Abstract
Theta and gamma frequency oscillations occur in the same brain regions and interact with each other, a process called cross-frequency coupling. Here, we review evidence for the following hypothesis: that the dual oscillations form a code for representing multiple items in an ordered way. This form of coding has been most clearly demonstrated in the hippocampus, where different spatial information is represented in different gamma subcycles of a theta cycle. Other experiments have tested the functional importance of oscillations and their coupling. These involve correlation of oscillatory properties with memory states, correlation with memory performance, and effects of disrupting oscillations on memory. Recent work suggests that this coding scheme coordinates communication between brain regions and is involved in sensory as well as memory processes.
Introduction
Multi-item messages must often be transmitted between brain regions. For instance, short-term memory may represent the last several events in the recent past; similarly, the sequence of events that constitute an episodic memory may be recalled from long-term memory. Handling such multi-item messages requires a neural code that specifies not only how items are represented, but also how different items are kept separate (e.g. the longer pauses that separate letters in the Morse code). Here, we evaluate the hypothesis that the neural code for multi-item messages is organized by brain oscillations. These oscillations can be observed in field potentials, a method of extracellular recording that provides a measure of average neural activity in a brain region (Buzsáki et al., 2012). Such recordings in rodents (Fig. 1A) have shown that gamma frequency (~40 Hz) oscillations are nested within slow theta frequency (~7 Hz) oscillations (Belluscio et al., 2012; Bragin et al., 1995; Colgin et al., 2009; Soltesz and Deschenes, 1993). A large number of experiments have investigated the role of theta/gamma oscillations, largely using physiological methods in rodents. More recently, the study of these oscillations in humans has become a focus of cognitive neuroscience (Axmacher et al., 2010; Canolty et al., 2006; Demiralp et al., 2007; Llinas and Ribary, 1993; Maris et al., 2011; Mormann et al., 2005; Sauseng et al., 2009; Voytek et al., 2010).
Fig. 1.
Neural code organized by theta and gamma oscillations. (A) Simultaneous extracellular (top) and intracellular (bottom) recordings from the hippocampus. Intracellular gamma is due to IPSPs, the amplitude of which is modulated by the phase of theta. From Fig. 4C of (Penttonen et al., 1998). (B) Schematic of the theta-gamma code. The ovals at top represent states of the same network during two gamma cycles (active cells are black and constitute the ensemble that codes for a particular item). Different ensembles are active in different gamma cycles.
The specific hypothesis that we will evaluate here is shown in Fig. 1B (Lisman and Buzsaki, 2008; Lisman and Idiart, 1995). According to this coding scheme, the subset of cells that fire during a given gamma cycle (sometimes referred to as a cell assembly or an ensemble) form a spatial pattern that represents a given item. Formatting of multiple items is organized by theta/gamma oscillations as follows: largely non-overlapping assemblies are active in different gamma cycles, i.e., at different theta phases. Given that there are four to eight gamma cycles nested within a theta cycle, multiple items can be represented in a defined order. Here, we will first describe the evidence that jointly occurring theta and gamma oscillations can organize information in the way hypothesized in Fig. 1B. We will then describe experiments that address the following questions: 1) Do the oscillations and their interaction vary with cognitive demands, and do these changes predict behavioral performance? 2) Does interfering with (or enhancing) the oscillations affect function? 3) Are the oscillations used to coordinate communication between brain regions? We then turn to an analysis of the mechanistic role of gamma oscillations in the context of the theta-gamma code. In the final section, we discuss outstanding issues, notably the relationship of alpha and theta frequency oscillations in cortex and the possibility that the theta-gamma code contributes not only to memory processes, but also to sensory processes.
Theta-gamma coding in the hippocampus
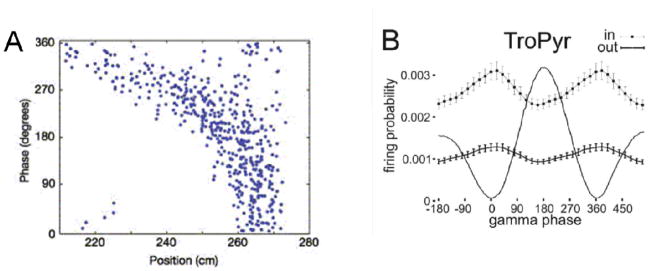
The first indication that theta oscillations have a role in neural coding came from the study of rat CA1 hippocampal place cells. Such cells increase their firing rate when the rat is in a subregion of the environment called the place field; different cells have different place fields (O’Keefe and Dostrovsky, 1971). As the rat crosses the place field of a cell, there are generally five to ten theta cycles. On each successive cycle, firing tends to occur with earlier and earlier theta phase (Fig. 2A), a phenomenon termed the phase precession (O’Keefe and Recce, 1993; Skaggs et al., 1996). These and related results (Lenck-Santini et al., 2008; Pastalkova et al., 2008) suggest that the hippocampus uses a code in which theta phase carries information. Further analysis showed that CA1 place cells fire at a preferred phase of the faster gamma oscillations (Fig. 2B) (Senior et al., 2008). Thus, during a given theta cycle, firing will tend to occur at a preferred theta phase and at a preferred gamma phase.
Fig. 2.
Spiking in the rat CA1 region depends on the phase of both theta and gamma oscillations.
(A) Phase precession: as the rat runs through a place field, a process that takes several seconds, theta phase becomes progressively earlier (closer to zero phase) on successive theta cycles. From Fig. 1A of (Mehta et al., 2002). The phase precession can be understood as cued, time-compressed readout of the sequences of places ahead of the animal. Such “sweeps” provide information to other brain regions of what is to come. Consider that the place field (210 cm to 270 cm) can be divided into seven subregions, a, b, c, d, e, f, and g. The rat enters the field from the left at position a, which cues the ordered prediction of upcoming positions (Battaglia et al., 2004), thereby producing a “sweep.” The “true” place field of this cell is the position g; the firing of this cell at position a represents a prediction that g is ahead of the animal. By the time of the second theta cycle, the rat has moved to b, so b is the cue and produces the sweep b, c, d, e, f, g, h in the second theta cycle. One can see from this example why the g cell fires with earlier theta phase on each successive theta cycle. According to one hypothesis, the successive activation of cells during a sweep results from simple chaining of excitation between the ensembles (Burgess and O’Keefe, 2011; Jensen and Lisman, 1996; Tsodyks et al., 1996). The results shown are combined data from many passages through the place field; the linkage of phase to position is even more exact when individual passages are analyzed (Schmidt et al., 2009), perhaps because theta can occur during different modes (see below) having different phase-position relationships.
(B) Many CA1 place cells fire at a preferred phase of gamma cycles (‘in’ and ‘out’ refer to the firing probability as a function of gamma phase when the rat is respectively inside or outside of the place field; the line with no error bars is the LFP filtered in the gamma band). From Fig. 6B of (Senior et al., 2008). Because the analysis averages over different functional modes (Colgin et al., 2009; de Almeida et al., 2012; Gupta et al., 2012; Senior et al., 2008) and gamma generators (Belluscio et al., 2012), the actual modulation by gamma phase may be stronger than shown.)
If a place cell fires at a particular phase during a theta cycle (i.e., in a particular gamma cycle), other place cells representing different information presumably fire at other theta phases, collectively forming a multi-part message. The ability to record simultaneously from >100 cells (Wilson and McNaughton, 1993) has made it possible to directly observe such messages. As illustrated in Figure 3, during an individual theta cycle, different place cells fire in a temporal sequence. These sequences are called “sweeps” because the firing order correspond to the cells’ place field centers along the track (Gupta et al., 2012) see also (Dragoi and Buzsaki, 2006; Gupta et al., 2012; Harris et al., 2003; Skaggs et al., 1996). Such data show directly that different cells representing different information (i.e. positions) fire at different theta phases. Given that firing is also modulated by gamma (Fig. 2B), there can be little doubt that a theta-gamma code is used in the hippocampus to represent ordered multi-item messages.
Fig. 3.
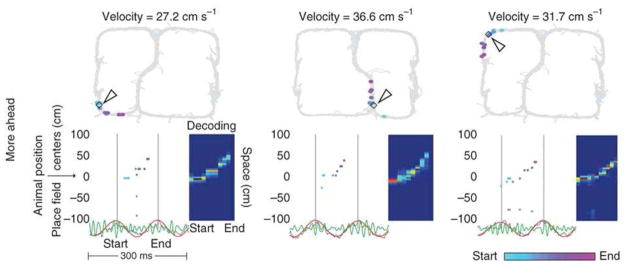
Cells that fire in sequence during a theta cycle form a sweep. Three examples are shown. (Top) Top panels show track. Current position of rat is marked by arrowhead, and it is at this position that the sweep shown below occurs. Different cells active during the sweep have place field centers marked by the colored dots. (Bottom) The spikes of different cells during a theta cycle (start to end). Cells are positioned on the y axis according to the position of their place field centers. In these examples, activity sweeps represent positions along the path ahead of the animal. From Fig. 3 of (Gupta et al., 2012).
An important property of sweeps is that that they are time compressed. Whereas the rat might take ~300 ms to move between positions a and b, cells representing these positions fire ~30 ms apart during a sweep (Skaggs et al., 1996). Furthermore, this time-compressed readout is often predictive (Battaglia et al., 2004), providing a way of rapidly informing downstream networks of the sequence of upcoming places (see Fig. 2 caption). The usefulness of such predictive readout is particularly evident for the sweeps that occur when a rat stops at the choice point of a familiar maze (Johnson and Redish, 2007). Under these conditions, one sweep may represent the sequence of positions down one arm of the maze (sweeps can turn corners and thus are dependent on memory rather than direct vision), and the next sweep may be down the other arm. Such rapid readout from memory presumably allows downstream brain regions to choose the arm leading to the goal.
