Abstract
Background
It is well accepted that exercise can decrease breast cancer risk. Limited clinical evidence suggests that this risk could be mediated through changes in estrogen metabolism in premenopausal women. Our objective was to investigate the effects of exercise on premenopausal estrogen metabolism pertinent to breast cancer risk.
Methods
Sedentary, healthy, young eumenorrheic women were randomized into an intervention of 30 minutes of moderate-to-vigorous aerobic exercise 5 times a week for approximately 16 weeks (n = 212), or into a usual-lifestyle sedentary control group (n = 179). Urinary levels of estrogens (estrone [E1], estradiol, and estriol) and nine estrogen metabolites were measured at baseline and at study end by liquid chromatography/tandem mass spectrometry. The ratios of 2-hydroxyestrone to 16α-hydroxyestrone (2-OHE1/16α-OHE1) and 2-OHE1 to 4-hydroxyestrone (2- OHE1/4-OHE1) were also calculated.
Results
The exercise intervention resulted in significant increases in aerobic fitness and lean body mass, and a significant decrease in percent body fat. For exercisers who completed the study (n = 165), 2-OHE1/16α-OHE1 increased significantly (P = 0.043), while E1 decreased significantly (P = 0.030) in control participants (n = 153). The change from baseline in 2-OHE1/16α-OHE1 was significantly different between groups (P = 0.045), even after adjustment for baseline values.
Conclusions
The exercise intervention resulted in a significant increase in the 2-OHE1/16α-OHE1 ratio, but no differences in other estrogen metabolites or ratios.
Impact
Our results suggest that changes in premenopausal estrogen metabolism may be a mechanism by which increased physical activity lowers breast cancer risk.
Key Terms: Aerobic Exercise, Estrogen Metabolism, Randomized Clinical Trial, Breast Cancer Risk
INTRODUCTION
It is well accepted that lifetime estrogen exposure increases the risk for breast cancer as a result of cumulative stimulation of epithelial cell division by estrogen (1). It has also been suggested that some metabolites resulting from the biotransformation and inactivation of estrogen can play a significant role in breast carcinogenesis (2). Specifically, the products resulting from the oxidation of estradiol (E2) and estrone (E1) known as hydroxyestrogens have been shown to display varying degrees of carcinogenicity. For example, 2-hydroxyestrone (2-OHE1) partially antagonizes the growth-stimulatory effect of E2 in cultured human MCF-7 breast cancer cells (3) while 2-hydroxyestradiol (2-OHE2)has little or no carcinogenic activity in Syrian hamsters (4, 5). In cultured mouse mammary epithelial cells, 16α-hydroxyestrone (16α-OHE1) increases unscheduled DNA synthesis and promoted anchorage-independent growth (6, 7). The metabolite 4-hydroxyestrone (4-OHE1) is considered genotoxic due to its redox cycling process, which generates reactive oxygen species (ROS) and highly cytotoxic semiquinone/quinone intermediates that react with DNA (2). The 2-hydroxyestrogens also undergo redox cycling, but appear to lack carcinogenic activity due to a more rapid clearance in vivo (8) associated with a faster rate of inactivation through O-methylation (9, 10). Finally, one product resulting from O-methylation, namely 2-methoxyestradiol (2-MeOE2), has been shown to be a potent inhibitor of cell proliferation and angiogenesis (11, 12).
Despite evidence suggesting the possible importance of other aspects of estrogen metabolism on breast cancer, human studies have largely focused on the ratio of 2-OHE1 to 16α-OHE1 (2-OHE1/16α-OHE1). Given the different genotoxic capacity of these metabolites, it has been hypothesized that metabolism favoring the production of 2-OHE1 over 16α-OHE1 may be inversely associated with breast cancer risk (13). In premenopausal women, the strongest evidence in favor of this hypothesis comes from two early prospective studies in which urine specimens were collected several years prior to diagnosis. In the Guernsey III cohort study, women in the highest tertile of urinary 2-OHE1/16α-OHE1 ratio had a non-significantly lower odds ratio (0.75) for breast cancer than women in the lowest two tertiles (14). Similarly, in a study reported by Muti et al., women in the highest quintile of the urinary 2-OHE1/16α-OHE1 ratio had an adjusted odds ratio for breast cancer of 0.58, although again this was not statistically significant (15). In contrast, in a more recent prospective study, a higher 2-OHE1/16α-OHE1 ratio was associated with an increase in premenopausal ER-positive breast cancer (16). However, the association was not statistically significant and estrogen metabolites were measured in serum and not in urine as in the two previous studies. Significant relationships between premenopausal breast cancer risk and urinary levels of estrogen metabolites and their ratios have been observed in some case-control studies, but findings have been inconsistent. The case-control studies of both Coker et al. (17) and Kabat et al. (18) found an increased risk in women with an increased 2-OHE1/16α-OHE1 ratio, but other studies did not (19–21). As for other measures, in two other studies, control women had significantly higher levels of 2-hydroxyestrogens, 4-hydroxyestrogens, 16α-OHE1 (22), and 2-OHE1/4-OHE1 ratio (23) than women with breast cancer.
