Abstract
To overcome the limitations of existing models, we developed a novel experimental in vivo platform for replacing mouse liver with functioning human liver tissue. To do this, a herpes simplex virus type 1 thymidine kinase (HSVtk) transgene was expressed within the liver of highly immunodeficient NOG mice (TK-NOG). Mouse liver cells gancyclovir (GCV), and transplanted human liver cells are stably maintained within the liver (humanized TK-NOG) without exogenous drug. The reconstituted liver was shown to be a mature and functioning “human organ” that had zonal position-specific enzyme expression and a global gene expression pattern representative of mature human liver; and could generate a human-specific profile of drug metabolism. The ‘humanized liver’ could be stably maintained in these mice with a high level of synthetic function for a prolonged period (8 months). This novel in vivo system provides an optimized platform for studying human liver physiology, including drug metabolism, toxicology, or liver regeneration.
Keywords: humanized mouse, liver reconstitution, herpes simplex virus type 1 thymidine kinase (HSVtk), drug metabolism
We [1] and other groups [2,3] have produced immunocompromised mice with human liver tissue as model system for analysis of drug metabolism and liver regeneration. In several models, human liver cells are transplanted into immunodeficient mice that express a urokinase-type plasminogen activator (uPA) transgene in their liver. Remarkably, human-specific hepatitis viruses can infect these mice [2,3,4]; their reconstituted livers express enzymes found in human liver cells [5]; and they can generate human-specific metabolites of test substrates, including steroids [6,7,8,9,10]. Although uPA expression facilitates the growth of transplanted human liver cells; it causes continuing and progressive damage to liver parenchymal cells, possibly via activation of plasminogen, which regulates the activity of matrix metalloproteinases that are critical for liver cell growth. Thus, these uPA-dependent models have very significant disadvantages that limit their utility for many applications, including a very poor breeding efficiency, renal disease, and a very narrow time window for transplantation before the mice succumb to their bleeding diathesis. A fumarylacetoacetate hydrolase (Fah) knockout mouse [11] has also been utilized for this purpose. In some instances, this model also requires (virus-mediated) uPA expression in liver to facilitate human hepatocyte transplantation, and thus has the same limitations. Fah mice also develop liver carcinomas as a consequence of their type I tyrosinemia, and continued or intermittent drug treatment after humanization is required to suppress the development of liver cancer, which enables their long term survival [4]. As a consequence, analyses of drug metabolism or liver regeneration in these models are confounded by the ongoing liver pathology or by the requirement for continued drug treatment. Therefore, we utilized a substantially different approach to overcome these limitations. The targeted expression of the a herpes simplex virus type 1 thymidine kinase (HSVtk) in the liver of severely immunodeficient NOG mice enabled mouse liver to be stably replaced with mature and functional human liver tissue in the absence of ongoing drug treatment.
Materials and Methods
Transgenic mice, human liver cell transplantation and drug biotransformation analysis
The herpes simplex virus type 1 thymidine kinase (UL23 or HSVtk) gene expression unit was constructed as in Fig. S1A. A vector-free 4.4-kb HSVtk expression fragment was microinjected into fertilized NOD/Shi strain mouse eggs using standard methods. For further information about the creation and breeding of the TK-NOG strain, human liver cell transplantation, and the drug biotransformation studies, see Supplementary Materials and Methods. This study was performed in accordance with institutional guidelines and was approved by the Animal Experimentation Committee of the Central Institute for Experimental Animals.
Histology and immunohistochemistry
Formalin-fixed and paraffin embedded (5-μm) sections were used for immunohistochemical staining with Cytokeratin (8/18) (h-CK8/18), HLA class I-A, B, C, asialoglycoprotein receptor 1 (ASGR1), albumin, glutamine synthase (GS) antibodies. To estimate the replacement index (RI), which is the percentage of donor human liver cells in recipient livers, the ratio of the area occupied by h-CK8/18-positive cells to the entire area examined in immunohistochemical sections of three to five lobes was measured.
