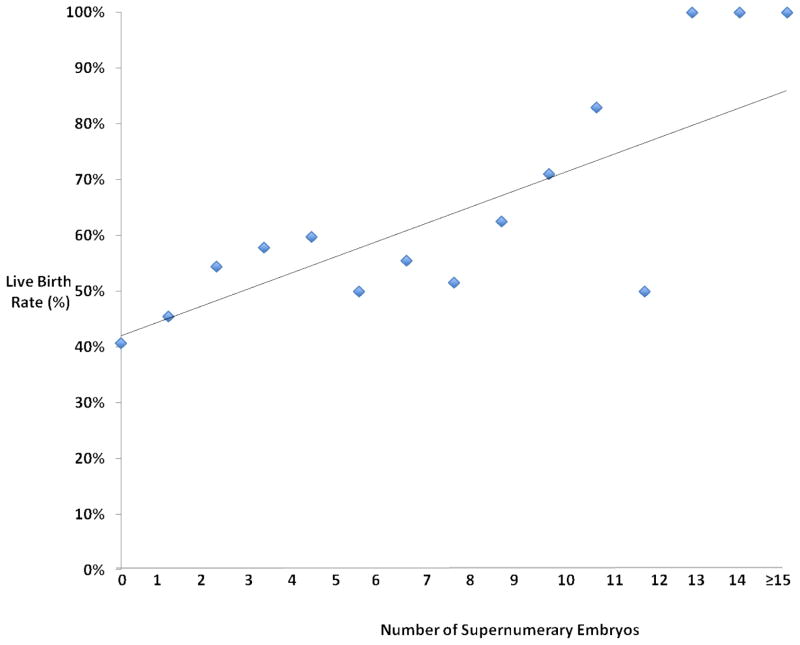
Chi-square analysis demonstrated that the implantation rate was significantly higher in patients with supernumerary vitrified blastocyst versus those without (65% versus 50%, p=0.01). Similarly, chi-square analysis demonstrated that the live birth rate was significantly higher in patients with supernumerary vitrified blastocyst versus those without (56% versus 41%, p=0.01). Patients without supernumerary embryos were similar to patients with supernumerary embryos in regard to age, BMI, and ART stimulation parameters (Table 1). Similarly, the day of embryo transfer and the percentage of patients receiving a day 6 embryo transfer was similar between the two groups. All embryos transferred in both groups were SART grade good with both groups having similar embryo expansion and ICM grades, however patients with supernumerary embryos were more likely to have a grade A trophectoderm in the actual embryo transferred (80.0% versus 60.2%, p<0.01). The implantation and live birth rates demonstrated a strong linear correlation with the number of supernumerary blastocysts (Figure 1 and 2). Univariate regression analysis demonstrated a positive correlation with the number of supernumerary vitrified blastocyst and implantation (OR 1.09, 95% CI 1.03–1.15) and live birth (OR 1.06, 95% CI 1.02–1.09). Multiple logistic regression analysis showed age and the number of supernumerary vitrified blastocyst to be significantly correlated with both implantation (p=0.04) and live birth (p=0.02) (Table 2). The trophectoderm score of the transferred embryo was positively correlated to implantation in the multiple regression analysis (OR 1.58, 95% CI 1.04–2.41). Embryo expansion and BMI were not correlated with ART outcomes in the multiple logistic regression analysis.
Table 1.
Baseline characteristics and stimulation parameters between patients without and with supernumerary embryos.
| No supernumerary embryos (n=98) | Yes supernumerary embryos (n=557) | P value | |
|---|---|---|---|
| Age | 32.4 ± 3.4 | 31.9 ± 3.4 | 0.23 |
| BMI | 25.3 ± 5.1 | 24.5 ± 5.1 | 0.2 |
| Amount of gonadotropins administered (IUs) | 2736 ± 1462 | 2830 ± 1462 | 0.58 |
| Estradiol on day of hCG (pg/ml) | 2830 ± 1597 | 2736 ± 1094 | 0.97 |
| Oocytes retrieved | 17.6 ± 8.2 | 18.7 ± 8.2 | 0.26 |
| MIIs retrieved | 14.4 ± 6.8 | 15.3 ± 6.8 | 0.24 |
| Fertilized oocytes | 11.0 ± 5.2 | 12.0 ± 5.2 | 0.19 |
| Day 6 transfers (%) | 20.40% | 16.50% | 0.43 |
Figure 1.
