Abstract
Two-thirds of American adults are overweight or obese, 75 million have hypertension and another 25 million have diabetes. Cardiovascular disease is the leading cause of morbidity and mortality and a major driver of health care costs in patients with type 2 diabetes. Observational studies suggest that insulin resistance, hypertension and hyperglycemia independently predict cardiovascular disease and chronic kidney disease. Indeed, coexisting hypertension appears to be a most powerful determinant of cardiovascular disease risk in diabetic patients. This update explores recent investigation which sheds light on our understanding of various metabolic and hemodynamic factors which promote vascular disease, as well as strategies to lessen cardiovascular disease in patients with diabetes.
Introduction
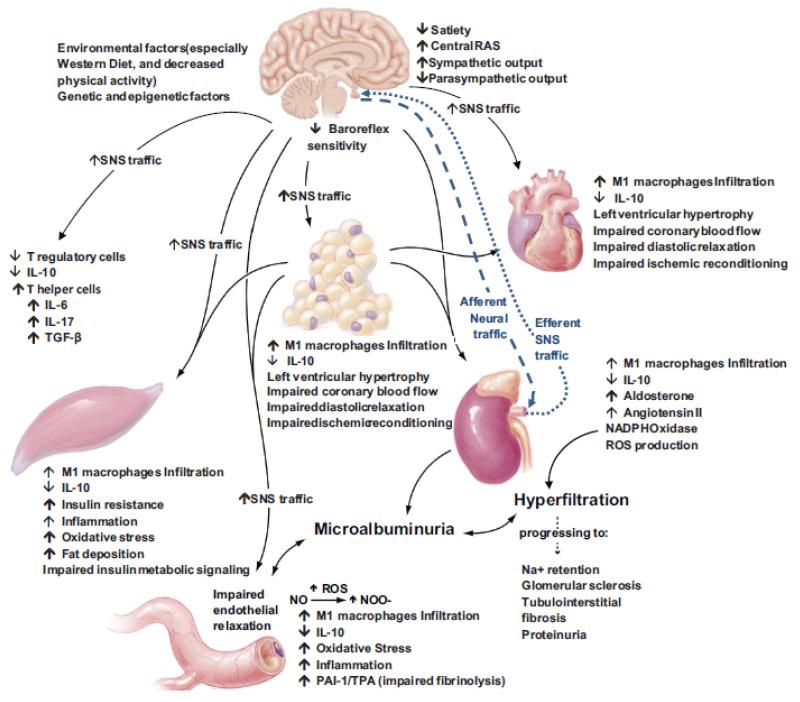
Obesity, impaired glucose tolerance and diabetes are associated with a substantially increased prevalence of hypertension, cardiovascular (CVD) and chronic renal disease. The prevalence of hypertension in patients who have type 2 diabetes is up to three times higher than in patients without diabetes Further, the coexistence of hypertension in diabetic patients greatly enhances their likelihood of developing CVD and chronic kidney disease (CKD) (1-4). Indeed, studies conducted in the Framingham population with diabetes (1) indicated that the presence of hypertension in these participants was a resulting risk factor for the presence of CVD. This data and other studies suggest a two-fold increased risk of CVD events and deaths in diabetic persons with hypertension compared to those with normal blood pressures. The increased association between hypertension and diabetes can be explained in part, by the presence of a maladaptive interaction of factors such as excessive caloric intake/decreased activity and associated insulin resistance (IR), chronic activation of the renin-angiotensin (Ang II)-aldosterone system (RAAS), the sympathetic nervous system and abnormalities of innate immunity, inflammation and oxidative stress (1-21) (Fig 1). The epidemic of obesity and sedentary lifestyle and the aging of populations worldwide have contributed to the current high prevalence of diabetes and hypertension.
Fig 1. Diabetes, Hypertension and Cardiovascular Disease.
Systemic and metabolic factors that promote co-existent diabetes, hypertension, cardiovascular and chronic kidney disease.