While the association between estrogen metabolism and breast cancer risk needs further investigation, epidemiological evidence strongly supports the association between higher levels of aerobic exercise and reduced risk for breast cancer (24). However, whether exercise in premenopausal women results in what may be favorable effects on estrogen metabolism is not clear. For example, in one small study highly fit women exercising strenuously for 368 minutes a week had similar values of 2-OHE1, 16α-OHE1, and 2-OHE1/16α-OHE1 ratio than those of women exercising recreationally for only 60 minutes a week (25). In contrast, in another small study higher levels of self-reported physical activity were associated with higher urinary concentrations of 2-OHE1 and a higher 2-OHE1/16α-OHE1 ratio (26). Recently, a large study of 603 women from the Nurses’ Health Study II found high levels of physical activity not only to be correlated with a higher 2-OHE1/16α-OHE1 ratio, but also significantly lower levels of E2 and 16α-OHE1 (27). In comparison, data from exercise intervention studies has been conflicting. For instance, in interventions lasting 12 weeks (28), 16 weeks (29), or even 6 months (30), moderate-to-vigorous intensity aerobic exercise in premenopausal women did not result in any significant changes in urinary concentrations of E1, E2, estriol (E3), 2-hydroxyestrogens, 4-hydroxyestrogens, or 16α-OHE1, or either 2-OHE1/16α-OHE1 or 2-OHE1/4-OHE1 ratios. In two small exercise interventions coupled with calorie restriction lasting 4 and 6 months, there were significant increases in urinary levels of luteal phase 16α-OHE1 and 2-OHE1/16α-OHE1, respectively (31, 32).
Overall, the data on the effects of aerobic exercise on premenopausal estrogen metabolism are not only conflicting but also narrow in scope. With the exception of two studies (27, 29), all published studies to date have focused on a limited number of estrogen metabolites, namely 2-OHE1 and 16α-OHE1, and their ratio. Furthermore, no study has yet investigated the levels of the 2- and 4- methylated catecholestrogens despite their purported role in breast carcinogenesis as suggested by culture and animal studies. The WISER (Women In Steady Exercise Research) study was a large, randomized, exercise-controlled, parallel-arm, clinical study investigating the effects of 16-weeks of moderate-to-vigorous intensity aerobic exercise on several parameters pertinent to breast cancer risk in sedentary, healthy, young eumenorrheic women. Here we report changes from baseline in urinary levels of estrogens (E1, E2, and E3), nine estrogen metabolites (2-OHE1, 2-OHE2, 16α-OHE1, 4-OHE1, 4-hydroxyestradiol [4-OHE2,], 2-methoxyestrone, [2-MeOE1], 2-MeOE2, 4-methoxyestrone [4-MeOE1], and 4-methoxyestradiol [4-MeOE2]), and two estrogen metabolite ratios (2-OHE1/16α-OHE1, and 2-OHE1/4-OHE1).
SUBJECTS AND METHODS
Study design
The WISER study was a randomized clinical trial investigating the effects of a 16-week aerobic exercise intervention on breast cancer biomarkers of healthy, premenopausal women. All procedures were approved by the Human Subjects Review Committee at the University of Minnesota (Institutional Review Board; IRB ID#0505M69867). Written informed consent was obtained from each participant prior to participation. A complete description of the study design, including participant recruitment, screening, randomization, and retention has been published (33).
Briefly, WISER study investigators emailed more than 100,000 female residents of the Minneapolis-St. Paul metropolitan area regarding participation. Women who were interested were screened online based on age (18–30 years old), physical activity (two or less weekly sessions of moderate intensity exercise), smoking status (non-smoking), body mass index (BMI) (18–40 kg/m2 inclusive), and self-reported menstrual cycle length (24 to 35 days). Women who met these criteria were further screened via telephone (n = 1684) and excluded based on previous hormonal contraception use (past three months or 12 months if depot-medroxyprogesterone acetate), gynecological problems, metabolic or endocrine-related diseases, current or recent (past 6 months) pregnancy, non-melanoma cancer in the past 5 years, alcohol consumption (more than 7 servings per week), and body weight changes (more than 10% over the past year).
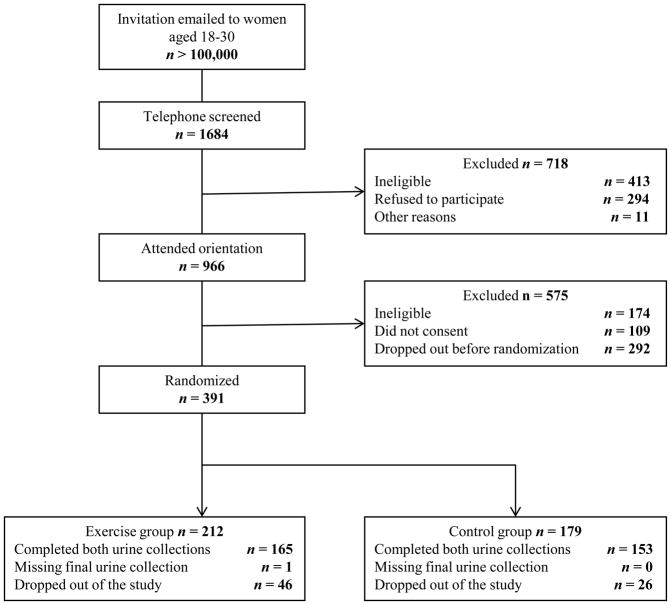
Of the 966 women who attended a 2-hour orientation, 391 provided written consent and were enrolled in the study. After baseline measurements, women were randomized into either an exercise intervention (n = 212) or a no-exercise, usual-lifestyle control group (n = 179) for approximately 16 weeks. Randomization was stratified on baseline BMI tertiles (≤ 22.8, 22.8–26.3, ≥ 26.3) based on the 50th and 75th percentiles from NHANES I data and age (18–24 vs. 25–30). Participants who failed to return for follow-up measures were dropped from of the study. Additionally, exercisers were subject to study exclusion if they missed 15 or more exercise sessions. Figure 1 shows the recruitment, screening, randomization, retention, and completion of WISER participants.
