Abstract
Objectives
To assess whether the composition and charge of microemulsions affect their ability to simultaneously deliver α-tocopherol and lipoic acid into viable skin layers.
Methods
α-Tocopherol and lipoic acid were added (1.1 and 0.5% w/w, respectively) to decylglucoside-based microemulsions containing mono-dicaprylin. Microemulsions containing surfactant : oil : water (w/w/w) at 60 : 30 : 10 (ME-O) and 46 : 23 : 31 (ME-W), as well as a cationic form of ME-W containing 1% phytosphingosine (ME-Wphy) were characterized, and their ability to disrupt the skin barrier and deliver the antioxidants in vitro in the skin was evaluated. Antioxidant activity in ME-Wphy-treated skin was assessed using the thiobarbituric acid-reactive substances (TBARS) assay.
Key findings
The internal phase diameters of microemulsions ranged between 42 and 55 nm; phytosphingosine addition and pH adjustment to 5.0 increased zeta potential from −4.3 to +29.1 mV. ME-O displayed w/o structure, whereas ME-W and ME-Wphy were consistent with o/w. Microemulsions affected skin electrical resistance and transepidermal water loss, but did not affect lipoic acid penetration. α-Tocopherol delivery increased following the order ME-O < ME-W < ME-Wphy. ME-Wphy presented suitable short-term stability. The antioxidants delivered by ME-Wphy decreased TBARS cutaneous levels.
Conclusions
Even though microemulsion structure only affected tocopherol penetration, delivered levels of both antioxidants were sufficient for a decrease in TBARS, supporting their use for enhanced protection.
Keywords: cutaneous delivery, lipoic acid, medium chain monoglycerides, microemulsions, phytosphingosine, α-tocopherol
Introduction
Continuous exposure of the skin to ultraviolet radiation results in several deleterious effects and disorders, including skin ageing and cancer.[1] Even though the mechanisms leading to these injuries are not fully elucidated, UV radiation seems to stimulate the generation of reactive oxygen species, which mediate oxidation reactions in the skin, and tissue damage.[1] Since the natural reserves of antioxidants are continuously depleted by UV exposure, delivery of antioxidants to the skin may be a promising strategy to enhance skin protection from oxidative stress.[2,3]
Co-delivery of antioxidants that display synergism has been studied as a strategy to maximize skin protection.[2] α-Tocopherol, one of the vitamin E compounds, represents one of the most studied non-enzymatic antioxidants for topical application.[2] It can be regenerated by several antioxidants, including lipoic acid, which in turn can be converted to the very potent dihydrolipoic acid in cells.[4,5] In addition, lipoic acid is able to directly scavenge reactive oxygen species and possesses metal-chelating activity, making it an excellent option for cutaneous co-administration with tocopherol for possible synergism and enhanced protection.[5]
For the desired enhanced protection, α-tocopherol and lipoic acid should be co-localized in viable skin layers. α-Tocopherol and lipoic acid have distinct structures, including molecular weight (MW), lipophilicity and dissociation characteristics (MW of 431 and 206, calculated log P = 7.8 and 2.3, and pKa above 10 and close to 4.7 for α-tocopherol and lipoic acid, respectively), making their co-localization difficult.[6–8] While the size and lipophilicity of lipoic acid is more suitable for penetration (although ionizable at skin pH), α-tocopherol is very lipophilic and prone to be retained within the stratum corneum (SC), and thus its penetration into viable skin layers might be more difficult to achieve than that of lipoic acid. However, if tocopherol does not reach viable skin layers, the co-localization of the antioxidants and their synergism is not possible.
In this study, we employed microemulsions to promote the concomitant delivery of tocopherol and lipoic acid into viable skin layers. Microemulsions present multiple advantages over other dermatological formulations, including thermodynamic stability, possibility to incorporate both hydrophilic and lipophilic compounds at the same time, and skin penetration-enhancing ability, which can be further modulated by incorporation of penetration enhancers.[2,9–11] Because of the ability of mono-dicaprylin (MCG, a mixture of mono and diglyceride from caprylic acid) to increase the cutaneous delivery of another lipophilic antioxidant,[9] this compound was used in the microemulsions studied here. The influence of water content on the internal structure of microemulsions and on their ability to co-localize the antioxidants was evaluated here. Moreover, because cationic systems seem to have a positive effect on skin permeation due to skin's negative surface charges,[12,13] we also evaluated the effect of phytosphingosine addition (to confer a positive charge to the formulations) on the penetration-enhancing effect of selected systems.
