Abstract
Introduction
Midazolam, a benzodiazepine, has a hypnotic effect and is widely used as an intravenous sedative. Past studies have clearly established that midazolam has beneficial effects in attenuating ischemia-reperfusion injury more than other currently used sedative drugs. However, the role of midazolam on chondroprotection via inhibition of matrix metalloproteinases (MMPs) is warrant investigation. The aim of this study was to examine the mechanisms of action of midazolam on MMP expression via nuclear factor κB (NF-κB) signaling in activated chondrosarcoma cells maintained in vitro.
Material and methods
Chondrocytes, SW1353 cells, were stimulated with phorbol 12-myristate 13-acetate (PMA) in the absence or presence of various concentrations of midazolam (5-20 µM). Release of MMP-9 into the culture media was determined by gelatin zymography. The expressions of MMP-1, MMP-9 and MMP-13, phosphorylation of extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinases and degradation of IκB-α were determined by western blotting assay.
Results
Midazolam significantly down-regulated PMA-induced MMP-9 protein expression at concentrations of 5, 10 and 20 µM, the values were 1.95 ±0.09 (p < 0.01), 1.71 ±0.12 (p < 0.01) and 1.35 ±0.20 (p < 0.001), respectively. At concentrations of 5, 10 and 20 µM, it was significantly inhibited the PMA-induced expressions of MMP-1 (2.27 ±0.10, 1.98 ±0.11 and 1.56 ±0.15; p < 0.001) and MMP-13 (0.89 ±0.04, 0.81 ±0.07, and 0.74 ±0.09; p < 0.001), respectively. Midazolam at concentrations of 10 and 20 µM for 15 min significantly reversed the rate of degradation (0.895 ±0.051; p < 0.05 and 0.926 ±0.060; p < 0.01, respectively) of IκB-α in PMA-chondrocyte cells. In addition, this sedative drug inhibited PMA-induced levels of phos-ERK (1.243 ±0.12, 1.108 ±0.16 and 0.903 ±0.19, respectively) and phos-p38 (1.146 ±0.10, 1.063 ±0.13 and 0.946 ±0.18, at concentrations of (5, 10 and 20 µM), respectively.
Conclusions
These results are important for understanding the mechanism of midazolam in inhibiting PMA-induced MMP expression through the signaling pathways of either NF-κB or ERK/p38 MAPKs down-regulation.
Keywords: human chondrosarcoma cells, midazolam, phorbol 12-myristate 13-acetate, matrix metalloproteinases, mitogen-activated protein kinases, IκB-α
Introduction
Osteoarthritis (OA) is a joint disease that involves degeneration of the articular cartilage and the formation of bone at the joint surface and margins. Although OA differs from rheumatoid arthritis (RA) in that it is not a systemic disease, synovitis in OA does occur, although more frequently in the advanced stages. Osteoarthritis cartilage is a rich source of inflammatory mediators, including cytokines, nitric oxide, and prostaglandins [1]. A role for proinflammatory cytokines in the pathogenesis of OA was proposed based on the data from studies using human OA cartilage and various animal models [2]. Cytokine production associated with degenerative changes in OA cartilage has been reported in a study [3], in which interlekin-1α (IL-1β) and tumour necrosis factor-α (TNF-α) were shown to co-localize with matrix metalloproteinases in the superficial zones of articular cartilage. Phorbol 12-myristate 13-acetate (PMA) was reported to be involved in expression of matrix metalloproteinases (MMPs) in activated human chondrocytes [4].
The metalloproteinases are a large group of enzymes that play a crucial role in tissue remodeling as well as in the destruction of cartilage and bone in an arthritic joint due to their ability to degrade a wide variety of extracellular matrix (ECM) components [5]. These proteolytic enzymes attack and degrade components of the extracellular matrix. The MMPs involved in endochondral ossification are: the collagenases, MMP-1 and MMP-13; the gelatinases, MMP-2 and MMP-9 (gelatinase A and B, respectively); the stromelysins, MMP-3 and MMP-10; and the membrane-type MMP-14 [6]. Inflammatory cytokines, such as IL-1β and TNF-α, have been reported to stimulate inducible expression of MMPs (1, 3, 9 and 13) in cartilage [7]. MMP-1 (collagenase-1) is expressed ubiquitously and is found in various cells, including chondrocytes [8]. MMP-13 (collagenase-3) has long been regarded as the major source of collagen degrading activity, since it has preferential capacity to degrade type II collagen [9]. Our recent study has demonstrated that PMA, as a protein kinase C (PKC) activator, stimulates human chondrocytes via expression of matrix metalloproteinases (MMPs) [10]. One study has well described that the mode of PKC mediated signaling involves transmission of signals from PKC to mitogen-activated protein kinases (MAPKs).
It is also well documented that the MAP kinase family members including extracellular signal-regulated kinase (ERK), p38 MAP kinases and c-Jun N-terminal kinase (JNK) are known to be activated in human chondrocytes [11]. IκB-α is reported to play a significant role in arthritis patients due to its inhibition of MMP-1 and MMP-13 production [12]. A variety of inhibitors of the MMPs are currently being used. However, adverse drug effects, particularly gastrointestinal ulceration, are commonly associated with these agents. Therefore, currently there is much interest in developing more effective and physiologic approaches such as therapeutic use of biological agents that block the activity of MMPs.
