Abstract
Background
The problem of obesity has risen to epidemic levels in the United States. A subset of patients with obesity will have metabolic syndrome. We sought to examine the impact of metabolic syndrome on the risk of morbidity and mortality among a large cohort of patients who underwent hepatic resection.
Methods
Patients included in the National Surgical Quality Improvement Program (NSQIP) dataset who underwent hepatic resection between January 2005 and December 2008 were identified. Data on clinical characteristics, comorbidities, operative details, as well as postoperative complications and mortality were collected and analyzed. Patients with BMI >30 kg/m2 who also had hypertension and diabetes were defined as having metabolic syndrome.
Results
A total of 3,973 patients who underwent a liver resection were identified. Overall mean body mass index was 28 kg/m2; 31.7% patients were obese (>30 kg/m2). Of the patients who were obese, 256 (20%) had metabolic syndrome. Patients with metabolic syndrome were less likely to have had a major hepatectomy (≥hemi-hepatectomy, 36% vs 43%; P = .01) but had a greater mean number of red blood cell transfusions (1.6 vs 1; P = .02). The incidence of postoperative complications after hepatectomy was 23%. Patients with metabolic syndrome had a greater risk for reintubation (odds ratio [OR] 1.9; P = .02), >48 hours ventilator dependence (OR 2.0; P = .003), myocardial infarction (OR 5.5; P = .01) and superficial surgical-site infections (OR 1. 7; P = .02) compared with nonmetabolic patients. Overall postoperative mortality was 3 %. Metabolic syndrome was associated with an increased risk of postoperative death (OR 2.7; P = .001).
Conclusion
The presence of metabolic syndrome was associated with a greater risk of perioperative complications. In addition, patients with metabolic syndrome had greater than a 2-fold increased risk of death after hepatic resection.
The indications and use of liver resection have increased during the last decade in the United States.1–3 Although advances in perioperative care, as well as intraoperative techniques, have led to a decrease in the morbidity and mortality associated with liver surgery, certain subsets of patients may remain at greater risk for complications and death. One such group of patients may be those patients who are obese. The problem of obesity has risen to epidemic levels in the United States, with estimates of 60% to 70% of the population being overweight or obese.4 A subset of patients with obesity can be characterized as having metabolic syndrome. Metabolic syndrome is a constellation of clinical features, including dyslipidemia, hypertension, elevated fasting glucose, and increased abdominal circumference.5,6 Metabolic syndrome can be associated with a range of metabolic disturbances, including increased free fatty acid levels, as well as low-grade systemic inflammation with an increase in proinflammatory cytokines such as TNF-α and IL-6 and a decrease in anti-inflammatory cytokines such as adiponection.7,8
Whether through oxidative stress secondary to lipid accumulation or growth signals from adipokines, metabolic syndrome has been associated with an increased risk of genitourinary and gastro-intestinal cancers, including hepatocellular carcinoma (HCC).9–12 Metabolic syndrome frequently can be found in individuals with nonalcoholic fatty liver disease (NAFLD) and may predispose patients not only to a greater incidence of NAFLD but also more severe disease.7 The effect of NALFD on outcomes after hepatic resection, which can include fatty liver disease in the absence (steatosis) or presence (steatohepatitis) of inflammation, has been reported.13–19 Similarly, the impact of obesity alone on perioperative outcomes after complex hepato-pancreaticobiliary surgery has been delineated previously.20–23 In contrast, the impact of the composite exposure of metabolic syndrome—obesity, hypertension, diabetes—on outcomes after liver resection remains ill defined. We sought to examine the perioperative outcomes of patients under-going hepatic resection by using the National Surgical Quality Improvement Program (NSQIP) database. Specifically, the objective of the current study was to investigate the impact of metabolic syndrome on the risk of morbidity and mortality among a large cohort of patients who underwent hepatic resection.
METHODS
Data source and patient population
We conducted a retrospective analysis of prospectively collected data from the NSQIP database.24 The NSQIP database represents an effort on behalf of the American College of Surgeons (ACS) that allows for the collection of risk-adjusted data to facilitate the assessment of outcome measures after surgery. The ACS NSQIP collects data on a variety of clinical variables, including preoperative risk factors, intraoperative variables, and 30-day postoperative morbidity and mortality outcomes for patients undergoing major surgical procedures. A participating site’s clinical nurse reviewer captures the data by using a variety of methods, including abstraction from the medical chart, as well as automatic electronic record extraction. The ACS intermittently audits the data to ensure its integrity. The ACS NSQIP and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
All patients included in the NSQIP dataset who underwent hepatic resection between January 2005 and December 2008 were evaluated for inclusion. Patients who had undergone hepatic resection were identified with the use of Current Procedural Terminology codes for hepatectomy, including codes 47120 (part lobectomy), 47122 (trisegmentectomy), 47125 (left hemi-hepatectomy), and 47130 (right hemi-hepatectomy). The International Classification of Diseases, 9th Revision, Clinical Modification diagnosis codes were used to identify and categorize the indications for hepatic surgery.