Figure 1.
CONSORT diagram showing participant recruitment, screening, randomization, and retention.
Exercise intervention
Women randomized to the exercise intervention trained aerobically five times a week for 30 minutes on a treadmill, stair-stepper, or elliptical machine, at a specified intensity based on age-predicted maximal heart rate (max HR) for 16 weeks (± 2 weeks). The exercise intensity was initially set at 65%–70% of the age-predicted max HR and was gradually increased by 5% every four weeks until 80%–85% of age-predicted max HR was reached (stage 1 = 65%–70%; stage 2 = 70%–75%; stage 3 = 75%–80%; stage 4 = 80%–85%).
All training sessions took place at the University of Minnesota’s Recreation Center. At the first training session, a certified personal trainer provided instruction on the proper use of the exercise machines, heart rate monitor and watch, and completion of an exercise log after each workout. Trainers supervised exercise sessions and reviewed the exercise logs at least once weekly to monitor adherence and safety. When not meeting with a trainer, participants were expected to complete the remaining of the workout sessions unsupervised. Exercise adherence was assessed using the data from the heart rate monitor (Polar Electro Inc., Lake Success, NY) and exercise logs.
Any physical activity performed after randomization and outside the prescribed exercise intervention was assessed at the end of the study with a physical activity questionnaire administered by a research staff member. All participants, regardless of randomization outcome, were asked to maintain their baseline body weight. Control participants were asked to not only to maintain their usual level of physical activity but also to not change their eating habits.
Anthropometrics
Body mass was measured to the nearest 0.1 kg using an electronic scale (Scale Tronix, White Plains, NY) 4 times throughout the study (baseline, intervention weeks 4 and 8, and follow-up). Height was measured without shoes to the nearest 0.1 cm (Scale Tronix, White Plains, NY) by a stadiometer at baseline. Body mass index was calculated by dividing body mass in kg by height in meters squared (kg/m2). Body composition was assessed at baseline and follow-up by dual energy x-ray absorptiometry (DXA) using a Lunar Prodigy DXA apparatus (Lunar Radiation Corp., Madison, WI).
Aerobic fitness and physical activity
Aerobic fitness was assessed at baseline and immediately after the intervention with a sub-maximal treadmill test described previously (33). This workload was then converted to metabolic equivalents (METs) by using a standard conversion formula (34). Self-reported physical activity performed a year prior to the study and during the 4-month follow-up period was assessed by a research staff using a modified version of the Modifiable Activity Questionnaire (35). This information was transformed into MET-hours per week (MET-hrs/wk) using commonly accepted MET values (36).
Dietary intake
Usual dietary intake was assessed through self-reported, 3-day food records completed concomitantly with the urine collections at baseline and follow-up. Nutrient intake was determined using The Food Processor SQL® by ESHA Research (Salem, OR).
Urine collection
Forty-eight hours prior to the urine collection, participants were asked to avoid moderate or vigorous exercise and abstain from alcohol. Urine was collected for three consecutive 24-hour periods in the mid-follicular phase (follicular days 7 - 9 of baseline menstrual cycle 2 and follow-up menstrual cycle 6). Throughout each day, urine was collected in a 1-liter bottle and kept cold with ice packs inside an insulated bag. At the end of each collection day, urine was transferred into a 3-liter bottle containing ascorbic acid (1 mg/mL) to prevent oxidation, and stored in a home refrigerator or cooler provided by the study. Once the urine collection was completed, collection bottles were retrieved by a research staff member and brought to the General Clinical Research Center at the University of Minnesota for processing. Urine was refrigerated and 0.1% sodium azide was added before the three 24-hour collections were pooled. Aliquots were taken and stored at −20 °C until analysis.
Estrogen metabolites
Urinary estrogens (E1, E2, and E3) and their metabolites (2-OHE1, 2-OHE2, 16α-OHE1, 4-OHE1, 4-OHE2, 2-MeOE1, 2-MeOE2, 4-MeOE1, and 4-MeOE2) were analyzed in the mid-follicular phase of baseline and follow-up cycles by liquid chromatography/tandem mass spectrometry (LC/MS-MS) performed using a Thermo Electron Quantum Discovery Max Triple Quadrupole LC-MS/MS instrument (37). Quantitative analysis was performed using Thermo Electron Xcalibur proprietary software.
Samples with non-detectable levels were assigned values of the lowest detectable standard (0.014 ng/mL urine). Concentrations were expressed both as nanomol per day (nmol/day) and nanograms per milligram of creatinine (ng/mg Cr). Urinary creatinine was analyzed at the Fairview University Diagnostic Laboratories.
Samples were run in duplicate and in batches such that each batch contained both baseline and follow-up samples from each participant and an equal number of exercise and control participants. One quality control sample was included in each batch. The mean intra-assay and inter-assay CVs were 5.1% and 13.4% for E1; 5.2% and 16.0% for E2; 5.6% and 11.4% for E3; 4.2% and 12.3% for 2-OHE1; 7.7% and 10.8% for 2-OHE2; 6.2% and 18.7% for 16α-OHE1; 4.3% and 12.2% for 4-OHE1; 14.0% and 51.2% for 4-OHE2; 7.0% and 11.3% for 2-MeOE1; 5.8% and 10.0% for 2-MeOE2; 7.4% and 10.3% for 4-MeOE1; and 6.9% and 7.9% for 4-MeOE2.
