Abstract
We conducted a cross-sectional analysis with 901 Parkinson’s disease (PD) patients in China to understand the epidemiological characteristics of PD-pain among Chinese patients. In addition, we searched PubMed and CHKD for epidemiological studies of pain and fatigue among PD patients from 1999 to 2011 to understand the prevalence of these symptoms around the world and associated clinical features. The 901 PD cases were recruited from 42 university affiliated hospitals randomly selected from seven provincial capitals across four economic-regions of China. We documented motor and non-motor symptoms via clinical examinations and questionnaire surveys. Using logistic regression models, we evaluated factors that were associated with PD-pain among these Chinese patients. Of the 901 Chinese patients, 269 (29.9%) had PD-related pain. After adjusting for confounders, only dyskinesias (OR =2.66) and depression (OR =2.88) were independently associated with PD-pain. The literature search identified a total of 16 eligible studies on PD-pain and 19 on PD-fatigue with various tools for symptom assessment. The data suggest a crude prevalence of 33.7% for PD-pain in Asia and 79.4% in Northern-Europe; the prevalence for PD-fatigue was 35.4% in Northern-Europe and 59.1% in Western-Europe. Interestingly, Northern-European PD patients reported the highest prevalence of pain, but the lowest prevalence of fatigue. These studies suggest that motor complications and depression are likely key predictors for PD-pain, while disease severity, depression and sleep disturbance are associated with PD-fatigue. More studies with standardized methods would be needed to better understand the prevalence of PD-pain and fatigue across various regions of the world.
Keywords: Parkinson’s disease, pain, fatigue, epidemiology
1. INTRODUCTION
Parkinson’s disease (PD) patients suffer from a variety of nonmotor symptoms which are often resistant to dopamine replacement therapies. Pain and fatigue are among these common non-motor symptoms but are often under recognized clinically. PD pain and fatigue could be driven by various etiologies (e.g. nociceptive, neurogenic psychogenic) and mechanisms (e.g. low dopa state pain, high dopa state pain) that often complicate etiological research and clinical management on these clinical features. Clinical research on PD pain and fatigue has also been affected by the use of different definitions and assessment tools which may explain some of the differences in the prevalence of PD pain and fatigue across various regions of the world. Finally, most research on PD pain and fatigue was conducted in Western countries, and little is known about the prevalence and associated factors of pain and fatigue among PD patients in China. We therefore analyzed the data of 901 PD patients in China to understand the epidemiological characteristics of pain among Chinese patients. In addition, we compared our results with those reported in other areas of the world as identified from a systematic literature search.
2. Methods
We conducted a cross-sectional study between 2005 and 2008 in China. We recruited a total of 901 PD patients from 42 university affiliated hospitals randomly selected from seven provincial capitals across four economic-regions of China, i.e., Gulf of Bohai (Beijing), Changjian River Delta (Shanghai and Hangzhou), Pearl River Delta (Guangzhou) and Mid-west area (Xian, Wuhan and Jinan). PD patients were diagnosed with the UK Parkinson’s Disease Society Brain Bank diagnostic clinical criteria for idiopathic PD. All patients were assessed for PD motor signs and non-motor symptoms, including a nonmotor symptom questionnaire that asked for unexplained pain in the past month [1]. The overall clinical assessment battery included the Unified Parkinson’s Disease Rating Scale (UPDRS) parts III (motor) and IV (complications), the Hoehn and Yahr (HY) stage, Epworth Sleepiness Scale (ESS) and Hamilton Depression Scale (HAMD-17). Categorical variables were compared with Chi square tests; factors associated with pain were analyzed with multivariate logistic regression models and odds ratios (ORs) and 95% confidence intervals (CIs) were reported. Highly correlated clinical factors (i.e., PD duration and HY stage) were not simultaneously included in the same regression mode to avoid co-linearity. All statistical tests were two sided with alpha of 0.05, the Ethics Committee on human experimentation at the Peking Union Medical College Hospital approved the study and written consent was obtained from all study participants.
To understand the prevalence of PD pain and fatigue in different areas of the world, we searched PubMed for English articles that reported pain and fatigue of PD from 1999 to 2011. Articles in Chinese were identified by searching the China Hospital Knowledge Database (CHKD). Epidemiological studies that reported the prevalence of pain or fatigue in more than 40 PD patients were reviewed. The overall crude prevalence of PD pain or fatigue in each geographic region (i.e., Northern Europe, Western Europe, America, and Asia) was calculated by combining data from individual studies.