Data collection and analysis
Standard demographic and clinicopathologic data were collected, including sex, age, and body mass index (BMI). As described previously,20,25 BMI was divided categorically in accordance with the World Health Organization definitions of obesity: underweight, BMI <18.5 kg/m2; normal, BMI 18.5 to 24.9 kg/m2; overweight, BMI 25.0 to 29.9 kg/m2; obese BMI ≥30 kg/m2. For BMI, there were 37 missing values (1%), and these patients were excluded. Patient information also was collected on existing pulmonary, cardiac, and renal comorbidities, as well as history of diabetes categorized as either insulin-dependent or noninsulin dependent. Operative details including operative time, receipt of transfusion, as well as extent of operation were recorded. Complications after hepatectomy were noted and included infectious (eg, superficial and organ-space surgical site infections, sepsis), pulmonary (eg, re-intubation, prolonged intubation, pneumonia), cardiac (eg, cardiac arrest, myocardial infarction), renal (eg, renal insufficiency, dialysis), as well as thromboembolic events, dehiscence, and reoperation.
The main objective of this analysis was to determine whether metabolic syndrome was associated with greater rates of complications and 30-day mortality. In general, metabolic syndrome can be defined by the presence of several factors, including increased abdominal circumference, hypertension, diabetes mellitus, and hypertriglyceridemia.5,6 For the purposes of this study, patients were classified as having metabolic syndrome if their BMI was ≥30 kg/m2 in the setting of concomitant diabetes and hypertension. Patient demographics and comorbidities were stratified by the presence or absence of metabolic syndrome.
Mean and median values were used to describe continuous data, with discrete variables displayed as totals and frequencies. For bivariate analyses, 2-tailed t tests and Mann-Whitney U tests were used to compare continuous data, whereas the Fisher exact or χ2 tests were used for categorical variables. Multivariate regression was used to control for co-variates noted to be significant on bivariate analysis (P < .05). Point estimates were expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs) were provided. Survival time was estimated by Cox proportional hazards models with cumulative hazards during the 30-day postoperative period and compared with log-rank tests. All statistical analysis was performed using Stata MP version 11 (College Park, TX).
RESULTS
Patient demographics and baseline comorbidity characteristics
Of the 635,265 patients contained in the NSQIP dataset from January 2005 to December 2008, 3,973 patients who underwent a liver resection were the focus of this study. The demographic and clinical characteristics of these patients are outlined in Table I. The majority of patients were white (n = 3,032, 76%), and the mean patient age was 58 years (range, 17–89). A minority of patients were smokers (15.6%), and 2.9% actively consumed >2 drinks daily. Although some patients did not have concomitant medical diseases (n = 1,904; 48%), 2,069 patients (52.1%) had at least one preoperative medical comorbidity. Preoperative comorbidities included cardiac (n = 312; 7.9%), pulmonary (n = 136; 3.4%), diabetes (n = 595; 15%), and renal (n = 23; 0.6%) disease (Table II). The presence of esophageal varices or ascites was noted in 17 (0.4%) and 56 (1.4%) patients, respectively.
Table I.
Description of study sample
| All patients (n = 3,973) | No metabolic syndrome (n =3,717) | Metabolic syndrome (n = 256) | P value* | |
|---|---|---|---|---|
| Age; mean ± SD | 58 ± 14 | 58 ± 0.2 | 63 ± 0.6 | <.001 |
| Age > 65; n (%) | 1,386 (35) | 1,269 (34) | 117 (46) | <.001 |
| Male sex; n (%) | 1,962 (49) | 1,806 (49) | 156 (61) | <.001 |
| Race; n (%)† | ||||
| White | 3,032 (83) | 2,838 (83) | 194 (81) | .86 |
| All other races | 637 (17) | 591 (17) | 46 (19) | |
| Smoker; n (%) | 621 (16) | 593 (16) | 28 (11) | .030 |
| Alcohol abuse; n (%) | 114 (3) | 109 (3) | 5 (2) | .48 |
| BMI; mean ± SD | 28 (0.1) | 27 (0.1) | 35 (0.3) | <.001 |
| Extent of resection; n (%) | ||||
| Partial hepatectomy | 2,341 (59) | 2,171 (58) | 170 (66) | .09 |
| Right hemihepatectomy | 838 (21) | 796 (21) | 42 (16) | |
| Left hemihepatectomy | 403 (10) | 381 (10) | 22 (9) | |
| Trisegmentectomy | 391 (10) | 369 (10) | 22 (9) | |
| Postoperative diagnosis | ||||
| Metastasis | 1,830 (46) | 1,717 (46) | 113 (44) | <.001 |
| HCC | 671 (17) | 596 (16) | 75 (29) | |
| ICC | 139 (4) | 130 (4) | 9(4) | |
| Other malignancy | 271 (7) | 253 (7) | 18(7) | |
| Other benign | 271 (7) | 441 (12) | 14(5) | |
P value for comparison between “no metabolic syndrome” and “metabolic syndrome” categories.
Information on race was missing for 304 (8%) individuals in the sample.
HCC, Hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma.
Table II.