Of the benzodiazepines (BDZ), midazolam is the most commonly administered intravenous sedative in clinical practice and in intensive care medicine. The analgesic property of midazolam has been reported to be mediated via the γ-aminobutyric acid (GABA) A receptor in the spinal cord [13]. Furthermore, both human and animal models have shown ubiquitous distribution of peripheral BDZ receptors (PBRs) in various organs and in joints [14]. A study has also proved that PBR is highly localized in chondrocytes [15]. Midazolam has gained widespread use in cardiac surgery; its cardioprotective effects have been increasingly recognized. Experimental and clinical studies have demonstrated that midazolam attenuated myocardial ischemia-reperfusion injury [16]. Although midazolam has more beneficial effects than other currently used sedative drugs [17], its effects on chondrocytes have not been investigated at the cellular or molecular levels.
Hence, the aim of this study was to use an in vitro model of human chondrocytes to study the effects of midazolam on MMP expression and test the hypothesis that midazolam antagonizes the effect of PMA by suppressing MAPK expression.
Material and methods
Material
Midazolam, sodium dodecyl sulfate (SDS), phenylmethylsulfonyl fluoride (PMSF), leupeptin, aprotinin, sodium fluoride, sodium orthovanadate, sodium pyrophosphate, diethyl pyrocarbonate (DEPC), phorbol 12-myristate 13-acetate (PMA), and bovine serum albumin (BSA) were all purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-mouse and anti-rabbit immunoglobulin G (IgG)-conjugated horseradish peroxidase (HRP) was purchased from Amersham Biosciences (Sunnyvale, CA) and/or Jackson-Immuno Research (West Grove, PA). A mouse monoclonal antibody (mAb) specific for human native 92-kDa MMP-9 was purchased from LabVision/NeoMarkers (Fremont, CA). A rabbit polyclonal antibody (pAb) specific for IκB-α was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-p38 MAPK and anti-phospho-c-Jun N-terminal kinase (JNK) (Thr183/Tyr185) mAbs, and the anti-phospho p44/p42 extracellular signal-regulated kinase (ERK) (Thr202/ Tyr204) polyclonal antibody were from Cell Signaling (Beverly, MA, USA); and the Hybond-P PVDF membrane, ECL Western blotting detection reagent and analysis system were from Amersham (Buckinghamshire, UK). All other chemicals used in this study were of reagent grade. Midazolam was dissolved in 0.5% dimethyl sulfoxide (DMSO) and stored at 4°C until use.
Cell cultivation
Human chondrosarcoma cells, SW1353, were obtained from American Type Culture Collection (Manassas, VA). Cells were cultured in Ham's F-12 and Dulbecco's Modified Eagle Medium (DMEM) (1: 1) supplemented with L-glutamine (3.65 mM), penicillin (90 U/ml), streptomycin (90 µg/ml), HEPES (18 mM), NaHCO3 (23.57 mM), and 10% heat-inactivated fetal bovine serum (FBS) at 37°C in humidified air with 5% CO2.
Stimulation experiments
For stimulation of PMA, chondrosarcoma SW1353 cells were seeded at 2.5 × 106 per well of Costar 6-well tissue culture plates in complete media until a confluence of 85% was reached (usually for 24 h). After 24 h, cells were changed to serum free media. Cells were treated with midazolam (5-20 µM) for 15 min after 24 h of changing serum free media and then treated with PMA (10 ng/ml) for an another 24 h. At the end of the incubation period, conditioned medium and cell supernatants were collected and stored at –80°C for gelatin zymography and Western blotting assay, respectively.
Cell viability assay
The cytotoxic effects of midazolam against the SW1353 cell line were determined by the MTT method as described previously [18]. Briefly, cells (2 × 106 cells/ml) were incubated in 12-well plates with different concentrations (5-30 µM) of midazolam for 24 h at 37°C. At the 22nd h, the MTT solution was added to each well at a final concentration of 0.5 mg/ml. After 2-h incubation at 37°C, the supernatant was discarded and replaced with DMSO to dissolve the formazan product, which was measured at 550 nm in a spectrophotometric plate reader. The following formula was used to calculate the % cell viability: Percentage cell viability = (absorbance of the experiment samples/absorbance of the control) × 100%.
Gelatin zymography
The expression of MMP-9 was detected by gelatin zymography as described by Chung et al. [19]. The conditioned medium was mixed with non-reducing buffer (500 mM Tris-HCl, 25% glycerol, 10% SDS, and 0.32% bromophenol blue; pH 6.8) and electrophoresed in 10% polyacrylamide gel containing gelatin (1 mg/ml). After electrophoresis, the gels were washed 2 times with 2.5% Triton X-100 to remove the SDS and then incubated with reacting buffer containing 50 mM Tris-base, 200 mM NaCl, 5 mM CaCl2, and 0.02% Brij 35 (pH 7.5) for 17 h in a closed container at 37°C. At the end of the incubation, the gels were fixed with a fixing solution (7% acetic acid and 40% methanol, v/v) for 30 min. Gels were stained with a solution of Colloidal Brilliant Blue G in 27% methanol for 30 min or longer. Finally, a destaining solution (10% acetic acid in 25% methanol) was used to adjust the clear conditions. Clear zones (bands) against the blue background indicated the presence of degradative activity of MMP-9.