Material and Methods
Materials
Propylene glycol (PG), isopropyl myristate, α-tocopherol, lipoic acid, ferrous sulfate, thiobarbituric acid and thrichloroacetic acid were obtained from Sigma (St Louis, MO, USA). MCG was a kind gift from Abitec Corporation (Janesville, WI). Acetonitrile, ethanol and methanol were purchased from Mallinckrodt Baker (Phillipsburg, NJ, USA) and decylglucoside was a kind gift from BASF (Florham Park, NJ, USA).
Methods
Pseudo-ternary phase diagram construction and sample preparation
Ternary phase diagrams were constructed using the water titration method at room temperature. Decylglucoside was chosen as surfactant and propylene glycol as co-surfactant because of their low irritation potential, and used at 1 : 1 (w/w).[14] The oil phase and surfactant blend were mixed at 1 : 9–9 : 1 ratios (w/w), titrated at room temperature with water under vortexing and formulations that were fluid, clear and did not undergo phase separation were assigned to a monophasic region in the phase diagram.[10]
Two microemulsions were chosen and subjected to further characterization: ME-O (containing more oil than water, 60 : 30 : 10 surfactant : oil : water, w/w/w) and ME-W (containing more water than oil, 46 : 23 : 31 surfactant : oil : water, w/w/w). Both contained the same surfactant : oil ratio, allowing us to study the influence of aqueous content on microemulsion characteristics. In both formulations MCG was used above 20% to favour cutaneous over transdermal delivery.[9,15] A third formulation was obtained by adding phytosphingosine to ME-W to generate a cationic system (ME-Wphy). The choice of phytosphingosine was based on the fact that it is a natural component of the SC and exhibits anti-inflammatory and antimicrobial effects, which can be advantageous for topical application.[12,16] The final composition of ME-Wphy was 45.3 : 22.7 : 1 : 31 (surfactant : oil : phytosphingosine : water, w/w/w). Lipoic acid was added at 0.5% and α-tocopherol at 1.1% for a 1 : 1 molar ratio.
The isotropicity of the selected formulations was confirmed by polarized light microscopy (Axiotop, Zeiss, Thornwood, NY, USA). The pH of the selected formulations was measured and adjusted to 5.0 with 0.25% of a phosphoric acid solution (85%). The internal phase diameter was determined using a Zetasizer Nano series instrument (Malvern, Westborough, MA, USA) at room temperature (using viscosities of 78 and 69 cp for ME-O and ME-W, as determined using a R/S-CPS rheometer at Brookfield, Middleboro, MA, USA). To evaluate the internal structure of the systems, the electrical conductivities of samples composed of a surfactant blend : oil (2 : 1, w/w) and variable amounts of water (1–35%, w/w) were determined at 25 ± 0.5°C using a Jenway 4520 conductivity/TDS meter (Techne Inc., Burlington, NJ, USA).
The short-term stability of ME-Wphy (as it delivered the largest amounts of tocopherol into the skin, see Results) was assessed next. In this study, formulation characteristics (internal phase diameter, microscopic characteristics and zeta potential) and drug content were assessed after storage of the formulation at 4°C for up to 7 days using HPLC (method described below). The formulation was diluted with methanol to obtain final lipoic acid and tocopherol concentrations of 5 and 11 μg/ml, respectively, prior to drug assay.
In-vitro skin penetration
Skin penetration of α-tocopherol and lipoic acid was studied using Franz diffusion cells (diffusion area of 1 cm2; Laboratory Glass Apparatus, Inc., Berkeley, CA, USA), with porcine ear skin and a receptor phase consisting of 100 mm phosphate buffer (pH 7.4) containing ethanol (10% v/v) and maintained at 37°C under constant stirring. Ethanol was added to aid α-tocopherol solubility in the aqueous medium (as solubility is <0.1 mg/ml).[17] The solubility of lipoic acid, on the other hand, is close to 0.6 mg/ml[18] and ethanol addition at 10% did not largely influence it. Considering that the maximum concentration of lipoic acid in the receptor phase after 12 h is over 100 times smaller than its solubility (15.8 μg/cm2 crossed the skin, which is equivalent to 5.3 μg/ml, see Results), and that this concentration of ethanol does not seem to change skin characteristics,[19] it is unlikely to affect lipoic acid penetration. Microemulsions (100 mg) were placed in the donor compartment of diffusion cells for 12 h; drug solutions in propylene glycol were used as control formulations.