Role of Increasing Overweight and Insulin Resistance (IR) in Promoting both Diabetes and Hypertension
Hypertension is more common in diabetic patients than in the general population (1-4). In the Strong Heart Study, baseline measurements which independently predicted incident hypertension included waist circumference, elevations in baseline systolic blood pressure (SBP) and left ventricular mass and diabetes (2). Further, a recent longitudinal Japanese study of 5198 subjects showed that parental hypertension has an age-independent impact on both male and female offspring in elevations in blood pressure, plasma glucose, and triglyceride levels (5). The authors of this study suggested that IR was the underlying pathophysiologic factor contributing to this array of abnormalities in the offspring as suggested in a prior publications in Hypertension regarding the relationship between IR and hypertension (3,6). In a longitudinal study of a northern Italian population, it was reported that an increase in body mass index (BMI) and waist circumference was associated with a linearly increased adjusted risk of developing impaired fasting glucose, diabetes, blood pressure and left ventricular hypertrophy (LVH) (7).
The risk of developing diabetes, hypertension and associated CVD risk factors is increased with obesity and inactivity (4-16). Indeed, the prevalence of childhood obesity is increasingly prevalent and is associated with metabolic abnormalities and CVD risk factors in childhood as well as during adulthood (12-16). For example, using carotid-femoral pulse wave velocity (PWV) as an index of arterial stiffness in children, investigators found that PWV was positively correlated with BMI, waist circumference and percentage body fat, and negatively correlated with cardiorespiratory fitness (12). In another study, Canadian adult (13) waist circumference was associated with greater elevations of SBP during exercise. In a recent study (14), an inverse association was shown between exercise capacity and the rate of progression from pre-hypertension to hypertension in middle aged and older men. Other significant factors predicting the progression from pre-hypertension to hypertension included SBP, age, BMI, and the presence of diabetes. These observations are important as it is increasingly recognized that pre-hypertension is a CVD risk factor. Recent findings in young diet or genetically induced obese rats and rats selectively bred for low aerobic capacity, indicate the exhibition of increased myocardial fibrosis and diastolic dysfunction in a pre-hypertension state (11-13). The translational relevance of these studies is highlighted by the fact that it is estimated that 37% of the adult US population has pre-hypertension and 40% of these people will progress to hypertension within a two year time frame (10). Collectively, these studies suggest that improved cardiorespiratory fitness in the young may greatly reduce the development of diabetes, hypertension and associated CVD.
Role of Hormonal/Neuroendocrine Factors in Promotion of Hypertension and Diabetes
Excessive caloric intake and a sedentary lifestyle promote IR, a condition in which the insulin metabolic signaling response in skeletal muscle, liver, and adipose tissue is impaired (6,15,16). This alteration in insulin metabolic signaling leads to increased vascular adhesion molecule expression, oxidative stress, inflammation and decreased vascular bioavailable nitric oxide (NO) (15-19). The decreased bioavailability of NO reduces endothelial-mediated vascular relaxation and promotes vascular stiffness (19). Obesity and IR are also associated with inappropriate activation of the RAAS and the sympathetic nervous system (20-32). A recent study (20) highlights the role of adipose tissue in promoting activity of the RAAS. This investigation was based on previous observations that adipocyte angiotensinogen (AGT) is important in the formation of Ang II. This study demonstrated that while a high fat diet substantially increased plasma levels of Ang II and blood pressure in wild type mice, it did not do so in adipocyte-specific AGT knockout mice showing the importance of adipose tissue AGT production in the development of hypertension in obesity. Increased adiposity has also been associated with high levels of plasma aldosterone (21-23) suggesting that obesity is a condition characterized by and activated in RAAS. Activation of the RAAS in obesity may, in turn, increase the propensity to IR, hypertension, diabetes and associated CVD (21,24). Ang II and aldosterone have been shown to inhibit insulin metabolic signaling in cardiovascular and classical insulin sensitive tissue (23-27) and this likely plays a role in reduced endothelial mediated vascular relaxation and development of hypertension. There is emerging evidence that Ang II and aldosterone, acting through non-genomic mechanisms, may promote IR through activation of serine kinases and increased serine phosphorylation of the critical insulin signaling/docking molecule, insulin receptor substrate protein 1 (IRS-1), leading to impaired phosphoinositol 3 kinase (PI3-K) engagement and protein kinase B stimulation, diminished insulin metabolic signaling and biological consequences, such as impaired NO-mediated vascular relaxation (23,25-27).
