Abstract
Acute lung injury (ALI) is a severe pulmonary disease causing high numbers of fatalities worldwide. Innate immune responses are an integral part of the pathophysiologic events during ALI. Interleukin-23 (IL-23) is a proinflammatory mediator known to direct the inflammatory responses in various settings of infection, autoimmunity and cancer. IL-23 has been associated with proliferation and effector functions in Th17 cells. Surprisingly, little is known about production of IL-23 during ALI. In this study we found expression of mRNA for IL-23p19 to be 10-fold elevated in lung homogenates of C57BL/6 mice after lipopolysaccharide (LPS)-induced ALI. Likewise, concentrations of IL-23, protein significantly increased in bronchoalveolar lavage fluids. Experiments with IL-23 deficient mice showed that endogenous IL-23 was required for production of IL-17A during LPS-ALI. CD11c-diphtheria toxin receptor transgenic mice were used to selectively deplete CD11c+ cells, the data suggesting that IL-23 production is dependent at least in part on CD11c+ cells during ALI. No alterations of IL-23 levels were observed in Rag-1 deficient mice as compared to wild type C57BL/6 mice following ALI. The mouse alveolar macrophage cell line, MH-S, as well as primary alveolar macrophages displayed abundant surface expression of CD11c. Activation of these macrophages by LPS resulted in release of IL-23 in vitro. Our findings identify CD11c+ macrophages in the lung are likely an important source of IL-23 during ALI, which may be helpful for better understanding of this disease.
Keywords: p19, MH-S cells, Rag-1, IL-17, diphtheria toxin, lipopolysaccharide
INTRODUCTION
It is estimated that 200,000 cases of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) occur annually in the United States (1, 2). Recent advances in supportive critical care medicine (e.g. low tidal volume ventilation) have reduced the severity and overall mortality rates to 20–30% (3–5). However, no FDA-approved treatments to specifically target ALI/ARDS are available to date. The pathogenesis of ALI/ARDS includes a dysregulated inflammatory response leading to a destruction of alveolar barrier and gas exchange functions (3, 6). Events leading to ALI are associated with activation of innate immune signaling pathways (such as toll-like receptors) by microbial products or endogenous ligands, which are released during tissue damage or destruction. Activation of the coagulation system results in deposits of fibrin and platelets in alveoli and adjacent pulmonary capillaries. Leukocytes (macrophages, polymorphonuclear neutrophils) rapidly accumulate in ALI/ARDS lungs (7). Such cells are essential for the release of proinflammatory mediators and other immune effector molecules, thereby driving the course of ALI/ARDS which in some cases may result in pulmonary fibrosis (3).
Interleukin-23 (IL-23) is a heterodimeric cytokine, which is composed of two disulfide-linked subunits, p40 and p19 (8). The subunit p40 is shared with IL-12, and, accordingly, IL-23 is classified as a member of the IL-12 family. IL-23 binds to a receptor consisting of IL-23R and the beta-1 subunit of the IL-12 receptor (IL-12Rβ1) (9, 10). Following engagement of the IL-23 receptor complex, which is highly expressed on T cell subsets, signaling pathways including JAK2, Tyk2, STAT1, STAT3, STAT4 and STAT5 are activated to regulate expression of target genes such as IL-17A, IL-1β, TNFα and IFNγ (11). By these mechanisms IL-23 plays a unique role for the promotion and stabilization of a Th17 cell phenotype. IL-23 is an initiating factor for the secretion of IL-17 by T cells and drives clonal proliferation of Th17 cells (12). While γδ T cells constitutively express the IL-23 receptor (IL-23R) (13), conventional αβ T cells require prior activation with IL-6 or IL-21 to up-regulate IL-23R (12). IL-23 has been described to be involved in the clearance of infectious pathogens, immune responses against malignancies, and development of autoimmune diseases. However, data on the implications of IL-23 during ALI are scant. In this report, we identify CD11c+ macrophages as a source of IL-23-producing cells during LPS-induced ALI in mice.
MATERIALS AND METHODS
Animals
All experiments with animals were in accordance with the U.S. National Institutes of Health guidelines and approved by the University Committee on Use and Care of Animals, University of Michigan. Male mice (8–12 weeks old) of the strains C57BL/6J, Rag-1−/− (B6.129S7-Rag1tm1Mom/J) and CD11c-DTR Tg (B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J) were purchased from the Jackson Laboratories (Bar Harbor, ME). IL-23p19−/− mice were obtained from Dr. G. Huffnagle, University of Michigan. All animals were housed under pathogen-free conditions.
