Abstract
Background
Cocaine dependence is a substantial public health problem, yet there are no clearly effective medication treatments. Amphetamine and topiramate have both shown promise for the treatment of cocaine dependence in preclinical and early-stage clinical studies.
Method
Eighty-one cocaine-dependent adults were randomized to receive a combination of mixed amphetamine salts extended-release (MAS-ER) and topiramate or placebo for twelve weeks under double-blind conditions. MAS-ER doses were titrated over two weeks to a maximum dose of 60 mg daily and topiramate doses were titrated over six weeks to a maximum dose of 150 mg twice daily. All participants received a supportive behavioral intervention. The primary outcome was the proportion of individuals who achieved three consecutive weeks of abstinence as measured by urine toxicology confirmed self-report.
Results
The overall proportion of participants who achieved three consecutive weeks of abstinence was larger in the extended-release mixed amphetamine salts and topiramate group (33.3%) than in placebo group (16.7%). There was a significant moderating effect of baseline total number of cocaine use days (Wald χ2=3.75, df =1, P=.05) on outcome suggesting that the combination treatment was most effective for participants with a high baseline frequency of cocaine use.
Conclusions
The results of this study supported our hypothesis that the combination of MASER and topiramate would be superior to placebo in achieving three weeks of consecutive abstinence. These findings provide evidence that the combination of MAS-ER and topiramate is efficacious in promoting abstinence in cocaine-dependent individuals.
Keywords: cocaine dependence, amphetamines, topiramate, treatment, clinical trial, substance dependence
INTRODUCTION
There are approximately 1.6 million current users of cocaine in the US,(1) and the past-year prevalence of cocaine dependence is estimated to be 1.1%,(2) yet there are no effective pharmacotherapies for cocaine dependence. Standard psychosocial treatments for cocaine are not effective for many cocaine-dependent patients with an average abstinence rate of approximately 30%.(3) Several dozen double-blind, randomized controlled trials for cocaine dependence have been conducted,(4–6) and while several agents have shown promise, none have been shown to be clearly effective.
The acute effects of cocaine are due to its central inhibition of catecholamine uptake, particularly dopamine, by binding to the dopamine transporter.(7) Substitution pharmacotherapy, which has been proven effective for opioid(8) and nicotine(9) dependence, is a plausible strategy for treating cocaine dependence. Findings with the positron emission tomography (PET) raclopride displacement procedure have shown that deficient dopamine transmission is associated with failure to respond to behavioral treatment.(10) Stimulant medication may correct this deficit, and by enhancing dopamine release, may improve the salience of competing reinforcers to cocaine. Psychostimulants, including amphetamine, methylphenidate, bupropion and modafinil, have been studied as treatments for cocaine dependence, both in patients with (11, 12) and without (13–19) co-occurring attention deficit hyperactivity disorder (ADHD). The results of these studies have been mixed with regard to effects on cocaine use outcomes, with promising therapeutic effects reported for dextroamphetamine.(20) Amphetamine and cocaine have similar pharmacological and clinical characteristics; they differ mainly in onset of action and half-life. The mechanism of action of amphetamine is to both block dopamine reuptake and promote dopamine release.
Gamma amino butyric acid (GABA) modulates the dopaminergic system and cocaine effects,(21) and anticonvulsant agents that facilitate GABA activity have increasingly become the focus of study as potential pharmacotherapy for cocaine dependence. As a medication class, anticonvulsants have not been shown to be effective for the treatment of cocaine dependence,(22) but individual agents, including topiramate(23) and vigabatrin(24) have demonstrated therapeutic benefit in preliminary controlled trials. Topiramate is an anticonvulsant agent that enhances GABA activity(25) and antagonizes glutamate transmission through effects at α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptors.(26) In addition to the evidence of promoting abstinence in cocaine-dependent patients,(23) topiramate has demonstrated evidence of efficacy in treating alcohol(27, 28) and nicotine(29) dependence. The presumed mechanism for the therapeutic action of topiramate across substance dependence classes is by augmenting GABAergic activity and inhibiting glutaminergic excitation, leading to decreased midbrain dopaminergic activity,(30, 31) while topiramate has also been shown to reduce stimulant-induced dopamine activation as well.(32)
For certain disorders the use of multiple drugs simultaneously, typically with distinct mechanisms of action, is essential to obtain a desired therapeutic outcome.(33) Promising separate lines of research of amphetamine and topiramate as single-agent pharmacotherapies for cocaine dependence suggested that studying the combined effects of these two medications was warranted. We hypothesized that by combining these two agents with distinct mechanisms of action, additive or synergistic effects would emerge and yield a more robust clinical benefit; that a combination of a psychostimulant and topiramate may help normalize striatal dopamine signaling by both increasing baseline levels of dopamine transmission and reducing cocaine-induced dopamine release, in theory both decreasing craving and cocaine-induced reinforcement.
