Abstract
Introduction
The influence of physical exercise on the parameters of the cardiovascular system of elderly persons has not been sufficiently investigated yet. The aim of the study was to assess the influence of regular 6-week physical exercise using the Nordic walking (NW) method in a group of elderly persons on their physical performance and regulation of selected parameters assessing the cardiovascular system.
Material and methods
Fifty patients over 65 years of age participated in the study. The study encompassed: medical interview, physical examination, resting ECG, spiroergometry examination, 6MWT (6-minute walk test) and 24-hour ambulatory blood pressure monitoring (ABPM). During the exercise programme, the pulse was monitored using pulsometers. After the completion of the training, check-up tests assessing the same parameters were performed. The control group consisted of 18 persons over 65 years of age with similar cardiovascular problems.
Results
In the test group, duration of the physical effort increased by 1.02 min (p = 0.0001), the maximum load increased by 10.68 W (p = 0.0001), values of VO2max by 2.10 (p = 0.0218), distance improved in 6MWT by 75.04 m (p = 0.00001), systolic blood pressure decreased by 5.50 mm Hg (p = 0.035) and diastolic blood pressure by 3.50 mm Hg (p = 0.054) as compared to the control group.
Conclusions
Systematic NW physical exercise limited by the pulse had a beneficial effect on the physical performance of elderly persons as assessed with main parameters. A short 6-week programme of endurance exercises had a hypotensive effect in elderly persons over 65 years of age.
Keywords: physical exercise, endurance, elderly persons, Nordic walking, blood pressure
Introduction
Ageing of society has been a permanent process. Since 1950, the percentage of elderly persons has been constantly on the rise, increasing from 8% in 1950 to 11% in 2009 and estimated to reach 22% in 2050, according to forecasts [1]. In the second half of the 20th century, the length of human life increased by approximately 20 years. According to the World Health Organization (WHO), the average length of human life will be 73 years in 2025 [2].
In 2004, the WHO presented its strategy in relation to physical activity, diet and health. The publication of the WHO contains information about the need to increase physical activity in the population of people all over the world. To decrease the risk of cardiovascular diseases, the report recommends at least 30 min of regular physical exercise with moderate intensity on most days [3]. Physical activity considerably lowers the risk of cardiovascular disease and the significance of this protective factor can be compared with giving up smoking [4, 5].
Cardiovascular disease (CVD) is the main cause of death in Europe. Almost a half (48%) of all deaths are caused by CVD (54% of deaths among women and 43% of deaths among men). It is estimated that over 4.3 million people die of CVD every year [6]. Lifestyle modification, especially regular physical exercise, is one of the simplest and most effective forms of preventing and improving the prognosis of the course of cardiovascular disease [7].
Material and methods
Sixty-eight persons over 65 years of age, studying at the University of the Third Age in Warsaw, participated in the study. Women constituted the majority of the subjects (88% women, 12% men). After giving informed consent for participation in the study, persons meeting the inclusion criteria were included in the study (Consent of the Bioethics Committee of Warsaw Medical University to conduct research study no. KB/68/2009). The inclusion criteria were the following: age over 65 years, efficient locomotor system enabling exercise, lack of serious disease limiting survival to 6 months, stable course of the heart disease: condition after a myocardial infarction, over 6 months, condition after cardiac and vascular surgery, over 6 months, no hazardous heart rhythm disorders, stable values of arterial pressure. The participants of the project were divided into two groups: the test group consisting of 50 randomly selected persons aged 65 to 84 years (average age 70.7 years) who were subject to a physical exercise programme using the Nordic walking (NW) method, and the control group consisting of 18 randomly selected persons aged 65 to 81 years (average age 69.9 years) who did not take part in the physical exercise programme. The exclusion criteria were the following: age under 65 years, locomotor diseases preventing the introduction of physical exercise, unstable course of cardiovascular disease (recent myocardial infarction, malignant ventricular rhythm disturbances, heart failure in NYHA class III-IV, high/unstable values of arterial pressure), disease limiting survival to 6 months, mental disorders disabling cooperation, condition of up to 6 months after myocardial infarction, vascular interventions after cardiac surgery, lack of informed consent to participate in the study. Each training session would consist of 10 min warming up, 30 min of proper training with Nordic walking methods and 10 min of cooling down. The proper training was followed by heart rate monitoring with pulsometers. The target heart rate was established during a spiroergometry test prior to the study and was defined as 60-70% of maximal heart rate. The training sessions took place three times a week.