Acute Lung Injury
Mice received a combination of ketamine (100 μg/g body weight, i.p.) and xylazine (8 μg/g body weight, i.p.). Thereafter, surgical access to the trachea was obtained by a 1 cm midline cervical incision. 40 μl sterile PBS containing 40 μg of LPS (E. coli, O111:B4, Sigma-Aldrich, St. Louis, MO) were slowly injected intra-tracheally during inspiration. Sham animals received 40 μl PBS without LPS. Immune complex-induced acute lung injury was done as described before (14). The surgical mortality of the procedures is <10% with typically no deaths following LPS-induced lung inflammation. After wound closure the mice were monitored every 2–4 hours. At the end of the experiments, bronchoalveolar lavages (BAL) were performed by repetitive i.t. injection of 1 ml PBS with approximately 700 μl of retrieval. BAL fluids were cleared of cells by centrifugation. All samples were stored at −80°C until further analysis. Diphtheria toxin for depletion of CD11c+ cells in CD11c-DTR Tg mice was from List Biological Laboratories (Campbell, CA).
Histopathology
Lungs were fixed in 4% formaldehyde solution overnight, paraffin-embedded, and 3 μm sections obtained and stained with hematoxylin & eosin. Microscopic images were obtained using a Zeiss Axio inverted microscope (Carl Zeiss Microscopy, Goettingen, Germany) and digital camera.
Isolation and Culturing of Cells
The SV40 transformed mouse alveolar macrophage cell line, MH-S, was a gift from Dr. J. Weinberg, University of Michigan (15). MH-S cells were maintained in RPMI 1640 media (25 mM HEPES together with 100 units/ml penicillin-streptomycin and 10% fetal calf serum) at 37°C with 5% CO2. MH-S cells were transferred to 24-well tissue culture plates 2–3 days before use in experiments.
For collection of primary alveolar macrophages, multiple bronchoalveolar lavages of lungs (20 × 1 ml) were done using ice-cold PBS plus 0.5 mM EDTA. Cells (2x105 cells/sample) were cultured in RPMI 1640 media (25 mM HEPES with 0.1% BSA and 100 units/ml penicillin-streptomycin) at 37°C in 5% CO2.
PMNs were obtained by peritoneal lavage (HBSS) following two injections with casein solution i.p. (9% w/v, casein sodium salt from bovine milk, Sigma) at -12h and -3h. PMNs were resuspended in RPMI 1640 (25 mM HEPES, 100 units/ml penicillin-streptomycin, 0.1% BSA) and plated at 5×106 cells/ml.
MLE-12 mouse type II like alveolar epithelial cells were a gift from Dr. J. Weinberg, University of Michigan, and were cultured in RPMI1640 (10% FCS, 100 U/ml penicillin-streptomycin, 10 nM β-estradiol, 10 nM hydrocortisone, 1x Insulin-Transferrin-Selenite, 5 μg/ml transferrin, 10 mM HEPES, 2 mM L-glutamine).
ELISA
Specific detection of IL-23, IL-17A and IL-6 was accomplished by sandwich ELISA according to instructions of the manufacturer (R&D Systems, Minneapolis, MN). The IL-23 ELISA exhibits no cross-reactivity or interference with mouse IL-12 p35 or IL-12/IL-23 p40.
Real Time PCR
Total RNA was extracted from mouse lung homogenates by the Trizol method. The TaqMan Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) and a GeneAmpR PCR System 9700 (Applied Biosystems) was used for generation of cDNA. PCRs were performed with the SYBR Green Mastermix and a 7500 Real Time PCR System (Applied Biosystems). Results were analyzed by the 2−ddCt method with normalization to GAPDH. The following primers were used (Life Technologies): mIL-23p19 (forward) 5′-AATAATGTGCCCCGTATCCAG-3′; mIL-23p19 (reverse) 5′-GAAGATGTCAGAGTCAAGCAG-3′ ; mGAPDH (forward) 5′-TACCCCCAATGTGTCCGTCGTG-3′; mGAPDH (reverse) 5′-CCTTCAGTGGGCCCTCAGATGC-3′.
Flow Cytometry
Flow cytometry staining protocols were done as described elsewhere (16). Briefly, cells were incubated for 30 min on ice with the following antibodies (all from eBioscience, San Diego, CA): anti-mouse CD11c-PE (clone N418), anti-mouse CD45-eFluor450 (clone 30-F11), anti-mouse CD11b-AF700 (clone M1/70), anti-mouse F4/80-APC (clone BM8) and matched fluorochrome labeled isotype controls. Cells were fixed in 2% paraformaldehyde solution. Data were acquired on a BD LSR II instrument (BD Biosciences, Franklin Lakes, NJ) and analyzed using FlowJo version X (Treestar).