Therefore, we conducted a randomized double-blind placebo-controlled trial of mixed amphetamine salts extended-release (MAS-ER) combined with topiramate for the treatment of cocaine dependence. We selected target doses of MAS-ER and topiramate based on studies of amphetamine for cocaine dependence(14) and topiramate for cocaine and alcohol dependence.(23, 27, 28) We hypothesized that the proportion of participants achieving three consecutive weeks of cocaine abstinence during the study would be greater for the combined pharmacotherapies group compared to the placebo group. Three consecutive weeks of abstinence is a clinically meaningful outcome associated with more favorable long-term outcomes.(34) We also explored secondary outcomes including treatment retention, abstinent weeks during the study period, and medication adherence, safety, and tolerability.
METHODS AND MATERIALS
Participants
All participants were seeking outpatient treatment for problems related to cocaine use and were recruited by local advertising or by clinical referrals in the New York City metropolitan area. We enrolled 81 participants who met Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, Text Revision (DSM-IV-TR)(35) criteria for cocaine dependence. Participants were 18–60 years old; reported using cocaine at least 4 days during the 28 days prior to study entry; and had a body mass index (BMI) ≥ 18 kg/m2. Participants were excluded if they had: a DSM-IV-TR diagnosis of major depressive disorder, bipolar disorder, schizophrenia or any psychotic disorder other than transient psychosis due to substance use; a DSM-IV-TR Axis I psychiatric disorder that was unstable or likely require treatment during the study period; physiological dependence on any substances (other than cocaine, nicotine or cannabis) that would require medical intervention; were prescribed psychotropic medication other than for insomnia; a current diagnosis of psychostimulant abuse or dependence; were at significant risk for suicide; coronary vascular disease as indicated by clinical history or electrocardiogram; unstable physical condition such as poorly controlled hypertension, acute hepatitis, or poorly controlled diabetes; a history of seizures; history of an allergic reaction to MAS-ER (or other amphetamine analogs) or topiramate; were pregnant or lactating; prescribed carbonic anhydrase inhibitors; a history of glaucoma or kidney stones; a history of failure to respond to either study medication for cocaine dependence or were compelled to receive treatment to avoid imprisonment or other penalties. This study was reviewed and approved by the Institutional Review Board of the New York State Psychiatric Institute and all participants gave written informed consent.
Treatment
The study was a single-site, randomized, double-blind, parallel-group, 14-week clinical trial comparing the combination of MAS-ER and topiramate to placebo, with a one-week single-blind placebo lead-in, a 12-week double blind study period, and a one-week taper. The clinical trial was conducted at the Substance Treatment and Research Service (STARS) of Columbia University Medical Center/New York State Psychiatric Institute. After determining study eligibility, participants began a one-week single-blind placebo lead-in phase to assess their baseline severity of cocaine use and the ability to comply with study procedures. Successful completers of the single-blind placebo lead-in were randomized to the combination of MAS-ER and topiramate or placebo in a 1:1 allocation. The randomization was stratified by a whether one of the three placebo lead-in week urine toxicology samples was positive for the cocaine metabolite benzoylecgonine (BE).
Following randomization, study medications were administered under double-blind conditions for 12-weeks. MAS-ER, topiramate, and matching placebo were packaged in matching gelatin capsules with a lactose filler and 12.5 mg of riboflavin. Each week, all participants were provided with two medication bottles under double-blind conditions, one labeled “MAS-ER or placebo” and the other labeled “topiramate or placebo”. If a participant could not tolerate at least 10 mg per day of the study medication labeled “MAS-ER or placebo”, all study medications were discontinued; no minimum was set for the medication labeled “topiramate or placebo”. MAS-ER doses began at 10 mg daily and were titrated over two weeks to a maximum dose of 60 mg daily and topiramate doses began at 25 mg daily were titrated over six weeks to a maximum dose of 150 mg twice daily. Reductions in dosing were made for intolerable adverse effects. Participants in each group received an identical number of capsules. Participants were instructed to take the MAS-ER or placebo capsules in the morning and the topiramate or placebo capsules in the morning and the evening. Each week, participants were asked to return all bottles and unused medication.
All patients had a weekly individual session with a study psychiatrist using a structured compliance enhancement manual designed for pharmacotherapy trials in subjects with substance use disorders.(36) The goal of compliance enhancement therapy is to achieve high quality supportive treatment that reinforces adherence to study procedures and the study medication regimen. Sessions were approximately 30 minutes in duration and were focused on: 1) setting abstinence from cocaine use as a goal; 2) participant compliance with study medications and procedures; and 3) current functioning. Mutual help support group (e.g., Narcotics Anonymous) attendance was encouraged.
Progressive incentive payments were provided for attendance to enhance compliance with study procedures and retention. Participants earned an additional $10 voucher each week that they returned their pill bottles and any remaining medication. Perfect attendance and compliance with returning pill bottles would result in a participant earning $592 in vouchers over the study period. Voucher earnings were redeemable for retail goods or services designated by the participant. Participants were also compensated in cash for transportation costs per visit.
