Abstract
Introduction
EsA was reported to have the effect of modulating immune response, cell proliferation and apoptosis as well as anti-inflammatory effects in acute and chronic experimental models. However, the effects of EsA on LN remain poorly understood. To investigate the roles of EsA in LN, the effects of EsA were tested on BXSB mice, a SLE model, in which male SB/Le mice and female C57BL/6 mice were hybridized through recombinant inbred species.
Material and methods
Twenty four BXSB mice were divided into three groups. After 4 weeks, blood samples, urine samples and kidney tissues were collected. Measurement of cytokine levels was carried out using sandwich ELISA reagent kits. Apoptotic scores were obtained with a TUNEL assay. PCNA and Caspase-3 mRNA was detected using the In Situ Hybridization Detection Kit.
Results
The results demonstrated that compared with the control group, EsA administration markedly controlled urine protein excretion, improved renal function, alleviated kidney damage and promoted the apoptosis of glomerular intrinsic cells and renal tubular epithelial cells in animals of the treated group (p < 0.05). Meanwhile, EsA reduced the serum IL-6 and TNF-α levels (p < 0.05), inhibited the expression of PCNA and promoted the expression of caspase-3, Fas and FasL in animals of the treated group (p < 0.05). The effects of EsA on BXSB mice were similar to dexamethasone.
Conclusions
All these findings indicated that EsA might play significant roles in the treatment of BXSB mice through modulation of inflammatory cytokines, inhibition of renal cell proliferation and induction of apoptosis. The special targets of EsA in lupus nephritis are worth further exploration.
Keywords: BXSB mice, esculentoside A, cytokine, apoptosis, proliferation
Introduction
Systemic lupus erythematosus (SLE) is a multi-organ autoimmune disease. Lupus nephritis (LN) is the most common and serious internal organ damage which is induced by SLE [1]. Glucocorticoids and cytotoxic drugs, as the cornerstone of the control of LN, improve the patients’ condition and prognosis to a large extent, but they inevitably induce serious side effects, such as secondary infection and even death. Many new therapies have been tried in the treatment of LN without definite superiority. In order to explore updated and more effective treatments, Chinese medicine or Chinese medicine extracts were frequently applied to treat LN both experimentally and clinically, which revealed hopeful effect [2, 3].
Phytolacca esculenta is a kind of perennial herb, and esculentoside A (EsA) is a triterpene saponin extracted from its roots. Recent studies [4–6] suggested that EsA had the effect of modulating the immune and inflammatory responses. It might also inhibit cell proliferation and induce apoptosis [4, 7]. BXSB mice were applied as an animal model of SLE. It is a recombinant inbred species hybridized by male SB/Le mice and female C57BL/6 mice. Because of carrying the Yaa gene in the Y chromosome, which can accelerate the autoimmune reaction, the male mice are prone to early onset and have severe organ damage compared with female mice [8, 9]. Male mice fall ill at about 3 months of age, and about half of them die at 5 months old [10]. They have proteinuria and the renal pathological finding is mesangial proliferative glomerulonephritis, which makes them an appropriate LN model.
In this study, BXSB mice were used to observe the effect of EsA on the changes of urinary protein, renal function, histology, serum cytokines, renal apoptosis and proliferation.
Material and methods
Mice
Male BXSB mice were purchased from the Jackson Laboratories (Bar Harbor, ME), maintained in our special pathogen-free facility, and had free access to water and food. The animal experiments were approved by the Ethics Committee for Animal Research of The Second Clinical Medical College of Jinan University. 24 BXSB mice were divided into 3 groups (8 mice in each group). The EsA-treated group was given intraperitoneal (i.p.) EsA at a dose of 20 mg/kg once a day for 4 weeks. The dexamethasone-treated group was given i.p. dexamethasone at a dose of 5 mg/kg once a day for 4 weeks. The control group was composed of model mice, which were given an equal volume of saline. At the end of the experiment, blood samples were centrifuged and serum was kept at –20°C until the assays were performed.
Reagent
RPMI-1640 was purchased from Sigma (Sigma, USA). EsA (molecular mass 826.0), with a purity of 99%, was purchased from Xian Wenyang Company. Esculentoside A was dissolved in RPMI 1640 medium for further use in experiments.