Statistical analyses
Unadjusted comparisons of baseline characteristics were performed using Student’s t-tests for continuous variables and chi-square tests for categorical variables.
Two estrogen metabolite ratios of interest, namely the 2-OHE1/16α-OHE1 ratio and 2-OHE1/4-OHE1 ratio, were calculated by dividing the concentration of 2-OHE1 by either 16α-OHE1 or 4-OHE1, respectively.
Baseline associations between urinary estrogens, their metabolites, and metabolite ratios and measures of body composition, adiposity, fitness, reproductive characteristics, and diet were determined using Spearman correlation coefficients.
The main study analysis assessed the intervention effects using only data from participants who completed the baseline and follow-up urine collection regardless of compliance level. Baseline and follow-up comparisons were conducted using log-transformed values and results are presented as geometric means with 95% confidence intervals. Changes from baseline comparisons were compared on the original scale. All comparisons were adjusted for study-design age and BMI strata with a general linear model. When there were significant differences at baseline in an outcome, follow-up and change from baseline comparisons were additionally adjusted for baseline values. Linear models were calculated using SAS software, version 9.2 (SAS institute Inc., 2008, Cary, NC). P < 0.05 was considered statistically significant.
Estrogen metabolite data were analyzed as both nmol/day and ng/mg Cr. Results from the two analyses did not differ significantly, and we report results as nmol/day.
RESULTS
Study participants
Of the 212 and 179 women randomized into the exercise and control groups, 165 (77.8%) and 153 (85.5%), respectively, completed the WISER study. With the exception of education (47% of drop-outs had some college education vs. 27% of study completers P = 0.002), drop-outs were no different from women who completed the study in any of the baseline demographic characteristic measured (data not shown). Also, there were no significant differences between exercisers and controls in baseline demographic characteristics (Table 1). In general, women who completed the study were mostly Caucasian (72%), single (82%), nulliparous (93%), had education beyond high school (96%), and had no first-degree relatives with breast cancer (97%).
Table 1.
Baseline Characteristics of Randomized WISER Participants (n = 318)
| Exercisers n = 165 | Controls n = 153 | |
|---|---|---|
| Demographics | ||
| Age, years (mean ± SE) | 25.4 ± 0.3 | 25.2 ± 0.3 |
| Not Married or Partnered (n, %) | 137 (83%) | 124 (81%) |
| Education beyond High School (n, %) | 157 (95.2%) | 148 (96.7%) |
| Caucasian (n, %) | 123 (75%) | 107 (70%) |
| Body Composition (mean ± SE) | ||
| Weight (kg) | 67.5 ± 1.1 | 67.6 ± 1.2 |
| BMI (kg/m2) | 24.8 ± 0.4 | 24.7 ± 0.4 |
| % Body Fat | 36.4 ± 0.7 | 36.1 ± 0.7 |
| Fat Mass (kg) | 24.3 ± 0.9 | 24.1 ± 0.9 |
| Lean Mass (kg) | 39.8 ± 0.4 | 40.1 ± 0.4 |
| Reproductive Characteristics | ||
| Age at Menarche, years (mean ± SE) a | 12.7 ± 0.12 | 12.7 ± 0.11 |
| Nulliparous (n, %) | 153 (93%) | 144 (94%) |
| Previous Contraceptive Use (n, %) | 84 (51%) | 82 (54%) |
| Family History of Breast Cancer b | ||
| No (n, %) | 129 (96%) | 114 (97%) |
| Physical Activity, Fitness & Diet (mean ± SE) | ||
| Moderate Exercise (MET-hrs/wk) | 1902 ± 421 | 1933 ± 525 |
| Aerobic Fitness (METs at 85% max HR) | 21.9 ± 1.3 | 21.8 ± 1.4 |
| Total calorie intake (kcal/day) c | 6.9 ± 0.1 | 7.1 ± 0.1 |
Note: There were no significant differences at baseline between study groups for any of these variables
n = 310,
n = 251,
n = 312
Baseline estrogen metabolism
With the exception of 2-OHE1 (P = 0.084) and 2-OHE1/16α-OHE1 ratio (P = 0.044), exercisers had similar levels of urinary estrogens, estrogen metabolites, and 2-OHE1/4-OHE1 ratio than control participants at baseline (Table 2). No significant baseline associations between any of the urinary endpoints and measures of body composition, adiposity, fitness, reproductive characteristics, and diet were found.
Table 2.