Statistical Analysis
All values are expressed as mean. Error bars represent SEM. Data sets were analyzed by two-tailed Student t test (GraphPad Prism 5.04 Software) and considered significant when P < 0.05. Representative results of 2–3 independent experiments with each sample in duplicates or triplicates are shown. For in vivo experiments the numbers of mice per group are indicated in the figure legends.
RESULTS
Expression and Release of IL-23 during LPS-induced ALI
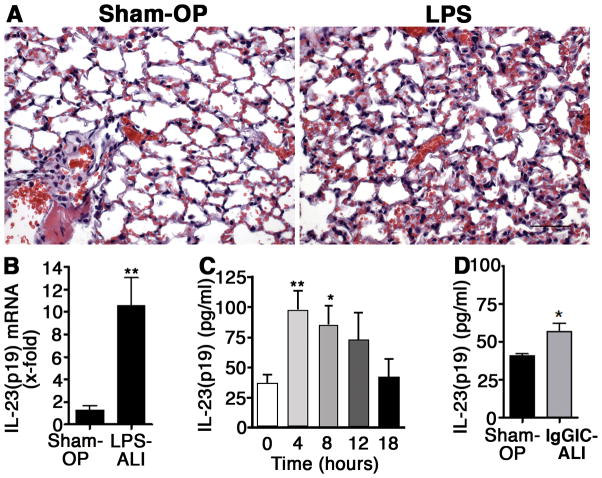
To investigate the expression of IL-23 during the acute inflammatory response in lungs, we used the model of LPS-induced ALI described above. LPS was slowly injected into the trachea of C57BL/6 mice and sham mice only received PBS. The histopathology of LPS-ALI is characterized by infiltration of alveolar spaces with PMNs, fibrin, edema and intra-alveolar hemorrhage (Fig. 1A). Following 8 h after LPS-ALI the lungs were collected and IL-23(p19) gene expression was analyzed in lung homogenates by RT-PCR (Fig. 1B). Expression of mRNA for IL-23(p19) was 10-fold enhanced above baseline levels during LPS-ALI. Concentrations of IL-23 were assessed in bronchoalveolar lavage fluids (BALF) at the end of LPS-ALI experiments (Fig. 1C). Low concentrations of IL-23 were detectable in lungs from sham mice (0 h). After 4 h in LPS-activated lungs, a 3–4-fold increase in levels of IL-23 in BALF was observed. IL-23 remained elevated at 8 h following LPS-ALI (Fig. 1C). When IL-23 was measured in BALF during immune-complex-induced ALI (IgGIC-ALI, a model dependent on complement activation rather than TLR4 activation), only minimal elevations of IL-23 were detected (Figure 1D). In addition, there was no induction of IL-17A after IgGIC-ALI (data not shown). Hence, we focused our further studies using the LPS-induced ALI model, where significant amounts of IL-23 were present in the BALF.
Figure 1.
Expression and release of IL-23(p19) during acute lung injury (ALI). A, Microscopic images of the alveolar compartment from normal lungs 8 h after sham operation (Sham-OP, 40 μl PBS i.t.) or 8 h after LPS-induced ALI (40 μg in 40 μl i.t.), H&E stains, x40, scale bar: 50 μm, n=3/group. B, Real time PCR for IL-23(p19) mRNA in lung homogenates of C57BL/6 mice 8 h after LPS- ALI or Sham-OP, n=4/group. C, Time course for presence of IL-23(p19) in BAL fluids after LPS-ALI in C57BL/6 mice (n=5/group), ELISA. D, Detection of IL-23(p19) in BAL fluids 8 h after Sham-OP (n=6) or immune complex-induced ALI (IgGIC-ALI; n=7). * P < 0.05, ** P < 0.01.