To be useful for transmitting information, a code must be consistent over cell populations and stable over time. A procedure for examining these requirements utilizes one part of the recording period to correlate potential coding variables (such as firing rate or theta phase) to the observed position of the rat. Different codes can then be quantitatively compared by their ability to predict the rat’s position from the firing patterns during the other part of the experiment (Harris et al., 2003; Jensen and Lisman, 2000). It was found that codes that take theta phase into account allowed the rat’s position to be predicted with an accuracy of about 3 cm, while codes that did not use theta phase had less accuracy. Together, these and related results (Harris et al., 2003) show that the theta-phase code carries information, is stable over time, and is used consistently by cell populations.
Information is represented by an ensemble of cells rather than by single cells. Can a cell assembly that codes for a particular place be observed, and do the cells fire together in a gamma cycle, as postulated? Testing this prediction is difficult because the “true” place field (~3 cm; see Fig. 2 caption) is only a small fraction of the environment. Therefore, finding two place cells that code for the same position is difficult, but this has been achieved (Dragoi and Buzsaki, 2006). It can be seen if Figure 4 that two cells (red, green) have nearly identical place fields (Fig. 4A). The key observation is that these cells tend to fire in the same gamma cycle, as indicated by the fact that the cross-correlation of spiking in the two cells (Fig. 4B; red) has a peak that is very near 0 ms and a half-width of ±15 ms. These results and similar data in (Shapiro and Ferbinteanu, 2006; Skaggs et al., 1996) provide the few glimpses available of actual cell assemblies.
Fig. 4.
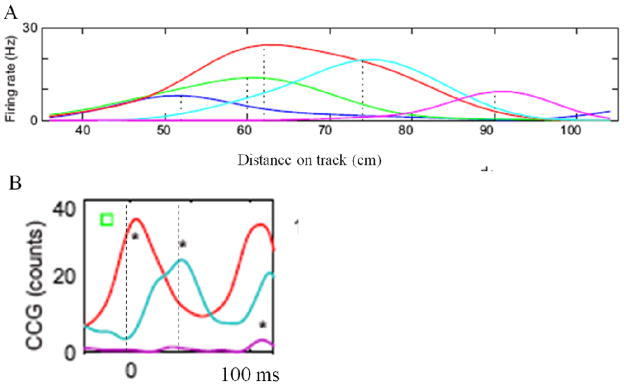
Cells that code for the same position (part of a cell assembly) and then fire in synchrony (within a gamma cycle). (A) Firing rate as a function of distance on a linear track for five place cells in CA1 (different colors). Green and red cells have peak firing rates at nearly the same position. (B) The cross-correlation between green and red cells (in A) has a peak very near 0 ms (red line), indicating that the cells tend to fire in the same gamma cycle. This is true even though the particular gamma subcycle changes during the theta-phase precession. Green and blue cells (in A) have different place field centers and tend to fire with a temporal offset that peaks at 40 ms (cyan line) (i.e., in different gamma cycles). From Fig. 1 of (Dragoi and Buzsaki, 2006).
The evidence we have described makes a strong case that the theta-gamma code is used to format multi-item messages about spatial information. We now turn to the question of whether this coding scheme can be linked to cognitive processes and to actual information transfer.
Changes in theta, gamma, and their interactions during tasks
Although the existence of brain oscillations has been known for many years, the idea that these oscillations provide a mechanistic framework for memory processes is relatively recent and has been controversial. One strategy has been to ask the simple question of whether oscillations are changed during memory processes. Another, more telling approach has been to test whether the observed change predicts the accuracy of subsequent memory performance. Initial studies focused on individual types of oscillation; more recent studies have examined the role of theta-gamma coupling. Below, we briefly review these studies---first those on long-term memory and then those on working memory.
Long-term memory
Gamma
Studies of long-term memory have focused on the hippocampus. It was found that gamma power and spike-gamma coherence in the monkey hippocampus were higher during successful encoding (Jutras et al., 2009). Similar correlations have been found in humans, both in the hippocampus (Sederberg et al., 2007) and cortical regions (Osipova et al., 2006; Sederberg et al., 2007)
Theta
In rats, hippocampal theta increases during locomotion or attention (Vanderwolf, 1969) and is necessary for memory function (Winson, 1978). In humans, the theta power preceding the stimulus predicted subsequent memory (Fell et al., 2011; Guderian et al., 2009). Using a somewhat different strategy, Rutishauser et al. showed that successful memory formation was predicted by how well spike timing was phase coupled to theta oscillations (Rutishauser et al., 2010). Recent work suggests that, in humans, “slow theta” (3–4 Hz) is predictive of correct recall (Lega et al., 2012; Watrous et al., In Press) and corresponds functionally to the 7 Hz theta of rats.
Theta-Gamma Coupling
Several signal processing tools have been developed to quantify theta-gamma coupling (more generally termed cross-frequency coupling) (Canolty et al., 2006; Cohen, 2008; Kramer et al., 2008; Onslow et al., 2011; Penny et al., 2008; Tort et al., 2010; Young and Eggermont, 2009). These measure the relationship between the phase of the theta oscillations and the envelope of the gamma power. Thus, high values of coupling indicate that gamma amplitude is a strong function of theta phase. Theta/gamma coupling has been shown to be functionally important for long-term memory processes (Tort et al., 2009). In this study, rats learned to associate contexts with the location of subsequent food reward (Fig. 5A). As learning progressed, there was an increase in cross-frequency coupling (Fig. 5B). Moreover, the strength of coupling predicted the probability of correct choice (Fig. 5C). These and related results (Shirvalkar et al., 2010) suggest that theta-gamma coupling in the rat hippocampus enables the recall of stored information. A recent study on humans points to a similar conclusion: long-term memory encoding was associated with increased cross-frequency coupling between frontal theta and posterior gamma oscillations (Friese et al., 2012).
Fig. 5.
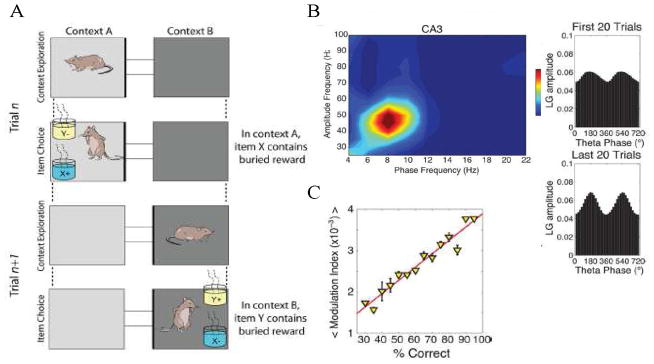
Theta-gamma cross-frequency coupling in CA3 predicts behavior. (A) Exposure to empty context A or B (light gray/dark gray) cues the rat about which cup (blue/yellow) when subsequently presented will contain reward. (B) While exploring context, cross-frequency coupling occurs (left) and can vary in the degree to which theta phase modulates gamma power (right). (C) Theta-gamma modulation occurs during recall of the association between the context and the type of rewarded cup; this modulation is predictive of whether the rat correctly goes to the rewarded cup. From Fig. 1 and 2 of (Tort et al., 2009).
Working memory
In a typical working memory experiment, subjects are presented with a list of several items. This is followed by a delay period (usually <10 s) during which no information is presented. Subjects are the shown a test item and must make a response based on the properties of the information stored in their working memory.
Gamma
In humans, MEG studies and intracranial recordings have reported an increase in gamma power during the delay period of working memory tasks. Importantly, this increase varies with the number of items being held in memory (Howard et al., 2003; Roux et al., 2012). An increase in spike-field coherence during the delay period has also been observed (Pesaran et al., 2002).
Theta
Several studies in humans have reported sustained theta band cortical activity during the delay period (Gevins et al., 1997; Jensen and Tesche, 2002; Raghavachari et al., 2001; Scheeringa et al., 2009), pointing to a role for theta in working memory. One objection to this conclusion is that single-unit recording of persistent firing during a working memory task did not reveal any obvious theta rhythmicity (Funahashi et al., 1991). However, rhythmicity can be difficult to detect by analysis of spikes alone and is more easily detected by determining whether spikes are phase locked to the oscillations in the local field potential (Wang, 2010). Consistent with this, experiments in monkeys have shown that persistent firing during a working memory task is phase locked to low-frequency (theta/delta) oscillations in the local field potential, both in extrastriate cortex (Lee et al., 2005; Liebe et al., 2012) and in prefrontal cortex (Siegel et al., 2009).
Theta-gamma coupling
Theta-gamma coupling during working memory has been demonstrated in humans both in cortex (Canolty et al., 2006; Lee et al., 2005) and in the hippocampus (van Vugt et al., 2010). Gamma power in the hippocampus is modulated by the phase of theta oscillations during working memory retention, and the strength of this cross-frequency coupling predicts individual working memory performance (Axmacher et al., 2010). Importantly, the particular cortical regions demonstrating cross-frequency coupling depend on the nature of the information being held in working memory. The spatial distribution of gamma band power in cortex can be used to predict whether working memory is maintaining an indoor or outdoor scene (Fuentemilla et al., 2010), and the gamma activity with this predictive capability is phase locked to the theta activity. In another study, gamma power at certain sites (primarily in occipital cortex) was shown to depend on the particular letter being viewed, and gamma was found to be phase locked to theta (Jacobs and Kahana, 2009).
The concept of the theta-gamma code (Lisman and Idiart, 1995) was originally proposed as a way of keeping multiple items active in working memory and there have been several related implementations of this idea (Jensen and Lisman, 1998; Koene and Hasselmo, 2007; Lisman and Idiart, 1995). Support for this class of models has come from the analysis of grid cells in the entorhinal cortex (de Almeida et al., 2012), a region that provides input to the hippocampus and has been previously implicated in working memory (Gaffan and Murray, 1992; Ranganath and D’Esposito, 2001; Stern et al., 2001; Suzuki et al., 1997; Young et al., 1997). It was found that the entorhinal cortex has a working memory mode in which grid cells represent the recent past (i.e., positions behind the animal). Consistent with the model of Fig. 1B, cells representing different positions fired in different gamma subcycles of the theta cycle.