Research procedures
The test group consisted of 50 persons over 65 years of age. Before qualification for the exercise programme using aerobic exercise, every person underwent preliminary procedures which involved: medical interview, physical examination, resting ECG, spiroergometry examination, echocardiogram, 6-minute walk test (6MWT) and 24-hour ambulatory blood pressure monitoring (ABPM). The NW exercise schedule was prepared individually for each person after an spiroergometry examination and determination of the maximum exercise pulse (MEP) defined as 60-70% of the maximum pulse for a given age, including the anaerobic threshold. During the exercise, the pulse of each subject was monitored using a pulsometer. The intensity of exercise was limited by the pulse and individual performance of each subject. After completion of the 6-week training, check-up tests assessing the same parameters were performed. The control group consisted of 18 patients over 65 years of age with similar cardiovascular problems. This group underwent the same tests as the group undertaking physical activity excluding the 6-week exercise programme using the NW method. The subjects in the 2 groups were receiving similar drug treatment. The characteristics of both groups are shown in Table I.
Table I.
Clinical characteristics of persons included in the study
| Features | Test group n (%) | Control group n (%) |
|---|---|---|
| Gender | ||
| Women | 44 (88) | 16 (88.24) |
| Men | 6 (12) | 2 (11.76) |
| Age (average) [years] | 70.68 | 69.89 |
| Body mass index (average) [kg/m2] | 26.38 | 27.46 |
| Body surface area (average) [m2] | 1.75 | 1.74 |
| Ischaemic heart disease | 11(22) | 3 (16.67) |
| Hypertension | 30 (60) | 11 (61.11) |
| Dyslipidaemia | 28 (56) | 9 (50) |
| Smoking | 3 (6) | 2 (11.11) |
| Diabetes | 4 (8) | 3 (16.67) |
| Chronic obstructive pulmonary disease | 1 (2) | 1 (5.56) |
| Asthma bronchiale | 2 (4) | 1 (5.56) |
| Stroke | 0 | 0 |
| History of myocardial infarction | 0 | 0 |
| Osteoporosis | 1 (2) | 1 (5.56) |
| Hypothyroidism | 12 (24) | 4 (22.22) |
| Atrial fibrillation | 3 (6) | 2 (11.11) |
| Heart failure | 0 | 2 (11.11) |
Statistical analysis
A generalized estimating equation was used in the analysis of intervention for continuous data with normal distribution. The model includes correlations between observations from the same subject; in this case, these observations were repeated after a period of time. The assumption of normality of distributions of the analysed variables was verified using the Shapiro-Wilk test. Due to deviations from the normality assumptions, the data were logarithmically transformed and/or deviating observations were omitted in the analysis. The data distributions after transformation did not deviate from the normal distribution. The group identifier (test group vs. control group), the time of measurement (follow-up vs. screening) and the interaction between the group and the time of measurement were taken into account in the models. By means of the stepwise elimination method with level 0.1 for staying in the model, variables which were statistically significant at the level of 0.05 were selected. The effect of group and time and the interaction between the group and time effect were left in the models regardless of their statistical significance.
Results
No person dropped out of the study in either of the groups. The results obtained during the spiroergometry examinations and ABPM are presented in Tables II and III.
Table II.
Table II. Parameters of preliminary screening and final tests assessing physical performance obtained in the ergospirometry test and the 6-minute walk test
| Features | Preliminary screening parameters | Final test parameters | |||
|---|---|---|---|---|---|
| Test group | Control group | Test group | Control group | ||
| Time to anaerobic threshold [min] | Min | 2.33 | 3.50 | 2.17 | 2.67 |
| Max | 9.50 | 7.17 | 9.67 | 7.00 | |
| Mean | 5.15 | 5.37 | 4.97 | 5.11 | |
| SD | 1.74 | 1.09 | 1.53 | 1.27 | |
| Median | 5.17 | 5.58 | 4.83 | 5.25 | |
| Load at the anaerobic point [W] | Min | 26.00 | 43.00 | 28.00 | 33.00 |
| Max | 110.00 | 82.00 | 104.00 | 79.00 | |
| Mean | 60.45 | 62.11 | 57.86 | 57.50 | |
| SD | 17.25 | 10.99 | 15.16 | 11.81 | |
| Median | 60.00 | 63.50 | 56.50 | 59.00 | |
| Duration of effort [min] | Min | 2.50 | 4.28 | 3.72 | 2.67 |
| Max | 10.95 | 14.33 | 11.95 | 13.87 | |
| Mean | 7.09 | 7.10 | 8.11 | 6.47 | |
| SD | 1.93 | 2.22 | 1.81 | 2.37 | |
| Median | 7.11 | 6.61 | 8.15 | 6.48 | |
| Maximum effort load [W] | Min | 26.00 | 53.00 | 47.00 | 33.00 |
| Max | 119.00 | 153.00 | 129.00 | 149.00 | |
| Mean | 80.14 | 80.67 | 90.82 | 74.56 | |
| SD | 19.77 | 22.31 | 18.27 | 24.07 | |
| Median | 80.00 | 76.00 | 93.50 | 74.50 | |
| O2-pulse | Min | 4.20 | 4.60 | 3.40 | 3.30 |
| Max | 13.00 | 15.30 | 27.50 | 13.60 | |
| Mean | 8.75 | 8.50 | 9.97 | 8.85 | |
| SD | 2.31 | 3.13 | 3.68 | 2.82 | |
| Median | 8.35 | 7.90 | 9.35 | 9.35 | |
| VO2max [ml/kg/min] | Min | 6.80 | 10.90 | 6.20 | 4.20 |
| Max | 24.30 | 24.40 | 26.20 | 25.70 | |
| Mean | 15.38 | 14.83 | 17.48 | 14.56 | |
| SD | 3.85 | 3.53 | 3.61 | 5.15 | |
| Median | 15.70 | 13.90 | 17.20 | 14.65 | |
| 6MWT [m] | Min | 422.00 | 452.00 | 452.00 | 411.00 |
| Max | 708.00 | 795.00 | 782.00 | 783.00 | |
| Mean | 537.90 | 519.83 | 612.94 | 522.22 | |
| SD | 68.00 | 77.07 | 80.88 | 81.62 | |
| Median | 525.50 | 492.00 | 603.50 | 511.50 | |
6MWT – 6-minute walk test
Table III.