Scatter plot with linear trend line showing the implantation rate versus the number of supernumerary vitrified blastocysts.
Figure 2.
Scatter plot with linear trend line showing the live birth rate versus the number of supernumerary vitrified blastocysts.
Table 2.
Multiple logistic regression analysis looking at correlations to implantation and live birth with variables of BMI, age, supernumerary vitrified blastocysts, and the quality of the transferred embryos expansion stage, and TE grade. Age, supernumerary vitrified blastocysts, and TE grade were significantly correlated with ART outcomes.
| Variable | Implantation | Live Birth | ||
|---|---|---|---|---|
| P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | |
| Age | 0.03 | 0.95 (0.90–0.99) | 0.03 | 0.95 (0.91–0.99) |
| Vitrified Embryos (n) | 0.04 | 1.07 (1.01–1.13) | 0.02 | 1.07 (1.01–1.14) |
| Transferred embryo TE grade | 0.03 | 1.58 (1.04–2.41) | 0.11 | 1.40 (0.93–2.13) |
| BMI | 0.08 | 0.94 (0.84–1.01) | 0.1 | 0.98 (0.95–1.01) |
| Transferred embryo stage | 0.86 | 1.04 (0.70–1.53) | 0.59 | 1.11 (0.76–1.63) |
To examine if the results held consistent in the best prognosis patients, a subgroup analysis of 451 single blastocyst transfers of an expanded or hatching blastocyst with a grade A trophectoderm and grade A inner cell mass was performed. The mean age was 32.0 ± 3.5. The subgroup implantation rate was 67% and the live birth rate was 60%, demonstrating that this was a very high prognosis subgroup. The average number of supernumerary vitrified blastocyst was 4.3 ± 3.1. In this subgroup, univariate regression analysis demonstrated a positive correlation with the number of supernumerary vitrified blastocyst and implantation (OR 1.09, 95%CI 1.02–1.16) and live birth (OR 1.09, 95%CI 1.02–1.15). Multiple logistic regression analysis controlling for age, BMI, and embryo stage demonstrated that the number of supernumerary vitrified blastocyst was significantly correlated with both implantation (p=0.02) and live birth (p=0.02).
Discussion
These data demonstrate that the number of supernumerary embryos was positively correlated to both implantation and live birth in single blastocyst transfers. Even when analyzing only patients receiving the highest quality blastocyst for embryo transfer, the number of supernumerary blastocysts was still a positive predictor of clinical outcomes. Patients with supernumerary embryos were able to achieve high success rates without the need to transfer additional embryos. Single embryo transfer can reduce the risk of multiple gestation and allow for an increase number of vitrified embryos for future frozen-thaw cycles (1). These data are consistent with a recent publication demonstrating that single blastocyst transfers with cryopreserved embryos had a higher live birth rate than single blastocyst transfers without cryopreserved embryos (75% versus 48%) (13). These data are also consistent with a national database study demonstrating that the presence of supernumerary embryos was a surrogate marker of embryo quality for the actual embryo transferred (2). The data in this current study furthered this knowledge by demonstrating that not only the presence of supernumerary embryos but also the number of those embryos is correlated to ART outcomes and that this correlation persists regardless of the quality of the actual embryo being transferred or the age of the patient.
Historically, implantation rates in ART have been low for individual embryos. In an effort to overcome this deficit and improve the chance of pregnancy, multiple embryos have been transferred. As a consequence of this practice, ART carries a significant risk of multiple gestation. Multiple gestation pregnancies are inherently at higher risk than singleton pregnancies to both mother and fetus. Maternal risks of multiple gestation pregnancies include pre-eclampsia, gestational diabetes, gestational hypertension, maternal hospital admission and cesarean delivery, while those risks to the neonate involve prematurity, neonatal intensive care unit admission, low birth weight, and increased morbidity and mortality (14).