Another way of asking whether the theta-gamma code underlies working memory is to relate the oscillations to the psychophysically measured properties of working memory. A classic result (Miller, 1956) is that working memory has a capacity limit (span) of 7±2 (see (Cowan, 2001) for a slightly lower value). The number of gamma cycles within a theta cycle may be what sets the capacity limitation for working memory (Lisman and Idiart, 1995). Initial efforts to test this concept sought to use the theta-gamma framework to quantitatively account for response time properties of the Sternberg task (i.e., time to respond to whether a given test item was on a short list presented several seconds before). The linear dependence of response time suggested that the list held in working memory was serially and exhaustively scanned at a rate of 20–30 ms per memory item (Sternberg, 1966), a time that approximately equals the duration of a gamma cycle. These and other quantitative results of the Sternberg task can be accounted for by models based on the theta-gamma code assuming either that theta phase is reset by stimuli or that theta frequency decreases with memory load (Jensen and Lisman, 1998). Experiments provide evidence for both effects (Axmacher et al., 2010; Moran et al., 2010; Mormann et al., 2005; Rizzuto et al., 2006). Recent work sought to determine whether properties of theta and gamma oscillations in individuals could explain their memory span. The ratio of theta to gamma (i.e., the maximum number of gamma cycles within a theta cycle) was found to correlate with span (Kaminski et al., 2011). However, the determinations of oscillation frequencies were very noise sensitive, raising doubts about the conclusion. Rigorous testing of this relationship will require resolution of the controversy about which brain regions are responsible for short-term memory maintenance and better methods for noninvasive measurement of the oscillatory frequencies at those locations.
Interfering with oscillations
The experiments described in the previous section make the strong case that theta and gamma oscillations are modified during memory tasks and that these modifications are predictive of performance. However, such experiments are only correlational; a stronger confirmation would require showing that disruption of the oscillations interferes with memory. Experiments of this kind are beginning to be possible because of increased understanding of the mechanisms that underlie oscillations and new optogenetic methods for perturbing the oscillations.
Gamma oscillations are generated by a feedback loop in which firing of pyramidal cells excites fast-spiking interneurons, which then rapidly inhibit the entire population of pyramidal cells (reviewed in (Buzsáki and Wang, 2012). Pyramidal cell firing is reinitiated after the GABAergic inhibition decays, thereby generating the next gamma cycle. Genetic methods have been used to block the excitation of fast-spiking interneurons by reducing their AMPAR-mediated input or by preventing firing using halorhodopsin. Both methods produce a decrease in gamma amplitude and result in memory deficits (Fuchs et al., 2007; Sohal et al., 2009).
There is substantial information about the processes that generate theta oscillations in the hippocampus. One important influence is the external input from the medial septal nucleus, a structure that shows theta rhythmicity (King et al., 1998; Zhang et al., 2011). This nucleus contains cholinergic and GABAergic neurons that impose a rhythmic drive onto the hippocampus. However, theta oscillations can also be generated by intrinsic mechanisms, as demonstrated by experiments on the isolated rat hippocampus (Goutagny et al., 2009) and on hippocampal slices (Fellous and Sejnowski, 2000; Fischer et al., 2002; Gloveli et al., 2005). The result of extrinsic and intrinsic influences is a theta frequency inhibition of pyramidal cells, which at its maximum strongly inhibits pyramidal cell firing and reduces the amplitude of gamma oscillations.
Shirvalkar et al. have shown that theta-gamma coupling increases during memory recall (Shirvalkar et al., 2010). They also found that inhibiting the medial septum with muscimol reduced theta-gamma coupling and interfered with recall. Remarkably stimulating the medial septum at theta frequency partially restored recall. Further evidence for the importance of theta-phase coding has come from experiments examining the effects of the cannabinoid receptor agonist CP5590 (Robbe et al., 2006). This agent strongly disrupted rats’ performance on a hippocampal-dependent delayed spatial alternation task. Recordings showed that hippocampal place fields were normal. However, the theta-phase precession normally seen in these cells was completely absent, suggesting that phase-coded information is necessary for this task.
Role of theta and gamma oscillations in communication between regions
A strong possibility is that the communication between regions is affected by the coherence of the oscillations in the sender and receiver networks (Buzsáki, 2010). Coherence occurs when the phase difference in a given frequency band is consistent over time. Coherence varies between 0 and 1, and high values are realistic. For instance, theta coherence can be 0.95 between different parts of the hippocampus (Penley et al., 2011), while theta coherence is in the range of 0.5 between hippocampus and interaction regions in the PFC, amygdala and striatum (Seidenbecher et al., 2003; Sirota et al., 2008; van der Meer and Redish, 2011). The theta coherence between two structures can be elevated in specific phases of a task (Benchenane et al., 2010; Kim et al., 2011; Young and Shapiro, 2011). Importantly, it has been found that during periods of decision, theta coherence between the hippocampus and striatum could be >0.8 and that the magnitude of coherence was predictive of learning (DeCoteau et al., 2007).
As we have argued, the importance of the theta rhythm is to provide a way of ordering multi-part messages, an ordering that is exemplified by the phase precession. Phase precession is found both in the structures that provide input to the hippocampus (e.g., the entorhinal cortex and subiculum) (Hafting et al., 2008; Kim et al., 2012; Mizuseki et al., 2009) and in structures to which to which the hippocampus projects (e.g., the PFC and striatum). Notably, firing in the rat mPFC shows phase precession coupled to that seen in the hippocampus (Jones and Wilson, 2005). Similarly, theta-phase precession can be seen in the striatum (Fig. 6; (Jones and Wilson, 2005; van der Meer and Redish, 2011). The strong coherence between the hippocampus and its targets and the existence of phase coding in target structures strongly suggest that theta oscillations organize communication between the hippocampus and distant brain regions.
Fig. 6.
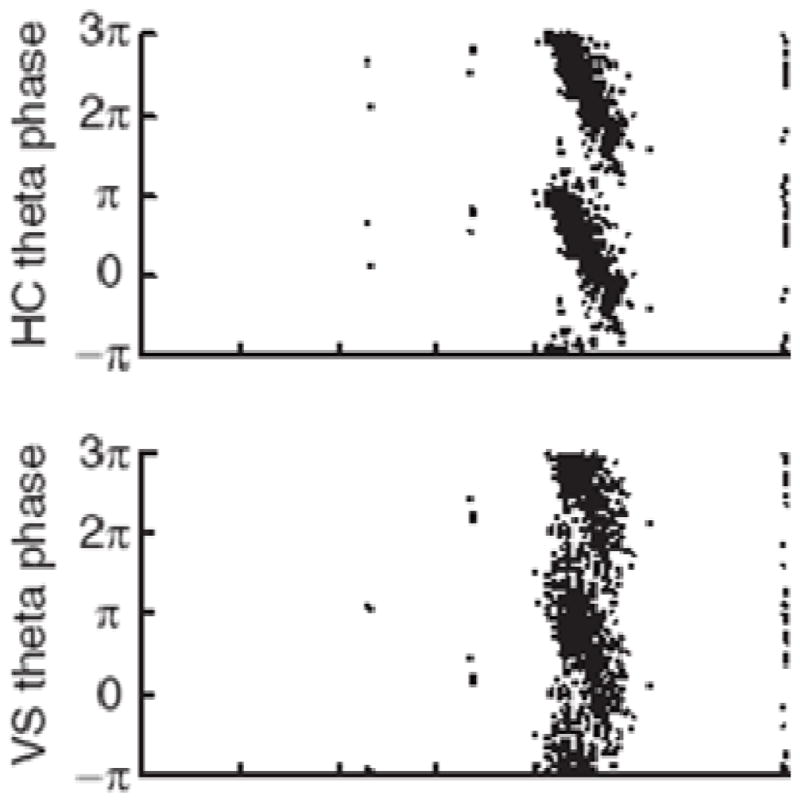
Phase precession, an indicator of theta-gamma coupling, is evident in the hippocampus (HC) (top) but is also evident in a hippocampal target area, the ventral striatum (bottom). The data are taken as the rat begins its approach to the reward site. Taken from Fig. 3B (van der Meer and Redish, 2011).
Theta-phase coding is also implicated in communication that does not involve the hippocampus. Theta-phase synchronization occurs between V4 and PFC and is predictive of task performance (Liebe et al., 2012). Although volume conduction confounds are often difficult to assess in human EEG and MEG studies, there are several encouraging findings indicating theta synchronization between frontal and posterior regions in working memory and error-monitoring tasks (Brzezicka et al., 2011; Cavanagh et al., 2009; Cohen and Cavanagh, 2011; Palva et al., 2010; Sarnthein et al., 1998; Sauseng et al., 2005; Schack et al., 2005).
The findings summarized above make the strong case that theta oscillations are important for long-range communication and that a signature of such communication is a high level of coherence, as originally proposed by (von Stein and Sarnthein, 2000). How this high level of coherence is achieved remains unclear. Within the hippocampus, high theta coherence occurs because the entire structure is driven by a theta generator in the medial septal nucleus of the basal forebrain. A nearby structure, the nucleus basalis, innervates cortex and is a good candidate mechanism for synchronizing cortical theta (Alonso et al., 1996; Lee et al., 2005). However, bidirectional interactions between cortex and thalamus (da Silva et al., 1973; Hughes and Crunelli, 2005; Lopes da Silva et al., 1980; Lorincz et al., 2009; Poulet et al., 2012; Saalmann and Kastner, 2011) and intrinsic mechanisms may also be involved (Alonso et al., 1996; Blatow et al., 2003; Flint and Connors, 1996; Jones, 2004; Raghavachari et al., 2006).