Parameters of the preliminary screening and final tests assessing blood pressure and heart rate in 24-hour ambulatory blood pressure monitoring
| Features | Preliminary screening parameters | Final test parameters | |||
|---|---|---|---|---|---|
| Test group | Control group | Test group | Control group | ||
| SBP [mm Hg] | Min | 110.00 | 101.00 | 108.00 | 103.00 |
| Max | 153.00 | 148.00 | 148.00 | 153.00 | |
| Mean | 134.61 | 122.04 | 129.67 | 122.26 | |
| SD | 13.11 | 12.76 | 11.79 | 13.79 | |
| Median | 136.00 | 121.00 | 133.00 | 119.00 | |
| DBP [mm Hg] | Min | 57.00 | 48.00 | 62.00 | 54.00 |
| Max | 88.00 | 93.00 | 95.00 | 90.00 | |
| Mean | 75.67 | 70.68 | 73.22 | 71.38 | |
| SD | 8.46 | 8.35 | 8.04 | 8.47 | |
| Median | 75.50 | 71.00 | 72.50 | 71.00 | |
| HR/24 h [beats/min] | Min | 57.00 | 57.00 | 49.00 | 60.00 |
| Max | 84.00 | 88.00 | 83.00 | 89.00 | |
| Mean | 70.24 | 74.56 | 69.14 | 71.56 | |
| SD | 6.02 | 8.95 | 6.68 | 7.94 | |
| Median | 71.00 | 77.00 | 70.00 | 71.00 | |
SBP – systolic blood pressure, DBP – diastolic blood pressure, HR/24 h – average heart rate in the 24-hour examination
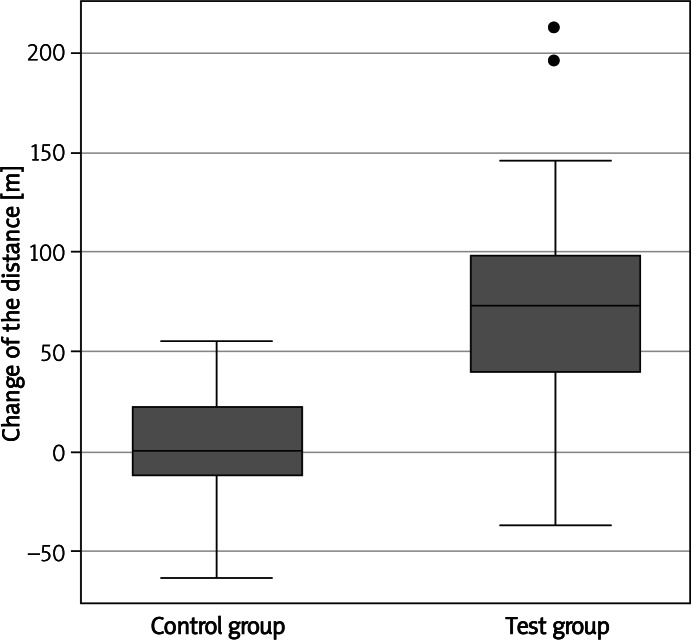
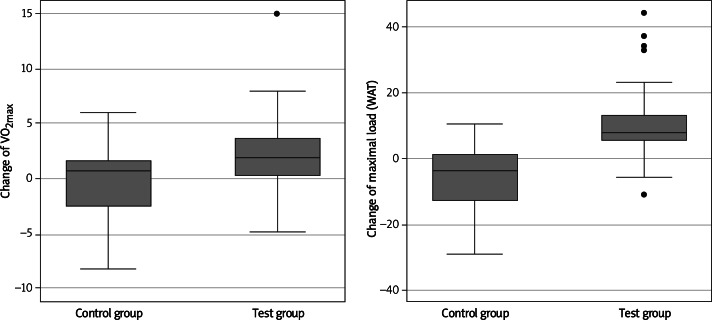
Persons participating in the NW exercise walked longer distances in the 6-minute walk test (on average by 75.04 m, median = 74.00, p = 0.00001) than persons from the control group (on average by 2.39, median = 0.50), Figure 1. Owing to the application of the exercise programme, in the test group, the duration of physical effort increased (on average by 1.02 min, median = 0.85, p = 0.0001), during the spiroergometry examination, compared with the control group, in which this time was slightly shortened (on average by 0.62 min, median = –0.42). The test group also achieved higher effort load values (on average by 10.68 W, median = –8.00, p = 0.0001) than the control group (on average, a decrease by 6.11 W, median = –4.00) (Figure 2) and the VO2max values changed significantly – the value of this parameter increased in the test group (on average by 2.10, median = 1.85, p = 0.0218) and decreased in the control group (mean value = –0.37, median = 0.70) (Figure 2).