Immunoblotting
Human albumin, complement C3 protein, transferrin and ceruloplasmin secretion was analyzed by Immunoblotting with specific primary antibodies and horseradish peroxidase-labeled secondary antibodies. The general information about the antiboodies used for detection of the human protein are provided in the Supplementary Materials and Methods.
Oligonucleotide array hybridization
Global gene expression was analyzed using the HG-U133A Plus 2 GeneChip array (Affymetrix Inc., Santa Clara, CA). Signal intensity for each transcript (background subtracted and adjusted for noise) and detection call (present, absent, or marginal) were determined using Affymetrix Expression Console Software (Affymetrix Inc.). The signal was normalized by house keeping gene, the human 18S rRNA gene (10098_M_at probe). The MIAME compliant microarray data was deposited in the Center for Information Biology gene EXpression database (CIBEX) at DDBJ (Japan)(CIBEX Accession: CBX102).
Statistical Analyses
Statistical analyses were performed with the Prism 5 software (GraphPad Software, CA, USA) and SAS preclinical package software ver. 5.0 (SAS Institute, Tokyo, Japan).
Results
A reconstituted ‘humanized liver’ in TK-NOG mice can be stably maintained
Targeted HSVtk expression has previously been used to ablate specific cell types in transgenic mice [12,13,14]. Therefore, we used an albumin promoter to drive the liver-specific expression of an HSVtk transgene in severely immunodeficient NOG mice [15] to produce TK-NOG mice. Administration of GCV, a drug that is not toxic to human or mouse tissues, induces tissue-specific ablation of transgenic liver parenchymal cells. Since HSVtk catalyzes GCV phosphorylation, which is the rate-limiting step that cannot be performed in mammalian cells lacking this transgene, liver cells expressing the transgene are selectively destroyed. The HSVtk transgene construct, mouse breeding, protocol variables, and the properties of TK-NOG mice are described in the Supplementary Information and in Fig. S1. We developed an initial protocol that enabled transplanted human liver cells to replace mouse liver. A dose of GCV (0.5 to 5 mg/kg I.P) that is not toxic to human or mouse tissues was administered on days 7 and 5 prior to transplantation, and 106 human liver cells were transplanted via intra-splenic injection. Despite using a non-optimized protocol in the initial pilot studies, a substantial amount of human albumin (hAlb) was detected in the plasma obtained from all 123 TK-NOG recipients after human liver cell transplantation, and the hAlb levels increased steadily to a maximal plasma concentration of 5.9 mg/mL (average 1.5 mg/mL; Table 1; Fig. S2A). The extent of human liver replacement was highly correlated with the measured hAlb levels (r2 = 0.9471; Fig. S2B), and the engrafted human liver cells were incorporated into the existing liver in recipient (‘humanized’ TK-NOG) mice (Fig. 1A). After optimization of the variables (age of mice at time of transplantation, dose/timing of GCV administration) as described in the Supplementary Information and Fig. S3, the hAlb concentration (average 3.3 mg/mL) and level of human engraftment (average 43%) in TK-NOG mice was substantially increased (Table 1). It has recently been reported that transplantation of an increased number of human cells increases the level of human liver chimerism in Fah−/− model [4]. However, the average level of human reconstitution (43%) in TK-NOG liver is already at or above that obtained when this modification was used in the Fah−/− mouse. However, it is possible that increasing the number of transplanted human cells could further increase liver chimerism in TK-NOG mice.
Table 1.