Preoperative comorbidities and operative details
| All patients (n = 3,973) | No metabolic syndrome (n = 3,717) | Metabolic syndrome (n = 256) | P value* | |
|---|---|---|---|---|
| Comorbidities | ||||
| Pulmonary | ||||
| Severe COPD; n (%) | 128 (8) | 116 (3) | 12(5) | .2 |
| Pneumonia; n (%) | 8 (0.2) | 8 (0.2) | 0(0) | |
| Cardiac | ||||
| CHF symptoms; n (%) | 10 (0.3) | 9 (0.2) | 1 (0.4) | .50 |
| MI within 6 months; n (%) | 8 (0.2) | 7 (0.2) | 1 (0.4) | .44 |
| Angioplasty/stenting; n(%) | 155 (4) | 126 (3) | 29 (11) | <.001 |
| Cardiac surgery; n (%) | 165 (4) | 138 (4) | 27 (11) | <.001 |
| Angina; n (%) | 20 (0.5) | 19 (0.5) | 1 (0.4) | .901 |
| Hypertension; n (%) | 1,818 (46) | 1,562 (42) | 256 (100) | <.001 |
| PVD; n (%) | 36 (0.9) | 32 (0.9) | 4(2) | .31 |
| Diabetes; n (%) | 595 (15) | 339 (9) | 256 (100) | <.001 |
| Renal failure; n (%) | 7 (0.2) | 5 (0.1) | 2 (0.8) | .07 |
| Dialysis dependent; n (%) | 20 (0.5) | 17 (0.5) | 3(1) | .17 |
| Esophageal varices; n (%) | 17 (0.4) | 13 (0.4) | 4(2) | .02 |
| Ascites; n (%) | 56 (1.4) | 52(1) | 4(2) | .81 |
| Chemotherapy; n (%) | 294 (7) | 282 (8) | 12(5) | .16 |
| Radiotherapy; n (%) | 41 (1) | 38(1) | 3(1) | .72 |
| Operative details | ||||
| Emergency procedure; n (%) | 36 (0.9) | 35(1) | 1 (0.4) | .48 |
| Operating room time, min; median | 228 | 228 | 229 | .42 |
| Resident involved; n (%) | 3,347 (84) | 3,141 (86) | 206 (80) | .09 |
| Resident ≥PGY 4 involved; n (%) | 2,950 (74) | 2,770 (75) | 180 (70) | .11 |
| Intraoperative transfusion (units); mean ± SD | 1 (3) | 1.1 (0.1) | 1.6 (0.3) | .02 |
P value for comparison between “no metabolic syndrome” and “metabolic syndrome” categories.
COPD, Chronic obstructive pulmonary disease; CHF, congestive heart failure; MI, myocardial infarction; PVD, peripheral vascular disease; PGY, postgraduate year.
The overall mean BMI was 28 kg/m2; 2.5% (n = 98) patients were underweight, 32% (n = 1,289) had a normal BMI score, 33.4% (n = 1,327) were overweight, and 31.7% (n= 1,259) were obese. Of the 1,259 patients who were obese, 256 (20%) had diabetes and hypertension and therefore were defined as having metabolic syndrome. The mean BMI in the nonmetabolic syndrome group was 27.5 kg/m2 vs 35.3 kg/m2 in the metabolic syndrome group (P < .001). Compared with nonmetabolic syndrome patients, patients with metabolic syndrome were older (57.9 years vs 62.8 years, P < .001) and more likely to be male (48.6% vs 60.9%, P < .001). With regard to preoperative medical comorbidities, patients with metabolic syndrome were more likely to have had previous coronary angioplasty (11.3% vs 3.4%) and cardiac surgery (10.6% vs 3.7%) compared with nonmetabolic syndrome patients (both P < .001). Other comorbidities were similar between the 2 groups (all P>.05).
Surgical and pathology details
At the time of operation, most patients underwent a partial hepatectomy (n= 2,341, 59%), whereas 838 (21%) and 403 (10%) underwent either a formal right or left hepatectomy, respectively. A minority of patients had an extended resection (ie, trisegmentectomy; n = 391, 10%). Of note, patients with metabolic syndrome were less likely to have had a major hepatectomy (ie, hemihepatectomy or extended resection). Specifically, only 34% (n = 86) patients with metabolic syndrome underwent a major hepatic resection compared with 43% of patients (n= 1,546) who did not have metabolic syndrome (P = .01). Despite being less likely to have undergone a major hepatic resection, the mean number of red blood cell transfusion units was greater in the metabolic syndrome group (1.6) compared with the nonmetabolic syndrome group (1.1; P = .02). The overall median operative time was 228 minutes and did not differ between the 2 groups (metabolic syndrome, 229 minutes compared with nonmetabolic syndrome, 228 minutes; P = .42).
On final pathology, most patients were noted to have metastatic/secondary neoplasms of the liver (n = 1,830; 46%), whereas fewer patients had a primary cancer of the liver (HCC: n = 671, 17%; intra-hepatic cholangiocarcinoma: n = 139, 4%). Benign lesions of the liver represented the pathologic diagnosis in only a minority of patients (n = 271, 7%). Of note, patients with metabolic syndrome were more likely to have HCC (n = 75, 29%) compared with patients without metabolic syndrome (n = 596, 16%; P< .001).