Figure 1.
Change of the duration of effort and distance in the 6-minute walk test
Figure 2.
Change of VO2max and change of maximum effort load in the spiroergometry test
No differences occurred between the test group and the control group with respect to the remaining analysed parameters, such as the time to the anaerobic threshold, load at the anaerobic point, O2-pulse, maximum effort heart rate, maximum minute ventilation, and maximum arterial pressure during effort.
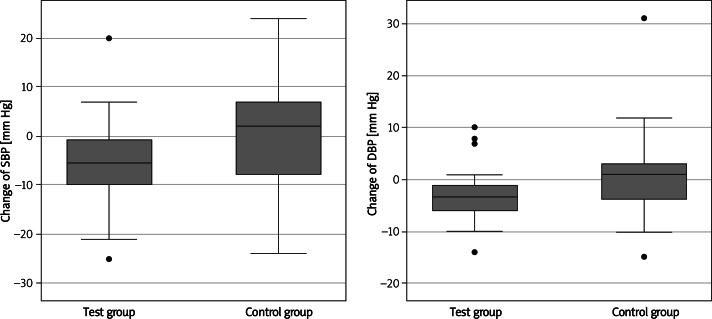
The analysis of results of the blood pressure parameters and the heart rate in an ABPM showed that the systolic blood pressure was lowered on average by 4.94 mm Hg (median = –5.50, p = 0.0352) in the test group, and it rose on average by 0.22 (median = 2.00) in the control group. The remaining parameters under analysis – change of the diastolic pressure and the average daily pulse – did not change significantly, although a tendency for lower values after the application of the NW exercise was observed in the active group (p = 0.054). The results of the blood pressure measurements are presented in Table III. The change in the tested parameters is shown in Figure 3.
Figure 3.
Change of the systolic (SBP) and diastolic blood pressure (DBP) in 24-hour ambulatory blood pressure monitoring
Discussion
Physical performance decreasing with age is a physiological phenomenon. There are more and more studies confirming the beneficial effect of physical exercise on the physical and mental spheres of elderly persons in the available literature [8–11].
The duration of effort and load increase after the application of a systematic physical exercise programme, as it is the body's adaptive response to physical effort, which is confirmed by the authors’ own study and by the results obtained by other researchers [8, 9, 12]. The results of the present authors’ research have shown an increase in the duration of effort in the spiroergometry examination after the completion of the exercise programme. Other researchers also noted the extension of the treadmill stress test (by 33%) among elderly persons (aged 80-92 years) after a 6-month exercise programme. Elderly persons taking part in the study were subjected to moderate-intensity endurance exercise 2 or 3 times a week. The training consisted of 20-30 min of proper effort preceded by a warm-up and finished by cool-down exercises [13].
The O2-pulse is considered to be a good parameter assessing the efficiency of the cardiovascular system during physical effort [14, 15]. In this study, no significant improvement in the O2-pulse was observed after the 6-week exercise programme. Other authors observed an increase in the O2-pulse parameter (by 8.6%) after a 6-month exercise programme in a study conducted in the United States and involving elderly persons aged over 80 years (80-92 years of age). No improvement in the O2-pulse parameter was observed during the first 4 stages of this study; only a 6-month training programme led to a significant improvement in this parameter [13]. Thus, it can be concluded that short-term physical training in elderly persons does not lead to an increase in the O2-pulse. Previously conducted research, which showed an increase in this index, used exercise programmes lasting 12 weeks and 6 months. There are too few studies including the O2-pulse index to make it possible to provide an unambiguous answer of what “dose” of exercise may have a significant effect on its improvement.