SDS-polyacrylamide gel electrophoresis and Western blot analysis
Western blot analyses were performed as previously described [18]. Lysates from each sample were mixed with 6× sample buffer (0.35 M Tris, 10% w/v SDS, 30% v/v glycerol, 0.6 M DTT, and 0.012% w/v bromophenol blue, pH 6.8) and heated to 95°C for 5 min. Proteins were separated by electrophoresis and transferred onto nitrocellulose membranes (for MMP-9) and polyvinylidene difluoride (PVDF) membranes (for MMP-1/-13, p38, pERK1/2, c-JUN and IκB-α). The membranes were blocked with 5% non-fat milk in TBS-0.1% Tween 20 and sequentially incubated with primary antibodies and HRP-conjugated secondary antibodies, followed by enhanced chemiluminescence (ECL) detection (Amersham Biosciences). The BIO-PROFIL Bio-1D light analytical software (Vilber Lourmat, Marue La Vallee, France) was used for the quantitative densitometric analysis. Data of specific protein levels are presented as relative multiples in relation to the control.
Statistical analysis
The experimental results are expressed as the mean ± SEM and are accompanied by the number of observations. For analysis of the results, a one-way analysis of variance (ANOVA) test was performed using the Sigma Stat v3.5 software. When group comparisons showed a significant difference, the Student-Newman-Keuls test was used. A value of p less than 0.05 was considered statistically significant.
Results
Effects of midazolam on MMP-9 activation and expression
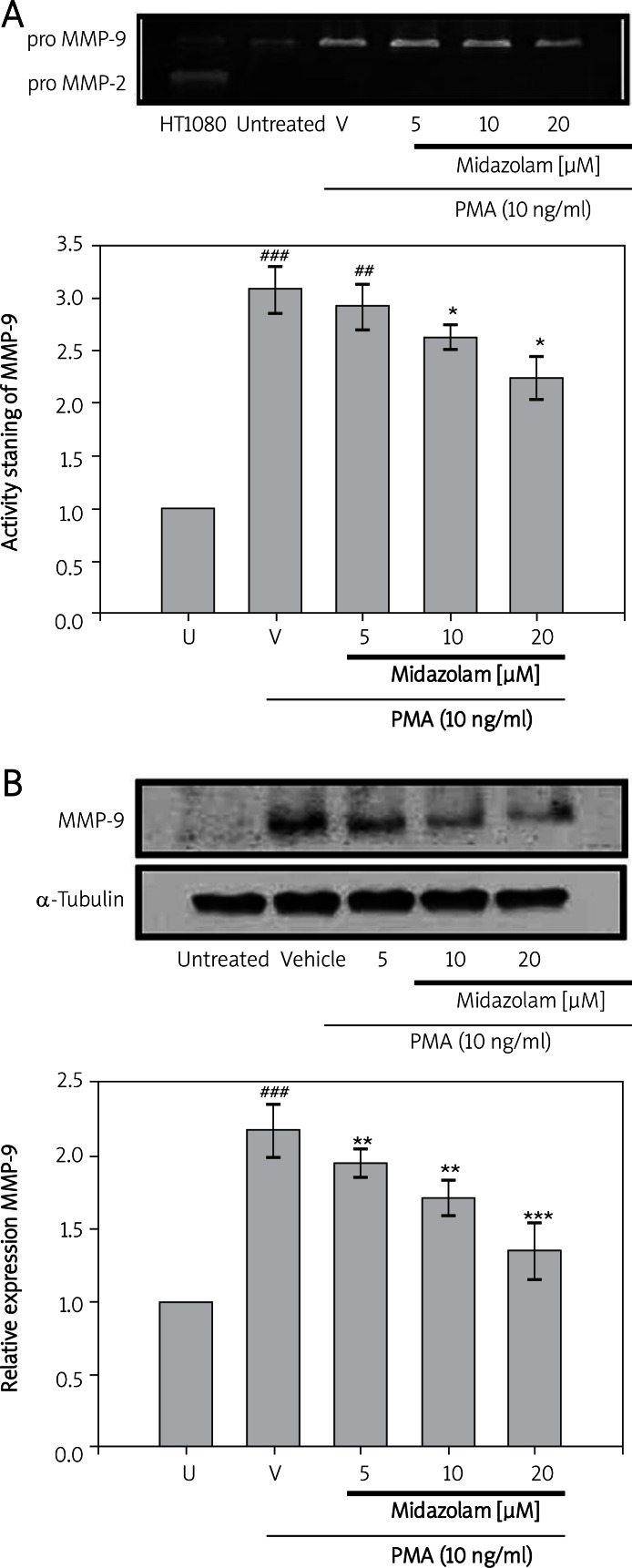
To investigate the effects of midazolam on MMP-9 expression and activation, the human chondrosarcoma cell line (SW1353) was exposed to various concentrations of midazolam (5-20 µM) and then stimulated with PMA (10 ng/ml). A marked increase (3.08 ±0.21; p < 0.001) in the activation of MMP-9 was noticed after 24 h of PMA stimulation as compared with the control (0.5%, DMSO). However, treatment of SW1353 cells with midazolam (5 µM, 10 µM and 20 µM) was found to down-regulate PMA-mediated MMP-9 gelatinolysis approximated at 2.91 ±0.19, 2.61 ±0.11 and 2.23 ±0.20, respectively (Figure 1A). Similarly, a significant induction of MMP-9 protein expression (2.18 ±0.17) by PMA (10 ng/ml) was found to be down-regulated in midazolam (5 µM, 10 µM and 20 µM) pretreated cells; the values were 1.95 ±0.09 (p < 0.01), 1.71 ±0.12 (p < 0.01) and 1.35 ±0.20 (p < 0.001), respectively (Figure 1B).
Figures 1.