There is emerging evidence that innate and adaptive immunity are involved in Ang II and aldosterone-induced hypertension and vascular disease (28-30). Infusion of both hormones has been shown to increase blood pressure, cause increases in vascular inflammation, oxidative stress, remodeling and endothelial dysfunction in wild type but not in mice deficient in T cells. These T cell mediated effects are accompanied by infiltration of T cells and macrophage and increases in NADPH oxidase in vessels, kidney and heart tissue. These studies also showed that Ang II and aldosterone reduced T regulatory cells, in part, by increasing the apoptosis of these cells. These adverse vascular responses to Ang II and aldosterone have been shown to be prevented by adoptive transfer of T regulatory cells (28-30). Data from one of these studies (29) showed that interleukin-10 released from these T regulatory cells reduced NADPH oxidase activity in vascular tissue. These data collectively suggest that adoptive transfer of T regulatory cells could be a mechanism for reducing vascular disease associated with diabetes.
There is an emerging body of evidence that central obesity, IR, sleep apnea and resistant hypertension are often accompanied by increased sympathetic nervous system activity (31,32). Further, activation of the sympathetic nervous system promotes IR, and the risk of developing diabetes (31,32). A percutaneous catheter based procedure has been developed for therapeutic renal denervation. This involves the application of low-dose radiofrequency energy to the renal artery endothelial surface causing selective reduction in renal sympathetic afferent and efferent signaling (31,32). This intervention is associated with reduced central sympathetic outflow, as evidenced by attenuation of muscle sympathetic nerve activity and plasma levels of norepinephrine (31). In recent studies this procedure has been reported to reduce fasting and post-prandial glucose levels and to improve insulin sensitivity in addition to having substantial blood pressure lowering effects (31,32). Collectively, these data suggest that this method of reducing sympathetic output has considerable utility in patients with IR, impaired glucose tolerance and resistant hypertension who are at high risk for CVD and chronic renal disease. Other prospective therapeutic targets for treatment in diabetic patients with hypertension include reductions in the urotensin system (33), uric acid (34) and endoplasmic reticulum stress (35).
Treatment of Hypertension in Diabetic Patients
There are evolving notions regarding the optimal treatment strategies for patients with hypertension and diabetes. Some of the controversies include the optimal blood pressure target in diabetic patients, the rapidity of achieving that target, the impact of various blood pressure lowering medications on cerebral perfusion, metabolism and progression of diabetes, CVD, and renal disease progression (36-40). While there is evidence that lowering blood pressures below the 140/90 mm Hg goal decreases stroke, there remains concerns about adequate cerebral perfusion in diabetic patients with microvascular disease and impaired cerebrovascular autoregulation (38). Thus, the optimal blood pressure goal for diabetic patients should be individualized just as for glycemic control. Various strategies such as more frequent patient visits (41) and home blood pressure monitoring (42) have been reported to improve achievement of blood pressure goals in patients with diabetes and hypertension.
One of the factors that may influence the choice of blood pressure lowering agents in diabetes is the influence of the agents on metabolic parameters, especially glucose metabolism. In this regard thiazide diurectics and conventional beta-blockers may worsen insulin sensitivity and glucose tolerance, especially in the setting of obesity (36,43,44). The impact of various predisposing factors on the worsening of glucose control is different for diurectics versus beta-blockers. For example, hypokalemia and hypomagnesemia have been attributed to worsening of glucose control with thiazide therapy (43). Low serum levels of these divalent cations has been observed to associated with both impaired beta-cell insulin secretion and insulin metabolic signaling in adipose tissue, liver and skeletal muscle tissue (43). Hyperuricemia has also been associated with development of worsening glucose metabolism in obese patients treated with diurectics and beta-blockers (36). There is also evidence that treatment with conventional beta-blockers are also associated with weight gain which, in turn, worsens glucose tolerance (43,44). In contrast to conventional beta-blockers, vasodilating beta-blockers such as nebivolol are not associated with weight gain and do not worsen glucose tolerance in states of obesity and impaired glucose tolerance (44-46).