The influence of ageing on the ability to undertake physical effort varies greatly and depends on the individual condition and regular physical activity [16]. The maximal oxygen uptake (VO2max) is the highest between the 20th and 30th year of life and then it begins to fall at a rate of approximately 1% per year and depends on the individual level of physical activity (it decreases at a faster rate in persons with a sedentary lifestyle than in physically active persons) [17, 18]. It was proven that controlled physical effort of appropriate intensity (≥ 60% of the training VO2max), frequency (≥ 3 days during a week) and length (≥ 16 weeks) may significantly increase VO2max in healthy middle-aged and elderly persons. An extensive meta-analysis performed by researchers showed that the average increase in the VO2max after 16 to 20 weeks of exercise amounted to 3.8 ml/kg/min or 16.3%, compared with the control group of persons who did not exercise in the period in question. Better results in the improvement of VO2max were observed after a longer exercise period (from 20 to 30 weeks), but higher-intensity effort does not necessarily bring positive effects (at the level of > 70% VO2max) [19].
This study shows that systematic physical exercise lasting for a shorter period of time (6 weeks) can significantly improve the VO2max index (an increase by 2.1 ml/kg/min). Similar results were obtained in a study involving elderly men (average age: 68 years) in which exercise significantly improved the VO2max after 6 weeks (an increase by 2.4 ml/kg/min). Medium-intensity training sessions (70% VO2max) using a cycloergometer were held three times a week and each session lasted 45 min. The test group consisted of only 8 participants [20]. Another meta-analysis focusing on elderly persons showed that the length of a single training session is another significant factor affecting the improvement in physical performance. Exercise lasting over 30 min resulted in a higher increase in the VO2max (1.59 ml/kg/min) [21]. This study revealed that medium-intensity physical exercise has a significant influence on the increase of the VO2max (by 2.1 ml/ kg/min). A decrease in the VO2max is an independent risk factor for all causes of death due to cardiovascular disease and may contribute to premature death in a population of middle-aged and elderly persons [22]. Therefore, increasing the VO2max value by the application of physical training may be of significant clinical importance for persons over 65 years of age.
6MWT is currently commonly used for assessing the functional capacity of patients suffering from pulmonary and cardiovascular diseases [23]. It is also used as a prognostic factor of the incidence and fatality rates in patients with left ventricular dysfunction [24, 25], advanced heart failure [26] and chronic obstructive pulmonary disease [27]. Owen and Croucher examined the influence of a 12-week physical exercise programme in elderly patients (average age of 81 years) with heart failure. The group of persons who exercised were able to walk a longer distance (40.1 m more) than the control group in the 6MWT [28]. Studies performed on healthy elderly subjects (age of the test group 77.2 ±3.6) confirm the body's adaptive response to physical effort, which is proved by increasing the walking distance (by 10%) in the 6MWT after one-year long physical training [29]. Another study in which the influence of systematic training on the distance covered in the 6MWT involved the participation of 98 elderly women (58 in the test group and 40 in the control group) (> 60 years old) suffering from hypertension. The exercise programme conducted by Cunha et al. was similar to that used in the present study, as it consisted of the application of exercise (mostly walking) 3 times a week at an intensity ranging from 55% to 75% of the theoretical maximal pulse. The main difference lay in the length of the programme used and the type of walk. In the aforementioned study, the training sessions lasted 16 weeks, while in the present study training lasted 6 weeks. Both programmes resulted in the improvement of the distance covered in the 6MWT. In the study by Cunha et al., between the preliminary test and the final test the distance increased by 70.58 m (p < 0001) [30]. In the present study, despite a shorter exercise programme, it was possible to extend the distance in the 6MWT on average by 75.04 m (p < 0.00001). The comparable positive effect probably results from the type of walk used and NW may be a more effective type of effort than ordinary walking, which still needs to be confirmed by other studies.
The ageing process is connected with a higher frequency of hypertension, ischaemic heart disease, heart failure and reduced effort tolerance [31]. In this study, hypertension was present in 60% of the subjects from the test group and 61.11% of the persons from the control group.
Treatment of hypertension is one of the most important goals of the prevention of cardiovascular events [32]. It has been proven by numerous studies that regular physical activity is a non-pharmacological method of achieving a hypotensive effect, regardless of the subjects’ age. Systematic endurance exercises lasting 6 weeks contributed to the lowering of ambulatory systolic blood pressure (ASBP) by 5.50 mm Hg (p = 0.035) (24-hour measurement) and lowering of the ambulatory diastolic blood pressure (ADBP) by 3.50 mm Hg (p = 0.054) (24-hour measurement), compared with the control group. Studies by numerous authors have attempted to explain the influence of physical exercise on lowering of blood pressure (BP) in persons at various ages as well as in persons suffering from cardiovascular disease. So far, the hypotensive effect of physical training in persons suffering from hypertension has been fully documented by researchers the most.