LPS-ALI: Albumin Leak and BALF IL-17A in Wt and IL-23−/− Mice
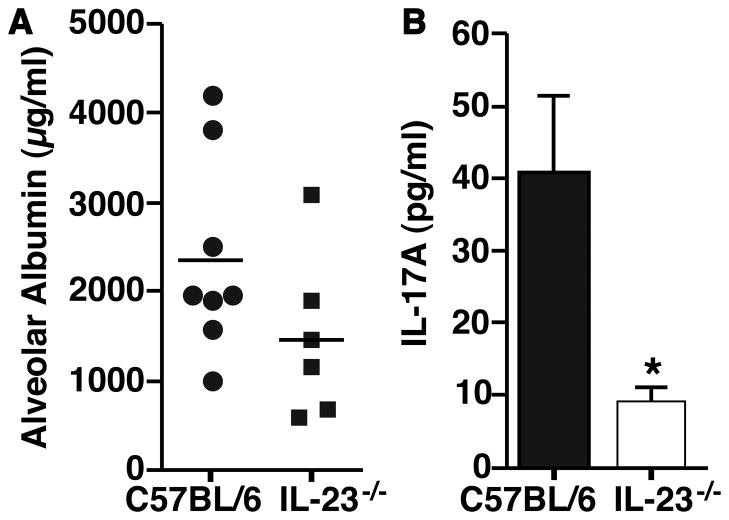
To study the biological effects of IL-23 during LPS-ALI, we used mice with genetic deficiency of the p19 subunit of IL-23. The alveolar albumin leakage reflects compromised endothelial/epithelial alveolar cell barrier, and was modestly reduced (P=0.14; Fig. 2A) when compared to Wt mice. Next, the concentrations of IL-17A in BALF from IL-23−/− mice were measured with C57BL/6 mice serving as controls (Fig. 2B). IL-17A was significantly reduced (40 pg/ml vs. 10 pg/ml) in BALF of IL-23−/− mice after LPS-ALI (Fig. 2B).
Figure 2.
Effects of genetic deficiency of IL-23 during LPS-ALI. A, LPS-ALI was performed with IL-23−/− mice (n=6) or C57BL/6 mice (n=8) as controls with measurements of albumin leakage after 10 h (student’s t test, P=0.14). B, Detection of IL-17A concentrations in BALF from the same experiments as in frame A, ELISA. * P < 0.05.
Reduction of IL-23 by Specific Depletion of CD11c+ Cells during ALI
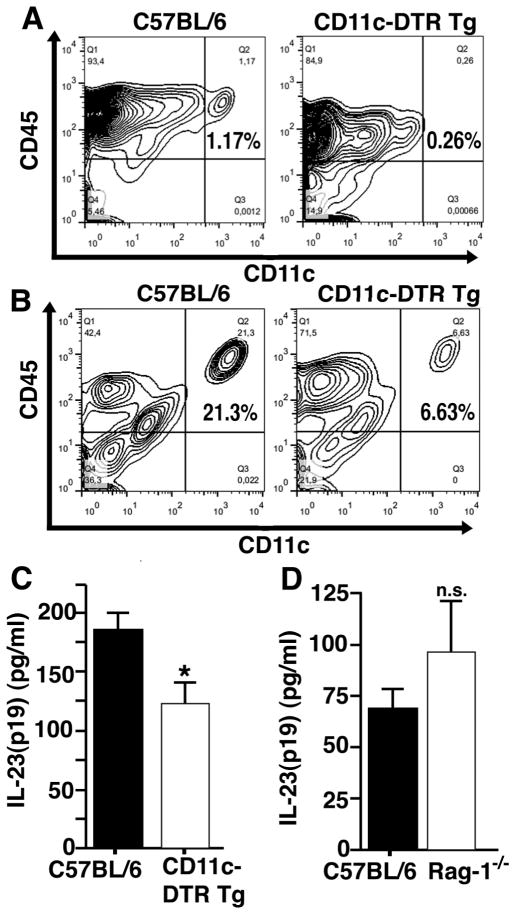
To characterize the possible cellular source(s) of IL-23 during LPS-induced ALI, we sought to specifically deplete CD11c+ cells using CD11c-DTR transgenic mice. In this strain the expression of CD11c is coupled to the diphtheria toxin receptor. Wildtype mice do not express a diphtheria toxin receptor and therefore are highly resistant to diphtheria toxin. Injection of diphtheria toxin 24 h prior to experiments with CD11c-DTR Tg mice has been reported to deplete CD11c+ cells, whereas no such phenomenon is observed in C57BL/6 wildtype mice (controls) receiving the same treatment with diphtheria toxin (17, 18).