At the end of the experiment, skin samples were rinsed to remove excess formulation, and the SC was separated from the epidermis (E) and dermis (D) by tape stripping, using 15 pieces of tape. The first piece was disposed of and the others were placed in conical tubes containing 4 ml of methanol : acetonitrile (1 : 1, v/v). The remaining skin (viable epidermis + dermis, ED) was cut into small pieces, placed into conical tubes containing 2 ml methanol : acetonitrile (1 : 1, v/v), homogenized using a hand-held tissue homogenizer (Biospec Products, Bartlesville, OK, USA) and bath sonicated for 20 min. Following this, the samples from SC, ED and aliquots of the receptor phase were filtered through a 0.45-μm pore cellulose membrane and assayed by HPLC. To standardize the extraction of drugs from the skin, the tape-stripping procedure was performed in untreated tissues. Following this, the tapes containing SC and the ED sections were spiked with 5–20 μg of each antioxidant, and extraction with methanol : acetonitrile (1 : 1, v/v) was performed as described above. Recovery of the antioxidants varied from 80 to 92%.
Microemulsion effect on the barrier function of the skin
Changes in skin electrical resistance and transepidermal water loss were assessed as an index of the ability of microemulsions to change skin permeability depending on their composition.[20,21] The electrical resistance of the tissue and the transepidermal water loss were measured before and after treatment with the control vehicle (propylene glycol) or microemulsions using a LCR multimeter (Mod. 179, accuracy 0.8%, Fluke, Everett, WA, USA) or a closed-chamber evaporimeter (Vapometer, Delfin Technologies Ltd, Kuopio, Finland) equipped with an adaptor to fit the diffusion cell opening.[9,21] To measure changes in resistance, skin samples were mounted in diffusion cells containing PBS in the donor and receptor compartments. After equilibration for 15 min, baseline skin resistance was measured by inserting electrodes into the donor and receptor compartments. The baseline transepidermal water loss was measured after equilibrating skin samples for 15 min in diffusion cells containing PBS in the receptor compartment. Following treatment with 100 mg of the control vehicle or the microemulsions for 12 h, tissues were carefully rinsed, mounted again on the diffusion cells, and after equilibration for 15 min measurements of the final electrical resistance or transepidermal water loss were performed.
In-vitro drug release
Microemulsions containing lipoic acid and α-tocopherol were placed in supports (1 cm2 area containing cellulose membranes) and mounted on the wells of a 12-well plate in contact with the receptor phase for 1, 2, 4, 6 and 8 h. As receptor phase, we chose 1.5% (w/v, 2 g) hydroxypropyl cellulose gel with 20% ethanol to avoid dilution of the microemulsion with liquid receptor phases, and because gels may better simulate the diffusion conditions in the skin.[22] The antioxidants were extracted from the receptor phase gel after polymer precipitation by acidification, dilution with acetonitrile (0.7 ml), centrifugation and filtration, and assayed in the supernatant by HPLC. The release rates were calculated from the slope of the linear portion of the plots of cumulative drug released against time.[23] The extraction procedure was standardized by spiking the receptor phase gel with known concentrations of the antioxidants (5–20 μg). Recovery varied from 80 to 89%.
Quantification of α-tocopherol and lipoic acid
Lipoic acid and α-tocopherol were assayed by HPLC using a Shimadzu Prominence HPLC system equipped with a pump model LC-20AB, an autosampler model SIL-20A and a photodiodoarray detector model SPD-M20A. Separation was performed on a Prevail C18 column (150 × 4.6 mm, Alltech, Deerfield, IL, USA) using mobile phases composed of acetonitrile : 0.05% phosphoric acid solution (50 : 50 or 35 : 65, v/v) at a flow rate of 0.8 ml/min for lipoic acid, and of acetonitrile (100%) at 1.2 ml/min for α-tocopherol.[24,25] UV detection wavelengths of 220 nm and 285 nm were used for lipoic acid and α-tocopherol, respectively. The specificity of the method was evaluated using untreated (blank) pieces of all skin sections employed. For this, untreated sections were mounted in diffusion cells for 12 h; homogenates of the SC, viable layers (homogenization and tape stripping performed as described in the ‘In-vitro skin penetration’ section) and the receptor phase were assayed to determine whether components of any of the skin sections employed would interfere with the assay method. Standard solutions of the drugs were prepared in methanol or acetonitrile, and linearity was achieved over the range of 0.1–100 μg/ml (r2 > 0.99). The detection and quantification limits of the methods were set at 50 and 100 ng/ml.