Effects of midazolam on phorbol-12-myristate-13-acetate (PMA)-induced activation and expression of MMP-9 in chondrosarcoma SW1353 cell lines. Cell-free conditioned medium and supernatants were assayed for MMP-9 activation and expression by ge la tin zymography (A) and western blotting (B), respecti vely. Lane 1 is the culture medium of HT1080 cells in which the constitutive proMMP-2 and proMMP- 9 were expressed (A). SW1353 cells (2 × 106 cells/ml in 6-well plates) were treated with various concentrations of midazolam (5, 10, and 20 μM) or vehicle (DMSO) for 15 min before treatment with PMA (10 ng/ml) for 24 h. α-tubulin is used as an internal control (B)
Data are shown as the mean (SEM of three independent experiments). ###p < 0.001 compared with the untreated group, *p < 0.05 **p < 0.01, and ***p < 0.001, compared with the vehicle group
To further evaluate whether midazolam inhibits MMP-9 activation in SW1353 cells through cytotoxic effects, cells were pre-incubated with midazolam (5-30 µM) for 24 h. Based on the MTT assay, it was found that this sedative drug had little effect on cell viability of SW1353 cells, even at a higher concentration (30 µM) at about 84.16 ±1.49% (data not shown).
Up-regulation of MMP-1 and MMP-13 expression by PMA in chondrocytes is inhibited by midazolam
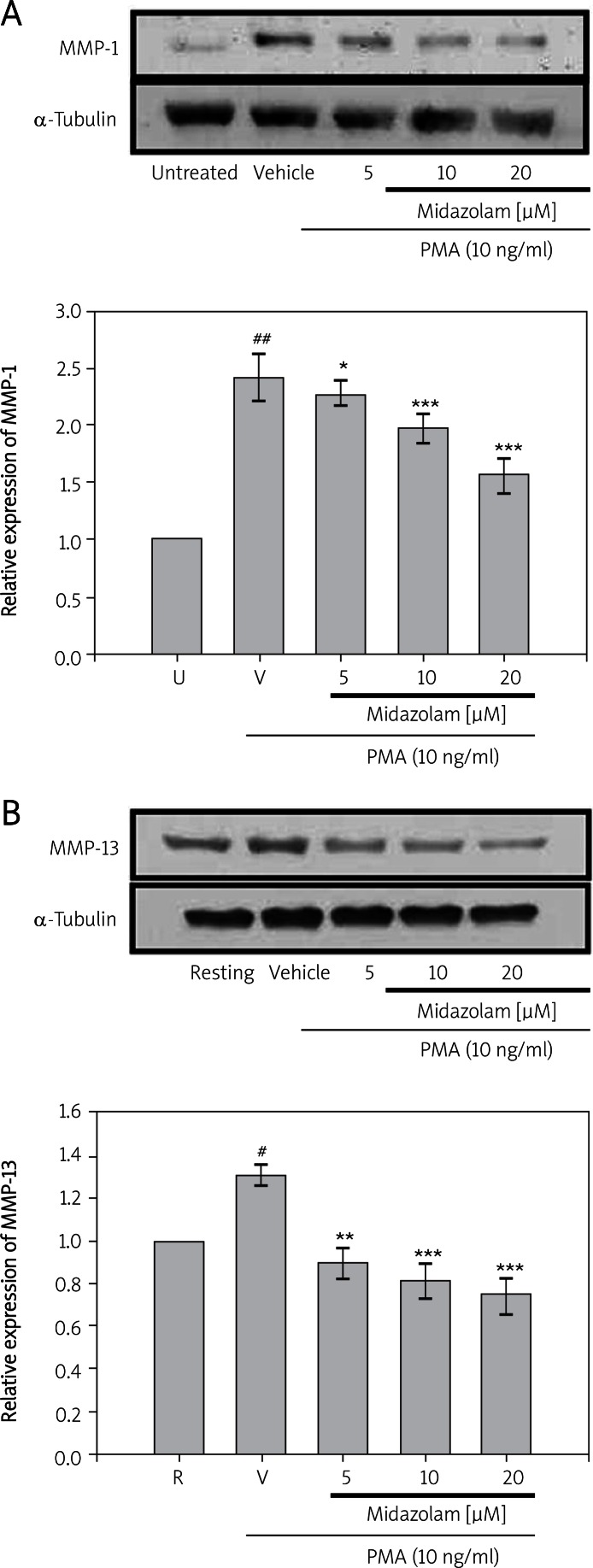
To evaluate MMP-1 and 13 expression in response to PMA followed by midazolam treatment, chondrocyte cultures were serum starved for 24 h and then stimulated with PMA (10 ng/ml) in the presence or absence of midazolam (5-20 µM). The results shown in Figure 2A indicate that, at concentrations of 5 µM, 10 µM and 20 µM, midazolam could significantly inhibit the activation of MMP-1 (2.27 ±0.10, 1.98 ±0.11 and 1.56 ±0.15; p < 0.001), respectively) induced by PMA (2.42 ±0.19). A similar significantly lower expression (0.89 ±0.04, 0.81 ±0.07, and 0.74 ±0.09; p < 0.001) of MMP-13 was noted in midazolam (5 µM, 10 µM and 20 µM, respectively) pretreated and PMA-induced (1.31 ±0.04) SW1353 cells (Figure 2B). These results indicate that midazolam down-regulates the stimulated expression of MMP-1 and MMP-13 in a concentration-dependent manner.
Figures 2.