Renal function parameters
Renal function was assessed by measuring serum creatinine and blood urea nitrogen (BUN), using a Biochemistry Auto-analyzer (Olympus). The animals were housed in metabolic cages every other week, and their urine was collected. The 24-hour proteinuria and proteinuria/urinary creatinine ratios were measured (Lab Test kit).
Measurement of serum cytokine levels
Measurement of cytokine levels of interleukin-6 (IL-6) and tumour necrosis factor α (TNF-α) in serum was carried out using sandwich ELISA reagent kits (R&D systems, MN, USA).
Histology and immunohistochemistry
Fragments of the renal cortex were fixed overnight in 10% neutral phosphate buffered formalin (Duksan Pure Chemical Co, Korea), dehydrated in alcohol, and then embedded in paraffin. The sections (3 µm thick) were stained with hematoxylin. The slides were viewed under an 80i light microscope (Nikon, Japan). For the immunohistochemistry study, the kidney specimens were incubated with an antibody against human Fas and FasL (Boster, China) respectively, which were operated for staining according to the manufacturer's instructions (Sun Biotech Co. Ltd., China). Apoptotic scores were obtained with a terminal transferase-mediated dUTP nick-end labeling (TUNEL) assay using an Apoptosis Detection Kit (Roche, Mannheim, Germany). Briefly, kidney sections were deparaffinized, rehydrated, and digested with protein K and labeled with TUNEL reaction mixture for 60 min at 37°C. Sections were screened for positive nuclei under a light microscope, and 10 random glomerular and tubular fields were counted for every kidney under 400× magnification. Data from all fields and all kidneys were pooled to obtain the apoptotic index, which is the percentage of TUNEL positive cells in total cells manually counted in 10 randomly selected fields.
In situ hybridization
Kidney tissue was fixed in formalin overnight and embedded in paraffin using standard procedures. Series sections 4 µm thick were deparaffinized with xylene, rehydrated in a graded series of ethanol, and washed in PBS. PCNA and caspase-3 mRNA was detected using the In Situ Hybridization Detection Kit (Boster, Wuhan, China) according to the manufacturer's instructions. Briefly, the sections were hybridized with PCNA and caspase-3 oligonucleotide probe for 16 h at 40°C and incubated with biotinylated mouse anti-digoxigenin antibody, then incubated with biotinylated peroxidase. Staining was developed with DAB. Slides were counterstained with hematoxylin, dehydrated and mounted.
The number of cells staining brown (indicating the presence of PCNA or caspase-3 mRNA) was assessed by light microscope (Olympus Hamburg, Germany) and micrographs obtained using an Olympus DP50 digital imaging system mounted on the microscope. Histological slides were semi-quantitatively evaluated using the software Image pro-plus 6.0 (Media Cybernetics, USA).
Statistical analysis
Results were presented as mean ± standard deviation and analyzed using one-way ANOVA with statistical analysis software (SPSS 13.0). The value of p < 0.05 was considered statistically significant.
Results
Effects of EsA on biochemical parameters
Before the experiment, the mice of each group had a high level of urine protein. At the end, urine protein and urine protein/urine creatinine ratio in the EsA-treated group and dexamethasone-treated group were significantly lower than those in the control group (p < 0.05). Also, the levels of serum creatinine and BUN were significantly lower than those in the control group (p < 0.05). There was no difference between the EsA-treated group and the dexamethasone-treated group (p > 0.05) (Table I).
Table I.
Laboratory parameters in each group
| Parameter | Control group | Dexamethasone-treated group | EsA-treated group |
|---|---|---|---|
| Pre-treatment urine protein [mg/l] | 752.79 ±64.29 | 761.57 ±66.84 | 742.4 ±49.63 |
| Post-treatment urine protein [mg/l] | 1071.25 ±134.09 | 726.76 ±109.05* | 773.75 ±128.80* |
| Creatinine [µmol/l] | 52.76 ±10.56 | 40.53 ±8.94* | 36.54 ±6.73* |
| BUN [mmol/l] | 9.69 ±1.58 | 7.04 ±1.29* | 7.73 ±1.45* |
| Post-treatment urine protein/urine creatinine [mg/g] | 14.13 ±4.96 | 10.77 ±4.49* | 10.54 ±3.72* |
Values presented as mean ± SEM.