There is now substantial evidence suggesting that gamma synchronization between regions can also change during a task. Gamma coherence between monkey parietal and prefrontal areas has been shown to increase from 0.1 to 0.18 during an attention task (Gregoriou et al., 2009). Colgin et al. found alternating modes in which CA1 became coherent either with entorhinal cortex (through the fast gamma characteristic of the entorhinal region) or with CA3 (through the slower gamma characteristic of CA3) (Colgin et al., 2009). A recent study by Bosman and colleagues provides compelling evidence that gamma coherence reflects the selectivity of attention-mediated communication (Bosman et al., 2012). Two regions in V1 were studied that converged onto V4. When attention was turned to one V1 region, the coherence of this region with V4 was increased from 0.02 to 0.12 (the other V1 region was not affected). Granger analysis indicated that this change was due to the influence of V1 on V4. Changes in coherence have been correlated with memory performance. A study analyzing data from depth electrodes in epileptic patients showed that gamma synchronization between rhinal cortex and hippocampus predicted memory formation (Fell et al., 2001). In a rat study, gamma band synchronization between CA3 and CA1 reflected performance in a spatial memory task of the behaving rat (Montgomery and Buzsaki, 2007).
An interesting possibility is that changes in gamma coherence may actually control the routing of information (Bressler, 1995; Fries, 2005; Siegel et al., 2012; Varela et al., 2001). This mechanism has been termed the communication through coherence (CTC) hypothesis (Fries, 2005). The general idea is that there are cycles of excitability in oscillatory networks; inputs will be most effective if they arrive at peaks of excitability. Thus, a mechanism that made gamma oscillations in two regions synchronous might selectively route information from one region to the other. However, several difficulties with this hypothesis must be noted, particularly. First, the measured levels of long-range gamma coherence are generally very low (0.1–0.2), so any matching of input with the local phase of gamma will be weak (it remains possible that coherence is high but is made low by signal-to-noise problems). Computational studies suggest that strong coherence is required for selective routing (Akam and Kullmann, 2012). Second, there is no indication of an external driver that can impose coherent gamma oscillations in two communicating regions; it is thus thought that coherence develops because of entrainment or resonance mechanisms, processes that develop over many gamma cycles. This process is thus too slow to explain the routing changes that occur during changes in covert attention, which occur in a time period of about a single gamma cycle (Buschman and Miller, 2009; Treisman and Gelade, 1980). These difficulties pose a challenge to the CTC hypothesis; perhaps, to the contrary, increases in gamma coherence are not the cause of selective routing but rather the consequence of some other routing mechanism (see also (Rolls et al., 2012). It is unclear what the alternative mechanism might be. One possibility is suggested by recent work showing that the thalamus (pulvinar) can coordinate coherent activity in the alpha bands between parts of visual cortex and produce the routing changes that are required for selective attention (Saalmann et al., 2012).
In summary, the presence of information coded by theta phase in the striatum and PFC, together with the strong coherence of theta between the hippocampus and these regions, point to the importance of theta phase coding in long-range communication between brain regions. The role of gamma coherence in long range communication is less clear. In the next section we discuss various postulated roles of gamma. This is followed by a summary section in which we attempt to bring the available information together.
What does gamma contribute to the theta-phase code?
Clocking
Gamma is sometimes referred to as the brain’s clock. According to this view, a given gamma cycle would occur at a predictable time that was a multiple of the gamma period. This view now seems unlikely. Gamma frequency in visual cortex changes with the contrast of visual stimuli (Ray and Maunsell, 2010). Moreover, the gamma period even varies from cycle to cycle (Burns et al., 2011; Henrie and Shapley, 2005; Ray and Maunsell, 2010). This variation occurs in a predictable way: the amplitude of gamma during one cycle (presumably reflecting the number of active cells) determines the duration of the next gamma cycle (Atallah and Scanziani, 2009). The variation of gamma frequency with intensity implies that two parts of the same object having different intensity cannot be synchronized in the same gamma cycles (Ray and Maunsell, 2010), as required if gamma has a binding role in perception (Engel and Singer, 2001). In the model of Fig. 1B, there is also a concept of binding (cells that represent the same item fire in the same gamma cycle), but it is not assumed that different cells represent different components of the item.
Synchronization and inter-item separation
Firing occurs during only part of the gamma cycle, a synchronization that allows downstream networks to use coincidence mechanisms to detect ensembles (Konig et al., 1996). Less recognized is the importance of the pauses that separate the firing that occurs in sequential gamma cycles. As noted earlier, information formatting is a critical aspect of coding that allows downstream “receiver” networks to interpret the coded message (Buzsáki, 2010). In Figure 7, we compare two schemes for organizing the multi-item message in the “sender” network. In one scheme, the sender rapidly switches from a state representing one item to a state representing the next item, i.e., there is no inter-item separation. In the other scheme, the sender clusters spikes so that they fire in about one-third of a gamma cycle, thereby creating inter-item separation. Now consider the fact that, for a cell to process input, there must be a window over which it integrates input. Experimentally, this window is shorter than a gamma cycle (Losonczy and Magee, 2006; Pouille and Scanziani, 2001). Without inter-item separation, receiver cells will integrate inputs that are part of different items. These cells may therefore detect conjunctions that do not correspond to those in either of the items present. Such false positives are avoided if the pauses generated by gamma frequency inhibition are present.
Fig. 7.
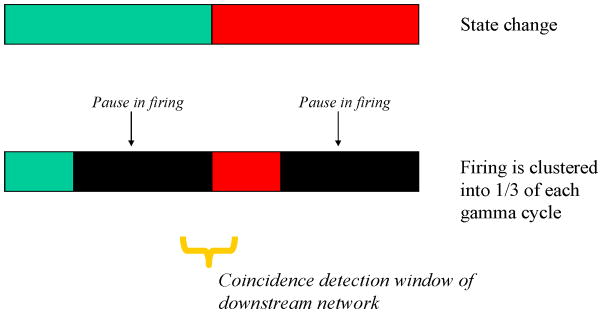
Role of the firing pause between items. Firing as a function of time for two sequential items in schemes with or without a firing pause between items. (Top) An ensemble representing one item (green) followed by rapid state change to an ensemble representing another item (red). (Bottom) Firing for the first item is clustered in one-third of a gamma cycle followed by a pause. Firing for the second item then occurs in the first part of the second gamma cycle, followed by another pause. Downstream networks detect patterns by integrating inputs that arrive within the coincidence detection window (yellow bar). If green is represented by cells 1,2,3, red by cells 4,5,6, and blue by 1,2,6, then the message detected in the state-change case is green, blue, red. In contrast, when firing is clustered, the message is correctly read as green, red.
Selecting which cells fire
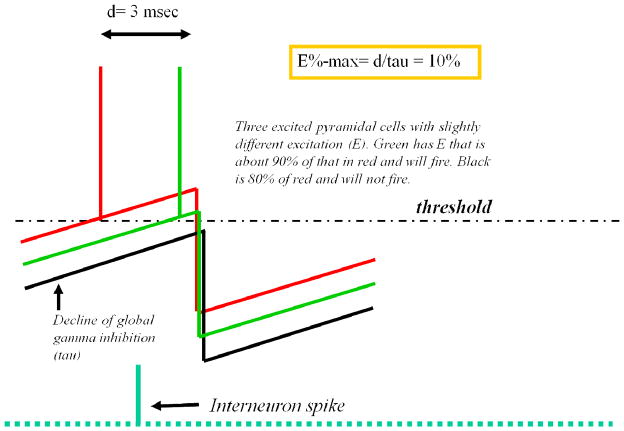
A second critical role for gamma is in the selection of which cells fire, thereby forming the representation that is active during a gamma cycle. According to one major theory (van Vreeswijk and Sompolinsky, 1996), cells receive large excitation that is balanced by a comparable inhibition; what determines whether a cell fires is stochastic deviations from this balance. However, this theory is not applicable to networks with oscillating inhibition, as discussed by (Isaacson and Scanziani, 2011). An alternative theory (“E%-max”) posits that, in networks with dynamic inhibition, a network process rather than a single-cell process determines which cells fire (de Almeida et al., 2009). According to this theory, the critical step is a “search” for the most excitable cells in the network that occurs as inhibition decays during a gamma cycle. The most excited cells in the network will reach threshold first and trigger global feedback inhibition of less-excited cells (Fig. 8). However, this inhibition occurs with a delay of a few milliseconds, allowing somewhat less-excited cells to fire before feedback inhibition arrives. Thus, gamma allows the firing of not only the most excited cell, but also somewhat less-excited cells. As a result of this process, the most excited cell (i.e., best tuned to the stimulus) will fire first in a gamma cycle followed by cells that are slightly less well-tuned. This prediction has been recently confirmed: it was found that orientation tuning in V1 is highest early in a gamma cycle but becomes slightly lower later in the cycle (Womelsdorf et al., 2012).
Fig. 8.
How inhibition during gamma oscillations determines which cells fire. Interneurons fired during a previous gamma cycle (left) and generated global inhibition in pyramidal cells. Inhibition decays with the time constant of the IPSP (tau; this equals the membrane time constant) (left). The voltage in three pyramidal cells having different tonic excitation is shown (red/green/black). As inhibition decays, the most excited cell (red) reaches threshold. This triggers an interneuron spike and feedback inhibition, which arrives at all pyramidal cells with delay, d. During this delay, the cell (green) with excitation that is close to that of the most excited cell will also fire.
Summary
There is now little doubt that multi-item information is formatted by a theta-gamma code in the hippocampus: different spatial locations are represented at different theta phases, and firing is clustered into discrete periods by the gamma rhythm. These oscillations and their interactions are altered during both long-term and working memory processes, as would be expected if different neural operations make different use of these oscillations. That said, we lack a clear understanding of why different tasks (or no task) result in the observed quantitative changes. A critical test of whether the theta-gamma code has an important role in memory is to determine whether inhibiting the oscillations interferes with memory. The available results suggest that this is the case, but more precise experiments are needed. Recently, optogenetic methods have been used to show that odor recognition can be disrupted by selectively interfering with information processes at particular phases of the sniff cycle (Smear et al., 2011). If the hypothesis we are proposing is correct, disrupting information at a particular theta phase should affect information represented at that phase, but not information represented at other phases.