Effects of midazolam on phorbol-12- myristate-13-acetate (PMA)-induced expression of MMP-1 (A) and MMP-13 (B) in chondrosarcoma SW1353 cell lines. Cell lysates were obtained and analyzed for MMP-1 and MMP-13 protein expressions by Western blotting. SW1353 cells (2 × 106 cells/ml in 6-well plates) were treated with various concentrations of midazolam (5 μM, 10 μM, and 20 μM) or vehicle (DMSO) for 15 min before treatment with PMA (10 ng/ml) for 24 h. α-Tubulin is used as an internal control
Data are shown as the mean (SEM of three independent experiments). ###p < 0.001 compared with the untreated group, *p < 0.05, **p < 0.01, and ***p < 0.001, compared with the vehicle group
Midazolam inhibits PMA-mediated degradation of IκB-α
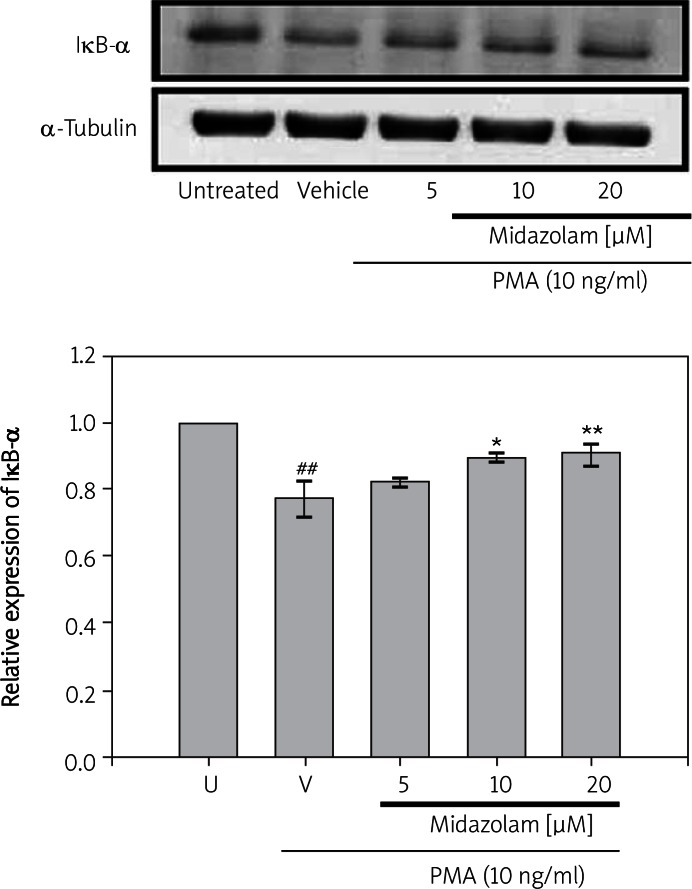
To determine whether the inhibitory action of midazolam was due to an effect on degradation, the cytoplasmic levels of IκB-α protein were examined by Western blot analysis. As shown in Figure 3, treatment of cells with PMA (for 30 min) could induce a rate of degradation of IκB-α of about 0.786 ±0.047. Interestingly, SW1353 cells that had been treated with midazolam at concentrations of 10 µM and 20 µM for 15 min significantly reversed the rate of degradation (0.895 ±0.051; p < 0.05 and 0.926 ±0.060; p < 0.01, respectively) of IκB-α induced by PMA (Figure 3). However, midazolam treatment at a concentration of 5 µM did not significantly recover the rate of PMA-mediated degradation of IκB-α.
Figure 3.
Effects of midazolam on phorbol-12- myristate-13-acetate (PMA)-induced degradation of IκB-α in chondrosarcoma SW1353 cell lines. Cell lysates were obtained and analyzed for IκB-α protein degradation by Western blotting. SW1353 cells (2 × 106 cells/ml in 6-well plates) were treated with various concentrations of midazolam (5 μM, 10 μM, and 20 μM) or vehicle (DMSO) for 15 min before treatment with PMA (10 ng/ml) for 24 h. α-Tubulin is used as an internal control
Data are shown as the mean (SEM of three independent experiments). ##p < 0.001 compared with the resting group, *p < 0.05, and **p < 0.01, compared with the vehicle group
Midazolam blocks PMA-mediated MAPK phosphorylation
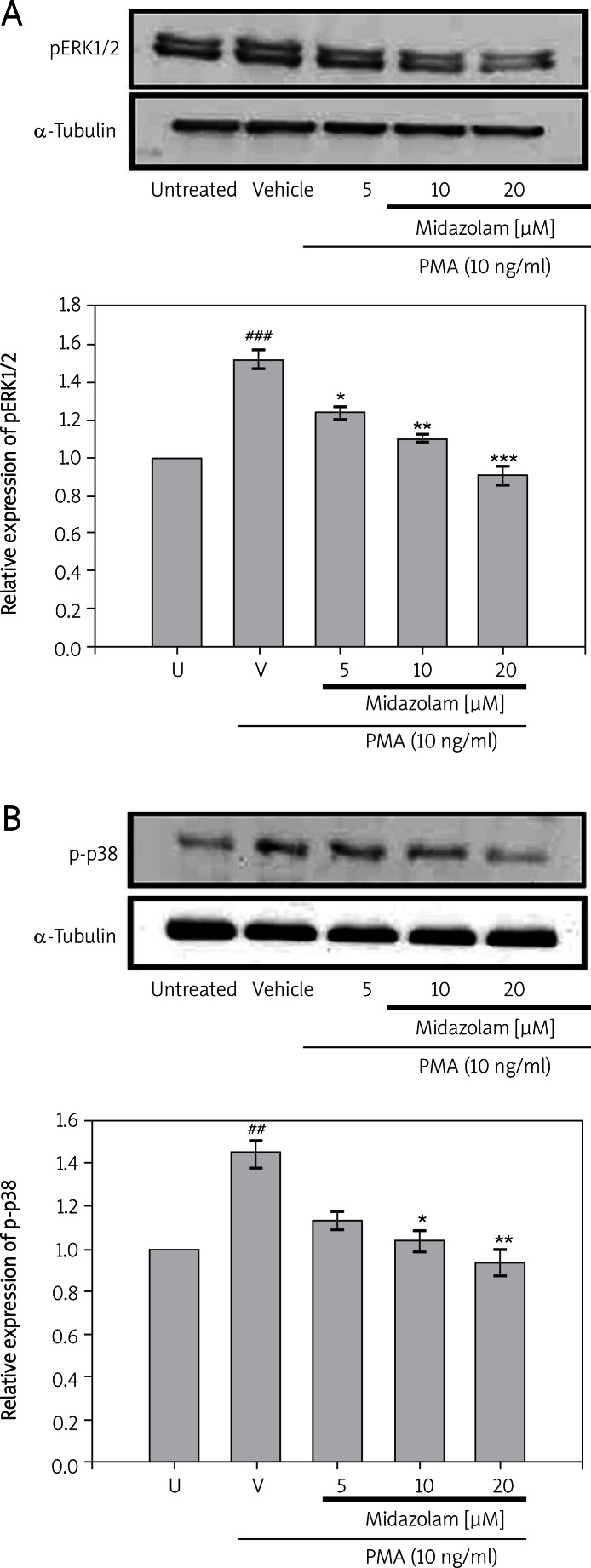
In order to examine whether midazolam affects stimulated ERK, p38, and JNK activation in SW-1353 cells, the phosphorylation levels of ERK, p38, and JNK were compared between the treatments with PMA in the presence or absence of midazolam. As shown in Figures 4A, B, PMA (10 ng/ml) was found to significantly stimulate phos-ERK (1.528 ±0.32; p < 0.001) and phos-p38 (1.463 ±0.41; p < 0.01) levels, but treatment of cells with midazolam (5 µM, 10 µM and 20 µM) suppressed the PMA-induced levels of phos-ERK (1.243 ±0.12, 1.108 ±0.16 and 0.903 ±0.19, respectively) and phos-p38 (1.146 ±0.10, 1.063 ±0.13 and 0.946 ±0.18, respectively). Moreover, the presence of midazolam at concentrations of 5 µM and 10 µM did not affect c-JNK levels and only a small impact was found at 20 µM concentration (Data not shown).
Figures 4.