There is evidence in experimental animals and man, that angiotensin receptor blockers (ARBs) may have beneficial effects on insulin secretion from beta-cells and insulin sensitivity (47,48). For example, treatment with an ARB was shown to protect against the adverse metabolic effects of a high fat diet in mice (47). Indeed ARB treatment not only improved glucose tolerance and insulin sensitivity, but it also protected against islet beta-cell destruction and improved glucose-induced insulin secretion. These beneficial metabolic effects of ARB treatment were accompanied by reduced systemic and tissue inflammation. In a study conducted in hypertensive patients with central obesity, adding an ARB to thiazide therapy attenuated the negative effects of thiazide treatment on pancreatic beta-cell glucose induced insulin secretion (48). Collectively these studies suggest that ARBs have beneficial metabolic effects when used alone or in combination with thiazide therapy in states of obesity and hypertension.
Another mechanism by which thiazide diurectics may worsen insulin sensitivity and glucose tolerance is by activation of the RAAS and the sympathetic nervous system. In a recently reported study (49), chlorthalidone therapy was associated with activation of the sympathetic nervous system as measured by recordings of sympathetic nerve traffic. This therapy was also associated with reduced insulin sensitivity as determined using a homeostasis model assessment of IR. However, the addition of the mineralocorticoid receptor antagonist spironolactone attenuated both the chlorthalidone-induced sympathetic activation and IR in hypertensive patients. Thus, thiazide diurectic therapy appears to worsen glucose metabolism via activation of the RAAS and sympathetic nervous system and via effects of islet beta cells as well as insulin sensitive tissues. Recently, it has been observed that dipeptidyl peptidase 4 inhibition, used in treating hyperglycemia, lowers blood pressure and improves endothelial function in hypertensive rodents (50). It will be interesting to see if these agents lower blood pressure while improving glucose tolerance in hypertensive patients with diabetes. It will also be important to determine if these agents also abrogate the negative metabolic effects of conventional beta-blockers and diurectics in hypertensive patients with diabetes.
Despite the differential effects of various antihypertensive agents on glucose metabolism, it is unclear which agents or combination of agents have the most beneficial effects on CVD and renal outcomes in patients with diabetes and hypertension. In the Nagoya Heart Study composite, CVD outcomes were comparable between valsartan- and amlodipine-based treatments in Japanese patients with glucose intolerance (51). However, admission to hospitals due to heart failure was significantly less in the valsartan treated group. The combination of angiotensin converting enzyme inhibitors and dihydropyridine calcium channel blockers was assessed in Italian patients with hypertension and type 2 diabetes. In these patients combined therapy did not slow glomerular filtration rate decline, but lessened CVD, retinopathy, neuropathy and stabilized insulin sensitivity, These results suggest that this may be an excellent combination therapy for treating hypertension in diabetic patients. Thus, RAAS blockers with either thiazide diurectics or dihydropyridine blockers appear to be a good combination to achieve optimal metabolic and CVD outcomes in this group of patients (50-51).
Acknowledgements
The author would like to thank Brenda Hunter for her assistance in editing the manuscript.
Sources of Funding
This research was supported by the NIH (R01 HL73101-01A1 and R01 HL107910-01 to J.R.S.) and the Veterans Affairs Merit System 0019 (to J.R.S.).