Assessment
During the screening period, a comprehensive psychiatric and medical evaluation, and the Structured Clinical Interview (SCID) for DSM-IV Axis I disorders(37) interview were performed. Self-reported cocaine use for the prior 28 days was measured using the timeline followback method.(38)
Participants were scheduled to attend three assessment visits per week. At each study visit, a urine sample for toxicology and ultra-violet detection of riboflavin was obtained, and vital signs and adverse effects were assessed. On a weekly basis, a timeline followback assessment of cocaine use was performed and medication compliance was assessed using a calendar procedure and pill count. Serum for topiramate levels were collected at weeks 6, 10, and 14. Female participants had serum pregnancy tests performed monthly. Electrocardiograms were performed at weeks 4 and 14.
Cocaine use was assessed by using urine toxicology confirmed self-report. Self-reported cocaine use data was collected using the timeline followback method(38) for the 28 days preceding study entry and each day of the study period. Urine samples were collected three times per week and creatinine-corrected quantitative BE levels were determined. We confirmed self-reported cocaine use with a modified version of a scheme developed by Winhusen et al.(39) A self-report of use was accepted as a “use day”. Urine samples with BE levels greater than 300 ng/ml were considered positive. A urine sample was considered negative for new use if it was preceded within two days by a sample with twice or more its BE level. If none of the three days preceding a positive urine sample has been self-reported as a use day, the day immediately preceding the sample was labeled a use day.
Data Analysis
The primary outcome measure was a dichotomous measure of a clinically significant treatment response, defined as three or more consecutive weeks of abstinence (no cocaine use based on self-report confirmed by urine toxicology) during the 12-week medication phase of the trial. Secondary outcomes were retention in treatment, weekly abstinence, and medication adherence, safety and tolerability. The dichotomous primary outcome was analyzed using logistic regression with independent predictors: treatment (MAS-ER and topiramate vs. placebo) and adjusted for baseline severity of cocaine use (total number of cocaine use days in the 28 days prior to randomization). Retention rates were compared using Kaplan-Meier curves and log-rank statistics. Weekly abstinence was analyzed using mixed effect model (MEM) with logistic link function, modeling abstinence as a function of treatment, time (weeks in the trial), and baseline level of cocaine use (days using). Interactions between treatment and baseline severity of cocaine use were assessed and included in the final model if found significant at a significance level of 15%. PROC LOGISTIC and PROC GLIMMIX in SAS were used to conduct these analyses.(40) All analyses were conducted on the intent-to-treat sample of all randomized patients. All statistical tests were 2-tailed and employed at a significance level of 5%, unless otherwise stated. The original sample size of 120 patients was chosen to ensure sufficient power (at least 80%) of a two-sided test with level of significance α=0.05 for detecting difference between the two experimental treatments with respect to the percentage of subjects who achieve continuous 3-weeks abstinence during the study, when the difference is of clinically meaningful and realistic magnitude. With 60 subjects per group there is 81% power to detect a difference in the proportions of 25% vs. 50% and there is 87% power to detect a difference of 15% vs. 40%.
Computer-generated randomized blocks of four were used (two medication and two placebo assignments per block in randomly permutated order) with stratification by presence of cocaine positive urine toxicology during the placebo lead-in week. Randomization was conducted by the research pharmacist with participants and all other study staff blind to treatment assignment.
A Data and Safety Monitoring Board met yearly to review study enrollment, overall medication tolerability, adverse and serious adverse events. Throughout the trial, no recommendations were made to alter the protocol or cease study enrollment.
RESULTS
Participant Progress in Study and Demographics
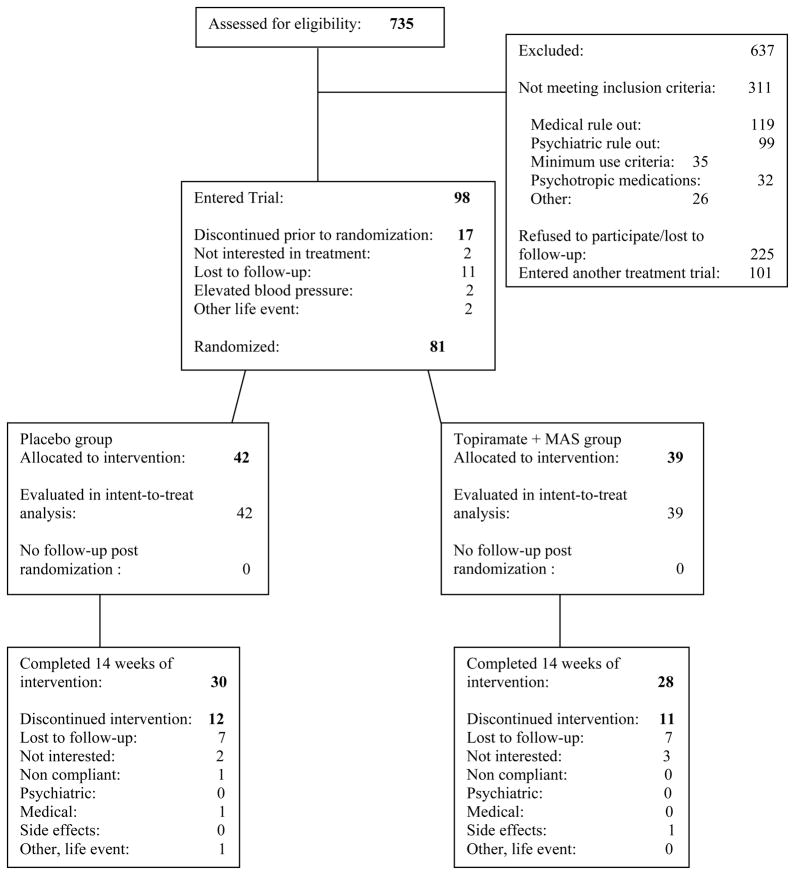
Seven-hundred and thirty-five participants were screened and a total of 81 participants were randomized between extended-release mixed amphetamine salts (MAS-ER) and topiramate (n=39; 48.1%) and placebo (n=42; 51.9%). Recruitment began in January of 2007 and all follow-up was completed in May of 2010. Trial enrollment was ended when resources to conduct the trial were depleted. Participants were screened for potential eligibility for multiple cocaine dependence clinical trials simultaneously. The most common reason for screen failure was not meeting eligibility criteria. The demographic characteristics of the randomized participants are shown in Table 1 and were not significantly different between the two treatment groups.