The amount of human albumin (hAlb) in plasma and extent of human liver replacement was measured after TK-NOG mice were transplanted with human liver cells using non-optimized pilot or optimized protocols. The extent of human liver chimerism was estimated as a function of the hAlb concentration, which was shown to correlate with the extent of human liver replacement. Protocol optimization (age at time of transplantation and GCV regimen) significantly increased the extent of humanization relative to that obtained in the pilot studies (***Mann-Whitney test, difference of P<0.0001).
| Pilot | Optimized | |||
|---|---|---|---|---|
|
| ||||
| Mice (n) | hAlb (mg/mL) | Mice (n) | hAlb (mg/mL) | Chimerism (%) |
| 39 | 0.2 | 5 | 0.8 | 13.4 |
| 26 | 0.7 | 14 | 1.6 | 22.5 |
| 29 | 1.5 | 8 | 3.3 | 41.9 |
| 18 | 3.2 | 9 | 4.5 | 55.7 |
| 8 | 4.7 | 2 | 6.9 | 83.1 |
| 3 | 5.7 | 4 | 7.8 | 93.4 |
| Total, 123 | Average, 1.5 | Total, 43 | Average, 3.3*** | Average, 42.5 |
Figure 1.
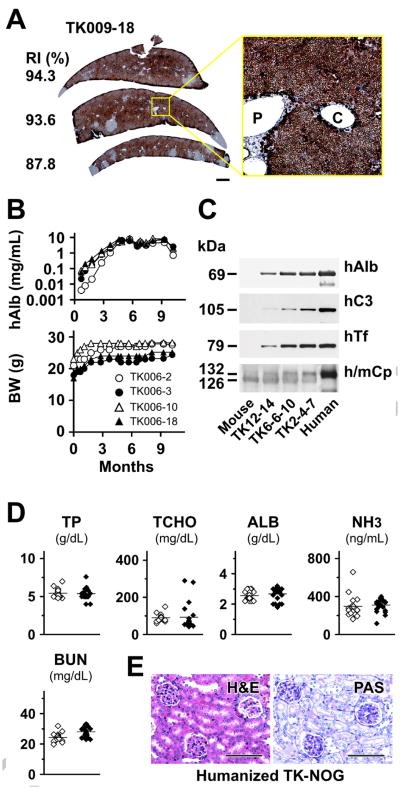
(A) Reconstituted ‘humanized liver’ in TK-NOG mouse. Immunohistochemical staining of sections obtained from TK-009-18 liver using h-CK8/18 antibody. The RI (%) for each section is indicated. Scale bar: 1 mm. An enlarged view of the boxed area is shown in the inset right. Scale bars: 100 μm. P, portal tract; C, central vein. (B) The human albumin (hAlb) level in the plasma of 4 TK-NOG mice and their body weight (BW) was measured over a 9-month period after GCV conditioning and human liver cell transplantation. (C) Immunoblot analyses of sera from 3 humanized TK-NOG mice with antibodies for specific for human albumin (hAlb), complement C3 proteins (hC3), transferrin (hTF), and for human and mouse ceruloplasmin (h/mCp). The RI of these mice (TK12-14, 6-6-10, and 2-4-7) mice were approximately 10, 30 and 85%, respectively. (D) Comparison of total protein (TP), total cholesterol (TCHO) albumin (ALB), ammonia (NH3), and serum blood urea nitrogen (BUN) levels between the control NOG and humanized TK-NOG mice. The hAlb level in all of the humanized mice is >3 mg/mL. Open rhomboid, control NOG mice; filled rhomboid, humanized TK-NOG mice. (E) H&E and PAS staining of kidney obtained from a humanized TK-NOG mouse (hAlb >5.0 mg/mL). Scale bars, 100 μm.
Of importance, the ‘humanized’ TK-NOG livers maintained their synthetic function for a prolonged period. Humanized TK-NOG mice maintained a very high plasma hAlb level over a 8-month period of observation, and did not experience any loss of body weight (Fig. 1B). The functioning human liver was maintained despite the fact these mice did not receive any medication other than the GCV, which was administered prior to transplantation. This prolonged period of human liver survival has not been achieved using uPA-dependent models. The Fah−/− model could provide a more stable humanization model; Bissig et al. achieved a long-term (34 weeks) reconstitution of Fah−/− mice as an experimental model for hepatitis C virus treatment [4]. However, long-term maintenance of these mice required repeated cycles of exposure to a drug (NTBC; 2-(2-nitro-4-trifluoro-methylbenzoyl)-1,3-cyclohexanedione) to prevent hepatocellular carcinomas, which will develop from the remaining mouse hepatocytes [4].