3. Results
We identified a total of 901 PD patients from four economic-regions in China and 562 (62.4%) of them were men. The average age at survey was 65.4±10.7 years with a mean disease duration of 5.3±4.4 years. Of these patients, 560 (62.2%) were classified as HY stage II, 737 (81.8%) received L-dopa with a median dose of 375 mg/day, 269 (29.9%) reported unexplained pain. Univariate analyses showed that PD pain was significantly associated with gender, L-dopa use, dyskinesias, depression, and the HY stage. However, in the multivariate regression model, only dyskinesias (OR =2.66, 95% CI 1.50–4.73) and depression (OR =2.88, 95% CI1.87–4.44) were independently associated with PD pain among Chinese patients (Table 1).
Table.
Demographic and clinical features of Parkinson patients with and without pain
| PD with Pain (n=269) | PD without Pain (n=632) | p value | Multivariate analysis | ||
|---|---|---|---|---|---|
|
|
|
||||
| No. (%) | No. (%) | OR (95%CI) | p value | ||
| Age at enrollment (vs. <65 years) | 128 (47.6) | 274 (43.4) | 0.37 | Reference | 0.46 |
|
| |||||
| 65–74 years | 92 (34.2) | 220 (34.8) | 0.88 (0.63–1.23) | ||
|
| |||||
| ≥75 years | 49 (18.2) | 135 (21.8) | 0.78 (0.52–1.17) | ||
|
| |||||
| Male, n (%) | 151 (56.1) | 411 (65.0) | 0.01 | 0.74 (0.54–1.01) | 0.06 |
|
| |||||
| Education, years (vs 0–6) | 54 (20.1) | 150 (23.7) | 0.48 | Reference | 0.13 |
|
| |||||
| 7–12 | 125 (46.5) | 277 (43.8) | 1.39 (0.93–2.09) | ||
|
| |||||
| ≥13 | 90 (33.5) | 205 (32.4) | 1.54 (1.00–2.37) | ||
|
| |||||
| PD duration, years (vs <5) | 151 (56.1) | 400 (63.3) | 0.10 | Reference | 0.51 |
|
| |||||
| 5–9 | 82 (30.5) | 153 (24.2) | 1.17 (0.82–1.66) | ||
|
| |||||
| ≥10 | 36 (13.4) | 79 (12.5) | 0.89 (0.55–1.45) | ||
|
| |||||
| HY stage (vs stage I) | 44 (20.4) | 129 (16.4) | 0.01 | ||
|
| |||||
| stage II | 159 (63.5) | 401 (59.1) | |||
|
| |||||
| stage III | 66 (16.1) | 102 (24.5) | |||
|
| |||||
| L-dopa use | 38 (14.1) | 126 (19.9) | 0.04 | Reference | 0.12 |
|
| |||||
| Yes | 231 (85.9) | 506 (80.1) | 1.40 (0.91–2.15) | ||
|
| |||||
| Motor fluctuation (vs no) | 218 (81.0) | 544 (86.4) | 0.26 | Reference | 0.75 |
|
| |||||
| Yes | 51 (19.0) | 86 (13.6) | 1.08 (0.69–1.68) | ||
|
| |||||
| Dyskinesias (vs no) | 135 (87.4) | 603 (95.4) | <0.001 | Reference | <0.001 |
|
| |||||
| Yes | 34 (12.6) | 29 (4.6) | 2.66 (1.50–4.73) | ||
|
| |||||
| Depression (vs no) | 44 (79.2) | 46 (91.5) | <0.001 | Reference | <.0001 |
|
| |||||
| Yes | 56 (20.8) | 54 (8.5) | 2.88 (1.87–4.44) | ||
HY stage was not included in the multivariate analysis due to colinearity. When HY stage, but not PD duration, was included in the multivariate logistic regression model, the OR comparing higher HY stage with stage I was 1.11 (95%CI 0.74–1.66) for stage II, 1.59 (95%CI 0.95–2.68) for stage III
Though literature search, a total of 50 articles on PD pain and 59 on PD fatigue was identified. From these, 16 articles on pain from 13 countries and 19 articles on fatigue from 16 countries met our selection criteria and were therefore reviewed. In these studies, the most frequently used instruments for pain assessment were visual analogue scale (VAS), Nonmotor Symptom Questionnaire (NMSQ), Brief Pain Inventory (BPI) and Leeds Assessment of Neuropathic Symptoms and Signs (LANSS). For PD fatigue, the most commonly used instruments included Fatigue Severity Scale (FSS), followed by Parkinson’s Fatigue Scale (PFS), Multidimensional Fatigue Inventory (MFI), and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F).