Value of p < 0.05 vs. control group
Effects of EsA on the levels of serum cytokines
The levels of serum TNF-α and IL-6 decreased significantly in the EsA-treated group and dexamethasone-treated group (p < 0.05) compared with the control group. There was no difference between the EsA-treated group and the dexamethasone-treated group (p > 0.05) (Table II).
Table II.
Comparison of TNF-α and IL-6 concentration in each group
| Group | N | TNF-α [pg/ml] | IL-6 [pg/ml] |
|---|---|---|---|
| Control group | 8 | 144.36 ±13.49 | 43.44 ±7.62 |
| Dexamethasone-treated group | 8 | 119.68 ±12.95* | 20.06 ±6.45* |
| EsA-treated group | 8 | 115.67 ±13.48* | 21.70 ±6.56* |
Values presented as mean ± SEM.
Value of p < 0.05 vs. control group
Effects of EsA on renal morphology
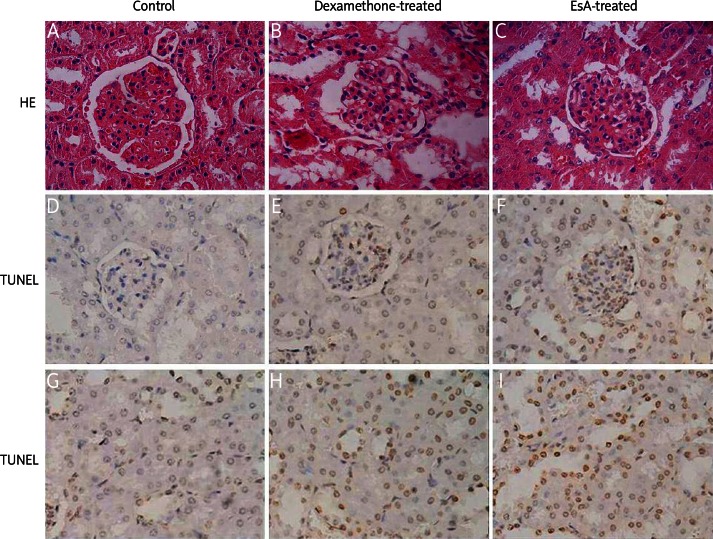
Renal pathology in the control group showed more than 50% of diffuse hyperplasia in glomerular mesangial cells and mesangial matrix. Capillary cavities were filled with proliferative cells, which was consistent with severe mesangial proliferative glomerulonephritis. Pathological changes improved in the EsA-treatment group and dexamethasone-treated group, which showed that the mesangial cell proliferation and mesangial matrix were reduced and the capillary opened. However, the pathological changes of interstitial area in each group were not obvious (Figures 1A-C).
Figure 1.
HE staining showed that the mesangial cell proliferation and mesangial matrix decreased and capillary opened in the dexamethasone-treated group and EsA-treated group (A-C). TUNEL positive cells (yellow particles in the nucleus) were apparent in glomerular cells and tubular epithelial cells (D-I). Magnification: 400×
Effects of EsA on renal cell apoptosis
TUNEL positive cells were apparent in every group, especially in glomerular cells and tubular epithelial cells (Figures 1D-I). There were significant changes in the glomerular and tubular apoptosis index among the three groups (p < 0.05). The glomerular and tubular apoptosis index values were the highest in the EsA-treated group, followed by the dexamethasone-treated group. Those of the control group were the lowest. The differences were statistically significant between every two groups (p < 0.05) (Table III).
Table III.
Apoptosis index of renal cells in each group
| Group | Fields (n) | Glomerular cells apoptosis index [%] | Tubular cells apoptosis index [%] |
|---|---|---|---|
| Control group | 60 | 14.34 ±2.66 | 27.35 ±4.44 |
| Dexamethasone-treated group | 60 | 22.82 ±4.29* | 35.81 ±5.71* |
| EsA-treated group | 60 | 29.42 ±3.94* # | 43.62 ±6.48* # |
Values presented as mean ± SEM.