Effects of 16 Weeks of Aerobic Exercise on Estrogen Metabolism of WISER participants
| Baseline Geometric Mean (95% CI) | P-value for Baseline Differences | Follow Up Geometric Mean (95% CI) | P-value for Differences in Mean Change | |
|---|---|---|---|---|
| Estrone, E1 (nmol/day) | ||||
| Exercisers | 23.1 (19.0 – 28.2) | 0.586 | 23.0 (18.9 – 28.0) | 0.042 |
| Controls | 25.0 (20.3 – 30.8) | 23.0 (18.8 – 28.2) a | ||
| Estradiol, E2 (nmol/day) | ||||
| Exercisers | 7.8 (6.8 – 8.8) | 0.786 | 8.3 (7.2 – 9.5) | 0.725 |
| Controls | 8.0 (7.0 – 9.1) | 8.0 (7.2 – 9.2) | ||
| Estriol, E3 (nmol/day) | ||||
| Exercisers | 21.3 (16.5 – 27.4) | 0.963 | 18.6 (14.4 – 24.2) | 0.715 |
| Controls | 21.1 (16.2 – 27.5) | 21.0 (16.0 – 27.5) | ||
| 16α-OHE1 (nmol/day) | ||||
| Exercisers | 2.9 (2.3 – 3.7) | 0.303 | 2.6 (2.1 – 3.4) | 0.971 |
| Controls | 2.5 (1.9 – 3.1) | 2.5 (1.9 – 3.2) | ||
| 2- OHE1 (nmol/day) | ||||
| Exercisers | 39.3 (33.7 – 45.8) | 0.084 | 44.2 (38.4 – 50.8) | 0.098 |
| Control | 47.6 (40.5 – 55.8) | 45.5 (39.3 – 52.6) | ||
| 4- OHE1 (nmol/day) | ||||
| Exercisers | 2.3 (2.1 – 2.7) | 0.647 | 2.4 (2.1 – 2.7) | 0.362 |
| Controls | 2.4 (2.1 – 2.8) | 2.4 (2.1 – 2.7) | ||
| 2-OHE2 (nmol/day) | ||||
| Exercisers | 3.4 (2.4 – 5.0) | 0.209 | 3.5 (2.4 – 5.1) | 0.194 |
| Controls | 2.5 (1.7 – 3.6) | 2.9 (1.9 – 4.2) | ||
| 4-OHE2 (nmol/day) | ||||
| Exercisers | 0.7 (0.4 – 1.0) | 0.767 | 0.8 (0.5 – 1.3) | 0.898 |
| Controls | 0.7 (0.5 – 1.2) | 0.5 (0.3 – 0.8) | ||
| 2-MeOE1 (nmol/day) | ||||
| Exercisers | 8.2 (6.5 – 10.4) | 0.312 | 9.3 (7.4 – 11.8) | 0406 |
| Controls | 6.9 (5.4 – 8.8) | 8.6 (6.8 – 10.9) | ||
| 4-MeOE1 (nmol/day) | ||||
| Exercisers | 1.9 (1.4 – 2.6) | 0.562 | 1.7 (1.2 – 2.4) | 0.488 |
| Controls | 2.2 (1.6 – 3.0) | 1.5 (1.1 – 2.1) | ||
| 2-MeOE2 (nmol/day) | ||||
| Exercisers | 7.1 (5.4 – 9.3) | 0.269 | 7.0 (5.4 – 9.1) | 0.556 |
| Controls | 5.8 (4.3 – 7.6) | 6.6 (5.1 – 8.7) | ||
| 4-MeOE2 (nmol/day) | ||||
| Exercisers | 1.6 (1.2 – 2.3) | 0.254 | 1.5 (1.0 – 2.1) | 0.552 |
| Controls | 1.2 (0.9 – 1.8) | 0.9 (0.7 – 1.4) | ||
| 2-OHE1/16α-OHE1 | ||||
| Exercisers | 13.4 (10.4 – 17.2) | 0.044 | 16.8 (13.1 – 21.4) b | 0.045 |
| Controls | 19.3 (14.8 – 25.1) | 18.5 (14.3 – 24.0) | ||
| 2-OHE1/4-OHE1 | ||||
| Exercisers | 16.8 (14.8 – 19.0) | 0.104 | 18.7 (16.8 – 20.9) | 0.123 |
| Controls | 19.5 (17.1 – 22.2) | 18.9 (16.9 – 21.2) | ||
NOTE: Values are age- and BMI-adjusted geometric means (95% CI). Follow-up and mean change from baseline comparisons in 2-OHE1 and 2-OHE1/16α-OHE1 were additionally adjusted for baseline values.
Within-group differences:
P-value = 0.030,
P-value = 0.043
Overall, the concentration of estrone and its metabolites were higher than their estradiol counterparts, especially for E1, 2-OHE1, 4-OHE1, and 4-MeOE1 as compared to E2, 2-OHE2, 4-OHE2, and 4-MeOE2, respectively. Estrogen hydroxylation showed an isomeric preference for the C-2 position. Specifically, concentrations of 2-OHE1 were about 14- and 20-fold higher than those of 16α-OHE1 and 4-OHE1, respectively, and concentration of 2-OHE2 about 40-fold those of 4-OHE2.
Exercise adherence
On average, exercise participants completed 127 minutes per week of the assigned 150 minutes of exercise intervention. Details about exercise adherence and compliance can be found elsewhere (38).
Intervention effects
The exercise intervention resulted in significant improvements in body composition and aerobic fitness without changes in body weight. As previously reported, exercisers experienced significant increases in aerobic fitness (0.90 METs reached at 85% of max HR vs. 0.12 METs in controls) and lean body mass (0.55kg vs. 0.07 kg), as well as significant decreases in fat mass (0.57 kg vs. 0.04 kg) and percent body fat (0.95% vs. 0.09%). In contrast, control participants experienced no changes in body composition, aerobic fitness, and body weight despite a significant reduction in daily caloric intake (−224 kcal/day). Exercisers also reduced their food consumption, but only by 18 kcal/day (P > 0.05). Details on the effects of this intervention on body composition, body weight, aerobic fitness, and energy intake have been published previously (39).
As previously reported, the exercise intervention resulted in no significant changes in endogenous levels of E2, estrone sulfate, progesterone, T, or SHBG (38). Exercisers, however, did experience a significant increase in urinary 2-OHE1/16α-OHE1 ratio (P = 0.043) while controls had a non-significant decrease in 2-OHE1/16α-OHE1 ratio. The difference in the change from baseline in 2-OHE1/16α-OHE1 ratio between groups was significant (P = 0.045), even after adjustment for baseline values. Figure 2 shows that many, but no all, of the participants who experienced an absolute change in 2-OHE1/16α-OHE1 ratio greater than 100 had a high baseline 2-OHE1/16α-OHE1 ratio. Levels of E1 remained unchanged in exercisers but decreased in controls resulting in a statistically significant change from baseline between the groups (P = 0.042). No significant within-group changes or between-group differences at follow-up were observed for other estrogens, estrogen metabolites, or ratios.