Serum proteins were analyzed in detail in three humanized TK-NOG mice by immunoblot analysis. Multiple different human proteins (albumin, complement C3, transferrin, and ceruloplasmin) were detected in their sera (Fig. 1C). The presence of the 105 kDa human C3 α chain in serum was of interest, since it indicates that the C3 precursor protein was processed by C3 convertase [16]. Although a high serum concentration of human C3 was thought to contribute to the renal failure that develops in uPA transgenic mice [5], humanized TK-NOG mice did not develop any biochemical or histologic evidence of kidney failure (Fig. 1D, E). Measurement of the serum total protein (TP), total cholesterol (TCHO) albumin (ALB), and ammonia (NH3) indicated that the humanized liver had normal synthetic and metabolic function (Fig. 1D).
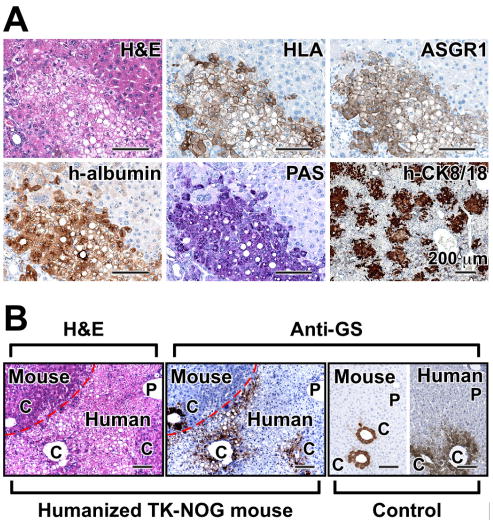
We further examined the histology of the humanized TK-NOG liver. In hematoxylin and eosin (H&E) stained sections, the human hepatocytes (HLA, ASGR1 and human albumin-positive) could be clearly distinguished by their size and pale cytoplasm from mouse hepatocytes (Fig. 2A), which is consistent with previous descriptions [11,17]. Periodic acid-Schiff (PAS) staining revealed that glycogen accumulation was restricted to the cytoplasm of the human hepatocytes. Most human hepatocytes (h-CK8/18-positive) were present as small foci that appeared to grow by clonal expansion within host parenchyma within 6 weeks after transplantation. There is a possibility that the reconstituted liver could be derived from a fusion between human and mouse hepatocytes [18]. However, double-immunofluorescent staining with antibodies specific for human or mouse albumin as well as PCR analysis of genomic segments within tissues isolated by laser-capture microdissection indicated that there was no evidence for fusion events between mouse and human cells (Fig. S4).
Figure 2.
(A) Histology and immunohistochemistry of the liver of humanized TK-NOG mice. Serial liver sections were stained for H&E, HLA, ASGR1, h-albumin, PAS, and h-CK8/18. Scale bars, 100 μm. (B) The position-specific pattern of GS expression within the liver lobule. H&E and immunohistochemical staining with anti-GS antibody in liver sections obtained from humanized TK-NOG mice 14 weeks after transplantation of human liver cells. The ‘Mouse’ and ‘Human’ hepatocyte boundary is indicated by a dashed line in the images on the left. Liver sections from control NOG or human livery sections that were stained with the anti-GS antibody are shown on the right. Scale bars: 100 μm. P, portal tract; C, central vein.