Value of p < 0.05 vs. control group
p < 0.05 vs. dexamethasone-treated group
Effects of EsA on the expression of Fas and FasL
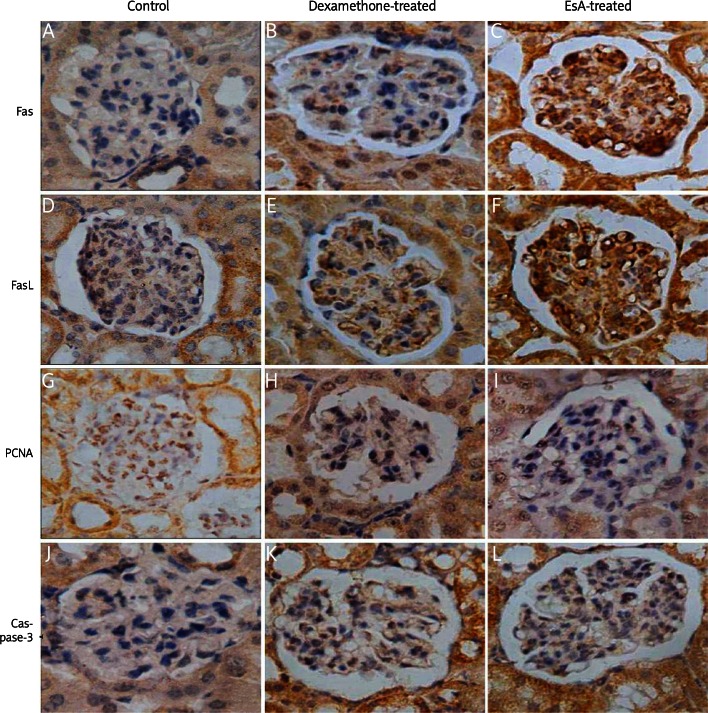
Fas and FasL were expressed in each group of kidney tissues. The number of positive cells in the glomerular and renal interstitial area of the dexamethasone-treated group and the EsA-treated group was more than that in the control group (Figures 2A-F). The mean optical density values were used for the semi-quantitative analysis; they moderately increased in the dexamethasone-treated group and the EsA-treated group compared with the control group (p < 0.05). There was no difference between the EsA-treated and dexamethasone-treated group (p > 0.05) (Table IV).
Figure 2.
Fas and FasL were expressed in each group. The number of positive cells (brown granules in the cytoplasm) in glomerular and renal interstitium of the dexamethasone-treated group and EsA-treated group was higher than that in the control group (A-F). PCNA mRNA was expressed in each group. The number of positive cells (brown granules in the cytoplasm) in glomerular and renal interstitium of the dexamethasone-treated group and EsA-treated group was lower than that in the control group (G-I). Caspase-3 mRNA was expressed in each group. The number of positive cells (brown granules in the cytoplasm) in glomerular and renal interstitium of the dexamethasone-treated group and EsAtreated group was higher than that in the control group (J-L). Magnification: 400×
Table IV.
Comparison of mean optical density values of Fas, FasL, PCNA-mRNA and caspase-3 mRNA in each group
| Group | Fields (n) | Fas | FasL | PCNA-mRNA | Caspase-3 mRNA |
|---|---|---|---|---|---|
| Control group | 60 | 0.215 ±0.010 | 0.196 ±0.013 | 0.363 ±0.025 | 0.200 ±0.038 |
| Dexamethasone-treated group | 60 | 0.253 ±0.012* | 0.257 ±0.021* | 0.241 ±0.075* | 0.265 ±0.024* |
| EsA-treated group | 60 | 0.271 ±0.023* | 0.277 ±0.016* | 0.216 ±0.015* | 0.282 ±0.017* |
Values presented as mean ±SEM.