Theta is critical for the transmission of multi-item messages because it provides a phase reference that signifies the onset of the message. This phase reference must be shared by sender and receiver; the high observed theta coherence between communicating regions appears to satisfy this requirement. The role of gamma is to define an item in a multi-item message. Gamma contributes to this in three ways: 1) it helps to form the message by allowing only the most excited cells to fire, 2) it synchronizes spikes (clustered spiking can be effectively detected in downstream regions), and 3) it creates pauses between items that prevent errors in decoding the message. The communication of the multi-part messages to downstream networks may be aided by coherence in the gamma band, but this is probably not required. We suspect that the small increases in gamma coherence that occur during communication are probably a result of effective communication rather than the cause. Because gamma cycles are not of the same duration, detection methods based on exact clocking are not plausible. Thus, although phase-dependent detectors (Jensen, 2001) can be used to detect early versus late items, detection of the information in a specific gamma subcycle does not appear possible. However, many useful functions do not require exact clocking. For instance, according to one model (Fukai, 1999), the sequence of actions to be executed is sent from the hippocampus to the striatum by a theta-gamma code; the striatum stores this sequence and then executes the actions in order, using other information to orchestrate the exact timing of each action. Another useful operation would be the recall of a sequence that contained a salient element such as reward. The detection of this element could be important to downstream networks even if the exact position of that element in the recalled sequence was uncertain. Finally, the entire recalled sequence may be processed (chunked) to represent a higher-level item. Network models that perform such chunking depend on the ordering of items rather than on exact timing (Sanders et al., 2012).
Outstanding Questions
How is the coherence of hippocampus, cortex, and basal ganglia produced?
As described above, when the hippocampus communicates with target regions, the theta in the two regions becomes high. Virtually nothing is known about how this coherence is produced. It seems likely that the basal forebrain and/or the thalamus may be involved, but this remains to be investigated.
What mechanism underlies changes in cross-frequency coupling?
The method for studying cross-frequency coupling are strictly operational and thus do not give insight into the underlying mechanism. For instance, a change in cross-frequency coupling could be explained either by a change in the number of gamma cycles per theta cycle (the duty cycle) or by a change in gamma amplitude with a constant number of gamma cycles per theta cycle. New analytical methods may be helpful in providing a clearer view of what is happening during the observed changes in theta-gamma coupling.
Is there also a super-fast gamma-phase code?
According to our hypothesis, a representation is formed by all of the cells that fire in a gamma cycle, regardless of their gamma phase. Thus, differences in gamma phase (amounting to only milliseconds) are thought to be unimportant and would be averaged over by the time window of integration in receiver networks. Consistent with the unimportance of gamma phase, position reconstruction methods did not show any advantage to using small theta phase differences that correspond to fractions of a gamma cycle (Harris et al., 2003; Jensen and Lisman, 2000). As noted above, V1 cells with millisecond differences in gamma phase have slightly different orientation tuning. We suspect that such millisecond differences are not important because they are integrated by downstream regions. In memory circuits, an important computation, pattern completion, can be performed during a gamma cycle (de Almeida et al., 2007); dealing with multiple input items within a gamma cycle would be disruptive of this processing. Still, the possibility that the brain uses a superfast code based on millisecond differences in gamma phase must be considered (Fries et al., 2007; Nikolic et al., 2013). Indeed, millisecond differences are important in specialized auditory circuits (Wagner et al., 2005; Yang et al., 2008). Furthermore, possible support for a gamma-phase code comes from single-unit recordings during multi-item working memory in monkeys (Siegel et al., 2009). It was found that neurons representing different memories fired maximally at a different gamma phases, consistent with a superfast gamma-phase code. However, given the analysis method used, it cannot be excluded that cells firing with a different gamma phase were actually firing in different gamma cycles (i.e., with a different low-frequency phase). Indeed, this would be consistent with results showing that hippocampal neurons that fire with different theta phase also fire with different gamma phase (Senior et al., 2008). Further work will thus be needed to clarify this important issue.
What is the signature of engaged neocortex (role of alpha and theta)?
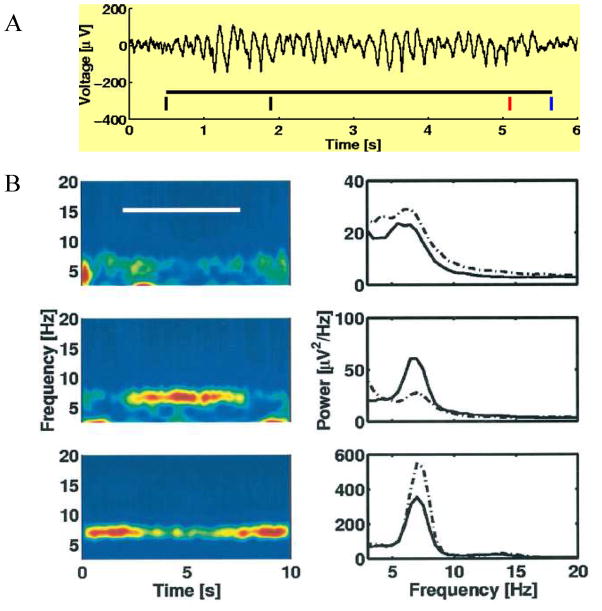
The properties of theta in the neocortex are much less understood than those in the hippocampus. In the hippocampus, increases in theta power are associated with engagement, but whether this is also true in neocortex is unclear. As shown in Figure 9, there are some sites where theta increases and others where it decreases during a working memory task. Which one is the engaged region? A further critical question is whether oscillations in the theta frequency range should be considered fundamentally different from those in the alpha range? In the hippocampus, these frequencies are lumped together as “theta” but is this also appropriate for cortex? In the following paragraphs, we discuss these questions.
Fig. 9.
Theta power is gated up at some cortical sites and down at others during the period of working memory. (A) With intracranial recordings, large-amplitude theta can be observed in single trials of the Sternberg task. First black bar is the time of cue presentation; second black bar indicates presentation of the item to be remembered; at the red bar, the test item is presented and the subject indicates (at the blue bar) whether it is the same as the remembered item. In other trials, more items were presented. (B) Recordings from three sites where task (white bar) had little effect on theta power (top), where it produced an increase in theta power (middle), or where it produced a decrease in theta power (bottom). Color code at right indicates theta power. From Fig. 8 of (Raghavachari et al., 2001).
A classic observation is that alpha power in occipital cortex is high when the visual system is not engaged (Adrian and Matthews, 1934). A cue indicating that attention must be turned to one hemifield leads to a sharp drop in alpha power in the engaged (contralateral) cortex (Worden et al., 2000). In contrast, alpha power in ipsilateral visual cortex may actually increase. This increase is associated with suppression of information that is irrelevant to the task (Thut et al., 2006) and with improved performance (Handel et al., 2011). Further evidence that high alpha power is associated with inhibition is that firing rates, phosphene detection, and the BOLD signal are all reduced (Haegens et al., 2011; Ritter et al., 2009; Romei et al., 2008). One characteristic of alpha is that neural activity is limited to only about half of the cycle (Bollimunta et al., 2008; Bollimunta et al., 2011; Lakatos et al., 2005). This low-duty cycle (for comparison, see high-duty cycle of firing in Fig. 2) probably accounts for why perception is dependent on alpha phase (Busch et al., 2009; Dugue et al., 2011; Mathewson et al., 2009). The suppression of alpha power has also been observed in other sensory and motor regions when they become engaged (Fontanini and Katz, 2005; Hari and Salmelin, 1997; Pfurtscheller et al., 1997). Importantly, the suppression is even specific to the particular motor subregion that is engaged (Pfurtscheller et al., 1997) (see also (Miller et al., 2009)). Thus, in cortex, high alpha power is an inhibited state, and low alpha power appears to be an indicator of engagement.
Do these “alpha rules” also apply to the only slightly slower cortical oscillations in the theta range? It is important to recall that the frequency bands corresponding to alpha and theta were assigned arbitrarily without consideration of function. Consider Figure 9. If we use hippocampal theta as a model, the elevated theta state would be the engaged state. But based on the “alpha rules”, the lowered theta state would be the engaged state. In favor of the latter, work combining EEG and fMRI has demonstrated that the BOLD signal is high when theta power is low (Michels et al., 2010; Scheeringa et al., 2009). Another perspective on this issue comes from consideration of gamma activity. Gamma is present during the high alpha power state and is phase locked to alpha. As alpha power is decreased, gamma power is increased (Spaak et al., 2012). Importantly, when alpha power falls during task engagement, gamma and its modulation by low-frequency oscillations remain (Sauseng et al., 2009). Indeed, the importance of this cross-frequency coupling was demonstrated by the fact that the coupling varied with memory load up to saturation and that this saturating load correlated with memory span.
Putting these experimental observations together, we suggest that a signature of engaged cortex is a reduction in the power of alpha/theta (perhaps with a lowering of frequency), modulation of gamma amplitude by the remaining alpha/theta oscillations, and an increase of the gamma duty cycle, as in Figure 9. Thus, the engaged sites in Figure 9 may be the ones at which alpha/theta decreases, but cross-frequency coupling is nevertheless important. Consistent with this view, cross-frequency coupling between gamma and alpha/theta correlates with the BOLD signal better than other available EEG variables (Wang et al., 2012).
Are there single theta cycle messages?
There are several reports showing brief periods of gamma activity (gamma bursts) in cortex and striatum (Berke, 2009; Edwards et al., 2005; Freeman, 2003; Howe et al., 2011; Jacobs and Kahana, 2009; Sirota et al., 2008; Yang et al., 2012). The duration of these “gamma bursts” is 100–200 ms. Gamma bursts of similar duration underlie sharp waves in the hippocampal CA3 region (Carr et al., 2012). It is curious that this duration is similar to that of a theta cycle. One could thus argue that, even though continuous theta oscillations are not present in these states, a single theta cycle (and associated gamma) is what is occurring. Indeed, during fast behavioral reactions, there may be time for only a single theta cycle. It will thus be of interest to determine whether perturbations that affect the frequency of continuous theta also affect the duration of gamma bursts.
Is there a role of theta-gamma in sensory processing?