Table 1.
Demographic characteristics of randomized sample (N=81)*
| Placebo | MAS-ER/Topiramate | ||
|---|---|---|---|
| Characteristic | (n=42) | (n=39) | p-value |
| Demographic characteristics | mean (SD) or n (%) | ||
| Age (years) | 42.4 (8.9) | 41.5 (8.2) | 0.63 |
| Male | 37 (88.1) | 33 (84.6) | 0.65 |
| Race/Ethnicity | |||
| Hispanic | 13 (31.0) | 12 (30.8) | 0.92 |
| Black | 15 (35.7) | 15 (38.5) | |
| White | 14 (33.3) | 11 (28.2) | |
| Asian | 0 (0) | 1 (2.6) | |
| Education | |||
| ≤ High school | 19 (47.5) | 13 (34.2) | 0.24 |
| Some college | 8 (20.0) | 14 (36.8) | |
| College & graduate school | 13 (32.5) | 11 (29.0) | |
| Employment status | |||
| Full-time | 17 (41.5) | 18 (47.4) | 0.60 |
| Unemployed/others | 24 (58.5) | 20 (52.6) | |
| Currently married | 10 (23.8) | 6 (15.8) | 0.37 |
| Mutual support meeting attendance | 8 (19) | 9 (23) | |
| Clinical characteristics | |||
| Total baseline use days (out of 28 days) | 12.7 (7.9) | 13.3 (6.9) | 0.72 |
| median (IQR) | |||
| Total dollars of use (in 28 days) | 678 (315–1158) | 750 (495–1303) | 0.31 |
Frequencies may not sum to N=81 due to missing values. Three patients did not report their education level, two patients did not report their employment status, and one patient did not report his marital status. Percentages may not add up to 100 due to rounding.
Primary Outcome: Three consecutive weeks of abstinence
Using the intent-to-treat (ITT) sample, the primary outcome, the proportion of patients who achieved any three consecutive weeks of abstinence in the trial, was twice as large for those receiving MAS-ER and topiramate (13/39, 33.3%), compared to placebo (7/42, 16.7%). The effect of treatment on the primary outcome was found to be significantly moderated by baseline severity of cocaine use (measured by cocaine use days at baseline; Wald χ2=3.75, df =1, P=.05) in the direction that the superiority of combination medication over placebo was significant among patients with greater frequency of cocaine use at baseline. To better understand the moderating effect of baseline frequency of cocaine use, the observed baseline total number of cocaine use days was divided into tertiles and plotted against the observed proportion of subjects with 3 weeks of abstinence (Figure 1). Within the first tertile (consisting of, the 33% of patients with the lowest number of cocaine use days at baseline: 0–8 days), 25% (3/12) of the ER-MAS and topiramate group achieved three-week abstinence versus 33% (5/15) in placebo. From the patients in the second tertile (patients 9–15 cocaine use days at baseline) 43% (6/14) were abstinent in the ER-MAS and topiramate group versus 13% (2/16) on placebo. From patients in the third tertile (16–28 baseline cocaine use days), 31% (4/13) achieved three-week abstinence in ER-MAS and topiramate group compared to 0% (0/11) in placebo group. Overall, for patients with baseline cocaine use days of at least 9 days or more (moderate to high severity), the proportion achieving abstinence in combination treatment was 37.0% compared to 7.4% achieving abstinence in placebo group, with the overall odds ratio of being abstinent on ER-MAS and topiramate versus placebo was 7.4 with 95% confidence interval (1.4, 37.8). The median time (interquartile range) to the first occurrence of the three weeks of consecutive abstinence was 5 weeks (4 weeks–6 weeks) for the MAS-ER and topiramate group and 3 weeks (3 weeks–4 weeks) for the placebo group.
Figure 1.