Morbidity and mortality
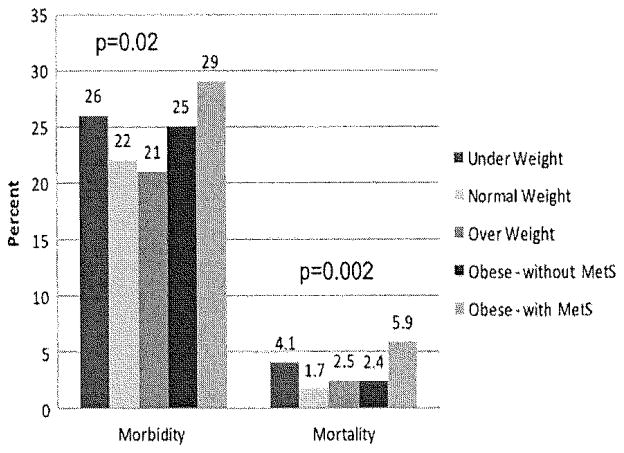
The overall incidence of postoperative complications after hepatectomy was 23% (n = 919) (Fig 1). Among those patients who had a postoperative complication, most morbidity was infectious in nature (n = 715; 18%; Table III). Superficial surgical-site infection occurred in 204 (5%) patients, whereas more severe infections such as deep organ-space infections (n = 265, 7%), systemic sepsis (n= 239, 6%), and septic shock (n= 143, 4%) also occurred in a subset of patients. Pulmonary complications included need for reintubation (n= 144, 4%), ventilator dependence for >48 hours (n= 177, 4%); cardiac complications were uncommon and included myocardial infarction (n = 11, 0.3%) and cardiac arrest (n = 34; 1%). Reoperation was required in 196 (5%) patients.
Fig. 1.
Overall morbidity and mortality stratified by BMI index, as well as the presence or absence of metabolic syndrome (MetS). Patients with metabolic syndrome had the greatest risk of perioperative morbidity and mortality.
Table III.
Postoperative morbidity and mortality after hepatectomy
| All patients (n = 3,973) | No metabolic syndrome (n = 3,717) | Metabolic syndrome (n = 256) | Odds ratio for event (MetS vs non-Met S) | P value* | |
|---|---|---|---|---|---|
| Any complication; n (%) | 919 (23) | 846 (23) | 73 (29) | 1.4 | .04 |
| Infection; n (%) | |||||
| Superficial SSI | 204 (5) | 183 (5) | 21 (8) | 1.7 | .02 |
| Deep SSI | 38 (1) | 38 (1) | 0(0) | — | — |
| Organ-space SSI | 265 (7) | 249 (7) | 16(6) | 0.9 | .82 |
| Pneumonia | 144 (4) | 134 (4) | 10(4) | 1.1 | .84 |
| Systemic sepsis | 239 (6) | 226 (6) | 13(5) | 0.9 | .79 |
| Septic shock | 143 (4) | 130 (3) | 13(5) | 1.5 | .23 |
| Pulmonary; n (%) | |||||
| Reintubation | 144 (4) | 128 (3) | 16(6) | 1.9 | .02 |
| Prolonged ventilation | 177 (4) | 156 (4) | 21 (8) | 2.0 | .003 |
| Cardiac; n (%) | |||||
| Cardiac arrest | 34(1) | 31 (1) | 3(1) | 1.4 | .63 |
| MI | 11 (0.3) | 8 (0.3) | 3(1) | 5.5 | .01 |
| Renal; n (%) | |||||
| Insufficiency | 36(1) | 31 (1) | 5 (2) | 2.4 | .08 |
| Dialysis | 55 (1) | 49(1) | 6(2) | 1.8 | .22 |
| Thromboembolism; n (%) | |||||
| Pulmonary embolism | 56(1) | 53(1) | 3(1) | 0.8 | .74 |
| Deep venous thrombosis | 80 (2) | 78 (2) | 2(1) | 0.4 | .22 |
| Dehiscence; n (%) | 37(1) | 35 (1) | 2(1) | 0.8 | .84 |
| Bleeding; n (%) | 36(1) | 32(1) | 4(2) | 1.8 | .31 |
| Return to the OR; n (%) | 196 (5) | 185 (5) | 11 (4) | 0.9 | .65 |
| Death; n (%) | 98 (3) | 83 (2) | 15 (6) | 2.7 | .001 |
P-value for comparison between “no metabolic syndrome” and “metabolic syndrome” categories.
MetS, Metabolic syndrome; MI, myocardial infarction; OR, operating room; SSI, surgical-site infection.
The presence of metabolic syndrome was associated with an increased risk of a postoperative complication (OR 1.4, 95% CI 1.02–1.8; P = .04; Table III). Specifically, patients with metabolic syndrome were at a greater risk for certain infectious, pulmonary, and cardiac complications. For example, compared with patients who did not have metabolic syndrome, patients with metabolic syndrome had a 2-fold increased risk for reintubation (OR 1.9, 95% CI 1.1–3.2; P = .02) and ventilator dependence for >48 hours (OR 2.0, P= .003). Patients with metabolic syndrome were also at a greater risk of a myocardial infarction (OR 5.5, 95% CI 1.4–20.8; P= .01) and superficial surgical-site infections (OR 1.7, 95% CI 1.1–2.8; P = .02). The risk for other complications, such as renal insufficiency, need for dialysis, deep venous thrombosis, pulmonary embolus, wound dehiscence, postoperative bleeding, and reoperation, was comparable between the 2 groups.