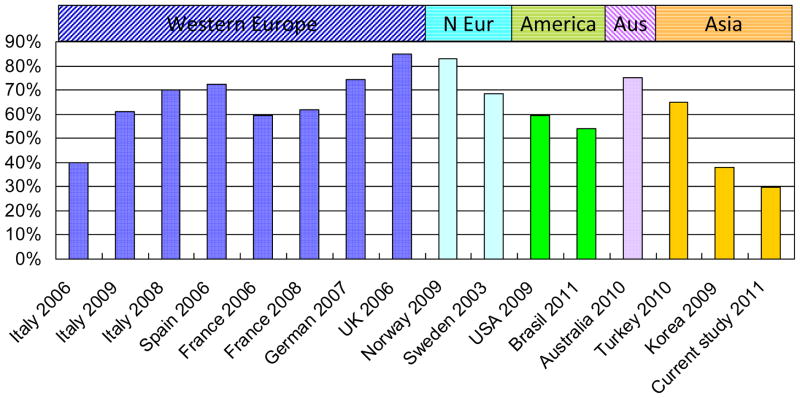
The 16 articles on PD pain included a total of 4,183 PD patients. Of these papers, the proportion of male patients range from 32.8% to 76.7%, and majority received L-dopa treatment (43.8–100%) and had motor complications (18–100%). Their average age of PD patients ranged from 54.8 to 74.3 years with mean PD durations from 5.1 to 11 years and average UPDRS β score from 10.2 to 27.7. The reported crude prevalence of pain varied from 29.9% in China (the current study) to >80% in Norway and UK [2–4] (Fig. 1). The prevalence of pain appeared to be higher in Northern Europe (68.4–83% for Norway and Sweden) [4–5] than in Asian countries (29.9–37.8% for China and Korea) [6–7]. Seven studies used the Ford pain category [8] to describe the types of pain and found musculoskeletal pain (36.8–74.8%) was most commonly reported, followed by dystonic pain (6.7–66%), radicular- neuropathic pain (4.7–24.3%), central neuropathic pain ( 4.3–12.7%) and akathisia (0–4.7%) [2–4,6–7,9–11]. More than one type of pain was reported by 12.7–29% of the patients.[2–4,6,7,11] The location of pain was most commonly noticed in the shoulder (10–80%), legs (19.9–78.7%), and back (15.7–74.3%) [2–3,5,11]. About one-quarter of PD patients who had experienced pain reported pain onset before the initiation of antiparkinsonian therapy [11].
Figure 1.
Worldwide prevalence of pain in PD patients
* N Eur: Northern Europe; Aus: Australia; References: 2–7,9–11,23–26
Several epidemiological studies found higher frequency of pain in PD patients than in controls. For example, in a large analysis of 450 PD cases and 98 controls with other chronic conditions in France, 39.3% of PD patients suffer from PD related pain, and 26% suffer from pain unrelated to PD; further PD patients were twice as likely to have pain than patients of other chronic illness [10]. In another case control study, pain was also twice more likely occurred after the reference age among PD patients than in controls, largely due to cramping and central neuropathic pain [11]. However, an international study failed to find that pain were more prevalent in PD patients compared to controls[1].
Eight studies [2–4,7,9–11], including the current one, used logistic regression analysis to examine clinical factors that were independently associated with PD-pain. A significant association with PD pain was reported for dyskinesia (OR=2.66–7.7 ) by 3 of 4 studies [2,11], for motor fluctuation (OR=2.8–6.3 ) by 3 of 5 studies [2,10,11], and for depression (OR=2–7.8) by 3 of 6 studies [9–10]. A few studies also showed that female gender [4], younger age [10], daily levodopa dose [2] and HY stage [3,11] were associated with PD pain. None of the studies however reported a significant association of PD pain with disease duration.