The influence of endurance exercises on ABPM in persons with normal BP has not been examined in a detailed manner. The ABPM is found to predict future cardiovascular risk better than conventional blood pressure [33]. It has been shown in numerous studies [34–38] that BP decreased after the application of endurance exercises, but in some cases, no such relation was observed [39]. A meta-analysis of the randomized controlled studies on endurance training has shown that a decrease in BP is most noticeable in groups of persons suffering from hypertension (–6.9 mm Hg/–4.9 mm Hg), but a statistically significant decrease in BP was also observed in persons with normal BP values (–2.4 mm Hg/–1.6 mm Hg) and those with pre-hypertension (–1.7 mm Hg/–1.7 mm Hg). The results obtained applied to endurance training lasting 40 min on average, 3 times a week with an intensity of 65% HRmax for 16 weeks [40]. One of the studies assessing the influence of 16-week endurance training focused on elderly persons (average age 68.5 years) with normal BP. The intensity of exercise ranged from 50% to 85% of the maximum HR. The results of the analysis confirm the hypotensive effect of physical exercise [35].
Numerous studies confirm the lowering of the systolic blood pressure (SBP) owing to the application of a walking exercise programme [41–44]. Diastolic blood pressure (DBP) was successfully lowered in elderly persons in research by Cononie et al. and in middle-aged women in a study by Palmer [45, 46]. Other studies revealed reduced SBP and DBP after the application of an endurance walking programme [47–49]. The analysis of studies concerning the influence of a walking exercise programme on the BP value showed that differences of BP between the test group and the control group, after the completion of the training programme, ranged from –5.2 mm Hg to –11.0 mm Hg for SBP and from –3.8 mm Hg to –7.7 mm Hg for DBP [50].
Numerous studies confirm a reduction in BP after the introduction of systematic endurance exercises in elderly people. However, exercises must be performed on a regular basis as abandoning this routine is connected with loss of the hypotensive effect [13]. The population of senior citizens suffering from hypertension should engage in physical activity combined with appropriate diet as an element of non-pharmacological activity, because, as shown by the TONE study (Trial Of Non-Pharmacological Intervention in the Elderly), it is more effective in elderly persons than in young ones [51]. Regular physical exercise may prevent an increase in hypertension connected with the normal ageing process of the body.
The exercise used in the present study did not have a significant influence on the elderly persons’ HR. As revealed by other studies, physical exercise must be used for an extended period of time to achieve a reduction in this parameter. It was found that the resting HR reduction among elderly persons was greater and more significant in a group of subjects who had exercised for more than 30 weeks [19].
The number of participants of the study is relatively small. Additionally, 18% of the patients received β-adrenolytic agents, which could have influenced the results.
In conclusion, physical training is an effective method to improve the function of the cardiovascular system in elderly persons, who experience unfavourable changes within this system as a result of the ageing process. Systematic physical exercise (Nordic walking) limited by the pulse has a beneficial effect on the main parameters assessing the physical performance of elderly persons. Even a short 6-week programme of endurance exercises has a hypotensive effect in elderly persons over 65 years of age.
Acknowledgments
This research was supported by the Medical University of Warsaw, Poland and thanks to a European Union grant for young doctors, “Mazovia PhD Grant”, financed by the European Union Social Fund together with the state budget in accordance with the Integrated Operational Programme of Regional Development. The authors would like to thank Interplastic® and Medic-Mar® for granting the equipment.
References
- 1.The 2009 Ageing Report. Economic and budgetary projections for the EU-27 Member States (2008-2060) European Economy. 2009. http://ec.europa.eu/economy_finance/publications/publication14992_en.pdf, 21.02.2011.
- 2.Alencar NA, Ferreira MA, Bezerra JC, Vale RGS, Dantas EHM. Levels of physical activity and quality of life in elderly women practitioners of formal and non-formal physical activities. Acta Medica Lituanica. 2009;16:155–8. [Google Scholar]
- 3.World Health Organization (WHO) Geneva: WHO; 2004. Global strategy on diet and physical activity. www.who.int/gb/ebwha/pdf_files/WHA57/A57_9-en.pdf. Accessed 06.03.2011. [Google Scholar]