Effects of midazolam on phorbol-12- myristate-13-acetate (PMA)-induced expression of pERK1/2 (A) and p38 (B) in chondrosarcoma SW1353 cell lines. Cell lysates were obtained and analyzed for pERK1/2 and p-p38 protein expression by Western blotting. SW1353 cells (2 × 106 cells/ml in 6-well plates) were treated with various concentrations of midazolam (5 μM, 10 μM, and 20 μM) or vehicle (DMSO) for 15 min before treatment with PMA (10 ng/ml) for 24 h. α-Tubulin is used as an internal control
Data are shown as the mean (SEM) of three independent experiments. ###p < 0.001 compared with the untreated group, *p < 0.05, **p < 0.01 and ***p < 0.001, compared with the vehicle group
Discussion
The present study is the first to highlight the molecular evidence that midazolam inhibits MMPs in PMA-activated chondrocyte cells by blocking pERK, p38 MAPK and/or NF-κB-mediated transcriptional regulation. Cartilage chondrocytes have shown to be activated during the progression of arthritis in producing MMPs, resulting in the decomposition of local connective matrix [20]. Therefore, MMPs play an important pathological role as decomposition factors to induce cartilage damage during inflammation of OA, in particular, MMP-1, MMP-9 and MMP-13 [21]. Interestingly, phorbol ester is reported to induce some pathological stimulation through activating various signaling pathways in chondrocytes, and also could induce MMP-9, MMP-1 and MMP-13 as described in our earlier study [10]. The present study revealed that midazolam (5-20 µM) markedly suppressed PMA-stimulated activation and expression of MMP-9 and expression of MMP-1 and MMP-13 in chondrocytes. In the range of approximately 3 µM to 30 µM, midazolam suppressed expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), which are known as proinflammatory mediators. Plasma concentrations of benzodiazepines that have been used clinically ranged between 0.1 µM and 50 µM [22]. A study also found that in the range of 3-30 µM midazolam suppresses VCAM-1 and monocyte adhesion in the TNF-α activated endothelial cell [23]. Midazolam at concentrations of 5-20 µM as used in the current study significantly inhibited activation and expression of MMP-9 and expression of MMP-1 and MMP-13. Amos et al. [24] demonstrated that the signaling of NF-κB and MAPKs could be activated under PKC stimulation.
The mechanism of down-regulating chondrocyte-specific matrix synthesis may also involve NF-κB activation [25]. It is apparent that OA synovial tissue has been considered as a typical marker of NF-κB [26] and the synthesis of inflammatory and destructive mediators from OA synovial tissue was reported to be NF-κB dependent [27]. The phosphorylation of IκB and its subsequent degradation allows translocation of NF-κB to the nucleus. Agents that can down-modulate the activation of NF-κB have potential for therapeutic intervention. Kim et al. [28] discovered that midazolam exerted anti-inflammatory action by inhibiting iNOS and COX-2 expression, possibly through the suppression of NF-κB and p38 mitogen-activated protein kinase activation. In the present study, midazolam that had been administered to the PMA-stimulated chondrocytes could significantly inhibit IκB-α degradation.