Even with multiple studies demonstrating the risks of multiple gestation, the United States lags behind in adoption of elective single embryo transfer (15, 16). Barriers to acceptance of single blastocyst transfers vary between patients and providers. In one study, the most common reason for providers to not accept single embryo transfer were perceived suboptimal success rates with cryopreservation, lack of a protocol to implement single embryo transfer, and not perceiving twins pregnancies as a complication (17). Among patients, common barriers to single blastocyst transfers included not perceiving that twin pregnancies were a complication, absence of a reimbursement system which favors single blastocyst transfers, perceiving the chance of pregnancy as a more important factor than the risk of multiple gestation, and inadequate options to select couples suitable for single blastocyst transfers (18). The data from this present study may help encourage patients with supernumerary blastocysts to proceed with elective single blastocyst transfers, rather than transferring additional available blastocysts.
The strengths of the current paper were the use of multiple logistic regression in single blastocyst transfers and this design allowed us to address the weaknesses of prior studies. Specifically, the use of regression analysis clearly demonstrated the correlation between the number of supernumerary vitrified blastocysts and ART clinical outcomes. Further, the use of multiple logistic regression analysis allowed this data to be controlled for other significant predictors of implantation and live birth, such as the age of the patient and the quality of the actual embryo being transferred. The quality of the trophectoderm was significantly associated with implantation and there was a trend towards an association with live birth. These findings are consistent with recent evidence correlating the trophectoderm grade with ART outcomes (19–21) Patients with supernumerary embryos were more likely to have an embryo transferred with a trophectoderm grade A, which could potentially bias the results in favor of patients with supernumerary embryos. However, when the trophectoderm grade was included in the multiple regression analysis, the number of supernumerary embryos remained highly correlated with live birth. All of the transferred embryos had an inner cell mass grade A, so correlations with inner cell mass and ART outcomes could not be evaluated. Weaknesses of this paper include the low number of embryos that were of poor quality. This design was employed to control for the quality of the embryo being transferred. These data represent good prognosis patients who are likely to achieve pregnancy from single blastocyst transfer and may not apply to poorer prognosis groups.
In conclusion, these data demonstrated for the first time that the number of supernumerary blastocysts available for cryopreservation positively correlate with both implantation and live birth. The data provide further evidence that the quality of the overall embryo cohort have an impact on the reproductive potential of the actual embryo transferred, even if that embryo is of the highest quality. Patients with two or more supernumerary embryos had a live birth rate over 50% from single blastocyst transfers. The excellent clinical outcomes in this cohort suggest that patients with supernumerary blastocyst are good candidates for elective single embryo transfer.
Acknowledgments
This research was supported, in part, by Intramural research program of the Program in Reproductive and Adult Endocrinology, NICHD, NIH.
Footnotes
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U. S. Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Csokmay JM, Hill MJ, Chason RJ, Hennessy S, James AN, Cohen J, et al. Experience with a patient-friendly, mandatory, single-blastocyst transfer policy: the power of one. Fertil Steril. 2011;96:580–4. doi: 10.1016/j.fertnstert.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern JE, Lieberman ES, Macaluso M, Racowsky C. Is cryopreservation of embryos a legitimate surrogate marker of embryo quality in studies of assisted reproductive technology conducted using national databases? Fertil Steril. 2012;97:890–3. doi: 10.1016/j.fertnstert.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 3.Tesarik J, Junca AM, Hazout A, Aubriot FX, Nathan C, Cohen-Bacrie P, et al. Embryos with high implantation potential after intracytoplasmic sperm injection can be recognized by a simple, non-invasive examination of pronuclear morphology. Hum Reprod. 2000;15:1396–9. doi: 10.1093/humrep/15.6.1396. [DOI] [PubMed] [Google Scholar]