Studies on PD fatigues included a total of 3,501 patients with average age at survey ranging from 56.9 to 73.6 years, mean disease duration from 3.9 to 12.7 years, mean HY stage from 1.9 to 3.8, and mean UPDRS motor score from 21.5 to 35.9. Of these patients, most (41.1–89%) used levodopa (mean dosage of 464.5–811mg / day) alone or with other antiparkinsonian agents. The prevalence of fatigue ranged from 20.3% in Netherlands [12] to 72.5% in Canada [13] (Fig. 2). The prevalence seems to be lower in Northern Europe (20.3–50% for Netherlands, Norway and Sweden) [12,14–15], than in Western Europe (56–67.6% for France, Switzerland, Italy, and Spain) [16–18]. In America, the prevalence was 37% in USA, 41.4–51.8% in Brazil and 72.5% in Canada [13]. Studies in Asia reported prevalence ranges from 27.1% to 64.8% [6,19]. Case-control studies also reported higher prevalence of fatigue among PD patients than among controls. In a large study in Norway, 44.2% of PD cases reported fatigue as compared with 18% controls [20].
Figure 2.
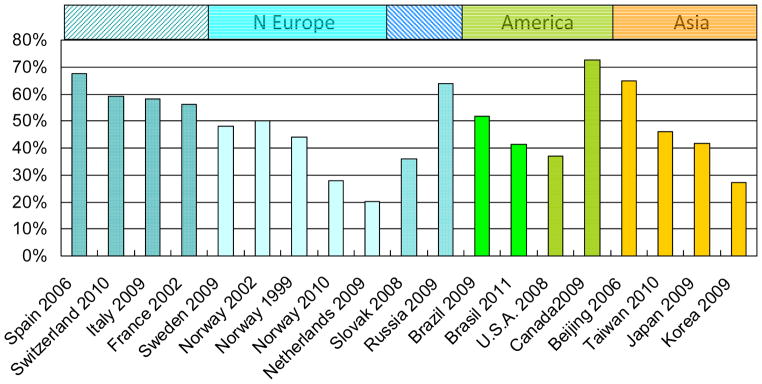
Worldwide prevalence of fatigue in PD patients
*W: Western; N: Northern; E Eur: Eastern Europe; References: 6,12–18,23,27–30
Nine studies further evaluated the associations between clinical factors and PD fatigue by comparing PD patients with and without fatigue [12,14–15,17–18,20]. Most of them found that depression [12,14–15,18,20] and disease severity [14–15,17] was significantly associated with PD fatigue, but a couple studies also reported associations with female gender and sleep disturbance[14]. Two studies further reported that the axial symptoms were associated with higher occurrence of PD fatigue, whereas amantadine treatment with a lower occurrence [15,18].
4. Discussion
It is increasingly recognized that a better understanding of PD nonmotor symptoms has great implications for understanding PD etiology, improving clinical management, and quality of life in PD patients. However, only a small number of studies have evaluated the prevalence of various nonmotor symptoms in PD patients or in the general elderly populations, and evaluated clinical and demographic factors that were associated with the presence of these symptoms. Available studies also often differed greatly in case identification and recruitments, sample size, symptom assessment strategies (e.g. PD-related pain or pain undefined), and detailed clinical classifications (e.g. mental and physical, overlaps of symptoms with neuropsychiatric disturbances) and analytical methods. Comparison of study results is further complicated by various cultural and clinical practice differences across different regions of the world.
With published data, we found that the prevalence of PD pain appeared to be lower among PD patients in Asia (33.7%) than in Northern Europe (79.4%), with a remarkable difference of 57.6%. For PD fatigue, the prevalence was the lowest in Northern Europe (35.4%) and the highest in Western Europe (59.1%) with a 40.1% difference. Interestingly, Northern European PD patients reported the highest prevalence of pain (79.4%) but the lowest prevalence of fatigue (35.4%). Although cross cultural comparison of these nonmotor symptoms is difficult for reasons discussed above, future studies should look into these remarkable differences.