The MAPKs, including c-Jun N-terminal kinase, extracellular signal-regulated kinase, and p38, have been reported to play a critical role in cytokines and PMA-mediated MMP expression [10]. Both ERK and p38 MAPK activation are reported to be involved in stimulating MMP-9 production in chondrocytes [29]. In the present study, our results revealed that midazolam markedly attenuated PMA-induced phosphorylation of ERK and p38 MAPK, and these results are consistent with the cartilage breakdown study which revealed that cytokine increased chondrocyte MMP expression through a p38 MAPK-mediated pathway [26]. It has previously been reported that midazolam inhibits N-formyl-methionyl-leucyl-phenylalanine-induced p38 MAP kinase activation in neutrophils [30]. Consequently, inhibition of ERK and p38 MAPK by midazolam in this study may contribute to the reduction of MMP expression. Furthermore, midazolam also attenuated PMA-stimulated phosphorylation of ERK1/2, and this result was consistent with the study which found that the treatment of ERK inhibitor could reduce the production of PMA-induced MMPs in different human cells. Particularly, there was inhibitory action of midazolam on the ERK and p38 MAPK pathway, but no significant effect on the JNK MAPK pathway.
In OA, up-regulated MMPs are considered critical to degrading ECM. Therefore, MMPs would be reasonable therapeutic targets to treat osteoarthritis. In this study, midazolam markedly and dose-dependently decreased MMP-1, MMP-9, and MMP-13 levels by down-regulating MAP kinases, such as ERK-1/-2 and p38 kinase, and recovering IκB-α degradation, which could make midazolam more effective in preventing cartilage destruction. Ozok et al. [31] reported the efficacy of supplementation of midazolam to periprostatic nerve blockage (PNB) during probe insertion and needle penetration in patients for pain reduction. A clinical study has also shown that intra-articular midazolam injection could dramatically improve the degree of pain after knee arthroscopy [32]. Therefore, it is considerably relevant to this study as the rapid action of midazolam and its modest effects on chondrocytes help make it an effective and safe alternative for the treatment of arthritis patients.
In conclusion, the results of this study confirm that midazolam suppressed PMA-induced expression of MMP-9, MMP-1 and MMP-13 and these effects might be mediated via inhibition of IκB-α degradation and ERK/p38 MAPK activation. Since these responses are possibly cell specific, further experiments need to be explored to evaluate the potency of midazolam through in vivo animal and clinical models of arthritis. Depending on the out-come of this study, it may be appropriate to assess further the potential for midazolam to be developed as an anti-inflammatory and chondroprotective therapeutic for use in OA.
Acknowledgments
Jen-Jui Wang and Steven Kuan-Hua Huan contributed equally to this work. This work was supported by grants from the National Science Council of Taiwan (NSC97-2320-B-038-016-MY3) and Chi-Mei Medical Center – Taipei Medical University (98CM-TMU-08).
References
- 1.LeGrand A, Fermor B, Fink C, et al. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001;44:2078–83. doi: 10.1002/1529-0131(200109)44:9<2078::AID-ART358>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi M, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Chondrocyte generated IL-1 and TNF are involved in matrix degradation of human osteoarthritis cartilage in explant culture. Arthritis Rheum. 2002;46:S80. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 3.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–94. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Shi J, Schmitt-Talbot E, DiMattia DA, Dullea RG. The differential effects of IL-1 and TNF-alpha on proinflammatory cytokine and matrix metalloproteinase expression in human chondrosarcoma cells. Inflamm Res. 2004;53:377–89. doi: 10.1007/s00011-004-1271-3. [DOI] [PubMed] [Google Scholar]
- 5.Mengshol JA, Mix KS, Brinckerhoff CE. Matrix metalloproteinases as therapeutic targets in arthritic diseases: bull's-eye or missing the mark? Arthritis Rheum. 2002;46:13–20. doi: 10.1002/1529-0131(200201)46:1<13::aid-art497>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Malemud CJ. Matrix metalloproteinases: role in skeletal development and growth plate disorders. Front Biosci. 2006;11:1702–15. doi: 10.2741/1916. [DOI] [PubMed] [Google Scholar]
- 7.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–11. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997;40:2065–74. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- 9.Knäuper V, Cowell S, Smith B, et al. The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem. 1997;272:7608–16. doi: 10.1074/jbc.272.12.7608. [DOI] [PubMed] [Google Scholar]
- 10.Lu YC, Jayakumar T, Duann YF, et al. Chondroprotective role of sesamol by inhibiting MMPs expression via retaining NF-kappaB signaling in activated SW1353 cells. J Agric Food Chem. 2011;59:4969–78. doi: 10.1021/jf1046738. [DOI] [PubMed] [Google Scholar]
- 11.Shikhman AR, Kuhn K, Alaaeddine N, Lotz M. N-acetylglucosamine prevents IL-1beta-mediated activation of human chondrocytes. J Immunol. 2001;166:5155–60. doi: 10.4049/jimmunol.166.8.5155. [DOI] [PubMed] [Google Scholar]