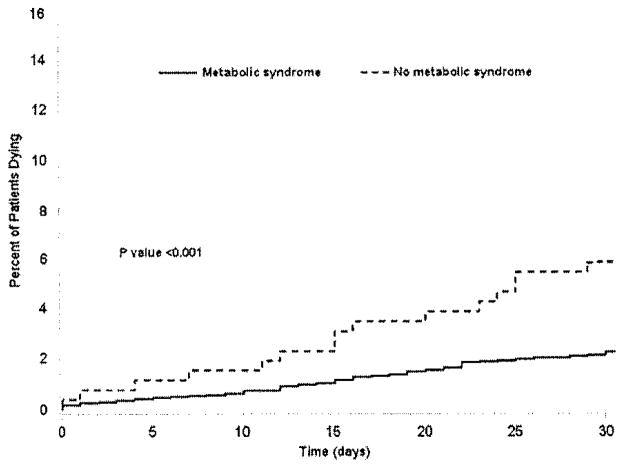
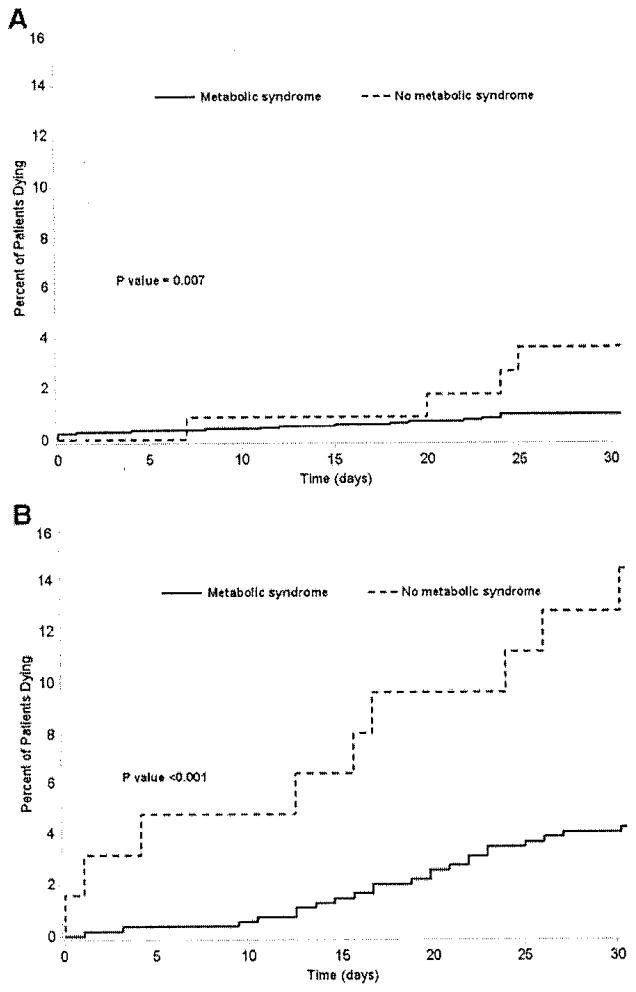
Overall, there were 98 deaths for a postoperative mortality of 3%. On bivariate analysis, the presence of metabolic syndrome was associated with an increased risk of postoperative death (OR 2.7, 95% CI 1.5–4.8; P = .001; Fig 2). After we controlled for competing risk factors, such as smoking status, previous coronary angioplasty or cardiac surgery, preoperative renal failure, esophageal varices, diagnosis of HCC, extent of resection and units of red blood cells transfused, we found that the presence of metabolic syndrome remained associated with risk of postoperative death (OR 2.1, 95% CI 1.1–3.9; P = .02; Table IV). Of note, however, was the finding that the risk of death was not associated with obesity alone (OR 1.4, 95% CI 0.95–2.2; P= .20). Specific subgroups of patients with metabolic syndrome continued to demonstrate a greater risk of death. The risk of postoperative death among patients with metabolic syndrome was increased for those patients undergoing liver resection to treat a hepatic malignancy (liver metastasis, OR 3.7, 95% CI 1.1–12.3, P= .04; HCC, OR 3.4, 95% CI 1.5–7.5, P= .002; Fig 3).
Fig. 2.
Proportion of patients dying within 30 days of hepatic surgery stratified by the presence or absence of metabolic syndrome. Although overall mortality was 3%, patients with metabolic syndrome were noted to have a greater mortality (5.9%) compared with patients without metabolic syndrome (2.2%; P < .001).
Table IV.