Footnotes
Conflict of Interest/Disclosure Statement
The author has nothing to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kivimäki M, Tabak AG, Batty GD, Ferrie JE, Nabi H, Marmot MG, Witte DR, Singh-Manoux A, Shipley MJ. Incremental predictive value of adding past blood pressure measurements to the Framingham hypertension risk equation: the Whitehall II Study. Hypertension. 2010;55:1058–1062. doi: 10.1161/HYPERTENSIONAHA.109.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Marco M, de Simone G, Roman MJ, Chinali M, Lee ET, Russell M, Howard BV, Devereux RB. Cardiovascular and metabolic predictors of progression of prehypertension into hypertension: the Strong Heart Study. Hypertension. 2009;54:974–980. doi: 10.1161/HYPERTENSIONAHA.109.129031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whaley-Connell A, Sowers JR. Hypertension and insulin resistance. Hypertension. 2009;54:462–464. doi: 10.1161/HYPERTENSIONAHA.109.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, McAlister FA, Walker RL, Hemmelgarn BR, Campbell NR. Cardiovascular outcomes in framingham participants with diabetes: the importance of blood pressure. Hypertension. 2011;57:891–897. doi: 10.1161/HYPERTENSIONAHA.110.162446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitsumata K, Saitoh S, Ohnishi H, Akasaka H, Miura T. Effects of parental hypertension on longitudinal trends in blood pressure and plasma metabolic profile: mixed-effects model analysis. Hypertension. 2012;60:1124–1130. doi: 10.1161/HYPERTENSIONAHA.112.201129. [DOI] [PubMed] [Google Scholar]
- 6.Whaley-Connell A, Sowers JR. Indices of obesity and cardiometabolic risk. Hypertension. 2011;58:6–991. doi: 10.1161/HYPERTENSIONAHA.111.180406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bombelli M, Facchetti R, Sega R, Carugo S, Fodri D, Brambilla G, Giannattasio C, Grassi G, Mancia G. Impact of body mass index and waist circumference on the long-term risk of diabetes mellitus, hypertension, and cardiac organ damage. Hypertension. 2011;58:1029–1035. doi: 10.1161/HYPERTENSIONAHA.111.175125. [DOI] [PubMed] [Google Scholar]
- 8.Sakuragi S, Abhayaratna K, Gravenmaker KJ, O’Reilly C, Srikusalanukul W, Budge MM, Telford RD, Abhayaratna WP. Influence of adiposity and physical activity on arterial stiffness in healthy children: the lifestyle of our kids study. Hypertension. 2009;53:611–616. doi: 10.1161/HYPERTENSIONAHA.108.123364. [DOI] [PubMed] [Google Scholar]
- 9.Huot M, Arsenault BJ, Gaudreault V, Poirier P, Pérusse L, Tremblay A, Bouchard C, Després JP, Rhéaume C. Insulin resistance, low cardiorespiratory fitness, and increased exercise blood pressure: contribution of abdominal obesity. Hypertension. 2011;58:1036–1042. doi: 10.1161/HYPERTENSIONAHA.111.180349. [DOI] [PubMed] [Google Scholar]
- 10.Faselis C, Doumas M, Kokkinos JP, Panagiotakos D, Kheirbek R, Sheriff HM, Hare K, Papademetriou V, Fletcher R, Kokkinos P. Exercise capacity and progression from prehypertension to hypertension. Hypertension. 2012;60:333–338. doi: 10.1161/HYPERTENSIONAHA.112.196493. [DOI] [PubMed] [Google Scholar]
- 11.DeMarco VG, Johnson MS, Ma L, Pulakat L, Mugerfeld I, Hayden MR, Garro M, Knight W, Britton SL, Koch LG, Sowers JR. Overweight female rats selectively bred for low aerobic capacity exhibit increased myocardial fibrosis and diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2012;302:H1667–H1682. doi: 10.1152/ajpheart.01027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majane OH, Vengethasamy L, du Toit EF, Makaula S, Woodiwiss AJ, Norton GR. Dietary-induced obesity hastens the progression from concentric cardiac hypertrophy to pump dysfunction in spontaneously hypertensive rats. Hypertension. 2009;54(6):1376–1383. doi: 10.1161/HYPERTENSIONAHA.108.127514. [DOI] [PubMed] [Google Scholar]