We confirmed depletion of CD11c+ cells in spleens from CD11c-DTR Tg mice 24 h after diphtheria toxin using flow cytometry staining for CD11c and the leukocyte common antigen, CD45 (Fig. 3A). In BALF after LPS-ALI using CD11c-DTR Tg mice the frequency of CD11c+ cells was partially reduced (21.3% vs. 6.63%, Fig. 3B). The depletion of CD11c+ cells in CD11c-DTR Tg mice in the alveolar compartments during LPS-ALI resulted in lower concentrations of IL-23 in BALF after 8 h as compared to C57BL/6 mice (Fig. 3C). This suggested that CD11c+ cells are a source of IL-23 in ALI. On the contrary, no significant changes in the concentrations of IL-23 were seen in experiments with Rag-1−/− mice, which are deficient in both T and B cells and would have few Th17 cells (Fig. 3D). Reasons for only partial depletion of IL-23 in the CD11c-DTR Tg mice given diphtheria toxin could be the inadequate depletion of CD11c+ cells in lung or other cell types also being involved in IL-23 production.
Figure 3.
CD11c+ cells are a source of IL-23(p19) during acute lung injury. A, CD11c-diphtheria toxin receptor transgenic mice (CD11c-DTR Tg) and C57BL/6 mice received diphtheria toxin (4 ng/g body weight i.t. together with 25 ng/g body weight i.p.) 24 h before LPS-ALI. Splenocytes were harvested 8 h after LPS-ALI and stained for CD45 and CD11c by flow cytometry. B, Cells in BALF from C57BL/6 and CD11c-DTR Tg mice were stained for CD45 and CD11c after LPS-ALI in mice from the same experiment as described above. C, Detection of IL-23(p19) by ELISA in BALF 8 h after LPS-ALI with depletion of CD11c+ cells in CD11c-DTR Tg (n=4) mice as compared to C57BL/6 mice (n=8) as controls. Both groups had received treatments with diphtheria toxin as described above. D, Comparison of IL-23(p19) concentrations in BAL fluids 8 h after LPS-ALI in Rag-1−/− mice (n=5, deficient in T and B cells) and C57BL/6 mice (n=7).* P < 0.05, n.s. indicates not significant.
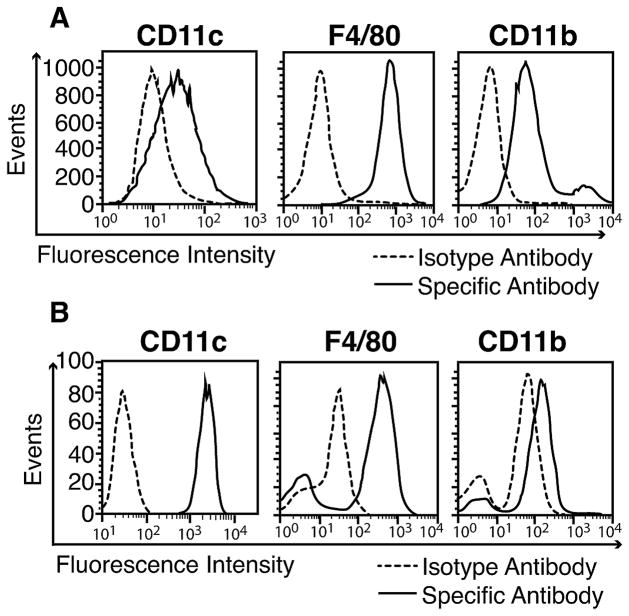
Phenotyping of MH-S Cells and Primary Alveolar Macrophages
To further study IL-23 production we first used MH-S macrophages which are an SV40 immortalized cell line derived from mouse lung alveolar macrophages. MH-S macrophages (esterase positive, peroxidase negative) retain many of the properties of alveolar macrophages including typical macrophage morphology, adherence and phagocytosis (15). Flow cytometric phenotyping of MH-S cells showed a clear expression of macrophage surface markers CD11c, F4/80 and CD11b when compared to isotype control antibodies (Fig. 4A). Next, primary alveolar macrophages were obtained by BAL from untreated healthy C57BL/6 mice. The purity of such preparations of nucleated cells was typically >98% macrophages as evaluated by light microscopy (19). We confirmed that these primary alveolar macrophages displayed a high surface expression of the CD11c marker together with expression of F4/80 and CD11b (CD11chighF4/80highCD11blow (20)). This phenotype is typical for alveolar macrophages as compared to tissue macrophages from the peritoneum (CD11clowF4/80highCD11bhigh) and other organs (data not shown).The higher abundancy of CD11c as compared to F4/80 and CD11b in mice make it an ideal target in cell depletion experiments (Fig. 3).
Figure 4.
Phenotyping of mouse alveolar macrophages. A, MH-S macrophages were stained with anti-CD11c, anti-F4/80 and anti-CD11b antibodies while control cells were exposed to fluorochrome labeled isotype antibodies. Cells were then analyzed by flow cytometry. B, Primary alveolar macrophages were obtained from normal C57BL/6 mice by broncho-alveolar lavage. The abundancy of CD11c, F4/80 and CD11b surface markers was detected by flow cytometry as described above. Data are representative of 2 independent experiments.