Adjusted risk of specific outcomes relative to presence or absence of metabolic syndrome
| Outcome | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Any complication | 1.3 | 0.97–1.8 | .08 |
| Any pulmonary complication | 1.6 | 1.02–2.5 | .04 |
| Pneumonia | 0.9 | 0.4–1.8 | .7 |
| Reintubation | 1.8 | 1.01–3.1 | .04 |
| Prolonged ventilation | 1.8 | 1.04–3.0 | .03 |
| Any cardiac complication | 1.8 | 0.7–4.6 | .2 |
| Any renal complication | 1.5 | 0.7–4.6 | .3 |
| Wound infection/dehiscence | 1.2 | 0.9–1.8 | .2 |
| Thromboembolism | 0.3 | 0.1–1.03 | .06 |
| Death | 2.1 | 1.1–3.9 | .02 |
Adjusted for extent of hepatectomy, diagnosis, previous coronary stent or cardiac surgery, renal failure, esophageal varices, tobacco use, and intraoperative red blood cell transfusion.
Fig. 3.
Specific subgroups of patients with metabolic syndrome demonstrated a greater risk of death. Specifically, the risk of postoperative death among patients with metabolic syndrome was increased for patients undergoing liver resection to treat either liver metastasis (A) or HCC (B).
The effect of metabolic syndrome on risk of postoperative death persisted even among patients undergoing less than a hemihepatectomy (OR 2.8, 95% CI 1.2–6.1; P = .01). Patients with metabolic syndrome had a greater cumulative risk of death over 30 days (log-rank P-value <.001). Specifically, the cumulative incidence of death was 6.9 deaths per 1,000 person-days among patients with metabolic syndrome compared with only 2.6 deaths per 1,000 person-days among patients without metabolic syndrome. After we controlled for competing covariates, the Cox proportional regression model revealed a hazard ratio (HR) of death of 2.2 among patients with metabolic syndrome (P = .008). This increased hazard of death persisted among patients treated for hepatic metastasis (HR 2.6, 95% CI 1.6–16.4; P = .01) and was even more pronounced among patients with HCC (HR 5.0, 95% CI 1.2–5.3; P= .007).
DISCUSSION
The problem of obesity has reached epidemic levels in the United States. More than two-thirds of the United States’ population is overweight (BMI 25.0–29.9 kg/m2), and one-third of individuals are obese (BMI >30 kg/m2).26 An increasing number of patients undergoing operative procedures are, therefore, likely to be obese. Although obesity may have implications for many different types of operative procedures, the impact of obesity on hepatic operation is of particular interest. NALFD is a major health burden, and its increasing prevalence is probably attributable to the great number of individuals with obesity, unhealthy dietary patterns, and a sedentary lifestyle.27 NAFLD can comprise a spectrum of diseases, including steatosis, steato-hepatitis, or even frank cirrhosis.28
In previous studies, authors have noted an association between obesity, NAFLD, and HCC.9,11,12 In fact, in the current study, we noted that there are more HCC cases in the metabolic syndrome group. The risk of NALD or HCC seems to be linked particularly to the presence of metabolic syndrome rather than obesity alone. Whether through the increased risk of cirrhosis or direct oxidative stress in the setting of a fatty liver, metabolic syndrome is associated with a greater risk of HCC. Metabolic syndrome is characterized by a constellation of clinical features including hypertension, elevated fasting glucose and high BMI. Metabolic syndrome has been associated with an increased risk of cardiovascular events, as well as possibly renal dysfunction.29,30 The effect of metabolic syndrome on perioperative outcomes after liver surgery has not been well examined. Our current study is important because we examined not only obesity but also the composite exposure of metabolic syndrome—obesity, hypertension, and diabetes—on outcomes after liver resection. We defined the incidence of metabolic syndrome to be approximately 6% among patients undergoing liver resection in a large, nationally representative cohort. Perhaps more importantly, we report that the presence of metabolic syndrome was not only associated with a greater risk of perioperative complications, but also more than a 2-fold increased risk of death after hepatic resection.
For major operations such as hepatectomy, morbidity remains a concern. Several investigators have noted an increased risk of perioperative complications among obese patients.20,21,31 In a case control study by Viganò et al31 that reported on 85 obese patients, the authors noted that obese patients had an increased risk of postoperative morbidity after liver resection compared with nonobese patients; however, only grade II morbidity was increased in obese patients, and there were no differences in more severe grade III or IV morbidity among obese compared with nonobese patients. In a separate study, Mathur et al21 reported an increased risk of wound and urinary tract infections as well as pulmonary complications.
In the current study, we noted that the presence of metabolic syndrome was associated with a 40% increased risk of a postoperative complication (Table III). Of note, patients with metabolic syndrome were at a greater risk for both minor and major complications. Specifically, patients with metabolic syndrome had a 70% increased risk of superficial surgical-site infection and were more likely to require blood transfusions. Moreover, patients with metabolic syndrome had a 2-fold increased risk of pulmonary complications as well as a 5-fold increased risk of myocardial infarction. These data suggest that, unlike obesity alone, metabolic syndrome is associated with an increased risk of both minor as well as major complications after hepatic surgery.