- 13.Demarco VG, Ford DA, Henriksen EJ, Aroor AR, Johnson MS, Habibi J, Ma L, Yang M, Albert CJ, Lally JW, Ford CA, Hayden MR, Whaley-Connell AT, Sowers JR. Obesity-related alterations in cardiac lipid profile and nondipping blood pressure pattern during transition to diastolic dysfunction in male db/db mice. Endocrinology. 2012 Nov; doi: 10.1210/en.2012-1835. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roman MJ, Kizer JR, Best LG, Lee ET, Howard BV, Shara NM, Devereux RB. Vascular biomarkers in the prediction of clinical cardiovascular disease: the Strong Heart Study. Hypertension. 2012;59:29–35. doi: 10.1161/HYPERTENSIONAHA.111.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Wang Y, Zhang J, Potter BJ, Sowers JR, Zhang C. Bariatric surgery reduces visceral adipose inflammation and improves endothelial function in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2011;31(9):2063–2069. doi: 10.1161/ATVBAHA.111.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sowers JR. Role of TRIB3 in diabetic and overnutrition-induced atherosclerosis. Diabetes. 2012;61(2):265–266. doi: 10.2337/db11-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Zwan LP, Scheffer PG, Dekker JM, Stehouwer CD, Heine RJ, Teerlink T. Hyperglycemia and oxidative stress strengthen the association between myeloperoxidase and blood pressure. Hypertension. 2010;55:1366–1372. doi: 10.1161/HYPERTENSIONAHA.109.147231. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa S, Nakayama K, Nakayama M, Mori T, Matsushima M, Okamura M, Senda M, Nako K, Miyata T, Ito S. Methylglyoxal is a predictor in type 2 diabetic patients of intima-media thickening and elevation of blood pressure. Hypertension. 2010;56:471–476. doi: 10.1161/HYPERTENSIONAHA.110.156786. [DOI] [PubMed] [Google Scholar]
- 19.Muniyappa R, Sowers JR. Endothelial insulin and IGF-1 receptors: when yes means NO. Diabetes. 2012;61:9–2225. doi: 10.2337/db12-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, Daugherty A, Cassis LA. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012;60:1524–1530. doi: 10.1161/HYPERTENSIONAHA.112.192690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150:11–776. doi: 10.7326/0003-4819-150-11-200906020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumagai E, Adachi H, Jacobs DR, Jr, Hirai Y, Enomoto M, Fukami A, Otsuka M, Kumagae S, Nanjo Y, Yoshikawa K, Esaki E, Yokoi K, Ogata K, Kasahara A, Tsukagawa E, et al. Plasma aldosterone levels and development of insulin resistance: prospective study in a general population. Hypertension. 2011;58:1043–1048. doi: 10.1161/HYPERTENSIONAHA.111.180521. [DOI] [PubMed] [Google Scholar]
- 23.Whaley-Connell A, Sowers JR. Aldosterone and risk for insulin resistance. Hypertension. 2011;58(6):998–1000. doi: 10.1161/HYPERTENSIONAHA.111.182782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, Quinkler M, Hanslik G, Lang K, Hahner S, Allolio B, Meisinger C, Holle R, Beuschlein F, Bidlingmaier M, et al. Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension. 2012;60:618–624. doi: 10.1161/HYPERTENSIONAHA.112.197111. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y, Whaley-Connell AT, Habibi J, Rehmer J, Rehmer N, Patel K, Hayden MR, Ferrario CM, Sowers JR. Mineralocorticoid receptor antagonism attenuates vascular apoptosis and injury via rescuing Akt activation. Hypertension. 2009;53(2):158–165. doi: 10.1161/HYPERTENSIONAHA.108.121954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohshima K, Mogi M, Jing F, Iwanami J, Tsukuda K, Min LJ, Higaki J, Horiuchi M. Roles of interleukin 17 in angiotensin II type 1 receptor-mediated insulin resistance. Hypertension. 2012;59:493–499. doi: 10.1161/HYPERTENSIONAHA.111.183178. [DOI] [PubMed] [Google Scholar]