CONSORT Flowchart. Participants’ progress through the screening, entry, randomization and medication phases of the treatment trial
Secondary outcomes
Weekly abstinence over time
As a confirmatory analysis, we used mixed effect model to predict longitudinal weekly abstinence (each week scored as abstinent if both urine and self-report were negative) as a function of treatment and time (week). There was no effect of time, but a significant main effect of treatment was detected favoring MAS-ER and topiramate as a covariate (p=.02). When baseline days using cocaine in the month prior enrollment were added to the model, there was a significant baseline by treatment interaction (p= .0062) and no significant effect of time. The likelihood of abstinence was significantly greater on medication than placebo beginning at a baseline of about 10 days using cocaine per month, with the superiority of medication over placebo increase as baseline level of use increases.
Retention in Treatment
Retention throughout the 12 week double blind phase was not significantly different between the MAS-ER and topiramate (29/39; 74.4%) and placebo (35/42, 83.3%) groups (χ2=.98, df =1, P=.32).
Medication Adherence, Safety and Tolerability
The median rates of self-reported medication adherence was not significantly different between the two treatment groups (Z=.46, P=.65). The median (interquartile range) percentage of MAS-ER pills taken was 97% (88%–100%) for the combined pharmacotherapy group and 97% (91%–100%) for the placebo group. The median (interquartile range) percentage of topiramate pills taken was 96% (86%–100%) for the combined pharmacotherapy group and 97% (90%–99%) for the placebo group (Z=.07, P=.94). The median (interquartile range) percentage of samples that fluoresced for riboflavin was 96% (92%–97%) for combined pharmacotherapy group and 96% (90%–100%) for placebo (Z=.05, P=.96). Ninety-three percent of the combined pharmacotherapy group participants had over 80% of their urine samples positive for amphetamine, and 89% of the combination medication group serum topiramate samples were positive.
The median (interquartile range) maintenance dose of MAS-ER was 50 mg/day (20 mg/day–60 mg/day) for the combined pharmacotherapy group, and 60 mg/day (60 mg/day–60 mg/day) for placebo (Z=2.41, P=.03). In the combined pharmacotherapy group, 46% of patients had their doses of MAS-ER reduced compared to 18% in the placebo group, (χ2=6.75, df =1, P=.01). The median (interquartile range) maintenance dose of topiramate was 150 mg/day (37.5 mg/day–300 mg/day) for the combined pharmacotherapy group, and 300 mg/day (150 mg/day– 300 mg/day) for placebo (Z=2.41, P=.02). Forty-seven percent patients in the combined pharmacotherapy group had their topiramate dose reduced during the trial versus 21% in placebo group (χ2=5.66, df =1, P=.02). In the combined pharmacotherapy group, 31% of patients had both medications reduced compared to 7% in the placebo group (χ2=33.9, df =1, P <.0001).
Moderate-to-severe adverse events reported by at least 5% of participants are described in table 2. Adverse effects that occurred significantly more frequently in the combined pharmacotherapy group included insomnia, changes in appetite, anxiety, irritability, parathesias, and itching. Two participants experienced a serious adverse event. One participant in the MASER and topiramate group developed a substance-induced mood syndrome and required hospitalization. The other participant in the placebo group voluntarily discontinued study participation during the final week of the trial and entered a residential substance abuse treatment center.
Table 2.
Moderate-to-severe adverse events reported by at least 5% of participants randomized to placebo and MAS-ER and topiramate during the trial (N=81).
| Placebo | ER-MAS and topiramate | X2 value | p-value | |
|---|---|---|---|---|
| Adverse event | (n=42) | (n=39) | ||
| Insomnia | 7 (16.7) | 16 (41.0) | 5.90 | 0.02 |
| Increased/decreased appetite | 4 (9.5) | 17 (43.6) | 12.22 | <.001 |
| Fatigue | 6 (14.3) | 11 (28.2) | 2.36 | 0.12 |
| GI upset | 5 (11.9) | 11 (28.2) | 3.39 | 0.07 |
| Headache | 5 (11.9) | 11 (28.2) | 3.39 | 0.07 |
| Nausea | 4 (9.5) | 10 (25.6) | 3.67 | 0.06 |
| Dizziness | 4 (9.5) | 10 (25.6) | 3.67 | 0.06 |
| Body ache | 4 (9.5) | 8 (20.5) | 1.94 | 0.16 |
| Dry mouth | 3 (7.1) | 8 (20.5) | 3.08 | 0.11 |
| Anxiety | 1 (2.4) | 9 (23.1) | 8.00 | 0.01 |
| Irritable | 2 (4.8) | 8 (20.5) | 4.64 | 0.04 |
| Paresthesia | 1 (2.4) | 9 (23.1) | 8.00 | 0.01 |
| Cold/flu | 3 (7.1) | 6 (15.4) | Fisher’s exact | 0.30 |
| Somnolence | 4 (9.5) | 4 (10.3) | Fisher’s exact | 0.91 |
| Hyperactive/jittery | 3 (7.1) | 5 (12.8) | Fisher’s exact | 0.48 |
| Diarrhea | 3 (7.1) | 4 (10.3) | Fisher’s exact | 0.71 |
| Disoriented | 1 (2.4) | 5 (12.8) | Fisher’s exact | 0.10 |
| Chest pains | 2 (4.8) | 3 (7.7) | Fisher’s exact | 0.67 |
| Depression | 2 (4.8) | 2 (5.1) | Fisher’s exact | 1.00 |