Advances in operative techniques and perioperative care have minimized mortality associated with hepatic surgery with most contemporary series from large academic centers reporting a 30-day mortality of less than 5%.32–36 Several factors may impact mortality after hepatectomy. In-hospital mortality after major hepatectomy has been noted to vary substantially by race, with black patients having a 2-fold greater population-level odds of surgical mortality than white patients.37 In contrast, mortality has not been shown to be greater in well-selected elderly patients, with several authors noting low rates of perioperative death even among patients older than 70 years of age. 38,39 Similarly, obesity, although associated with an increased risk of morbidity, has not been associated with an increased risk of mortality after liver surgery.20
In the current study, we also report that the risk of perioperative death was not associated with obesity alone (OR 1.4, 95% CI 0.95–2.2; P= .20); of note, however, was our finding that metabolic syndrome was associated with risk of postoperative death. In fact, patients with metabolic syndrome had more than a 2-fold increased risk of death after hepatic resection. Stratification of patients by diagnosis revealed that perioperative mortality risk was increased among patients undergoing liver resection for metastatic disease as well as HCC, although the risk was greater in the latter group (Fig 3). Given differences in perioperative mortality risk, screening for metabolic syndrome rather than obesity alone has important implications for risk stratification of patients undergoing liver surgery. Similar to other risk factors such as age, performance status, medical comorbidities, metabolic syndrome should be considered in the preoperative setting to help stratify patients with regard to perioperative risk.
Using a large, nationally representative cohort of patients, we found that approximately one-third of patients undergoing liver resection were obese. Although the overall prevalence of metabolic syndrome was 6% among the entire cohort, about 1 in 5 obese patients had metabolic syndrome for a prevalence of 20%. Ervin,40 using the National Health and Nutrition Examination Survey, reported a prevalence of metabolic syndrome of about 30% among obese patients. Epidemiologic studies have demonstrated differences in prevalence of metabolic syndrome by age, sex, and ethnicity. Razzouk and Muntner41 reported that the prevalence of metabolic syndrome increased with age through the sixth decade of life among men and seventh decade among women. We similarly noted that patients with metabolic syndrome were older (57.9 years vs 62.8 years, P < .0001).
In addition, metabolic syndrome also seemed to be more common among male non-Hispanic whites than male non-Hispanic blacks, with the reverse being true among women.41 In the current study, most patients were non-Hispanic white (>80%), and we noted that patients with metabolic syndrome were more likely to be male (48.6% vs 60.9%, P < .001). Together, these data suggest that metabolic syndrome has a relatively high prevalence, especially among those who are obese, older, and from certain ethnic backgrounds.
There are several limitations of the current study that should be considered. The current configuration of the NSQIP dataset does not provide assessment of liver-specific outcomes. As such, we were not able to ascertain differences in outcomes of certain complications such as bile leak or hepatic insufficiency. Several investigators have advocated for the creation of an hepato-pancreato-biliary NSQIP to improve quality, as well as provide risk-adjusted registries with hepato-pancreato-biliary-specific data.42 Although these efforts are under way, the current study provides important data that defines overall morbidity and mortality among patients with metabolic syndrome undergoing liver surgery. Another limitation of the NSQIP dataset is the lack of accurate data on dyslipidemia, which can be part of the constellation of clinical factors associated metabolic syndrome. Therefore, dyslipidemia was not included as part of the criteria to define metabolic syndrome in the current study. This consideration may have lead to an underestimation of the prevalence of metabolic syndrome in our study population; however, as noted, the prevalence reported in the current study was comparable to other population-based reports.40,41
In conclusion, we noted an overall prevalence of 6% in our large, population-based dataset, but the prevalence of metabolic syndrome among obese patients was even greater at approximately 20%. The impact of metabolic syndrome on outcomes after liver resection was clinically relevant. Both morbidity and mortality after hepatic resection were markedly greater among patients with metabolic syndrome. These data should help guide future management of this challenging group of patients.