p < 0.05 vs. control group
Effects of EsA on expression of PCNA mRNA and caspase-3 mRNA
PCNA mRNA was expressed in kidney tissues of each group. The number of positive cells in the glomerular and renal interstitial area of the dexamethasone-treated group and the EsA-treated group was less than that of the control group (Figures 2G-I). The mean optical density values were used for the semi-quantitative analysis; they significantly decreased in the dexamethasone-treated group and EsA-treated group compared with the control group (p < 0.05). There was no difference between the EsA-treated and dexamethasone-treated group (p > 0.05) (Table IV). Caspase-3 mRNA was also expressed in kidney tissues of each group. The number of positive cells in the glomerular and renal interstitial area of the dexamethasone-treated group and EsA-treated group was higher than that of the control group (Figures 2J-L). The mean optical density values were used for the semi-quantitative analysis; they significantly increased in the dexamethasone-treated group and EsA-treated group compared with the control group (p < 0.05). There was no difference between the EsA-treated and dexamethasone-treated group (p > 0.05) (Table IV).
Discussion
Nearly all SLE patients had renal involvement in varying degrees. Kidney damage is the main indicator of prognosis in SLE [11].
Before the experiment, the urine protein excretion in every group was high, as reported [12]. The proteinuria in these mice was controlled by EsA treatment, which was similar to dexamethasone treatment. In treated mice of both groups, the levels of serum creatinine and BUN were significantly lower than those in the control group after treatment. The renal pathological results also suggested that EsA could alleviate the renal damage of LN.
Tumour necrosis factor-α plays an important role in the triggering and maintenance of the inflammation. Avramescu's studies showed that the TNF-α level of SLE patients’ serum sample was higher than in normal people. The TNF-α level of active SLE patients was significantly higher, while inactive SLE patients’ TNF-α level was close to a normal level, or even lower [13]. Some studies found that TNF-α related to SLE renal pathological changes. They also discovered the transcription of TNF in mice kidney [14]. The LN would be aggravated with TNF-α injection [15]. Our results showed that TNF-α concentration in treated groups was significantly lower than in the control group. This suggested that EsA significantly inhibited the secretion of TNF-α, which might reduce the inflammatory response and prevent disease progression.
Avramescu's study showed that the serum IL-6 level in SLE patients was significantly high, especially in patients in the active state [13]. Dobbelsteen's study showed that the polymeric IgG and immune complexes could promote mesangial cells to produce IL-6 in rats [16]. The IL-6 also promoted proliferation of mesangial cells [17]. Therefore, the increased level of IL-6 might play an important role in the occurrence of LN. We found that EsA could decrease the IL-6 level, which might partly explain the effect of EsA on proteinuria and renal function.
The apoptosis pathway is divided into the non-caspase-dependent pathway and the caspase-dependent pathway. The caspase-dependent pathway can be divided into the non-innate pathway (also called the death receptor pathway) and the intrinsic pathway (also called the mitochondrial pathway) [18]. The death receptor pathway is mainly mediated by the death receptor, which mainly includes the Fas/FasL and tumor necrosis factor receptor (TNFR). The Fas/FasL system was involved in renal damage induced by autoimmune disease [19, 20]. Fas and FasL are expressed in the glomerular and tubulointerstitial cells in different degrees. They are also expressed in LN patients [21]. Caspase plays an important role in apoptosis signaling transduction as part of the cysteine protease family [22]. One of the earliest apoptosis characteristics is occurrence of protease-caspase enzymes. Active caspase breaks down intracellular enzyme that leads to specific changes of apoptosis. The key step is the activation of caspase-3 [18]. It is the most important effective protease in the caspase cascade reaction, considered as an irreversible sign of apoptosis activation. Therefore, the detection of caspase-3 mRNA is generally used as a signal of early apoptotic change [23]. Many studies have confirmed that caspase-3 participates in LN and other kidney diseases [24–26]. Our results indicated that both EsA and dexamethasone can induce apoptosis of renal cells. The glomerular and tubular cell apoptosis index values of the EsA-treated group increased significantly compared to those of the dexamethasone-treated group. Under the experimental dosage, renal cell apoptosis induced by EsA was more obvious than dexamethasone. In addition, EsA and dexamethasone up-regulated the expression of caspase-3 and Fas, FasL protein in renal glomerular and interstitial cells to a similar degree.