- 27.Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab. 2012;302(2):E201–E208. doi: 10.1152/ajpendo.00497.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 29.Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol. 2011;31:2534–2542. doi: 10.1161/ATVBAHA.111.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59:324–330. doi: 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]
- 31.Witkowski A, Prejbisz A, Florczak E, Ka̧dziela J, Śliwiński P, Bieleń P, Michałowska I, Kabat M, Warchoł E, Januszewicz M, Narkiewicz K, Somers VK, Sobotka PA, Januszewicz A. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 32.Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA, Krum H, Esler M, Böhm M. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940–46. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 33.Vogt L, Chiurchiu C, Chadha-Boreham H, Danaietash P, Dingemanse J, Hadjadj S, Krum H, Navis G, Neuhart E, Parvanova AI, Ruggenenti P, Woittiez AJ, Zimlichman R, Remuzzi G, de Zeeuw D, PROLONG (PROteinuria Lowering with urOteNsin receptor antaGonists) Study Group Effect of the urotensin receptor antagonist palosuran in hypertensive patients with type 2 diabetic nephropathy. Hypertension. 2010;55:1206–1209. doi: 10.1161/HYPERTENSIONAHA.109.149559. [DOI] [PubMed] [Google Scholar]
- 34.Miao Y, Ottenbros SA, Laverman GD, Brenner BM, Cooper ME, Parving HH, Grobbee DE, Shahinfar S, de Zeeuw D, Lambers Heerspink HJ. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58:2–7. doi: 10.1161/HYPERTENSIONAHA.111.171488. [DOI] [PubMed] [Google Scholar]
- 35.Galán M, Kassan M, Choi SK, Partyka M, Trebak M, Henrion D, Matrougui K. A novel role for epidermal growth factor receptor tyrosine kinase and its downstream endoplasmic reticulum stress in cardiac damage and microvascular dysfunction in type 1 diabetes mellitus. Hypertension. 2012;60:71–80. doi: 10.1161/HYPERTENSIONAHA.112.192500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper-DeHoff RM, Wen S, Beitelshees AL, Zineh I, Gums JG, Turner ST, Gong Y, Hall K, Parekh V, Chapman AB, Boerwinkle E, Johnson JA. Impact of abdominal obesity on incidence of adverse metabolic effects associated with antihypertensive medications. Hypertension. 2010;55:61–68. doi: 10.1161/HYPERTENSIONAHA.109.139592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruggenenti P, Lauria G, Iliev IP, Fassi A, Ilieva AP, Rota S, Chiurchiu C, Barlovic DP, Sghirlanzoni A, Lombardi R, Penza P, Cavaletti G, Piatti ML, Frigeni B, Filipponi M, et al. Effects of manidipine and delapril in hypertensive patients with type 2 diabetes mellitus: the delapril and manidipine for nephroprotection in diabetes (DEMAND) randomized clinical trial. Hypertension. 2011;58:776–783. doi: 10.1161/HYPERTENSIONAHA.111.174474. [DOI] [PubMed] [Google Scholar]
- 38.Kim YS, Davis SC, Truijen J, Stok WJ, Secher NH, van Lieshout JJ. Intensive blood pressure control affects cerebral blood flow in type 2 diabetes mellitus patients. Hypertension. 2011;57:738–45. doi: 10.1161/HYPERTENSIONAHA.110.160523. [DOI] [PubMed] [Google Scholar]
- 39.Torp-Pedersen C, Jeppesen J. Diabetes and hypertension and atherosclerotic cardiovascular disease: related or separate entities often found together. Hypertension. 2011;57:887–888. doi: 10.1161/HYPERTENSIONAHA.110.168583. [DOI] [PubMed] [Google Scholar]