Early work in the olfactory cortex provided evidence that theta-gamma interactions are involved in cortical sensory processing (Woolley and Timiras, 1965), and there has recently been renewed interest in this possibility. Recordings from awake, behaving rodents have been made from mitral cells, the output neurons of the olfactory bulb. It was found that a given odor produced brief bursts at a particular phase in the sniff (theta) cycle; different cells had different preferred phases (Shusterman et al., 2011). Firing in the bulb is also phase locked to gamma phase (Sanders et al., 2012). These results and related work in insects (Laurent et al., 2001) suggest that the signature of an odor is a discrete sequence of ensembles organized by theta-gamma oscillations.
In the auditory system, a model has been proposed in which ongoing syllables and words are segmented by an oscillatory cortical decoder based on nested theta and gamma oscillations (Ghitza, 2011; Giraud and Poeppel, 2012; Peelle and Davis, 2012). This process may involve yet slower oscillations tied to larger lexical units, a hypothesis that relates to an important study (Lakatos et al., 2005) showing that theta oscillations can be nested within slower delta frequency oscillations. This and other recent findings (Fujisawa and Buzsaki, 2011) raise the possibility that a hierarchy of oscillations that include gamma, theta, and delta oscillations organizes sensory and memory functions.
Interest in oscillations in the visual system has been stimulated by the finding that the phase of a stimulus with respect to ongoing cortical alpha/theta oscillations is predictive of the perception threshold, at least for attended stimuli (Busch et al., 2009; Mathewson et al., 2009). This finding was corroborated by a TMS/EEG study in which the perception of phosphenes was modulated by the phase of both frontal and posterior rhythms in the theta and alpha band (Dugue et al., 2011). Furthermore, the phase of oscillations in the theta-alpha band has been implicated in saccade onset (Drewes and VanRullen, 2011; Hamm et al., 2012). Given that covert attention can move very rapidly (Buschman and Miller, 2009), one possibility is that a theta cycle may be subdivided by faster oscillations, with each of these subcycles representing a different component of the visual scene (Miconi and Vanrullen, 2010).
Conclusion
Neurophysiological studies have provided evidence that a theta-gamma code is used to organize information in the hippocampus and to transmit it to targets in the PFC and striatum. Cognitive studies have shown a strong linkage between theta-gamma oscillations and successfully recalled memories. This body of work lays a foundation for understanding the general problem of how multi-item messages are sent between brain regions, including sensory and possibly motor areas. There is now little doubt that brain oscillations will have an important role in these processes, but important questions remain. The ongoing integration of neurophysiological and cognitive approaches is likely to provide new insights into how the theta-gamma code contributes to brain function.
Acknowledgments
The authors gratefully acknowledge The Netherlands Organization for Scientific Research (NWO) VICI grant number: 453-09-002, NIH grants awarded to JL - 5R01MH086518 from the National Institute Of Mental Health and 1R01DA027807 from the National Institute On Drug Abuse. The authors thank Michael Kahana, John Maunsell, Don Katz, Nikolai Axmacher, Dan Pollen, and Honi Sanders for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John E. Lisman, Email: Lisman@brandeis.edu.
Ole Jensen, Email: ole.jensen@donders.ru.nl.
References
- Adrian ED, Matthews BHC. The Berger rhythm: potential changes from the occipital lobes in man. Brain. 1934;57:355–385. doi: 10.1093/brain/awp324. [DOI] [PubMed] [Google Scholar]
- Akam TE, Kullmann DM. Efficient “Communication through Coherence” Requires Oscillations Structured to Minimize Interference between Signals. PLoS Comput Biol. 2012;8:e1002760. doi: 10.1371/journal.pcbi.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Khateb A, Fort P, Jones BE, Muhlethaler M. Differential oscillatory properties of cholinergic and noncholinergic nucleus basalis neurons in guinea pig brain slice. Eur J Neurosci. 1996;8:169–182. doi: 10.1111/j.1460-9568.1996.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Atallah BV, Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron. 2009;62:566–577. doi: 10.1016/j.neuron.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci U S A. 2010;107:3228–3233. doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Sutherland GR, McNaughton BL. Local sensory cues and place cell directionality: additional evidence of prospective coding in the hippocampus. J Neurosci. 2004;24:4541–4550. doi: 10.1523/JNEUROSCI.4896-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsaki G. Cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. J Neurosci. 2012;32:423–435. doi: 10.1523/JNEUROSCI.4122-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Berke JD. Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. European Journal of Neuroscience. 2009;30:848–859. doi: 10.1111/j.1460-9568.2009.06843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- Bollimunta A, Chen Y, Schroeder CE, Ding M. Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. J Neurosci. 2008;28:9976–9988. doi: 10.1523/JNEUROSCI.2699-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, Ding M. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J Neurosci. 2011;31:4935–4943. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, De Weerd P, Fries P. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 2012;75:875–888. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL. Large-scale cortical networks and cognition. Brain Res Brain Res Rev. 1995;20:288–304. doi: 10.1016/0165-0173(94)00016-i. [DOI] [PubMed] [Google Scholar]
- Brzezicka A, Kaminski M, Kaminski J, Blinowska K. Information transfer during a transitive reasoning task. Brain Topogr. 2011;24:1–8. doi: 10.1007/s10548-010-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, O’Keefe J. Models of place and grid cell firing and theta rhythmicity. Curr Opin Neurobiol. 2011;21:734–744. doi: 10.1016/j.conb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SP, Xing D, Shapley RM. Is gamma-band activity in the local field potential of V1 cortex a “clock” or filtered noise? J Neurosci. 2011;31:9658–9664. doi: 10.1523/JNEUROSCI.0660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Serial, covert shifts of attention during visual search are reflected by the frontal eye fields and correlated with population oscillations. Neuron. 2009;63:386–396. doi: 10.1016/j.neuron.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Wang XJ. Mechanisms of Gamma Oscillations. Annu Rev Neurosci. 2012 doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr Margaret F, Karlsson Mattias P, Frank Loren M. Transient Slow Gamma Synchrony Underlies Hippocampal Memory Replay. Neuron. 2012;75:700–713. doi: 10.1016/j.neuron.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. Assessing transient cross-frequency coupling in EEG data. J Neurosci Methods. 2008;168:494–499. doi: 10.1016/j.jneumeth.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Cavanagh JF. Single-trial regression elucidates the role of prefrontal theta oscillations in response conflict. Front Psychol. 2011;2:30. doi: 10.3389/fpsyg.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. discussion 114–185. [DOI] [PubMed] [Google Scholar]
- da Silva FH, van Lierop TH, Schrijer CF, van Leeuwen WS. Organization of thalamic and cortical alpha rhythms: spectra and coherences. Electroencephalogr Clin Neurophysiol. 1973;35:627–639. doi: 10.1016/0013-4694(73)90216-2. [DOI] [PubMed] [Google Scholar]
- de Almeida L, Idiart M, Lisman JE. Memory retrieval time and memory capacity of the CA3 network: role of gamma frequency oscillations. Learn Mem. 2007;14:795–806. doi: 10.1101/lm.730207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida L, Idiart M, Lisman JE. A second function of gamma frequency oscillations: an E%-max winner-take-all mechanism selects which cells fire. J Neurosci. 2009;29:7497–7503. doi: 10.1523/JNEUROSCI.6044-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida L, Idiart M, Villavicencio A, Lisman J. Alternating predictive and short-term memory modes of entorhinal grid cells. Hippocampus. 2012 doi: 10.1002/hipo.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoteau WE, Thorn C, Gibson DJ, Courtemanche R, Mitra P, Kubota Y, Graybiel AM. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc Natl Acad Sci U S A. 2007;104:5644–5649. doi: 10.1073/pnas.0700818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiralp T, Bayraktaroglu Z, Lenz D, Junge S, Busch NA, Maess B, Ergen M, Herrmann CS. Gamma amplitudes are coupled to theta phase in human EEG during visual perception. Int J Psychophysiol. 2007;64:24–30. doi: 10.1016/j.ijpsycho.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Buzsaki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Drewes J, VanRullen R. This is the rhythm of your eyes: the phase of ongoing electroencephalogram oscillations modulates saccadic reaction time. J Neurosci. 2011;31:4698–4708. doi: 10.1523/JNEUROSCI.4795-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugue L, Marque P, VanRullen R. The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. J Neurosci. 2011;31:11889–11893. doi: 10.1523/JNEUROSCI.1161-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Deouell L, Berger M, Knight R. High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. J Neurophysiol. 2005;94:4269–4280. doi: 10.1152/jn.00324.2005. [DOI] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, Fernandez G. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci. 2001;4:1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- Fell J, Ludowig E, Staresina BP, Wagner T, Kranz T, Elger CE, Axmacher N. Medial temporal theta/alpha power enhancement precedes successful memory encoding: evidence based on intracranial EEG. J Neurosci. 2011;31:5392–5397. doi: 10.1523/JNEUROSCI.3668-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous JM, Sejnowski TJ. Cholinergic induction of oscillations in the hippocampal slice in the slow (0.5–2 Hz), theta (5–12 Hz), and gamma (35–70 Hz) bands. Hippocampus. 2000;10:187–197. doi: 10.1002/(SICI)1098-1063(2000)10:2<187::AID-HIPO8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Fischer Y, Wittner L, Freund TF, Gahwiler BH. Simultaneous activation of gamma and theta network oscillations in rat hippocampal slice cultures. J Physiol. 2002;539:857–868. doi: 10.1113/jphysiol.2001.013050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint AC, Connors BW. Two types of network oscillations in neocortex mediated by distinct glutamate receptor subtypes and neuronal populations. J Neurophysiol. 1996;75:951–957. doi: 10.1152/jn.1996.75.2.951. [DOI] [PubMed] [Google Scholar]
- Fontanini A, Katz DB. 7 to 12 Hz activity in rat gustatory cortex reflects disengagement from a fluid self-administration task. J Neurophysiol. 2005;93:2832–2840. doi: 10.1152/jn.01035.2004. [DOI] [PubMed] [Google Scholar]