- 12.Bondeson J, Brennan F, Foxwell B, Feldmann M. Effective adenoviral transfer of IkappaBalpha into human fibroblasts and chondrosarcoma cells reveals that the induction of matrix metalloproteinases and proinflammatory cytokines is nuclear factor-kappaB dependent. J Rheumatol. 2000;27:2078–89. [PubMed] [Google Scholar]
- 13.Nishiyama T, Tamai H, Hanaoka K. Serum and cerebrospinal fluid concentrations of midazolam after epidural administration in dogs. Anesth Analg. 2003;96:159–62. doi: 10.1097/00000539-200301000-00032. [DOI] [PubMed] [Google Scholar]
- 14.Bazzichi L, Betti L, Giannaccini G, Rossi A, Lucacchini A. Peripheral-type benzodiazepine receptors in human mononuclear cells of patients affected by osteoarthritis, rheumatoid arthritis or psoriasic arthritis. Clin Biochem. 2003;36:57–60. doi: 10.1016/s0009-9120(02)00408-3. [DOI] [PubMed] [Google Scholar]
- 15.Bribes E, Bourrie B, Esclangon M, Galiegue S, Vidal H, Casellas P. Involvement of the peripheral benzodiazepine receptor in the development of rheumatoid arthritis in Mrl/lpr mice. Eur J Pharmacol. 2002;452:111–22. doi: 10.1016/s0014-2999(02)02231-8. [DOI] [PubMed] [Google Scholar]
- 16.Bartosikova L, Necas J, Bartosik T, Frana P, Pavlik M. Changes in biomechanical parameters during heart perfusion and after midazolam pre-medication – experimental pilot study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:79–82. doi: 10.5507/bp.2008.012. [DOI] [PubMed] [Google Scholar]
- 17.Kelbel I, Weiss M. Anaesthetics and immune function. Curr Opin Anaesthesiol. 2001;14:685–91. doi: 10.1097/00001503-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao G, Huang HY, Fong TH, et al. Inhibitory mechanisms of YC-1 and PMC in the induction of iNOS expression by lipoteichoic acid in RAW 264.7 macrophages. Biochem Pharmacol. 2004;67:1411–9. doi: 10.1016/j.bcp.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Chung CL, Sheu JR, Liu HE, et al. Dynasore, a dynamin inhibitor, induces PAI-1 expression in MeT-5A human pleural mesothelial cells. Am J Respir Cell Mol Biol. 2009;40:692–700. doi: 10.1165/rcmb.2008-0087OC. [DOI] [PubMed] [Google Scholar]
- 20.Caterson B, Flannery CR, Hughes CE, Little CB. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000;19:333–44. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 21.Yoshihara Y, Nakamura H, Obata K, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455–61. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilms H, Claasen J, Röhl C, Sievers J, Deuschl G, Lucius R. Involvement of benzodiazepine receptors in neuroinflammatory and neurodegenerative diseases: evidence from activated microglial cells in vitro. Neurobiol Dis. 2003;14:417–24. doi: 10.1016/j.nbd.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Joo HK, Oh SC, Cho EJ, et al. Midazolam inhibits tumor necrosis factor-alpha-induced endothelial activation: involvement of the peripheral benzodiazepine receptor. Anesthesiology. 2009;110:106–12. doi: 10.1097/ALN.0b013e318190bc69. [DOI] [PubMed] [Google Scholar]
- 24.Amos N, Lauder S, Evans A, Feldmann M, Bondeson J. Adenoviral gene transfer into osteoarthritis synovial cells using the endogenous inhibitor IkappaBalpha reveals that most, but not all, inflammatory and destructive mediators are NFkappaB dependent. Rheumatology. 2006;45:1201–9. doi: 10.1093/rheumatology/kel078. [DOI] [PubMed] [Google Scholar]
- 25.Murakami S, Lefebvre V, de Crombrugghe B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-alpha. J Biol Chem. 2000;275:3687–92. doi: 10.1074/jbc.275.5.3687. [DOI] [PubMed] [Google Scholar]
- 26.Marok R, Winyard PG, Coumbe A, et al. Activation of the transcription factor nuclear factor-kappaB in human inflamed synovial tissue. Arthritis Rheum. 1996;39:583–91. doi: 10.1002/art.1780390407. [DOI] [PubMed] [Google Scholar]
- 27.Baeuerle PA, Henkel T. Function and activation of NF-kappaB in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 28.Kim SN, Son SC, Lee SM, et al. Midazolam inhibits proinflammatory mediators in the lipopolysaccharide-activated macrophage. Anesthesiology. 2006;105:105–10. doi: 10.1097/00000542-200607000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Grilli M, Chiu JJ, Lenardo MJ. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- 30.Ghori K, O'Driscoll J, Shorten G. The effect of midazolam on neutrophil mitogen-activated protein kinase. Eur J Anaesthesiol. 2010;27:562–5. doi: 10.1097/EJA.0b013e3283328442. [DOI] [PubMed] [Google Scholar]
- 31.Ozok HU, Sagnak L, Ates MA, Karakoyunlu N, Topaloglu H, Ersoy H. The efficiency of a sedative or analgesic supplement to periprostatic nerve blockage for pain control during transrectal ultrasound-guided prostate biopsy – a prospective, randomized, controlled, double blind study. Arch Med Sci. 2010;6:787–92. doi: 10.5114/aoms.2010.17096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batra YK, Mahajan R, Kumar S, Rajeev S, Singh Dhillon M. A dose-ranging study of intraarticular midazolam for pain relief after knee arthroscopy. Anesth Analg. 2008;107:669–72. doi: 10.1213/ane.0b013e3181770f95. [DOI] [PubMed] [Google Scholar]