- 4.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295:1412–9. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 5.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 6.Allender S, Scarborough P, Peto V, et al. European cardiovascular disease statistics. Brussels: European Heart Network; 2008. p. 2008. [Google Scholar]
- 7.Kappagoda CT, Amsterdam EA. Improving guidelines for the management of coronary heart disease risk factors. Arch Med Sci. 2011;7:923–4. doi: 10.5114/aoms.2011.26599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallinen M, Sipilä S, Alen M, Suominen H. Improving cardiovascular fitness by strength or endurance training in women aged 76-78 years. A population-based, randomized controlled trial. Age Ageing. 2002;31:247–54. doi: 10.1093/ageing/31.4.247. [DOI] [PubMed] [Google Scholar]
- 9.Pogliaghi S, Terziotti P, Cevese A, Balestreri F, Schena F. Adaptations to endurance training in the healthy elderly: arm cranking versus leg cycling. Eur J Appl Physiol. 2006;97:723–31. doi: 10.1007/s00421-006-0229-2. [DOI] [PubMed] [Google Scholar]
- 10.Huang G, Gibson CA, Tran ZV, Osness WH. Controlled endurance exercise training and VO2max changes in older adults: a meta-analysis. Prev Cardiol. 2005;8:217–25. doi: 10.1111/j.0197-3118.2005.04324.x. [DOI] [PubMed] [Google Scholar]
- 11.Devereux K, Robertson D, Briffa NK. Effects of a water-based program on women 65 years and over. A randomised controlled trial. Austral J Physiother. 2005;51:102–8. doi: 10.1016/s0004-9514(05)70038-6. [DOI] [PubMed] [Google Scholar]
- 12.Delagardelle C, Feiereisen P, Autier P, Shita R, Krecke R, Beissel J. Strength/endurance training versus endurance training in congestive heart failure. Med Sci Sports Exerc. 2002;34:1868–72. doi: 10.1097/00005768-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Vaitkevicius PV, Ebersold C, Shah MS, et al. Effects of aerobic exercise training in community-based subjects aged 80 and older: a pilot study. J Am Geriatr Soc. 2002;50:2009–13. doi: 10.1046/j.1532-5415.2002.50613.x. [DOI] [PubMed] [Google Scholar]
- 14.Patterson RP, Remole WD. The response of the oxygen pulse during a stress test in patients with coronary artery disease. Cardiology. 1981;67:52–62. doi: 10.1159/000173228. [DOI] [PubMed] [Google Scholar]
- 15.Holverda S, Bogaard HJ, Groepenhoff H, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Cardiopulmonary exercise test characteristics in patients with chronic obstructive pulmonary disease and associated pulmonary hypertension. Respiration. 2008;76:160–7. doi: 10.1159/000110207. [DOI] [PubMed] [Google Scholar]
- 16.Amra B, Kelishadi R, Golshan M. Peak oxygen uptake of healthy Iranian adolescents. Arch Medi Sci. 2009;5:69–73. [Google Scholar]
- 17.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine position stand, Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–30. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 18.Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1:253–60. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang G, Shi X, Davis-Brezette JA, Osness WH. Resting heart rate changes after endurance training in older adults: a meta-analysis. Med Sci Sports Exerc. 2005;37:1381–6. doi: 10.1249/01.mss.0000174899.35392.0c. [DOI] [PubMed] [Google Scholar]
- 20.Murias JM, Kowalchuk JM, Paterson DH. Time course and mechanisms of adaptations in cardiorespiratory fitness with endurance training in older and young men. J Appl Physiol. 2010;108:621–7. doi: 10.1152/japplphysiol.01152.2009. [DOI] [PubMed] [Google Scholar]
- 21.Lemura LM, von Duvillard SP, Mookerjee S. The effects of physical training of functional capacity in adults. Ages 46 to 90: a meta-analysis. J Sports Med Phys Fitness. 2000;40:1–10. [PubMed] [Google Scholar]
- 22.Posner JD, McCully KK, Landsberg LA, et al. Physical determinants of independence in mature women. Arch Phys Med Rehabil. 1995;76:373–80. doi: 10.1016/s0003-9993(95)80664-4. [DOI] [PubMed] [Google Scholar]
- 23.Swisher A, Goldfarb A. Use of the six-minute walk/run test to predict peak oxygen consumption in older adults. Cardiopulm Phys Ther. 1998;9:3–5. [Google Scholar]
- 24.Milligan NP, Havey J, Dossa A. Using a 6-minute walk test to predict outcomes in patients with left ventricular dysfunction. Rehabil Nurs. 1997;22:177–81. doi: 10.1002/j.2048-7940.1997.tb02095.x. [DOI] [PubMed] [Google Scholar]
- 25.Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA. 1993;270:1702–7. [PubMed] [Google Scholar]
- 26.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–32. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 27.Kadikar A, Maurer J, Kesten S. The six-minute walk test: a guide to assessment for lung transplantation. J Heart Lung Transplant. 1997;16:313–9. [PubMed] [Google Scholar]
- 28.Owen A, Croucher L. Effect of an exercise programme for elderly patients with heart failure. Eur J Heart Fail. 2000;2:65–70. doi: 10.1016/s1388-9842(99)00067-7. [DOI] [PubMed] [Google Scholar]