Figure 2.
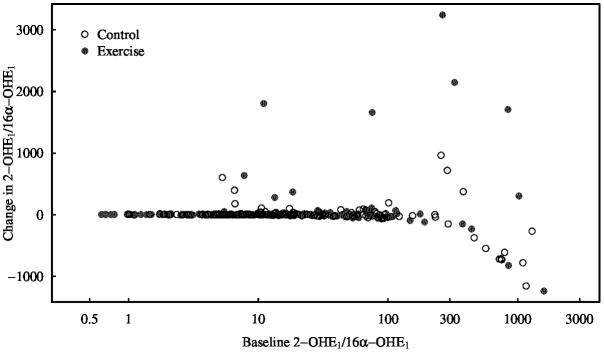
Changes in Baseline vs. Baseline in 2-OHE1/16α-OHE1 Ratio
DISCUSSION
We found that in healthy premenopausal women, an exercise regimen of 150 minutes of moderate-to-vigorous aerobic exercise per week for 16 weeks resulted in significant changes in estrogen metabolism in a direction consistent with reduction of breast cancer risk. Specifically, exercise participants experienced a significant increase in urinary levels of 2-OHE1 and a small non-significant decrease in 16α-OHE1 levels. These changes resulted in a significant increase in the 2-OHE1/16α-OHE1 ratio. In contrast, women in the control group had a non-significant decrease in 2-OHE1/16α-OHE1 ratio largely attributable to those few controls with large baseline ratio having large decreases in the ratio. Controls also had an unexplained significant decrease in E1. We did not find evidence for exercise resulting in changes in the 4-hydroxylation pathway or other differences that conceivably could have been found.
Overall, our results differ from those of other exercise intervention studies investigating the effects of aerobic exercise on premenopausal estrogen metabolism. For instance, in a small 5-month weight-loss clinical trial involving moderate-intensity exercise, both controls and exercisers had significant increases in the urinary 2-OHE1/16α-OHE1 ratio, but in contrast to our results, the change in ratio between the groups was not statistically significant (32). In a small pre-post design study, 4 months of moderate exercise coupled with calorie restriction resulted in non-significantly higher urinary levels of 2-OHE1 and 16α-OHE1 and the ratio (31). In our study, both 2-OHE1 and the 2-OHE1/16α-OHE1 ratio increased significantly, whereas 16α-OHE1 decreased non-significantly. Both of these studies differed from our study in that aerobic exercise was coupled with significant calorie restriction making it impossible to discern whether the changes reported were the result of the exercise or diet. When compared to exercise-only interventions, our study remains the only one to report significant changes in estrogen metabolism. For example, in a moderate-to-vigorous aerobic exercise intervention lasting 12 weeks (30–45 minutes, 4 days per week), Campbell and colleagues reported no significant changes in urinary premenopausal levels of 2-OHE1, 16α-OHE1, or 2-OHE1/16α-OHE1 ratio (28). Similarly, in a pilot study carried out by our research group, total levels of 2-OHE (2-OHE1 + 2-OHE2), 4-OHE (4-OHE1 + 4-OHE2), and the 2-OHE1/16α-OHE1 ratio remained unchanged in 15 young women exercising aerobically for 30 minutes a day, 5 times a week for 16 weeks (29). Similarly, Robles-Gil et al. did not find significant changes in E1, E2, or E3 in 20 premenopausal women after 6 months of 60 minutes of moderate-intensity exercise 3 days/week (30).
A possible explanation for the disparity between the results reported by these exercise intervention studies and our study may be found in the choice of study design and methodology. The WISER study had many methodological advantages over previously published research. First, the sample size in our study (n = 318) was an order of magnitude or more larger than those of the other three studies. Second, our study design utilized randomized controls (only Campbell et al. study was randomized). Third, unlike the studies of Campbell et al. and Robles-Gil et al., in which first morning urine samples were used, WISER participants collected three 24-hour urine collections allowing for a more robust and representative analysis of the chronic effect of aerobic exercise on estrogen metabolism. Finally, our study provides the most comprehensive analysis on the effects of exercise on estrogen metabolism to date. We have analyzed urinary levels of the major parent estrogens (E1, E2 and E3) and nine of their estrogen metabolites by LC/MS-MS. This newer methodology is considered to be superior not only to the ELISA methods employed by these studies but also to the current gold standard GC-MS due to its increased sensitivity and sample throughput (37, 40). Unlike Xu and colleagues, we were able to quantify and report 4-OHE2, concentrations, although its lack of detection in 53.4% of our samples resulted in a higher-than-expected inter-assay CV.
Altogether, the findings of the WISER study are significant because they provide the first clinical evidence that aerobic exercise can significantly change estrogen metabolism in premenopausal women. Specifically, our results show that such an exercise intervention can lead to increases in 2-OHE1 and possible decreases in 16α-OHE1 ultimately resulting in significant increases in the 2-OHE1/16α-OHE1 ratio. Importantly, increases in this ratio have been associated with a significant reduction in breast cancer risk. From a clinical point of view, the assessment of urinary 2-OHE1/16α-OHE1 ratio is also relevant as it has been found to be a good approximation to the 2-OHE1/16α-OHE1 ratio of breast tissue (41). Perhaps one mechanism by which exercise mediates estrogen metabolism is through the regulation of P-450 cytochrome enzymes responsible for controlling estrogen hydroxylation and catecholestrogen methylation. Given the implication these results have for breast cancer prevention efforts, future studies should not only attempt to corroborate our results, but also investigate the exact mechanisms by which exercise leads to these favorable estrogen metabolism changes.