In Vitro Production of IL-23 by MH-S Cells and Primary Alveolar Macrophages
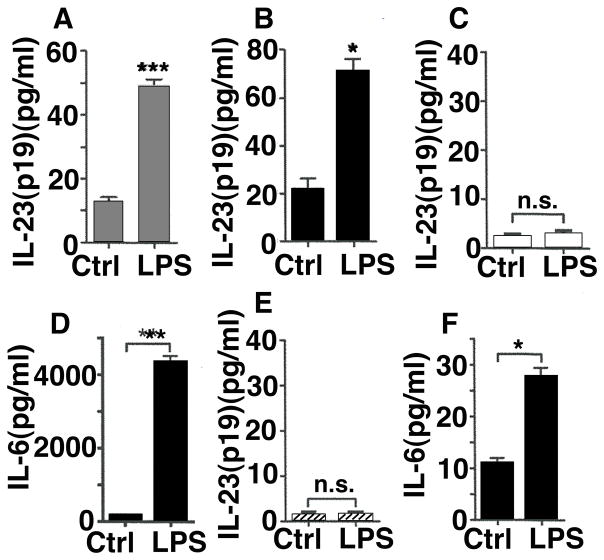
Since MH-S cells and primary alveolar macrophages were phenotyped as CD11c+ (Fig. 4) and depletion of CD11c during LPS-induced ALI reduced the production of IL-23 (Fig. 3), we measured the in vitro release of IL-23 by MH-S macrophages and primary alveolar macrophages. After in vitro incubation of MH-S with LPS the concentrations of IL-23 in cell culture supernatant fluids increased 4–5-fold after 12 h (Fig. 5A). Similar results were observed, when primary alveolar macrophages were activated with LPS in vitro (Fig. 5B).
Figure 5.
Production of IL-23(p19) by alveolar macrophages, but not PMNs or alveolar epithelial cells, in vitro. A, MH-S macrophages were untreated (Ctrl) or incubated with LPS for 12 h followed by detection of IL-23 by ELISA in cell culture supernatants. B, Primary alveolar macrophages were obtained by broncho-alveolar lavage from healthy C57BL/6 mice and activated with LPS for 24 h in vitro with IL-23 detection. C, PMNs (C57BL/6) were incubated with LPS for 6 h followed by analysis of IL-23. D, Detection of IL-6 from PMNs, 6h. E, MLE-12 mouse type II like alveolar epithelial cells as unstimulated controls or in the presence of LPS with undetectable IL-23 after 20 h. F, Detection of IL-6 from MLE-12 cells, 20 h. LPS was used as 1 μg/ml in all experiments and all results were obtained by ELISA. * P < 0.05, ** P < 0.01, *** P < 0.001, n.s. indicates not significant. Data represent 3 independent experiments.
When PMNs from C57BL/6 (Wt) mice were incubated with LPS, IL-23 in supernatant fluids was extremely low (Fig. 5C). PMNs were confirmed to be responsive to LPS by observing abundant release of IL-6 (Fig. 5D). Similarly, the mouse alveolar epithelial cell line, MLE-12, failed to generate IL-23 despite IL-6 production in response to LPS (Fig. 4E, 4F). Collectively, our findings suggest that CD11c+ alveolar macrophages (rather than PMNs or the alveolar epithelium) are a source of IL-23 during experimental ALI, induced by LPS.
DISCUSSION
Our data provide clear evidence for expression and release of IL-23 in lungs following intra-tracheal challenge with LPS. Bronchoalveolar lavages with 1 ml of instilled PBS per mouse typically resulted in substantial dilution of the intra-alveolar exudate. The concentrations of IL-23 found in BALF (100–200 pg/ml) in our experiments are in a comparable range as observed for other inflammatory mediators (e.g. IFNγ, IL-12) in LPS-induced ALI (21). We observed clearly reduced concentrations of IL-17A in IL-23−/−mice in the setting of LPS-ALI, which is consistent with the well established role of IL-23 as an IL-17-inducing factor in other experimental models (22, 23).