Available studies on clinical factors associated with pain or fatigue were mostly cross-sectional analyses, making it impossible to deduce their causal relationships with either pain or fatigue [21]. Nevertheless majority of the studies found that motor fluctuation, dyskenisias, depression were most significantly associated with the prevalence of PD pain, with little difference across study regions. This may in part explained the lower pain prevalence among Asian patients as they often took levodopa at lower doses and thus had fewer motor complications. On the other hand, disease severity, depression and excessive daytime sleepiness were often associated with PD fatigue across various studies. These results suggest that the cause of fatigue may be central probably as a result of decreased physical functions or external stimulation followed by motor impairments. However, pain may be more neurogenic [8], which could be improved through the relief of motor symptom and motor complications. It was also reported that PD patients might have a reduced pain threshold which could be affected by levodopa [22].
Future studies should further examine the prevalence and incidence of nonmotor symptoms in PD patients as well as in older adults without PD, and to understand their etiological and clinical relevance in PD research. These studies are preferably done with more standardized instruments, larger sample sizes, and a prospective design.
References
- 1.Chaudhuri KR, Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord. 2006;21:916–23. doi: 10.1002/mds.20844. [DOI] [PubMed] [Google Scholar]
- 2.Tinazzi M, Del Vesco C, Fincati E, Ottaviani S, Smania N, Moretto G, et al. Pain and motor complications in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:822–5. doi: 10.1136/jnnp.2005.079053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee MA, Walker RW, Hildreth TJ, Prentice WM. A survey of pain in idiopathic Parkinson’s disease. J Pain Symptom Manage. 2006;32:462–9. doi: 10.1016/j.jpainsymman.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Beiske AG, Loge JH, Rønningen A, Svensson E. Pain in Parkinson’s disease: Prevalence and characteristics. Pain. 2009;141:173–7. doi: 10.1016/j.pain.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Broetz D, Eichner M, Gasser T, Weller M, Steinbach JP. Radicular and nonradicular back pain in Parkinson’s disease: a controlled study. Mov Disord. 2007;22:853–6. doi: 10.1002/mds.21439. [DOI] [PubMed] [Google Scholar]
- 6.Cheon SM, Park MJ, Kim WJ, Kim JW. Non-motor off symptoms in Parkinson’s disease. J Korean Med Sci. 2009;24:311–4. doi: 10.3346/jkms.2009.24.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanagasi HA, Akat S, Gurvit H, Yazici J, Emre M. Pain is common in Parkinson’s disease. Clin Neurol Neurosurg. 2011;113:11–3. doi: 10.1016/j.clineuro.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Ford B. Pain in Parkinson’s disease. Mov Disord. 2010;25 (Issue S1):S98–S103. doi: 10.1002/mds.22716. [DOI] [PubMed] [Google Scholar]
- 9.Santos-García D, Abella-Corral J, Aneiros-Díaz Á, Santos-Canelles H, Llaneza-González MA, Macías-Arribi M. Pain in Parkinson’s disease: prevalence, characteristics, associated factors, and relation with other non motor symptoms, quality of life, autonomy, and caregiver burden. Rev Neurol. 2011;52:385–93. [PubMed] [Google Scholar]
- 10.Nègre-Pagès L, Regragui W, Bouhassira D, Grandjean H, Rascol O DoPaMiP Study Group. Chronic pain in Parkinson’s disease: the cross-sectional French DoPaMiP survey. Mov Disord. 2008;23:1361–9. doi: 10.1002/mds.22142. [DOI] [PubMed] [Google Scholar]
- 11.Defazio G, Berardelli A, Fabbrini G, Martino D, Fincati E, Fiaschi A, et al. Pain as a nonmotor symptom of Parkinson disease: evidence from a case-control study. Arch Neurol. 2008;65:1191–4. doi: 10.1001/archneurol.2008.2. [DOI] [PubMed] [Google Scholar]
- 12.Elbers R, van Wegen EE, Rochester L, Hetherington V, Nieuwboer A, Willems AM, et al. Is Impact of Fatigue an Independent Factor Associated with Physical Activity in Patients with Idiopathic Parkinson’s Disease? Mov Disord. 2009;24:1512–8. doi: 10.1002/mds.22664. [DOI] [PubMed] [Google Scholar]