| Itching | 0 (0) | 4 (10.3) | Fisher’s exact | 0.05 |
| Rash | 2 (4.8) | 2 (5.1) | Fisher’s exact | 1.00 |
| Vomiting | 0 (0) | 3 (7.7) | Fisher’s exact | 0.11 |
| Cough | 0 (0) | 3 (7.7) | Fisher’s exact | 0.11 |
| Sore throat | 1 (2.4) | 2 (5.1) | Fisher’s exact | 0.61 |
| Distracted | 0 (0) | 3 (7.7) | Fisher’s exact | 0.11 |
| Racing heart | 1 (2.4) | 2 (5.1) | Fisher’s exact | 0.61 |
| Memory loss | 0 (0) | 3 (7.7) | Fisher’s exact | 0.11 |
| Fever | 0 (0) | 2 (5.1) | Fisher’s exact | 0.23 |
| Tooth infection | 0 (0) | 2 (5.1) | Fisher’s exact | 0.23 |
DISCUSSION
The results indicate that the combination of mixed amphetamine salts extended-release (MAS-ER) and topiramate is superior to placebo in achieving three weeks of consecutive abstinence, with the effect most evident in participants with greater baseline frequency of cocaine use. In psychopharmacology, the effect of a medication often depends on the baseline level of severity of the disorder being treated,(41) prompting us to test the baseline by treatment interaction here. The combination of MAS-ER and topiramate was more likely than placebo to promote abstinence among participants using cocaine more than 9 days during the 28 days prior to study enrollment.
Cocaine dependence is associated with blunted striatal dopamine signaling,(10) and targeting dopamine regulation is logical treatment strategy. MAS-ER increases dopamine transmission and topiramate has been shown to reduce stimulant-induced dopamine activation.(32) We hypothesize that this combination of medications normalizes striatal dopamine signaling by both increasing baseline dopamine levels and reducing cocaine-induced dopamine release; a modulatory pharmacological effect that may simultaneously decrease baseline craving and decrease cocaine-induced reinforcement. The combination of an agent promoting dopamine release such as MAS-ER with a dopamine modulating agent such as topiramate may mimic the partial agonist effects of other successful substance use disorder pharmacotherapy approaches such as buprenorphine or varenicline.
The finding that the more frequent users of cocaine were most helped by the combination medication treatment is consistent with this hypothesized mechanism of action of the combination of MAS-ER and topiramate; higher severity users are more likely to have dysregulated dopamine signaling. In contrast, low severity cocaine dependence is predictive of spontaneous abstinence,(42, 43) and for this group of patients psychosocial treatment alone may be effective with less need for a medication treatment to correct the neurophysiological imbalances associated with more severe cocaine dependence. The ability to abstain at entry into a clinical trial is predictive of outcome regardless of clinical trial treatment assignment,(42, 43) and arguably, these individuals are better candidates for behavioral treatments.
Presuming that the observed results are due to an additive or synergistic interaction between MAS-ER and topiramate, a possible mechanism for this interaction is that topiramate, in addition to reducing cocaine use, may also favorably modulate the effect of MAS-ER. Topiramate has been reported to modulate the reinforcing effects and adverse effects associated with methamphetamine.(44) In this study, the combination of MAS-ER and topiramate was associated with a relatively high rate of adverse effects and dose reductions. One cocaine-dependent participant developed a substance-induced mood disorder after exposure to MAS-ER and topiramate, a known potential adverse effect of stimulant pharmacotherapy. However, the rate of medication non-compliance and investigator discontinuation of study medication was relatively low and retention did not significantly differ between treatment groups, supporting the feasibility of combination pharmacotherapy for cocaine dependence.
Cocaine dependence pharmacotherapy trials do not use uniform outcome measures. In our judgment, a three-week period of abstinence, in the context of a 12-week study period, is a clinically significant outcome, and is similar to outcomes used in other recently-conducted cocaine dependence trials.(16, 23, 24) We also examined weekly urine-confirmed abstinence over the study period with mixed effect modeling, another typical strategy for analyzing outcome of cocaine dependence trials. The lack of an effect of time on the achievement of weeks of abstinence in conjunction with the significant treatment effect suggests that the onset of the treatment effect was relatively rapid. In contrast, a study of topiramate treatment for cocaine dependence,(23) did not show a benefit until after the titration phase was completed (eight weeks). The trial enrolled individuals with mild cocaine withdrawal symptoms who were able to maintain a three-day period of abstinence prior to trial initiation, while the combination of MASER and topiramate tested in this study was found to be effective for higher severity users.