Depletion of CD11c+ macrophages significantly reduced (by 34%) the BALF levels of IL-23 after LPS-ALI. While CD11c (integrin αX) may play a redundant role in leukocyte adhesive interactions, it was used in this study to identify and target the cell population of alveolar macrophages. The transgenic mouse strain used in our experiments (CD11c DTR Tg) has been described by others to display a 1 log reduction in numbers of CD11c+ cells for 2 days following treatment with diphtheria toxin, after which counts of CD11c+ cells were gradually restored (18). In our study, we observed a less efficient cell depletion in the alveolar compartment. CD11c is widely considered a surface marker for dendritic cells but also alveolar macrophages. Notably, primary mouse alveolar macrophages display a unique phenotype, which includes expression of much higher amounts of CD11c as compared to macrophages in other tissues in which they are predominantly CD11b/CD18 positive. It has been suggested that these differences are explained by higher levels of GM-CSF in lungs and may potentially also be due to the presence of surfactant protein D (24). Alveolar macrophages are the major antigen-presenting cell type in lungs. During ALI, these cells have essential functions for initiation of the acute inflammatory response by release of proinflammatory cytokines and chemokines to direct polymorphonuclear leukocytes (PMNs), T cells and other macrophages. Later stages of ALI depend on the phagocytosis of debris and apoptotic PMNs (efferocytosis) by alveolar macrophages to facilitate resolution of inflammatory responses (25, 26). We observed a release of IL-23 by isolated primary alveolar macrophages and MH-S cells after in vitro stimulation with LPS. It is well established that IL-23 can be produced by dendritic cells from various organs and thioglycollate-elicited macrophages from the peritoneum (8, 19). While little IL-17 production was found following severe injuries (non-infectious trauma) (27), the IL-23-induced cytokine, IL-17, exacerbates production of mucus and mediators following lung infection with respiratory syncytial virus (28). IL-23 aggravates the severity of T cell-dependent colitis in a mouse model of inflammatory bowel disease (29). Patients with psoriasis vulgaris have increased expression of IL-23 in skin lesions. Antibodies to neutralize IL-23 in vivo have recently been used as a novel therapy (30, 31). Treatment with IL-23 decreases the proliferation of malignant cells isolated from patients with leukemia (B-ALL) (32). Studies with p19 deficient mice showed that IL-23 is essential during the development of experimental autoimmune encephalitis (33). Furthermore, IL-23 is involved in the pathogenesis of endotoxic shock (34). In an elegant study, transgenic over-expression of p19 in mice has been described to induce severe systemic inflammation and death before 3 months of age (35). Collectively, these reports indicate that IL-23 is expressed in a variety of pathologic conditions and that IL-23 can be linked to disease induction. Our findings that IL-23 is produced by CD11c+ macrophages in lungs suggest the need for further investigation of the role of this cytokine during experimental ALI. Future studies may also aim to measure IL-23 levels in BALF samples from humans developing ALI.
In summary, our results demonstrate gene expression and release of IL-23 in lungs during experimental ALI, induced by LPS. In mice, a relevant cellular source of IL-23 appears to be CD11c+ alveolar macrophages. Such cells also produce significant amounts of IL-23 after LPS incubation in vitro.
Acknowledgments
We thank Dr. Gary B. Huffnagle (University of Michigan, Ann Arbor) for providing the IL-23p19−/− mice for this study. We would like to thank Beverly Schumann, Sue Scott and Robin Kunkel for excellent assistance in the preparation of the manuscript, as well as Mikel D. Haggadone for technical assistance.
This study was supported by grants from the National Institutes of Health, USA (GM-29507, GM-61656 to P.A.W.), the Deutsche Forschungsgemeinschaft (Project 571701, BO 3482/1-1 to M.B.), by the Federal Ministry of Education and Research (BMBF, 01EO1003 to M.B.) and the Boehringer Ingelheim Fonds (to N.F.R. and R.R.). The authors are responsible for the contents of this publication.
Footnotes
The authors have no commercial or financial conflicts of interests.