References
- 1.Lyratzopoulos G, Tyrrell C, Smith P, Yelloly J. Recent trends in liver resection surgery activity and population utilization rates in English regions. HPB (Oxford) 2007;9:277–80. doi: 10.1080/13651820701504165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimick JB, Wainess RM, Cowan JA, Upchurch GR, Jr, Knol JA, Colletti LM. National trends in the use and outcomes of hepatic resection. J Am Coll Surg. 2004;199:31–8. doi: 10.1016/j.jamcollsurg.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Feldstein AE. Nonalcoholic steatohepatitis: risk factors and diagnosis. Expert Rev Gastroenterol Hepatol. 2010;4:623–35. doi: 10.1586/egh.10.56. [DOI] [PubMed] [Google Scholar]
- 8.Gade W, Schmit J, Collins M, Gade J. Beyond obesity: the diagnosis and pathophysiology of metabolic syndrome. Clin Lab Sci. 2010;23:51–61. quiz 62–5. [PubMed] [Google Scholar]
- 9.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–61. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arano T, Nakagawa H, Tateishi R, et al. Serum level of adiponectin and the risk of liver cancer development in chronic hepatitis C patients. Int J Cancer. 2010 Dec 17; doi: 10.1002/ijc.25861. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Wang SN, Lee KT, Ker CG. Leptin in hepatocellular carcinoma. World J Gastroenterol. 2010;16:5801–9. doi: 10.3748/wjg.v16.i46.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu JM, Skill NJ, Maluccio MA. Evidence of aberrant lipid metabolism in hepatitis C and hepatocellular carcinoma. HPB (Oxford) 2010;12:625–36. doi: 10.1111/j.1477-2574.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vetelainen R, van Vliet A, Gouma DJ, van Gulik TM. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;245:20–30. doi: 10.1097/01.sla.0000225113.88433.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for post-operative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007;245:923–30. doi: 10.1097/01.sla.0000251747.80025.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–72. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 16.Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Pre-operative chemotherapy for colorectal liver metastases: impact on hepatic histology and post-operative outcome. J Gastrointest Surg. 2007;11:860–8. doi: 10.1007/s11605-007-0149-4. [DOI] [PubMed] [Google Scholar]
- 17.Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–8. doi: 10.1016/s1091-255x(98)80025-5. [DOI] [PubMed] [Google Scholar]
- 18.Gomez D, Malik HZ, Bonney GK, et al. Steatosis predicts post-operative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395–402. doi: 10.1002/bjs.5820. [DOI] [PubMed] [Google Scholar]
- 19.Kooby DA, Fong Y, Suriawinata A, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–44. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Mathur AK, Ghaferi AA, Osborne NH, et al. Body mass index and adverse perioperative outcomes following hepatic resection. J Gastrointest Surg. 2010;14:1285–91. doi: 10.1007/s11605-010-1232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathur AK, Ghaferi AA, Sell K, Sonnenday CJ, Englesbe MJ, Welling TH. Influence of body mass index on complications and oncologic outcomes following hepatectomy for malignancy. J Gastrointest Surg. 2010;14:849–57. doi: 10.1007/s11605-010-1163-5. [DOI] [PubMed] [Google Scholar]
- 22.Benns M, Woodall C, Scoggins C, McMasters K, Martin R. The impact of obesity on outcomes following pancreatectomy for malignancy. Ann Surg Oncol. 2009;16:2565–9. doi: 10.1245/s10434-009-0573-7. [DOI] [PubMed] [Google Scholar]
- 23.House MG, Fong Y, Arnaoutakis DJ, et al. Pre-operative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg. 2008;12:270–8. doi: 10.1007/s11605-007-0421-7. [DOI] [PubMed] [Google Scholar]
- 24.Overview. Available at: http://site.acsnsqip.org/ACoSN-SQIPANDC.
- 25.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894(i–xii):1–253. [PubMed] [Google Scholar]
- 26.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 27.Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. 2011;17:3377–89. doi: 10.3748/wjg.v17.i29.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Jarvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010;42:320–30. doi: 10.1016/j.dld.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Cirillo P, Sautin YY, Kanellis J, et al. Systemic inflammation, metabolic syndrome and progressive renal disease. Nephrol Dial Transplant. 2009;24:1384–7. doi: 10.1093/ndt/gfp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–32. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 31.Viganò L, Kluger MD, Laurent A, et al. Liver resection in obese patients: results of a case-control study. HPB (Oxford) 2011;13:103–11. doi: 10.1111/j.1477-2574.2010.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18. doi: 10.1097/00000658-199909000-00004. discussion 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayo SC, Shore AD, Nathan H, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB (Oxford) 2011;13:473–82. doi: 10.1111/j.1477-2574.2011.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asiyanbola B, Chang D, Gleisner AL, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842–51. doi: 10.1007/s11605-008-0494-y. [DOI] [PubMed] [Google Scholar]
- 35.de Jong MC, Mayo SC, Pulitano C, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13:2141–51. doi: 10.1007/s11605-009-1050-0. [DOI] [PubMed] [Google Scholar]
- 36.Vauthey JN, Pawlik TM, Abdalla EK, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–30. doi: 10.1097/01.sla.0000124385.83887.d5. discussion 30–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathan H, Frederick W, Choti MA, Schulick RD, Pawlik TM. Racial disparity in surgical mortality after major hepatectomy. J Am Coll Surg. 2008;207:312–9. doi: 10.1016/j.jamcollsurg.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Portolani N, Baiocchi GL, Coniglio A, et al. Limited liver resection: a good indication for the treatment of hepatocellular carcinoma in elderly patients. Jpn J Clin Oncol. 2011;41:1358–65. doi: 10.1093/jjco/hyr154. [DOI] [PubMed] [Google Scholar]
- 39.Di Benedetto F, Berretta M, D’Amico G, et al. Liver resection for colorectal metastases in older adults: a paired matched analysis. J Am Geriatr Soc. 2011;59:2282–90. doi: 10.1111/j.1532-5415.2011.03734.x. [DOI] [PubMed] [Google Scholar]
- 40.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Rep. 2009;13:1–7. [PubMed] [Google Scholar]
- 41.Razzouk L, Muntner P. Ethnic, gender, and age-related differences in patients with the metabolic syndrome. Curr Hypertens Rep. 2009;11:127–32. doi: 10.1007/s11906-009-0023-8. [DOI] [PubMed] [Google Scholar]
- 42.Pitt HA, Kilbane M, Strasberg SM, et al. ACS-NSQIP has the potential to create an HPB-NSQIP option. HPB (Oxford) 2009;11:405–13. doi: 10.1111/j.1477-2574.2009.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]