- Freeman W. The wave packet: an action potential for the 21st century. Journal of integrative neuroscience. 2003;2:3–30. doi: 10.1142/s0219635203000214. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Friese U, Koster M, Hassler U, Martens U, Trujillo-Barreto N, Gruber T. Successful memory encoding is associated with increased cross-frequency coupling between frontal theta and posterior gamma oscillations in human scalp-recorded EEG. Neuroimage. 2012;66C:642–647. doi: 10.1016/j.neuroimage.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Fuentemilla L, Penny WD, Cashdollar N, Bunzeck N, Duzel E. Theta-coupled periodic replay in working memory. Curr Biol. 2010;20:606–612. doi: 10.1016/j.cub.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Buzsaki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72:153–165. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai T. Sequence generation in arbitrary temporal patterns from theta-nested gamma oscillations: a model of the basal ganglia-thalamo-cortical loops. Neural Netw. 1999;12:975–987. doi: 10.1016/s0893-6080(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Neuronal activity related to saccadic eye movements in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1991;65:1464–1483. doi: 10.1152/jn.1991.65.6.1464. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Murray EA. Monkeys (Macaca fascicularis) with rhinal cortex ablations succeed in object discrimination learning despite 24-hr intertrial intervals and fail at matching to sample despite double sample presentations. Behav Neurosci. 1992;106:30–38. doi: 10.1037//0735-7044.106.1.30. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Ghitza O. Linking speech perception and neurophysiology: speech decoding guided by cascaded oscillators locked to the input rhythm. Front Psychol. 2011;2:130. doi: 10.3389/fpsyg.2011.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci. 2012;15:511–517. doi: 10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloveli T, Dugladze T, Rotstein HG, Traub RD, Monyer H, Heinemann U, Whittington MA, Kopell NJ. Orthogonal arrangement of rhythm-generating microcircuits in the hippocampus. Proc Natl Acad Sci U S A. 2005;102:13295–13300. doi: 10.1073/pnas.0506259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutagny R, Jackson J, Williams S. Self-generated theta oscillations in the hippocampus. Nat Neurosci. 2009;12:1491–1493. doi: 10.1038/nn.2440. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guderian S, Schott BH, Richardson-Klavehn A, Duzel E. Medial temporal theta state before an event predicts episodic encoding success in humans. Proc Natl Acad Sci U S A. 2009;106:5365–5370. doi: 10.1073/pnas.0900289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Segmentation of spatial experience by hippocampal theta sequences. Nat Neurosci. 2012;15:1032–1039. doi: 10.1038/nn.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Nacher V, Luna R, Romo R, Jensen O. alpha-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci U S A. 2011;108:19377–19382. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Bonnevie T, Moser MB, Moser EI. Hippocampus-independent phase precession in entorhinal grid cells. Nature. 2008;453:1248–1252. doi: 10.1038/nature06957. [DOI] [PubMed] [Google Scholar]
- Hamm JP, Dyckman KA, McDowell JE, Clementz BA. Pre-cue fronto-occipital alpha phase and distributed cortical oscillations predict failures of cognitive control. J Neurosci. 2012;32:7034–7041. doi: 10.1523/JNEUROSCI.5198-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel BF, Haarmeier T, Jensen O. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J Cogn Neurosci. 2011;23:2494–2502. doi: 10.1162/jocn.2010.21557. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci. 1997;20:44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsaki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Henrie JA, Shapley R. LFP power spectra in V1 cortex: the graded effect of stimulus contrast. J Neurophysiol. 2005;94:479–490. doi: 10.1152/jn.00919.2004. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma Oscillations Correlate with Working Memory Load in Humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Howe MW, Atallah HE, McCool A, Gibson DJ, Graybiel AM. Habit learning is associated with major shifts in frequencies of oscillatory activity and synchronized spike firing in striatum. Proceedings of the National Academy of Sciences. 2011;108:16801–16806. doi: 10.1073/pnas.1113158108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;11:357–372. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ. Neural Representations of Individual Stimuli in Humans Revealed by Gamma-Band Electrocorticographic Activity. The Journal of neuroscience. 2009;29:10203–10214. doi: 10.1523/JNEUROSCI.2187-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O. Information transfer between rhythmically coupled networks: reading the hippocampal phase code. Neural Comput. 2001;13:2743–2761. doi: 10.1162/089976601317098510. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Hippocampal CA3 region predicts memory sequences: accounting for the phase precession of place cells. Learn Mem. 1996;3:279–287. doi: 10.1101/lm.3.2-3.279. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. An oscillatory short-term memory buffer model can account for data on the Sternberg task. J Neurosci. 1998;18:10688–10699. doi: 10.1523/JNEUROSCI.18-24-10688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Position reconstruction from an ensemble of hippocampal place cells: contribution of theta phase coding. J Neurophysiol. 2000;83:2602–2609. doi: 10.1152/jn.2000.83.5.2602. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res. 2004;145:157–169. doi: 10.1016/S0079-6123(03)45011-5. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Phase precession of medial prefrontal cortical activity relative to the hippocampal theta rhythm. Hippocampus. 2005;15:867–873. doi: 10.1002/hipo.20119. [DOI] [PubMed] [Google Scholar]
- Jutras MJ, Fries P, Buffalo EA. Gamma-band synchronization in the macaque hippocampus and memory formation. J Neurosci. 2009;29:12521–12531. doi: 10.1523/JNEUROSCI.0640-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski J, Brzezicka A, Wrobel A. Short-term memory capacity (7 +/− 2) predicted by theta to gamma cycle length ratio. Neurobiol Learn Mem. 2011;95:19–23. doi: 10.1016/j.nlm.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Kim J, Delcasso S, Lee I. Neural correlates of object-in-place learning in hippocampus and prefrontal cortex. J Neurosci. 2011;31:16991–17006. doi: 10.1523/JNEUROSCI.2859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Ganguli S, Frank LM. Spatial Information Outflow from the Hippocampal Circuit: Distributed Spatial Coding and Phase Precession in the Subiculum. The Journal of neuroscience. 2012;32:11539–11558. doi: 10.1523/JNEUROSCI.5942-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Recce M, O’Keefe J. The rhythmicity of cells of the medial septum/diagonal band of Broca in the awake freely moving rat: relationships with behaviour and hippocampal theta. Eur J Neurosci. 1998;10:464–477. doi: 10.1046/j.1460-9568.1998.00026.x. [DOI] [PubMed] [Google Scholar]
- Koene RA, Hasselmo ME. First-in-first-out item replacement in a model of short-term memory based on persistent spiking. Cereb Cortex. 2007;17:1766–1781. doi: 10.1093/cercor/bhl088. [DOI] [PubMed] [Google Scholar]
- Konig P, Engel AK, Singer W. Integrator or coincidence detector? The role of the cortical neuron revisited. Trends Neurosci. 1996;19:130–137. doi: 10.1016/s0166-2236(96)80019-1. [DOI] [PubMed] [Google Scholar]
- Kramer MA, Tort AB, Kopell NJ. Sharp edge artifacts and spurious coupling in EEG frequency comodulation measures. J Neurosci Methods. 2008;170:352–357. doi: 10.1016/j.jneumeth.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Laurent G, Stopfer M, Friedrich RW, Rabinovich MI, Volkovskii A, Abarbanel HD. Odor encoding as an active, dynamical process: experiments, computation, and theory. Annu Rev Neurosci. 2001;24:263–297. doi: 10.1146/annurev.neuro.24.1.263. [DOI] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, Rainer G. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron. 2005;45:147–156. doi: 10.1016/j.neuron.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22:748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini PP, Fenton AA, Muller RU. Discharge properties of hippocampal neurons during performance of a jump avoidance task. J Neurosci. 2008;28:6773–6786. doi: 10.1523/JNEUROSCI.5329-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebe S, Hoerzer GM, Logothetis NK, Rainer G. Theta coupling between V4 and prefrontal cortex predicts visual short-term memory performance. Nat Neurosci. 2012;15:456–462. S451–452. doi: 10.1038/nn.3038. [DOI] [PubMed] [Google Scholar]
- Lisman J, Buzsaki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull. 2008;34:974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Llinas R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci U S A. 1993;90:2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva FH, Vos JE, Mooibroek J, Van Rotterdam A. Relative contributions of intracortical and thalamo-cortical processes in the generation of alpha rhythms, revealed by partial coherence analysis. Electroencephalogr Clin Neurophysiol. 1980;50:449–456. doi: 10.1016/0013-4694(80)90011-5. [DOI] [PubMed] [Google Scholar]
- Lorincz ML, Kekesi KA, Juhasz G, Crunelli V, Hughes SW. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron. 2009;63:683–696. doi: 10.1016/j.neuron.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Magee JC. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:291–307. doi: 10.1016/j.neuron.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Maris E, van Vugt M, Kahana M. Spatially distributed patterns of oscillatory coupling between high-frequency amplitudes and low-frequency phases in human iEEG. Neuroimage. 2011;54:836–850. doi: 10.1016/j.neuroimage.2010.09.029. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: prestimulus alpha phase predicts visual awareness. J Neurosci. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MR, Lee AK, Wilson MA. Role of experience and oscillations in transforming a rate code into a temporal code. Nature. 2002;417:741–746. doi: 10.1038/nature00807. [DOI] [PubMed] [Google Scholar]
- Michels L, Bucher K, Luchinger R, Klaver P, Martin E, Jeanmonod D, Brandeis D. Simultaneous EEG-fMRI during a working memory task: modulations in low and high frequency bands. PLoS One. 2010;5:e10298. doi: 10.1371/journal.pone.0010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miconi T, Vanrullen R. The gamma slideshow: object-based perceptual cycles in a model of the visual cortex. Front Hum Neurosci. 2010;4:205. doi: 10.3389/fnhum.2010.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA. The magical number seven plus or minus two: some limits on our capacity for processing information. Psychol Rev. 1956;63:81–97. [PubMed] [Google Scholar]