- 29.Deley G, Kervio G, Van Hoecke J, Verges B, Grassi B, Casillas JM. Effects of a one-year exercise training program in adults over 70 years old: a study with a control group. Aging Clin Exp Res. 2007;19:310–5. doi: 10.1007/BF03324707. [DOI] [PubMed] [Google Scholar]
- 30.Cunha CRL, Ferreira MA, Bezerra JCP, Guerral I, Dantas EHM. Aerobic capacity of elderly women engagedin controlled physical activity. J Hum Kinet. 2010;23:63–9. [Google Scholar]
- 31.Wei JY. Age and the cardiovascular system. N Engl J Med. 1992;327:1735–9. doi: 10.1056/NEJM199212103272408. [DOI] [PubMed] [Google Scholar]
- 32.Barylski M, Małyszko J, Rysz J, Mysliwiec M, Banach M. Lipids, blood pressure, kidney – what was new in 2011? Arch Med Sci. 2011;7:1055–66. doi: 10.5114/aoms.2011.26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sethi A, Arora R. Ambulatory blood pressure as a predictor of cardiovascular risk. Arch Med Sci. 2009;5:3–9. [Google Scholar]
- 34.Fagard RH. Exercise is good for your blood pressure: effects of endurance training and resistance training. Clin Exp Pharmacol Physiol. 2006;33:853–6. doi: 10.1111/j.1440-1681.2006.04453.x. [DOI] [PubMed] [Google Scholar]
- 35.Jessup JV, Lowenthal DT, Pollock ML, Turner T. The effects of endurance exercise training on ambulatory blood pressure in normotensive older adults. Geriatr Nephrol Urol. 1998;8:103–9. doi: 10.1023/a:1008287320868. [DOI] [PubMed] [Google Scholar]
- 36.Ohkubo T, Hozawa A, Nagatomi R, et al. Effects of exercise training on home blood pressure values in older adults: a randomized controlled trial. J Hypertens. 2001;19:1045–52. doi: 10.1097/00004872-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Fortmann SP, Haskell WL, Wood PD. Effects of weight loss on clinic and ambulatory blood pressure in normotensive men. Am J Cardiol. 1988;62:89–93. doi: 10.1016/0002-9149(88)91370-7. [DOI] [PubMed] [Google Scholar]
- 38.Zemva A, Rogel P. Gender differences in athlete's heart: association with 24-h blood pressure. A study of pairs in sport dancing. Int J Cardiol. 2001;77:49–54. doi: 10.1016/s0167-5273(00)00417-4. [DOI] [PubMed] [Google Scholar]
- 39.Cox KL, Puddey IB, Morton AR, Burke V, Beilin LJ, McAleer M. Exercise and weight control in sedentary overweight men: effects on clinic and ambulatory blood pressure. J Hypertens. 1996;14:779–90. doi: 10.1097/00004872-199606000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46:667–75. doi: 10.1161/01.HYP.0000184225.05629.51. [DOI] [PubMed] [Google Scholar]
- 41.Lee LL, Arthur A, Avis M. Evaluating a community-based walking intervention for hypertensive older people in Taiwan: a randomized controlled trial. Prevent Med. 2007;44:160–6. doi: 10.1016/j.ypmed.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Moreau KL, Degarmo R, Langley J, et al. Increasing daily walking lowers blood pressure in postmenopausal women. Med Sci Sports Exerc. 2001;33:1825–30. doi: 10.1097/00005768-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Murphy MH, Murtagh EM, Boreham CA, Hare LG, Nevill AM. The effect of a worksite based walking programme on cardiovascular risk in previously sedentary civil servants. BMC Public Health. 2006;6:136. doi: 10.1186/1471-2458-6-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nemoto K, Genno H, Masuki S, Okazaki K, Nose H. Effects of high-intensity interval walking training on physical fitness and blood pressure in middle-aged and older people. Mayo Clinic Proc. 2007;82:803–11. doi: 10.4065/82.7.803. [DOI] [PubMed] [Google Scholar]
- 45.Cononie CC, Graves JE, Pollock ML, Phillips MI, Sumners C, Hagberg JM. Effect of exercise training on blood pressure in 70- to 79-yr-old men and women. Med Sci Sports Exerc. 1991;23:505–11. [PubMed] [Google Scholar]
- 46.Palmer LK. Effects of a walking program on attributional style, depression, and self-esteem in women. Percept Mot Skills. 1995;81:891–8. doi: 10.2466/pms.1995.81.3.891. [DOI] [PubMed] [Google Scholar]
- 47.Braith RW, Pollock ML, Lowenthal DT, Graves JE, Limacher MC. Moderate- and high-intensity exercise lowers blood pressure in normotensive subjects 60 to 79 years of age. Am J Cardiol. 1994;73:1124–8. doi: 10.1016/0002-9149(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 48.Higashi Y, Sasaki S, Sasaki N, et al. Daily aerobic exercise improves reactive hyperemia in patients with essential hypertension. Hypertension. 1999;33:591–7. doi: 10.1161/01.hyp.33.1.591. [DOI] [PubMed] [Google Scholar]
- 49.Lin JH, Fang CL. Effects of 12 weeks different walking training in borderline hypertensive adolescents. Bulletin of Physical Education. 2000;29:115–25. [Google Scholar]
- 50.Lee LL, Watson MC, Mulvaney CA, Tsai CC, Lo S. The effect of walking intervention on blood pressure control: a systematic review. Int J Nurs Stud. 2010;47:1545–6. doi: 10.1016/j.ijnurstu.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279:839–46. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]