- 40.Roman MJ, Howard BV, Howard WJ, Mete M, Fleg JL, Lee ET, Devereux RB. Differential impacts of blood pressure and lipid lowering on regression of ventricular and arterial mass: the Stop Atherosclerosis in Native Diabetics Trial. Hypertension. 2011;58:367–371. doi: 10.1161/HYPERTENSIONAHA.111.172486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turchin A, Goldberg SI, Shubina M, Einbinder JS, Conlin PR. Encounter frequency and blood pressure in hypertensive patients with diabetes mellitus. Hypertension. 2010;56:68–74. doi: 10.1161/HYPERTENSIONAHA.109.148791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Logan AG, Irvine MJ, McIsaac WJ, Tisler A, Rossos PG, Easty A, Feig DS, Cafazzo JA. Effect of home blood pressure telemonitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension. 2012;60:51–57. doi: 10.1161/HYPERTENSIONAHA.111.188409. [DOI] [PubMed] [Google Scholar]
- 43.Manrique C, Johnson M, Sowers JR. Thiazide diuretics alone or with beta-blockers impair glucose metabolism in hypertensive patients with abdominal obesity. Hypertension. 2010;55:15–17. doi: 10.1161/HYPERTENSIONAHA.109.142620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou X, Ma L, Habibi J, Whaley-Connell A, Hayden MR, Tilmon RD, Brown AN, Kim JA, Demarco VG, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension. 2010;55(4):880–888. doi: 10.1161/HYPERTENSIONAHA.109.145136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ayers K, Byrne LM, DeMatteo A, Brown NJ. Differential effects of nebivolol and metoprolol on insulin sensitivity and plasminogen activator inhibitor in the metabolic syndrome. Hypertension. 2012;59:893–888. doi: 10.1161/HYPERTENSIONAHA.111.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stears AJ, Woods SH, Watts MM, Burton TJ, Graggaber J, Mir FA, Brown MJ. A double-blind, placebo-controlled, crossover trial comparing the effects of amiloride and hydrochlorothiazide on glucose tolerance in patients with essential hypertension. Hypertension. 2012;59:934–942. doi: 10.1161/HYPERTENSIONAHA.111.189381. [DOI] [PubMed] [Google Scholar]
- 47.Cole BK, Keller SR, Wu R, Carter JD, Nadler JL, Nunemaker CS. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high-fat diet in mice. Hypertension. 2010;55:715–721. doi: 10.1161/HYPERTENSIONAHA.109.148049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sowers JR, Raij L, Jialal I, Egan BM, Ofili EO, Samuel R, Zappe DH, Purkayastha D, Deedwania PC. Angiotensin receptor blocker/diuretic combination preserves insulin responses in obese hypertensives. J Hypertens. 2010;28:1761–1769. doi: 10.1097/HJH.0b013e32833af380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raheja P, Price A, Wang Z, Arbique D, Adams-Huet B, Auchus RJ, Vongpatanasin W. Spironolactone prevents chlorthalidone-induced sympathetic activation and insulin resistance in hypertensive patients. Hypertension. 2012;60:319–325. doi: 10.1161/HYPERTENSIONAHA.112.194787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muramatsu T, Matsushita K, Yamashita K, Kondo T, Maeda K, Shintani S, Ichimiya S, Ohno M, Sone T, Ikeda N, Watarai M, Murohara T, NAGOYA HEART Study Investigators Comparison between valsartan and amlodipine regarding cardiovascular morbidity and mortality in hypertensive patients with glucose intolerance: NAGOYA HEART Study. Hypertension. 2012;59:580–586. doi: 10.1161/HYPERTENSIONAHA.111.184226. [DOI] [PubMed] [Google Scholar]
- 51.Egan BM, Bandyopadhyay D, Shaftman SR, Wagner CS, Zhao Y, Yu-Isenberg KS. Initial monotherapy and combination therapy and hypertension control the first year. Hypertension. 2012;59:1124–1131. doi: 10.1161/HYPERTENSIONAHA.112.194167. [DOI] [PMC free article] [PubMed] [Google Scholar]