Acknowledgments
The project was funded by the National Institutes of Health/National Cancer Institute grant 1U54CA116849-010003, the Department of Defense/U.S. Army Medical Research and Materiel Command Congressionally Directed Medical Research Programs award #W81XWH-08-1-0301, and the National Institutes of Health/National Center for Research Resources grant M01-RR00400.
The authors wish to acknowledge the General Clinical Research Center at the University of Minnesota, the Minneapolis YWCAs, and the study participants. We would also like to thank the WISER administrative and laboratory staff, especially Mike Wachter and Steve McColley.
Footnotes
For reprint requests, please contact Dr. Mindy S. Kurzer.
Disclosure Summary: The authors have nothing to disclose.
Clinical Trial Registration Number: NCT00393172
References
- 1.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008 Mar;8(3):205–11. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 2.Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000;(27):67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 3.Schneider J, Huh MM, Bradlow HL, Fishman J. Antiestrogen action of 2-hydroxyestrone on MCF-7 human breast cancer cells. J Biol Chem. 1984 Apr 25;259(8):4840–5. [PubMed] [Google Scholar]
- 4.Li JJ, Li SA. Estrogen carcinogenesis in Syrian hamster tissues: role of metabolism. Fed Proc. 1987 Apr;46(5):1858–63. [PubMed] [Google Scholar]
- 5.Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J Steroid Biochem. 1986 Jan;24(1):353–6. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 6.Suto A, Bradlow HL, Wong GY, Osborne MP, Telang NT. Experimental down-regulation of intermediate biomarkers of carcinogenesis in mouse mammary epithelial cells. Breast Cancer Res Treat. 1993 Sep;27(3):193–202. doi: 10.1007/BF00665689. [DOI] [PubMed] [Google Scholar]
- 7.Telang NT, Suto A, Wong GY, Osborne MP, Bradlow HL. Induction by estrogen metabolite 16 alpha-hydroxyestrone of genotoxic damage and aberrant proliferation in mouse mammary epithelial cells. J Natl Cancer Inst. 1992 Apr 15;84(8):634–8. doi: 10.1093/jnci/84.8.634. [DOI] [PubMed] [Google Scholar]
- 8.Lipsett MB, Merriam GR, Kono S, Brandon DD, Pfeiffer DG, Loriaux DL. Catechol Estrogens. New York: Raven Press; 1983. [Google Scholar]
- 9.Li SA, Purdy RH, Li JJ. Variations in catechol O-methyltransferase activity in rodent tissues: possible role in estrogen carcinogenicity. Carcinogenesis. 1989 Jan;10(1):63–7. doi: 10.1093/carcin/10.1.63. [DOI] [PubMed] [Google Scholar]
- 10.Roy D, Weisz J, Liehr JG. The O-methylation of 4-hydroxyestradiol is inhibited by 2-hydroxyestradiol: implications for estrogen-induced carcinogenesis. Carcinogenesis. 1990 Mar;11(3):459–62. doi: 10.1093/carcin/11.3.459. [DOI] [PubMed] [Google Scholar]
- 11.Fotsis T, Zhang Y, Pepper MS, et al. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature. 1994 Mar 17;368(6468):237–9. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- 12.Klauber N, Parangi S, Flynn E, Hamel E, D’Amato RJ. Inhibition of angiogenesis and breast cancer in mice by the microtubule inhibitors 2-methoxyestradiol and taxol. Cancer Res. 1997 Jan 1;57(1):81–6. [PubMed] [Google Scholar]
- 13.Bradlow HL, Hershcopf RJ, Martucci CP, Fishman J. Estradiol 16 alpha-hydroxylation in the mouse correlates with mammary tumor incidence and presence of murine mammary tumor virus: a possible model for the hormonal etiology of breast cancer in humans. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6295–9. doi: 10.1073/pnas.82.18.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meilahn EN, De Stavola B, Allen DS, et al. Do urinary oestrogen metabolites predict breast cancer? Guernsey III cohort follow-up. Br J Cancer. 1998 Nov;78(9):1250–5. doi: 10.1038/bjc.1998.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muti P, Bradlow HL, Micheli A, et al. Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16alpha-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000 Nov;11(6):635–40. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Arslan AA, Shore RE, Afanasyeva Y, Koenig KL, Toniolo P, Zeleniuch-Jacquotte A. Circulating estrogen metabolites and risk for breast cancer in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2009 Aug;18(8):2273–9. doi: 10.1158/1055-9965.EPI-09-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coker AL, Crane MM, Sticca RP, Sepkovic DW. Re: Ethnic differences in estrogen metabolism in healthy women. J Natl Cancer Inst. 1997 Jan 1;89(1):89–90. doi: 10.1093/jnci/89.1.89. [DOI] [PubMed] [Google Scholar]
- 18.Kabat GC, O’Leary ES, Gammon MD, et al. Estrogen metabolism and breast cancer. Epidemiology. 2006 Jan;17(1):80–8. doi: 10.1097/01.ede.0000190543.40801.75. [DOI] [PubMed] [Google Scholar]
- 19.Fowke JH, Qi D, Bradlow HL, et al. Urinary estrogen metabolites and breast cancer: differential pattern of risk found with pre- versus post-treatment collection. Steroids. 2003 Jan;68(1):65–72. doi: 10.1016/s0039-128x(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 20.Kabat GC, Chang CJ, Sparano JA, et al. Urinary estrogen metabolites and breast cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 1997 Jul;6(7):505–9. [PubMed] [Google Scholar]