Cell proliferation can be considered as a pathological state and is closely related to the cell cycle. PCNA is a cell cycle-dependent protein gathered in the cell nucleus of S phase, also acting as a DNA polymerase accessory protein. The PCNA is directly involved in DNA replication in cell proliferation [27, 28]. The PCNA is also involved in DNA synthesis and repair, acting as an auxiliary factor of DNA polymerase δ. The number of PCNA-positive stained cells is recognized as an important index reflecting cell proliferation [29, 30]. Previous studies suggested that expression of glomerular PCNA in LN increased, and the degree was positively correlated with glomerular proliferation [29]. Our results showed that PCNA mRNA was expressed in renal tissues of all groups and the expression decreased in the EsA-treated group and dexamethasone-treated group. There was no significant difference between the EsA-treated group and dexamethasone-treated group, which indicated that EsA under the experimental dosage has a similar effect as dexamethasone. They could block or inhibit the proliferation of LN cells by down-regulating the PCNA expression in renal glomerular and interstitial cells.
In conclusion, we found that with application of EsA to BXSB mice, the urinary protein excretion, renal function and renal pathology were significantly improved. The results suggested that EsA had therapeutic value for LN. Meanwhile, we found that it could affect IL-6 and TNF-α levels, inhibit renal cell proliferation and induce apoptosis just like dexamethasone. We speculate that these effects might be the mechanisms of its beneficial actions on LN in mice. The special target of EsA in lupus nephritis is worth further exploration.
Acknowledgments
This study was supported by a grant from Zunyi Medical College (no. 200407).
References
- 1.Avihingsanon Y, Hirankarn N. Major lupus organ involvement: severe lupus nephritis. Lupus. 2010;19:1391–8. doi: 10.1177/0961203310376522. [DOI] [PubMed] [Google Scholar]
- 2.Ding ZX, Yang SF, Wu QF, et al. Therapeutic effect of total glucosides of paeony on lupus nephritis in MRL/lpr mic. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31:656–60. [PubMed] [Google Scholar]
- 3.Wu X, Zhang W, Shi X, An P, Sun W, Wang Z. Therapeutic effect of artemisinin on lupus nephritis mice and its mechanisms. Acta Biochim Biophys Sin. 2010;42:916–23. doi: 10.1093/abbs/gmq101. [DOI] [PubMed] [Google Scholar]
- 4.Xiao ZY, Zheng QY, Zhang JP, Jiang YY, Yi YH. Effect of esculentoside A on autoimmunity in mice and its possible mechanisms. Acta Pharmacol Sin. 2002;23:638–44. [PubMed] [Google Scholar]
- 5.Xiao ZY, Zheng QY, Jiang YY, et al. Effects of esculentoside A on production of interleukin-1, 2, and prostaglandin E2. Acta Pharmacol Sin. 2004;25:817–21. [PubMed] [Google Scholar]
- 6.Xiong N, Zhang Z, Huang J, et al. VEGF-expressing human umbilical cord mesenchymal stem cells, an improved therapy strategy for Parkinson's disease. Gene Therapy. 2010;18:394–402. doi: 10.1038/gt.2010.152. [DOI] [PubMed] [Google Scholar]
- 7.Hu Z, Qiu L, Xiao Z, et al. Effects of esculentoside A on autoimmune syndrome induced by Campylobacterjejuni in mice and its modulation on T-lymphocyte proliferation and apoptosis. Int Immunopharmacol. 2010;10:65–71. doi: 10.1016/j.intimp.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Hogarth MB, Slingsby JH, Allen PJ, et al. Multiple lupus susceptibility loci map to chromosome 1 in BXSB mice. J Immunol. 1998;161:2753–61. [PubMed] [Google Scholar]
- 9.Haywood ME, Rogers NJ, Rose SJ, et al. Dissection of BXSB lupus phenotype using mice congenic for chromosome 1 demonstrates that separate intervals direct different aspects of disease. J Immunol. 2004;173:4277–85. doi: 10.4049/jimmunol.173.7.4277. [DOI] [PubMed] [Google Scholar]