References
- 1.Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Critical care medicine. 2003;31:1607–11. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. The Journal of clinical investigation. 2012;122:2731–40. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 5.Su F, Nguyen ND, Creteur J, Cai Y, Nagy N, Anh-Dung H, Amaral A, Bruzzi de Carvalho F, Chochrad D, Vincent JL. Use of low tidal volume in septic shock may decrease severity of subsequent acute lung injury. Shock. 2004;22:145–50. doi: 10.1097/01.shk.0000131488.89874.8a. [DOI] [PubMed] [Google Scholar]
- 6.Bosmann M, Ward PA. Role of C3, C5 and anaphylatoxin receptors in acute lung injury and in sepsis. Adv Exp Med Biol. 2012;946:147–59. doi: 10.1007/978-1-4614-0106-3_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao H, Neff T, Ward PA. Regulation of lung inflammation in the model of IgG immune-complex injury. Annual review of pathology. 2006;1:215–42. doi: 10.1146/annurev.pathol.1.110304.100155. [DOI] [PubMed] [Google Scholar]
- 8.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 9.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–42. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 10.Bosmann M, Ward PA. Therapeutic potential of targeting IL-17 and IL-23 in sepsis. Clinical and Translational Medicine. 2012;1:1–5. doi: 10.1186/2001-1326-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lankford CS, Frucht DM. A unique role for IL-23 in promoting cellular immunity. Journal of leukocyte biology. 2003;73:49–56. doi: 10.1189/jlb.0602326. [DOI] [PubMed] [Google Scholar]
- 12.Croxford AL, Mair F, Becher B. IL-23: one cytokine in control of autoimmunity. Eur J Immunol. 2012;42:2263–73. doi: 10.1002/eji.201242598. [DOI] [PubMed] [Google Scholar]
- 13.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Bosmann M, Grailer JJ, Zhu K, Matthay MA, Sarma JV, Zetoune FS, Ward PA. Anti-inflammatory effects of beta2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 2012;26:2137–44. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mbawuike IN, Herscowitz HB. MH-S, a murine alveolar macrophage cell line: morphological, cytochemical, and functional characteristics. Journal of leukocyte biology. 1989;46:119–27. doi: 10.1002/jlb.46.2.119. [DOI] [PubMed] [Google Scholar]
- 16.Bosmann M, Russkamp NF, Patel VR, Zetoune FS, Sarma JV, Ward PA. The outcome of polymicrobial sepsis is independent of T and B cells. Shock. 2011;36:396–401. doi: 10.1097/SHK.0b013e3182295f5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett CL, Clausen BE. DC ablation in mice: promises, pitfalls, and challenges. Trends in immunology. 2007;28:525–31. doi: 10.1016/j.it.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosmann M, Sarma JV, Atefi G, Zetoune FS, Ward PA. Evidence for anti-inflammatory effects of C5a on the innate IL-17A/IL-23 axis. FASEB J. 2011 doi: 10.1096/fj.11-199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, Frelinger JA, Kawula TH. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensisSchu S4, LVS, or U112. Infect Immun. 2008;76:5843–52. doi: 10.1128/IAI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosmann M, Russkamp NF, Ward PA. Fingerprinting of the TLR4-induced acute inflammatory response. Exp Mol Pathol. 2012;93:319–23. doi: 10.1016/j.yexmp.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–7. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nature Reviews Immunology. 2009;9:556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2009;296:L936–46. doi: 10.1152/ajplung.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bratton DL, Henson PM. Neutrophil clearance: when the party is over, clean-up begins. Trends in immunology. 2011;32:350–7. doi: 10.1016/j.it.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med. 2011;184:547–60. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frangen TM, Bogdanski D, Schinkel C, Roetman B, Kalicke T, Muhr G, Koller M. Systemic IL-17 after severe injuries. Shock. 2008;29:462–7. doi: 10.1097/shk.0b013e3181598a9d. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee S, Allen RM, Lukacs NW, Kunkel SL, Carson WFt. STAT3-Mediated IL-17 Production by Postseptic T Cells Exacerbates Viral Immunopathology of the Lung. Shock. 2012;38:515–23. doi: 10.1097/SHK.0b013e31826f862c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. The Journal of clinical investigation. 2006;116:1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, Dhodapkar M, Krueger JG. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. The Journal of experimental medicine. 2004;199:125–30. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, Guzzo C, Xia Y, Zhou B, Li S, Dooley LT, Goldstein NH, Menter A. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–28. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- 32.Cocco C, Canale S, Frasson C, Di Carlo E, Ognio E, Ribatti D, Prigione I, Basso G, Airoldi I. Interleukin-23 acts as antitumor agent on childhood B-acute lymphoblastic leukemia cells. Blood. 2010;116:3887–98. doi: 10.1182/blood-2009-10-248245. [DOI] [PubMed] [Google Scholar]
- 33.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 34.Belladonna ML, Vacca C, Volpi C, Giampietri A, Fioretti MC, Puccetti P, Grohmann U, Campanile F. IL-23 neutralization protects mice from Gram-negative endotoxic shock. Cytokine. 2006;34:161–9. doi: 10.1016/j.cyto.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Wiekowski MT, Leach MW, Evans EW, Sullivan L, Chen SC, Vassileva G, Bazan JF, Gorman DM, Kastelein RA, Narula S, Lira SA. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. Journal of immunology (Baltimore, Md : 1950) 2001;166:7563–70. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]