- 21.Ursin G, London S, Yang D, et al. Urinary 2-hydroxyestrone/16alpha-hydroxyestrone ratio and family history of breast cancer in premenopausal women. Breast Cancer Res Treat. 2002 Mar;72(2):139–43. doi: 10.1023/a:1014896417653. [DOI] [PubMed] [Google Scholar]
- 22.Adlercreutz H, Fotsis T, Hockerstedt K, et al. Diet and urinary estrogen profile in premenopausal omnivorous and vegetarian women and in premenopausal women with breast cancer. J Steroid Biochem. 1989;34(1–6):527–30. doi: 10.1016/0022-4731(89)90138-6. [DOI] [PubMed] [Google Scholar]
- 23.Gaikwad NW, Yang L, Muti P, et al. The molecular etiology of breast cancer: evidence from biomarkers of risk. Int J Cancer. 2008 May 1;122(9):1949–57. doi: 10.1002/ijc.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008 Aug;42(8):636–47. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 25.Campbell KL, Westerlind KC, Harber VJ, Friedenreich CM, Courneya KS. Associations between aerobic fitness and estrogen metabolites in premenopausal women. Med Sci Sports Exerc. 2005 Apr;37(4):585–92. doi: 10.1249/01.mss.0000158185.23595.24. [DOI] [PubMed] [Google Scholar]
- 26.Bentz AT, Schneider CM, Westerlind KC. The relationship between physical activity and 2-hydroxyestrone, 16alpha-hydroxyestrone, and the 2/16 ratio in premenopausal women (United States) Cancer Causes Control. 2005 May;16(4):455–61. doi: 10.1007/s10552-004-6256-6. [DOI] [PubMed] [Google Scholar]
- 27.Matthews CE, Fortner RT, Xu X, Hankinson SE, Eliassen AH, Ziegler RG. Association between physical activity and urinary estrogens and estrogen metabolites in premenopausal women. J Clin Endocrinol Metab. 2012 Oct;97(10):3724–33. doi: 10.1210/jc.2012-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell KL, Westerlind KC, Harber VJ, Bell GJ, Mackey JR, Courneya KS. Effects of aerobic exercise training on estrogen metabolism in premenopausal women: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2007 Apr;16(4):731–9. doi: 10.1158/1055-9965.EPI-06-0784. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz KH, Warren M, Rundle AG, Williams NI, Gross MD, Kurzer MS. Exercise effect on oxidative stress is independent of change in estrogen metabolism. Cancer Epidemiol Biomarkers Prev. 2008 Jan;17(1):220–3. doi: 10.1158/1055-9965.EPI-07-0058. [DOI] [PubMed] [Google Scholar]
- 30.Robles Gil MC, Timon R, Toribio AF, et al. Effects of aerobic exercise on urinary estrogens and progestagens in pre and postmenopausal women. Eur J Appl Physiol. 2012 Jan;112(1):357–64. doi: 10.1007/s00421-011-1982-4. [DOI] [PubMed] [Google Scholar]
- 31.Westerlind KC, Williams NI. Effect of energy deficiency on estrogen metabolism in premenopausal women. Med Sci Sports Exerc. 2007 Jul;39(7):1090–7. doi: 10.1097/mss.0b013e3180485727. [DOI] [PubMed] [Google Scholar]
- 32.Pasagian-Macaulay A, Meilahn EN, Bradlow HL, et al. Urinary markers of estrogen metabolism 2- and 16 alpha-hydroxylation in premenopausal women. Steroids. 1996 Aug;61(8):461–7. doi: 10.1016/0039-128x(96)00089-x. [DOI] [PubMed] [Google Scholar]
- 33.Arikawa AY, O’Dougherty M, Kaufman B, et al. Women in Steady Exercise Research (WISER): Study design and methods. Contemp Clin Trials. 2010 Sep;31(5):457–65. doi: 10.1016/j.cct.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American College of Sports Medicine. Guidelines for Exercise Testing and Prescription. 6. Philadelphia, PA: Lippincott, Williams & Wilkins; 2000. [Google Scholar]
- 35.Kriska AM, Knowler WC, LaPorte RE, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990 Apr;13(4):401–11. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 36.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000 Sep;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 37.Xu X, Veenstra TD, Fox SD, et al. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005 Oct 15;77(20):6646–54. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 38.Arikawa AY, O’Dougherty M, Kaufman BC, Schmitz KH, Kurzer MS. Attrition and adherence of young women to aerobic exercise: lessons from the WISER study. Contemp Clin Trials. Mar;33(2):298–301. doi: 10.1016/j.cct.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith AJ, Phipps WR, Arikawa AY, et al. Effects of aerobic exercise on premenopausal sex hormone levels: results of the WISER study, a randomized clinical trial in healthy, sedentary, eumenorrheic women. Cancer Epidemiol Biomarkers Prev. Jun;20(6):1098–106. doi: 10.1158/1055-9965.EPI-10-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson RE, Grebe SK, DJOK, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004 Feb;50(2):373–84. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 41.Taioli E, Im A, Xu X, Veenstra TD, Ahrendt G, Garte S. Comparison of estrogens and estrogen metabolites in human breast tissue and urine. Reprod Biol Endocrinol. 2010 Aug 2;8:93. doi: 10.1186/1477-7827-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]