- 10.Cheung YH, Loh C, Pau E, Kim J, Wither J. Insights into the genetic basis and immunopathogenesis of systemic lupus erythematosus from the study of mouse models. Semin Immunol. 2009;21:372–82. doi: 10.1016/j.smim.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Mok CC. Biomarkers for lupus nephritis: a critical appraisal. J Biomed Biotechnol. 2010;2010:638413. doi: 10.1155/2010/638413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Tong H, Zhang Y, et al. An experimental study on the effect of Leflunomide in BXSB lupus mice. Chin J Dermatol. 2004;37:662–5. [Google Scholar]
- 13.Avrǎmescu C, Biciuşcǎ V, Dǎianu T, et al. Cytokine panel and histopathological aspects in the systemic lupus erythematosus. Rom J Morphol Embryol. 2010;51:633–40. [PubMed] [Google Scholar]
- 14.Jacob CO, McDevitt HO. Tumour necrosis factor-alpha in murine autoimmune ‘lupus’ nephritis. Nature. 1988;331:356–8. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- 15.Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988;141:3050–4. [PubMed] [Google Scholar]
- 16.Makarov SS. NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today. 2000;6:441–8. doi: 10.1016/s1357-4310(00)01814-1. [DOI] [PubMed] [Google Scholar]
- 17.Coleman DL, Ruef C. Interleukin-6: an autocrine regulator of mesangial cell growth. Kidney Int. 1992;41:604–6. doi: 10.1038/ki.1992.91. [DOI] [PubMed] [Google Scholar]
- 18.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue C, Lan-Lan W, Bei C, Jie C, Wei-Hua F. Abnormal Fas/FasL and caspase-3-mediated apoptotic signaling pathways of T lymphocyte subset in patients with systemic lupus erythematosus. Cell Immunol. 2006;239:121–8. doi: 10.1016/j.cellimm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Singh R, Pradhan V, Patwardhan M, Ghosh K. APO-1/Fas gene: Structural and functional characteristics in systemic lupus erythematosus and other autoimmune diseases. Indian J Hum Genet. 2009;15:98–102. doi: 10.4103/0971-6866.60184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fathi NA, Hussein MR, Hassan HI, Mosad E, Galal H, Afifi NA. Glomerular expression and elevated serum Bcl-2 and Fas proteins in lupus nephritis: preliminary findings. Clin Exp Immunol. 2006;146:339–43. doi: 10.1111/j.1365-2249.2006.03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2665–72. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonomini M, Dottori S, Amoroso L, Arduini A, Sirolli V. Increased platelet phosphatidylserine exposure and caspase activation in chronic uremia. J Thromb Haemost. 2004;2:1275–81. doi: 10.1111/j.1538-7836.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Huang JP, Ding J. Apoptosis and expressions of PDCD5 and Caspase-3 in renal tissues of children with lupus nephritis. Zhonghua Er Ke Za Zhi. 2005;43:517–20. [PubMed] [Google Scholar]
- 25.Jani A, Ljubanovic D, Faubel S, Kim J, Mischak R, Edelstein CL. Caspase inhibition prevents the increase in caspase-3, -2, -8 and -9 activity and apoptosis in the cold ischemic mouse kidney. Am J Transplant. 2004;4:1246–54. doi: 10.1111/j.1600-6143.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang B, El Nahas AM, Thomas GL, et al. Caspase-3 and apoptosis in experimental chronic renal scarring. Kidney Int. 2001;60:1765–76. doi: 10.1046/j.1523-1755.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 27.Kochaniak AB, Habuchi S, Loparo JJ, et al. Proliferating cell nuclear antigen uses two distinct modes to move along DNA. J Biol Chem. 2009;284:17700–10. doi: 10.1074/jbc.M109.008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strzalka W, Ziemienowicz A. Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann Bot. 2011;107:1127–40. doi: 10.1093/aob/mcq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker AJ, Mooney A, Hughes J, Lombardi D, Johnson RJ, Savill J. Mesangial cell apoptosis: the major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis. J Clin Invest. 1994;94:2105–16. doi: 10.1172/JCI117565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee DW, Kwak IS, Lee SB, et al. Post-treatment effects of erythropoietin and nordihydroguaiaretic acid on recovery from cisplatin-induced acute renal failure in the rat. J Korean Med Sci. 2009;24(Suppl):S170–5. doi: 10.3346/jkms.2009.24.S1.S170. [DOI] [PMC free article] [PubMed] [Google Scholar]