The gene expression profile in reconstituted TK-NOG liver resembles human liver
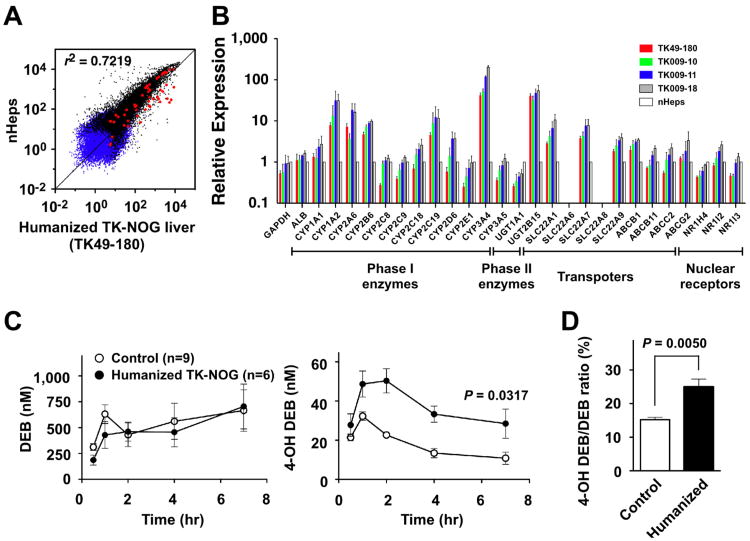
We also evaluated global gene expression patterns in humanized TK-NOG liver using microarrays. There was a very high level of correlation between the gene expression profiles in TK-NOG humanized liver tissue and that in the transplanted donor human liver cells (r2 = 0.7219; Fig. 3A). There was only a minimal level (8%) of probes on the human gene expression array that cross-hybridized with murine mRNA (Fig. S5A). Since these mice could be used to evaluate drug metabolism, the level of expression of 26 drug metabolism-related mRNAs were also evaluated by quantitative PCR (qPCR) analyses, including: 12 CYP450 and 2 phase II enzymes, 5 SLC and 4 ABC transporters, and 3 nuclear hormone receptors. All of the mRNAs that were expressed in donor liver cells were reproducibly expressed at comparable levels in the livers obtained from four humanized TK-NOG mice (Fig. 3B). The microarray and qPCR results were highly correlated (r2 = 0.9784; Fig. S5B). In addition, there was abundant expression of human CYP3A4 in the liver of humanized TK-NOG mice (Fig. S6).
Figure 3.
(A) Gene expression within the reconstituted ‘humanized liver’ of TK-NOG mice. Global gene expression profiles within a fully humanized TK-NOG liver (RI of >85%) and donor human liver cells were compared by microarray analysis. The blue and black triangles indicate ‘absent or marginal’ and ‘present’ detection calls, respectively. The red circles indicate the probe sets for mRNAs related to drug metabolism. (B) The relative expression of 26 human drug metabolism related mRNAs in four independent ‘humanized’ TK-NOG livers and in donor human liver cells (nHeps) was assessed by qPCR. Each bar represents the average of 3 independent determinations and the standard error is shown. (C) Human (CYP2D6)-specific drug biotransformation in humanized TK-NOG mice. The serum concentration of DEB (left panel) and 4-OH DEB (right panel) in control NOG (n = 9) and humanized TK-NOG (n = 6) mice were measured 0, 0.5, 1, 2, 4 and 7 hrs after administration of a single oral dose of DEB (2 mg/kg). Each data point represents the average ± SD of 6-9 independent mice tested. (D) Urine samples were collected between 0 and 7 hrs after DEB administration. The % ratio of 4-OH DEB/DEB excreted into urine within 7 hr was compared between control NOG (n = 9) and humanized TK-NOG mice (n = 6). By all of these measures, humanized TK-NOG mice had significantly increased amounts of 4-OH DEB in the plasma and higher 4-OH DEB excretion than control NOG mice.
Functional analyses of the reconstituted ‘humanized liver’ in TK-NOG mice
We also investigated whether the reconstituted ‘humanized liver’ had the three-dimensional architecture characteristic of mature human liver. Since glutamine synthetase (GS) is normally expressed within a narrow zone around the central vein of the liver lobule [19], its pattern of expression in control NOG and in humanized TK-NOG livers was examined. There was no evidence for zonal GS expression within liver lobules containing human hepatocytes 6 weeks after transplantation, which suggested an immature lobular organization at this time (data not shown). In contrast, at 14 weeks after transplantation, GS had pericentral expression in the human-derived regions of the reconstituted TK-NOG liver (Fig. 2B). Thus, after 14 weeks, a mature hepatic architecture is formed in the reconstituted humanized liver in TK-NOG mice, which is in agreement with prior studies indicating that zonation occurs 8 weeks after autologous liver cell transplantation [20].