The design and results of this study have implications for future trial designs. The use of a supportive psychosocial intervention is focused on setting abstinence as a treatment goal and encouraging adherence with study medication and compliance with other study procedures. In addition, incentives contingent on study procedure compliance, yielded a high rate of study retention as well as high rates of medication adherence. A similar psychosocial platform, focused on adherence and abstinence, was more successful than cognitive-behavioral therapy in supporting naltrexone efficacy among alcohol-dependent patients.(45)
The results of this study confirm that pharmacotherapy for cocaine dependence is most useful for frequent users of cocaine while the behavioral therapy may be sufficient for lower frequency users. In future trials, eligibility criteria should be considered that set as a baseline a minimum frequency of cocaine use that will enroll participants who are more likely to benefit from pharmacotherapy and to minimize the potential for a “floor effect” among the more mild severity cocaine users who are likely to achieve and sustain abstinence without needing any assistance from a medication. The findings also suggest the importance of exploring the interaction between medication treatment and baseline severity as part of the data analytic plan.
This study has several limitations. The two-arm placebo controlled design does not allow us to address whether both medications (both MAS-ER and topiramate) are necessary for efficacy. Large sample size multi-arm trials would be needed to disentangle and confirm contributions of both medications. Another important limitation of the study is the potential limited treatment masking that occurred when giving two medications with distinctive adverse effect profiles simultaneously. Additionally, clinical trial participants at a university-based research clinic, recruited primarily via advertising, represent a subgroup of cocaine-dependent patients, and trials in other settings and populations would be needed to determine the extent to which the finding of this trial generalize.
The combination of MAS-ER and topiramate appears promising as a treatment for cocaine dependence. The positive results observed in this study need to be replicated in a larger, multi-center clinical trial. The findings also provide encouragement for the strategy of testing medication combinations, rather than single agents, for cocaine dependence.
Supplementary Material
Acknowledgments
This research was supported by NIDA grants R01 DA022217 (Dr. Levin), K23 DA021209 (Dr. Mariani) and K24 029647 (Dr. Levin), and K24 DA022412 (Dr. Nunes). Dr. Mariani had full access to all of the data of the study and takes full responsibility for the integrity of the data and for the accuracy of the data analysis. We would like to thank the staff of the Substance Treatment and Research Service (STARS) of the Columbia University Medical Center/New York State Psychiatric Institute for their clinical support. The results of this clinical trial were presented at the 73rd Annual Meeting of the College on Problems of Drug Dependence, June 18–23, 2011 in Hollywood, Florida.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00421603
FINANCIAL DISCLOSURES
Drs. Mariani, Pavlicova, Bisaga, and Nunes, reported no biomedical financial interests or potential conflicts of interest. Mr. Brooks reported no biomedical financial interestes of potential conflicts of interest. Dr. Levin is a past consultant for Eli Lily and Company, Shire Pharmaceuticals Group, AstraZeneca, and OrthoMcNeil Pharmaceutical Inc. Dr. Levin has received research support from Eli Lily and Company, UCB Pharma Inc, Shire Pharmaceuticals Group, AstraZeneca and OrthoMcNeil Pharmaceutical Inc. Dr. Levin currently receives medication from US WorldMed for an ongoing study that is sponsored by the National Institute on Drug Abuse.
References
- 1.United State Department of Health and Human Services. Substance Abuse and Mental health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Office of Applied Studies; Rockville, MD: 2011. [Google Scholar]
- 2.Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64(5):566–76. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- 3.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179–87. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 4.de Lima MS, de Oliveira Soares BG, Reisser AA, Farrell M. Pharmacological treatment of cocaine dependence: a systematic review. Addiction. 2002;97(8):931–49. doi: 10.1046/j.1360-0443.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- 5.Leiderman DB, Shoptaw S, Montgomery A, Bloch DA, Elkashef A, LoCastro J, et al. Cocaine Rapid Efficacy Screening Trial (CREST): a paradigm for the controlled evaluation of candidate medications for cocaine dependence. Addiction. 2005;100(Suppl 1):1–11. doi: 10.1111/j.1360-0443.2005.00988.x. [DOI] [PubMed] [Google Scholar]
- 6.Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, et al. New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol. 2008;11(3):425–38. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- 7.White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51(1–2):141–53. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 8.Amato L, Davoli M, Ferri APCM, Faggiano F, PMR An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abuse Treat. 2005;28(4):321–9. doi: 10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Berrettini WH, Lerman CE. Pharmacotherapy and pharmacogenetics of nicotine dependence. Am J Psychiatry. 2005;162(8):1441–51. doi: 10.1176/appi.ajp.162.8.1441. [DOI] [PubMed] [Google Scholar]