- Miller KJ, Zanos S, Fetz EE, den Nijs M, Ojemann JG. Decoupling the cortical power spectrum reveals real-time representation of individual finger movements in humans. J Neurosci. 2009;29:3132–3137. doi: 10.1523/JNEUROSCI.5506-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Sirota A, Pastalkova E, Buzsáki G. Theta Oscillations Provide Temporal Windows for Local Circuit Computation in the Entorhinal-Hippocampal Loop. Neuron. 2009;64:267–280. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci U S A. 2007;104:14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran RJ, Campo P, Maestu F, Reilly RB, Dolan RJ, Strange BA. Peak frequency in the theta and alpha bands correlates with human working memory capacity. Front Hum Neurosci. 2010;4:200. doi: 10.3389/fnhum.2010.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Fell J, Axmacher N, Weber B, Lehnertz K, Elger CE, Fernandez G. Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15:890–900. doi: 10.1002/hipo.20117. [DOI] [PubMed] [Google Scholar]
- Nikolic D, Fries P, Singer W. Gamma oscillations: precise temporal coordination without a metronome. Trends Cogn Sci. 2013;17:54–55. doi: 10.1016/j.tics.2012.12.003. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Onslow AC, Bogacz R, Jones MW. Quantifying phase-amplitude coupling in neuronal network oscillations. Prog Biophys Mol Biol. 2011;105:49–57. doi: 10.1016/j.pbiomolbio.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Palva S, Monto S, Palva JM. Graph properties of synchronized cortical networks during visual working memory maintenance. Neuroimage. 2010;49:3257–3268. doi: 10.1016/j.neuroimage.2009.11.031. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Davis MH. Neural Oscillations Carry Speech Rhythm through to Comprehension. Front Psychol. 2012;3:320. doi: 10.3389/fpsyg.2012.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penley SC, Hinman JR, Sabolek HR, Escabi MA, Markus EJ, Chrobak JJ. Theta and gamma coherence across the septotemporal axis during distinct behavioral states. Hippocampus. 2011 doi: 10.1002/hipo.20962. [DOI] [PubMed] [Google Scholar]
- Penny WD, Duzel E, Miller KJ, Ojemann JG. Testing for nested oscillation. J Neurosci Methods. 2008;174:50–61. doi: 10.1016/j.jneumeth.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Acsady L, Buzsaki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci. 1998;10:718–728. doi: 10.1046/j.1460-9568.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat Neurosci. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G. Foot and hand area mu rhythms. Int J Psychophysiol. 1997;26:121–135. doi: 10.1016/s0167-8760(97)00760-5. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Poulet JF, Fernandez LM, Crochet S, Petersen CC. Thalamic control of cortical states. Nat Neurosci. 2012;15:370–372. doi: 10.1038/nn.3035. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol. 2006;95:1630–1638. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron. 2010;67:885–896. doi: 10.1016/j.neuron.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum Brain Mapp. 2009;30:1168–1187. doi: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto DS, Madsen JR, Bromfield EB, Schulze-Bonhage A, Kahana MJ. Human neocortical oscillations exhibit theta phase differences between encoding and retrieval. Neuroimage. 2006;31:1352–1358. doi: 10.1016/j.neuroimage.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Webb TJ, Deco G. Communication before coherence. Eur J Neurosci. 2012 doi: 10.1111/j.1460-9568.2012.08188.x. [DOI] [PubMed] [Google Scholar]
- Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, Thut G. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex. 2008;18:2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ. Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci. 2012;32:12411–12420. doi: 10.1523/JNEUROSCI.0421-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464:903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71:209–223. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders H, Kolterman B, Rinberg D, Koulakov A, Lisman J. S.f. Neuroscience, editor. 2012 Annual Society for Neuroscience Meeting. New Orleans: 2012. Temporal-spatial transformation in the piriform cortex. [Google Scholar]
- Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, von Stein A. Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci U S A. 1998;95:7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC. Brain oscillatory substrates of visual short-term memory capacity. Curr Biol. 2009;19:1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, Doppelmayr M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int J Psychophysiol. 2005;57:97–103. doi: 10.1016/j.ijpsycho.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Schack B, Klimesch W, Sauseng P. Phase synchronization between theta and upper alpha oscillations in a working memory task. Int J Psychophysiol. 2005;57:105–114. doi: 10.1016/j.ijpsycho.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Petersson KM, Oostenveld R, Norris DG, Hagoort P, Bastiaansen MC. Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage. 2009;44:1224–1238. doi: 10.1016/j.neuroimage.2008.08.041. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Diba K, Leibold C, Schmitz D, Buzsaki G, Kempter R. Single-trial phase precession in the hippocampus. J Neurosci. 2009;29:13232–13241. doi: 10.1523/JNEUROSCI.2270-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex. 2007;17:1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Senior TJ, Huxter JR, Allen K, O’Neill J, Csicsvari J. Gamma oscillatory firing reveals distinct populations of pyramidal cells in the CA1 region of the hippocampus. J Neurosci. 2008;28:2274–2286. doi: 10.1523/JNEUROSCI.4669-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M, Ferbinteanu J. Relative spike timing in pairs of hippocampal neurons distinguishes the beginning and end of journeys. Proc Natl Acad Sci U S A. 2006;103:4287–4292. doi: 10.1073/pnas.0508688103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvalkar PR, Rapp PR, Shapiro ML. Bidirectional changes to hippocampal theta-gamma comodulation predict memory for recent spatial episodes. Proc Natl Acad Sci U S A. 2010;107:7054–7059. doi: 10.1073/pnas.0911184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman R, Smear MC, Koulakov AA, Rinberg D. Precise olfactory responses tile the sniff cycle. Nat Neurosci. 2011;14:1039–1044. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13:121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Siegel M, Warden MR, Miller EK. Phase-dependent neuronal coding of objects in short-term memory. Proc Natl Acad Sci U S A. 2009;106:21341–21346. doi: 10.1073/pnas.0908193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Smear M, Shusterman R, O’Connor R, Bozza T, Rinberg D. Perception of sniff phase in mouse olfaction. Nature. 2011;479:397–400. doi: 10.1038/nature10521. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Deschenes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol. 1993;70:97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- Spaak E, Bonnefond M, Maier A, Leopold DA, Jensen O. Layer-Specific Entrainment of Gamma-Band Neural Activity by the Alpha Rhythm in Monkey Visual Cortex. Curr Biol. 2012 doi: 10.1016/j.cub.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus. 2001;11:337–346. doi: 10.1002/hipo.1048. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104:1195–1210. doi: 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cogn Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Skaggs WE, Sejnowski TJ, McNaughton BL. Population dynamics and theta rhythm phase precession of hippocampal place cell firing: a spiking neuron model. Hippocampus. 1996;6:271–280. doi: 10.1002/(SICI)1098-1063(1996)6:3<271::AID-HIPO5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- van der Meer MA, Redish AD. Theta phase precession in rat ventral striatum links place and reward information. J Neurosci. 2011;31:2843–2854. doi: 10.1523/JNEUROSCI.4869-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vreeswijk C, Sompolinsky H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science. 1996;274:1724–1726. doi: 10.1126/science.274.5293.1724. [DOI] [PubMed] [Google Scholar]
- van Vugt MK, Schulze-Bonhage A, Litt B, Brandt A, Kahana MJ. Hippocampal gamma oscillations increase with memory load. J Neurosci. 2010;30:2694–2699. doi: 10.1523/JNEUROSCI.0567-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Voytek B, Canolty RT, Shestyuk A, Crone N, Parvizi J, Knight RT. Shifts in gamma phase-amplitude coupling frequency from theta to alpha over posterior cortex during visual tasks. Front Hum Neurosci. 2010:4. doi: 10.3389/fnhum.2010.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H, Brill S, Kempter R, Carr CE. Microsecond precision of phase delay in the auditory system of the barn owl. J Neurophysiol. 2005;94:1655–1658. doi: 10.1152/jn.01226.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Saalmann YB, Pinsk MA, Arcaro MJ, Kastner S. Electrophysiological Low-Frequency Coherence and Cross-Frequency Coupling Contribute to BOLD Connectivity. Neuron. 2012;76:1010–1020. doi: 10.1016/j.neuron.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiological reviews. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrous AJ, Lee DJ, Izadi A, Gurkoff GG, Shahlaie K, Ekstrom AD. A comparative study of human and rat hippocampal low frequency oscillations during spatial navigation. Hippocampus. doi: 10.1002/hipo.22124. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Lima B, Vinck M, Oostenveld R, Singer W, Neuenschwander S, Fries P. Orientation selectivity and noise correlation in awake monkey area V1 are modulated by the gamma cycle. Proc Natl Acad Sci U S A. 2012;109:4302–4307. doi: 10.1073/pnas.1114223109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley DE, Timiras PS. Prepyriform Electrical Activity in the Rat During High Altitude Exposure. Electroencephalogr Clin Neurophysiol. 1965;18:680–690. doi: 10.1016/0013-4694(65)90112-4. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-W, An S, Sun J-J, Reyes-Puerta V, Kindler J, Berger T, Kilb W, Luhmann HJ. Thalamic Network Oscillations Synchronize Ontogenetic Columns in the Newborn Rat Barrel Cortex. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs103. [DOI] [PubMed] [Google Scholar]
- Yang Y, DeWeese MR, Otazu GH, Zador AM. Millisecond-scale differences in neural activity in auditory cortex can drive decisions. Nat Neurosci. 2008;11:1262–1263. doi: 10.1038/nn.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. J Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CK, Eggermont JJ. Coupling of mesoscopic brain oscillations: recent advances in analytical and theoretical perspectives. Prog Neurobiol. 2009;89:61–78. doi: 10.1016/j.pneurobio.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Young JJ, Shapiro ML. Dynamic coding of goal-directed paths by orbital prefrontal cortex. J Neurosci. 2011;31:5989–6000. doi: 10.1523/JNEUROSCI.5436-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lin SC, Nicolelis MA. A distinctive subpopulation of medial septal slow-firing neurons promote hippocampal activation and theta oscillations. J Neurophysiol. 2011;106:2749–2763. doi: 10.1152/jn.00267.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]