To determine whether human-specific drug metabolism could occur in the humanized TK-NOG liver, we measured the metabolism of debrisoquine (DEB), a prototypical CYP2D6 substrate that is converted to its 4-OH metabolite (4-OH DEB) by CYP2D6. Since mice are relatively deficient in this activity [21], an increase of 4-OH DEB production indicates that human (CYP2D6-mediated) drug metabolism is occurring in the humanized liver. A single oral dose of DEB (2.0 mg/kg) was administered to control NOG and to humanized TK-NOG mice ( >4.5 mg/mL hAlb), and the amount of DEB and 4-OH DEB in serum was measured as a function of time after dosing (Fig. 3C). The area under the plasma concentration-time curve (AUC) for DEB showed no significant difference between humanized TK-NOG (n = 6) and NOG (n = 9) mice (3,211 ± 524.1 and 3,587 ± 854.0 nM, respectively; p = 0.7139). However, humanized TK-NOG mice had significantly increased amounts of 4-OH DEB in their sera, the AUC for 4-OH DEB was 2.2-fold higher in humanized TK-NOG mice than in NOG mice (247.7 ± 26.7 and 112.9 ± 12.6 nM, respectively; p = 0.0028). Consistent with this difference, an increased amount of the 4-OH DEB metabolite was excreted into the urine of humanized TK-NOG mice (Fig. 3D). These results indicate that the humanized liver can mediate a human CYP2D6-specific drug biotransformation.
Discussion
The molecular, histological and functional studies demonstrate that the reconstituted ‘humanized liver’ in TK-NOG mice is a mature and functional “human organ.” Because of these unique properties, we believe that this model could become a preferred platform for studying many aspects of human liver physiology. A unique advantage is that the TK-NOG humanized liver and its synthetic function could be stably maintained for a long period after transplantation, without the use of exogenous drugs. The TK-NOG mice did not develop systemic morbidity (liver disease, renal disease, bleeding diathesis), nor were drug treatments required to suppress the liver tumor development. This has not previously been achieved by any of the other (uPA and Fah−/−) liver reconstitution models. The longer survival and stable humanization pattern in TK-NOG mice provides a wide time-window that facilitates drug metabolism, toxicology or other longer-term studies. In fact, their prolonged survival has enabled us to ‘re-use’ mice in different studies.
CYP3A4 is the most abundant CYP450 enzyme in human liver, and it is able to metabolize nearly 50% of all marketed drugs [22]. The metabolism of ∼30% and ∼10% of marketed drugs is mediated by CYP2D6 and CYP2C19, respectively [23]. We have demonstrated that reconstituted TK-NOG mice can carry out a human-specific (CYP2D6-mediated) biotransformation of a test drug. Furthermore, a large number of human phase I and II enzymes, transporters and nuclear receptors also expressed in the humanized TK-NOG liver. The murine organs mediate many important extra-hepatic components of drug clearance (renal clearance, intestinal absorption and metabolism, etc) in this model system. Nevertheless, this data indicates that the humanized liver in TK-NOG mice holds great promise as a model system for studying the hepatic component of human drug metabolism. The ability to maintain these mice for prolonged time periods without requiring maintenance drugs indicates that these mice could be especially valuable for toxicological studies that require a prolonged period of drug administration.
The TK-NOG model also has another unique advantage; additional GCV doses can be administered after human cells have re-populated the liver. This enables a ‘mop up’ strategy to be developed to ablate residual mouse hepatic cells after human cell reconstitution, which could ensure that a very high level of human replacement is reproducibly achieved.
Supplementary Material
Research highlights.