- 10.Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. Imaging Dopamine Transmission in Cocaine Dependence: Link Between Neurochemistry and Response to Treatment. Am J Psychiatry. 2011 doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10(3):286–94. doi: 10.1037//1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- 12.Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: Double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2006 doi: 10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Grabowski J, Roache JD, Schmitz JM, Rhoades H, Creson D, Korszun A. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol. 1997;17(6):485–8. doi: 10.1097/00004714-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, et al. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21(5):522–6. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98(8):1137–41. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- 16.Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30(1):205–11. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- 17.Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63(2):219–28. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- 18.Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, et al. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104(1–2):133–9. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101(1–2):34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castells X, Casas M, Perez-Mana C, Roncero C, Vidal X, Capella D. Efficacy of psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev. 2010;(2):CD007380. doi: 10.1002/14651858.CD007380.pub3. [DOI] [PubMed] [Google Scholar]
- 21.Sofuoglu M, Dudish-Poulsen S, Poling J, Mooney M, Hatsukami DK. The effect of individual cocaine withdrawal symptoms on outcomes in cocaine users. Addict Behav. 2005;30(6):1125–34. doi: 10.1016/j.addbeh.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez Y, Farre M, Fonseca F, Torrens M. Anticonvulsant drugs in cocaine dependence: a systematic review and meta-analysis. J Subst Abuse Treat. 2010;38(1):66–73. doi: 10.1016/j.jsat.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75(3):233–40. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Brodie JD, Case BG, Figueroa E, Dewey SL, Robinson JA, Wanderling JA, et al. Randomized, double-blind, placebo-controlled trial of vigabatrin for the treatment of cocaine dependence in Mexican parolees. Am J Psychiatry. 2009;166(11):1269–77. doi: 10.1176/appi.ajp.2009.08121811. [DOI] [PubMed] [Google Scholar]
- 25.White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia. 2000;41(Suppl 1):S17–20. [PubMed] [Google Scholar]
- 26.Skradski S, White HS. Topiramate blocks kainate-evoked cobalt influx into cultured neurons. Epilepsia. 2000;41(Suppl 1):S45–7. doi: 10.1111/j.1528-1157.2000.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677–85. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- 28.Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. Jama. 2007;298(14):1641–51. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- 29.Johnson BA, Ait-Daoud N, Akhtar FZ, Javors MA. Use of oral topiramate to promote smoking abstinence among alcohol-dependent smokers: a randomized controlled trial. Arch Intern Med. 2005;165(14):1600–5. doi: 10.1001/archinte.165.14.1600. [DOI] [PubMed] [Google Scholar]
- 30.Johnson BA, Swift RM, Addolorato G, Ciraulo DA, Myrick H. Safety and efficacy of GABAergic medications for treating alcoholism. Alcohol Clin Exp Res. 2005;29(2):248–54. doi: 10.1097/01.alc.0000153542.10188.b0. [DOI] [PubMed] [Google Scholar]
- 31.Johnson BA, Swift RM, Ait-Daoud N, DiClemente CC, Javors MA, Malcolm RJ., Jr Development of novel pharmacotherapies for the treatment of alcohol dependence: focus on antiepileptics. Alcohol Clin Exp Res. 2004;28(2):295–301. doi: 10.1097/01.alc.0000113409.47937.6c. [DOI] [PubMed] [Google Scholar]
- 32.Schiffer WK, Gerasimov MR, Marsteller DA, Geiger J, Barnett C, Alexoff DL, et al. Topiramate selectively attenuates nicotine-induced increases in monoamine release. Synapse. 2001;42(3):196–8. doi: 10.1002/syn.10000. [DOI] [PubMed] [Google Scholar]
- 33.Nies AS. Principles of Therapeutics. In: Hardman JG, Limbard EL, Gilman AG, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 10. New York: The McGraw-Hill Companies, Inc; 2001. p. 54. [Google Scholar]
- 34.Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51(12):989–97. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- 35.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 36.Carroll KMOMS, Nuro K. Yale University Psychotherapy Development Center Training Series No 4. West Haven, CT: 1999. Compliance Enhancement: A Manual for the Psychopharmacology of Drug Abuse and Dependence. [Google Scholar]
- 37.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 38.Litten R, Allen J. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: The Humana Press Inc; 1992. pp. 41–72. [Google Scholar]
- 39.Winhusen T, Somoza E, Ciraulo DA, Harrer JM, Goldsmith RJ, Grabowski J, et al. A double-blind, placebo-controlled trial of tiagabine for the treatment of cocaine dependence. Drug Alcohol Depend. 2007;91(2–3):141–8. doi: 10.1016/j.drugalcdep.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 40.SAS 9.2. SAS Institute, Inc; Cary, NC: 2009. [Google Scholar]
- 41.Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, et al. Utility of lead-in period in cocaine dependence pharmacotherapy trials. Drug Alcohol Depend. 2005;77(1):7–11. doi: 10.1016/j.drugalcdep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Kampman KM, Volpicelli JR, Mulvaney F, Rukstalis M, Alterman AI, Pettinati H, et al. Cocaine withdrawal severity and urine toxicology results from treatment entry predict outcome in medication trials for cocaine dependence. Addict Behav. 2002;27(2):251–60. doi: 10.1016/s0306-4603(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 44.Johnson BA, Roache JD, Ait-Daoud N, Wells LT, Wallace CL, Dawes MA, et al. Effects of acute topiramate dosing on methamphetamine-induced subjective mood. Int J Neuropsychopharmacol. 2007;10(1):85–98. doi: 10.1017/S1461145705006401. [DOI] [PubMed] [Google Scholar]
- 45.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jama. 2006;295(17):2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.