- 13.Mendonca DA, Menezes K, Jog MS. Methylphenidate Improves Fatigue Scores in Parkinson Disease: A Randomized Controlled Trial. Mov Disord. 2007;22:2070–6. doi: 10.1002/mds.21656. [DOI] [PubMed] [Google Scholar]
- 14.Beiske AG, Svensson E. Fatigue in Parkinson’s disease: a short update. Acta Neurol Scand. 2010;122 (Suppl 190):78–81. doi: 10.1111/j.1600-0404.2010.01381.x. [DOI] [PubMed] [Google Scholar]
- 15.Hagell P, Brundin L. Towards an understanding of fatigue in Parkinson disease. J Neurol Neurosurg Psychiatry. 2009;80:489–492. doi: 10.1136/jnnp.2008.159772. [DOI] [PubMed] [Google Scholar]
- 16.Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, et al. Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology. 2002;59:408–13. doi: 10.1212/wnl.59.3.408. [DOI] [PubMed] [Google Scholar]
- 17.Valko PO, Waldvogel D, Weller M, Bassetti CL, Held U, Baumann CR. Fatigue and excessive daytime sleepiness in idiopathic Parkinson’s disease differently correlate with motor symptoms, depression and dopaminergic treatment. European Journal of Neurology. 2010;17:1428–36. doi: 10.1111/j.1468-1331.2010.03063.x. [DOI] [PubMed] [Google Scholar]
- 18.Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The Priamo Study: A Multicenter Assessment of Nonmotor Symptoms and Their Impact on Quality of Life in Parkinson’s disease. Mov Disord. 2009;24:1641–9. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Feng T, Liu P, Wang XM, Chen B. Relationship between fatigue and motor dysfunction for Parkinson’s disease. Chin J Rehabil Theory Pract. 2009;15:1150–2. [Google Scholar]
- 20.Karlsen K, Larsen JP, Tandberg E, Jørgensen K. Fatigue in patients with Parkinson’s disease. Mov Disord. 1999;14:237–41. doi: 10.1002/1531-8257(199903)14:2<237::aid-mds1006>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P. Parkinson’s disease: The non-motor issues. Parkinsonism Relat Disord. 2011 Jul 7; doi: 10.1016/j.parkreldis.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Stoessl AJ. Functional imaging studies of non-motoric manifestations of Parkinson’s Disease. Parkinsonism Relat Disord. 2009;15 (Suppl 3):S13–6. doi: 10.1016/S1353-8020(09)70771-0. [DOI] [PubMed] [Google Scholar]
- 23.Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24:1641–9. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 24.Quittenbaum BH, Grahn B. Quality of life and pain in Parkinson’s disease: a controlled cross-sectional study. Parkinsonism Relat Disord. 2004;10:129–36. doi: 10.1016/j.parkreldis.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Ehrt U, Larsen JP, Aarsland D. Pain and its relationship to depression in Parkinson disease. Am J Geriatr Psychiatry. 2009;17:269–75. doi: 10.1097/jgp.0b013e31818af7ef. [DOI] [PubMed] [Google Scholar]
- 26.LETRO Grace Helena, QUAGLIATO, VIANA MAB Elizabeth, Aparecida Maura. Pain in Parkinson’s disease. Arq Neuropsiquiatr. 2009;67(3-A):591–4. doi: 10.1590/s0004-282x2009000400003. [DOI] [PubMed] [Google Scholar]
- 27.Schifitto G, Friedman JH, Oakes D, Shulman L, Comella CL, Marek K, et al. Fatigue in levodopa-naive subjects with Parkinson disease. Neurology. 2008;71:481–5. doi: 10.1212/01.wnl.0000324862.29733.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okuma Y, Kamei S, Morita A, Yoshii F, Yamamoto T, Hashimoto S, et al. Fatigue in Japanese patients with Parkinson’s disease: a study using Parkinson fatigue scale. Mov Disord. 2009;24:1977–83. doi: 10.1002/mds.22731. [DOI] [PubMed] [Google Scholar]
- 29.Kummer A, Scalzo P, Cardoso F, Teixeira AL, et al. Evaluation of fatigue in Parkinson’s disease using the Brazilian version of Parkinson’s Fatigue Scale. Acta Neurol Scand. 2011;123:130–6. doi: 10.1111/j.1600-0404.2010.01364.x. [DOI] [PubMed] [Google Scholar]
- 30.Havlikova E, van Dijk JP, Rosenberger J, Nagyova I, Middel B, Dubayova T, et al. Fatigue in Parkinson’s disease is not related to excessive sleepiness or quality of sleep. J Neurol Sci. 2008;170:107–13. doi: 10.1016/j.jns.2008.02.013. [DOI] [PubMed] [Google Scholar]