A novel experimental in vivo platform for replacing mouse liver with human cells. Administration of GCV ablates the liver of HSVtk transgenic immunodeficient NOG mice. Transgenic TK-NOG mice maintaine the transplanted human liver cells over 8 months. Reconstituted liver shows humanized profiles of gene expression and drug metabolism. This novel humanized model provides many facets of human liver biology.
Acknowledgments
We thank Dr. R.D. Palmiter for providing the plasmid p2335A-1 containing the mouse albumin enhancer/promoter gene, and M. Kuronuma, S. Inoue, Y. Ando, N. Ogata, T. Mizushima, E. Hayakawa, K. Hioki, T. Sugioka, T. Ogura, T. Kamisako, and T. Etoh for outstanding technical assistance with the animal experiments. We thank C. Yagihashi and N. Omi for technical assistance with molecular analyses, and Drs. M. Kajimura, M. Ohmura, and Y. Ohnishi for helpful discussions. This work was supported by a Grant-in-Aid for Scientific Research (17300136 and 21240042) to H.S. Design of metabolome analysis in humanized NOG mice was supported by Research and Development of the Next-Generation Integrated Simulation of Living Matter, a part of the Development and Use of the Next-Generation Supercomputer Project of MEXT. M.S. is supported by JST, ERATO, Suematsu Gas Biology Project, Tokyo 160-8582, Japan. GP was supported by funding from a transformative RO1 award (1R01DK090992-01) from the NIDDK.
Abbreviations
- CK8/18
human Cytokeratin (8/18)
- DEB
Debrisoquine
- Fah
fumarylacetoacetate hydrolase
- GCV
ganciclovir
- H&E
hematoxylin and eosin
- hAlb
human albumin
- HSVtk
herpes simplex virus type 1 thymidine kinase
- MIAME
Minimum Information About a Microarray Experiment
- nHeps
normal human liver cells
- RI
replacement index
- RT-PCR
Reverse Transcription-PCR
- uPA
urokinase-type plasminogen activator
Footnotes
Additional Supplementary Information and a full description of the methods may be found in the online version of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suemizu H, Hasegawa M, Kawai K, Taniguchi K, Monnai M, Wakui M, Suematsu M, Ito M, Peltz G, Nakamura M. Establishment of a humanized model of liver using NOD/Shi-scid IL2Rg(null) mice. Biochem Biophys Res Commun. 2008;377:248–252. doi: 10.1016/j.bbrc.2008.09.124. [DOI] [PubMed] [Google Scholar]
- 2.Dandri M, Burda MR, Torok E, Pollok JM, Iwanska A, Sommer G, Rogiers X, Rogler CE, Gupta S, Will H, Greten H, Petersen J. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- 3.Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR, Tyrrell DL, Kneteman NM. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 4.Bissig KD, Wieland SF, Tran P, Isogawa M, Le TT, Chisari FV, Verma IM. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120:924–930. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tateno C, Yoshizane Y, Saito N, Kataoka M, Utoh R, Yamasaki C, Tachibana A, Soeno Y, Asahina K, Hino H, Asahara T, Yokoi T, Furukawa T, Yoshizato K. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am J Pathol. 2004;165:901–912. doi: 10.1016/S0002-9440(10)63352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katoh M, Sawada T, Soeno Y, Nakajima M, Tateno C, Yoshizato K, Yokoi T. In vivo drug metabolism model for human cytochrome P450 enzyme using chimeric mice with humanized liver. J Pharm Sci. 2007;96:428–437. doi: 10.1002/jps.20783. [DOI] [PubMed] [Google Scholar]
- 7.Lootens L, Van Eenoo P, Meuleman P, Leroux-Roels G, Delbeke FT. The uPA(+/+)-SCID mouse with humanized liver as a model for in vivo metabolism of 4-androstene-3,17-dione. Drug Metab Dispos. 2009;37:2367–2374. doi: 10.1124/dmd.109.028183. [DOI] [PubMed] [Google Scholar]