- 4.Van Royen E, Mangelschots K, De Neubourg D, Laureys I, Ryckaert G, Gerris J. Calculating the implantation potential of day 3 embryos in women younger than 38 years of age: a new model. Hum Reprod. 2001;16:326–32. doi: 10.1093/humrep/16.2.326. [DOI] [PubMed] [Google Scholar]
- 5.Racowsky C, Ohno-Machado L, Kim J, Biggers JD. Is there an advantage in scoring early embryos on more than one day? Hum Reprod. 2009;24:2104–13. doi: 10.1093/humrep/dep198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern JE, Brown MB, Luke B, Wantman E, Lederman A, Hornstein MD, et al. Cycle 1 as predictor of assisted reproductive technology treatment outcome over multiple cycles: an analysis of linked cycles from the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System online database. Fertil and Steril. 2011;95:600–5. doi: 10.1016/j.fertnstert.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Stern JE, Goldman MB, Hatasaka H, MacKenzie TA, Racowsky C, Surrey ES, et al. Optimizing the number of blastocyst stage embryos to transfer on day 5 or 6 in women 38 years of age and older: a Society for Assisted Reproductive Technology database study. Fertil and Steril. 2009;91:157–66. doi: 10.1016/j.fertnstert.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Stern JE, Goldman MB, Hatasaka H, MacKenzie TA, Surrey ES, Racowsky C, et al. Optimizing the number of cleavage stage embryos to transfer on day 3 in women 38 years of age and older: a Society for Assisted Reproductive Technology database study. Ferti and Steril. 2009;91:767–76. doi: 10.1016/j.fertnstert.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 9.Sjogren A, Sjoblom P, Hamberger L. CULTURE OF HUMAN SPARE PREEMBRYOS - ASSOCIATION BETWEEN BLASTOCYST FORMATION AND PREGNANCY. Journal of Assisted Reproduction and Genetics. 1992;9:41–4. doi: 10.1007/BF01204113. [DOI] [PubMed] [Google Scholar]
- 10.Stillman RJ, Richter KS, Banks NK, Graham JR. Elective single embryo transfer: A 6-year progressive implementation of 784 single blastocyst transfers and the influence of payment method on patient choice. Fertil and Steril. 2009;92:1895–906. doi: 10.1016/j.fertnstert.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Gardner DKSW. In vitro culture of human blastocyst. In: Mortimer JR, editor. Towards Reproductive Certainty: Infertility and Genetics Beyond. Pathenon Press; 1999. pp. 378–88. [Google Scholar]
- 12.Liebermann J, Tucker MJ. Comparison of vitrification and conventional cryopreservation of day 5 and day 6 blastocysts during clinical application. Fertil Steril. 2006;86:20–6. doi: 10.1016/j.fertnstert.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Mullin CBA, Grifo JA. Journal of assisted reproduction and genetics. Supernumerary blastocyst cryopreservation: a key prognostic indicator for patients opting for an elective single blastocyst transfer (eSBT) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinborg A. IVF/ICSI twin pregnancies: risks and prevention. Hum Reprod Update. 2005;11:575–93. doi: 10.1093/humupd/dmi027. [DOI] [PubMed] [Google Scholar]
- 15.Maheshwari A, Griffiths S, Bhattacharya S. Global variations in the uptake of single embryo transfer. Hum Reprod Update. 2011;17:107–20. doi: 10.1093/humupd/dmq028. [DOI] [PubMed] [Google Scholar]
- 16.Elective single-embryo transfer. Fertility and sterility. 2012;97:835–42. doi: 10.1016/j.fertnstert.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 17.van Peperstraten AM, Hermens R, Nelen W, Stalmeier PFM, Scheffer GJ, Grol R, et al. Perceived barriers to elective single embryo transfer among IVF professionals: a national survey. Hum Reprod. 2008;23:2718–23. doi: 10.1093/humrep/den327. [DOI] [PubMed] [Google Scholar]
- 18.van Peperstraten AM, Nelen W, Hermens R, Jansen L, Scheenje E, Braat DDM, et al. Why don’t we perform elective single embryo transfer? A qualitative study among IVF patients and professionals. Hum Reprod. 2008;23:2036–42. doi: 10.1093/humrep/den156. [DOI] [PubMed] [Google Scholar]
- 19.Ahlstrom A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod. 2011;26:3289– 96. doi: 10.1093/humrep/der325. [DOI] [PubMed] [Google Scholar]
- 20.Honnma H, Baba T, Sasaki M, Hashiba Y, Ohno H, Fukunaga T, et al. Trophectoderm morphology significantly affects the rates of ongoing pregnancy and miscarriage in frozen-thawed single-blastocyst transfer cycle in vitro fertilization. Fertil Steril. 2012 doi: 10.1016/j.fertnstert.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Hill MJ, Richter KS, Heitmann RJ, Graham JR, Tucker MJ, DeCherney AH, et al. Trophectoderm grade predicts outcomes of single blastocyst transfers. Fertil Steril. 2013 doi: 10.1016/j.fertnstert.2012.12.003. in press. [DOI] [PubMed] [Google Scholar]