- 8.Zhao M, Amiel SA, Ajami S, Jiang J, Rela M, Heaton N, Huang GC. Amelioration of streptozotocin-induced diabetes in mice with cells derived from human marrow stromal cells. PLoS One. 2008;3:e2666. doi: 10.1371/journal.pone.0002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pozo OJ, Van Eenoo P, Deventer K, Lootens L, Grimalt S, Sancho JV, Hernandez F, Meuleman P, Leroux-Roels G, Delbeke FT. Detection and structural investigation of metabolites of stanozolol in human urine by liquid chromatography tandem mass spectrometry. Steroids. 2009;74:837–852. doi: 10.1016/j.steroids.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Kamimura H, Nakada N, Suzuki K, Mera A, Souda K, Murakami Y, Tanaka K, Iwatsubo T, Kawamura A, Usui T. Assessment of chimeric mice with humanized liver as a tool for predicting circulating human metabolites. Drug Metab Pharmacokinet. 2010;25:223–235. doi: 10.2133/dmpk.25.223. [DOI] [PubMed] [Google Scholar]
- 11.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heyman RA, Borrelli E, Lesley J, Anderson D, Richman DD, Baird SM, Hyman R, Evans RM. Thymidine kinase obliteration: creation of transgenic mice with controlled immune deficiency. Proc Natl Acad Sci USA. 1989;86:2698–2702. doi: 10.1073/pnas.86.8.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borrelli E, Heyman R, Arias C, Sawchenko P, Evans R. Transgenic mice with inducible dwarfism. Nature. 1989;339:538–541. doi: 10.1038/339538a0. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Huang SZ, Wang S, Zeng YT. Development of an HSV-tk transgenic mouse model for study of liver damage. FEBS J. 2005;272:2207–2215. doi: 10.1111/j.1742-4658.2005.04644.x. [DOI] [PubMed] [Google Scholar]
- 15.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 16.Bokisch VA, Dierich MP, Muller-Eberhard HJ. Third component of complement (C3): structural properties in relation to functions. Proc Natl Acad Sci USA. 1975;72:1989–1993. doi: 10.1073/pnas.72.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meuleman P, Libbrecht L, De Vos R, de Hemptinne B, Gevaert K, Vandekerckhove J, Roskams T, Leroux-Roels G. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology. 2005;41:847–856. doi: 10.1002/hep.20657. [DOI] [PubMed] [Google Scholar]
- 18.Fujino H, Hiramatsu H, Tsuchiya A, Niwa A, Noma H, Shiota M, Umeda K, Yoshimoto M, Ito M, Heike T, Nakahata T. Human cord blood CD34+ cells develop into hepatocytes in the livers of NOD/SCID/gamma(c)null mice through cell fusion. FASEB J. 2007;21:3499–3510. doi: 10.1096/fj.06-6109com. [DOI] [PubMed] [Google Scholar]
- 19.Gebhardt R, Mecke D. Heterogeneous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. EMBO J. 1983;2:567–570. doi: 10.1002/j.1460-2075.1983.tb01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun KM, Degen JL, Sandgren EP. Hepatocyte transplantation in a model of toxin-induced liver disease: variable therapeutic effect during replacement of damaged parenchyma by donor cells. Nat Med. 2000;6:320–326. doi: 10.1038/73179. [DOI] [PubMed] [Google Scholar]
- 21.Masubuchi Y, Iwasa T, Hosokawa S, Suzuki T, Horie T, Imaoka S, Funae Y, Narimatsu S. Selective deficiency of debrisoquine 4-hydroxylase activity in mouse liver microsomes. J Pharmacol Exp Ther. 1997;282:1435–1441. [PubMed] [Google Scholar]
- 22.Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002;34:83–448. doi: 10.1081/dmr-120001392. [DOI] [PubMed] [Google Scholar]
- 23.Zuber R, Anzenbacherova E, Anzenbacher P. Cytochromes P450 and experimental models of drug metabolism. J Cell Mol Med. 2002;6:189–198. doi: 10.1111/j.1582-4934.2002.tb00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.