Antioxidant potential
The antioxidant potential of skin was evaluated using the thiobarbituric acid-reactive substances assay (TBARS assay).[26] Freshly excised skin samples, treated for 12 h with plain (not containing drug) ME-Wphy or ME-Wphy containing only α-tocopherol, only lipoic acid or both antioxidants, were rinsed with distilled water, homogenized with 2 ml of ethanol, and the homogenates were combined with ferrous sulfate to initiate the lipid peroxidation reaction for 30 min at 37°C with mixing. Following this, 40% thrichloroacetic acid was added, and tubes were centrifuged at 15 000 rpm for 5 min. The supernatant was incubated with thiobarbituric acid solution (1%) at 90°C for 20 min, and samples were analysed by spectrophotometry at 532 nm using a standard curve generated from malondialdehyde (5–500 μm).
Statistical analysis
The results were reported as means ± standard deviation. The results of skin penetration of tocopherol into viable skin layers comparing microemulsions and control were analysed based on chi-squared comparisons of the number of samples containing detectable/non-detectable levels of the antioxidant (GraphPad Prism software). All other results were analysed using one-way ANOVA (followed by the Tukey post-hoc test, GraphPad Prism software). Values were considered significantly different when P < 0.05.
Results
Formulation development and characterization
The phase behaviour of mixtures without phytosphingosine is depicted in Figure 1a, and the behaviour of those containing phytosphingosine at a final concentration of 1% is depicted in Figure 1d. Isotropic, single phase and fluid formulations were assigned to the monophasic grey-shaded region in the diagrams, which corresponded to approximately 55% of the diagrams. The diagrams are similar to those described in one of our previous studies, in which monocaprylin was mixed with isopropyl myristate in the oil phase.[27] Based on the phase behaviour of samples, we chose the formulations ME-O (60 : 30 : 10 surfactant : oil : water, w/w/w), ME-W (46 : 23 : 31 surfactant : oil : water, w/w/w) and ME-Wphy (45.3 : 22.7 : 1 : 31 surfactant : oil : phytosphingosine : water, w/w/w/w) for further characterization. Their composition is represented as black dots in the diagrams.
Figure 1.
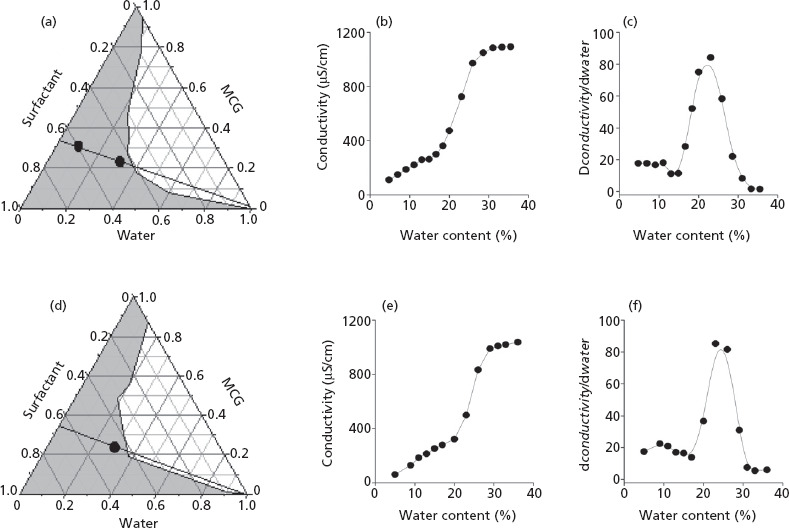
Phase diagrams and conductivity as a function of aqueous content of mixtures composed of decylglucoside : propylene glycol (1:1 w/w), water and mono- and dicaprylin (MCG) (A–C) or decylglucoside : propylene glycol (1 : 1 w/w), water MCG and phytosphingosine (D–F). The phase diagrams (A and D) show the dilution lines investigated in the conductivity studies (black lines in the diagrams), and the composition of microemulsions ME-O, ME-W and ME-Wphy. Panels C and F show the conductivity change (dconductivity/dwater) as a function of water content to determine the percolation threshold.
Figures 1b and 1e show the influence of water content on the electrical conductivity of systems along the dilution lines (black lines) depicted in panels A and D. In the percolation model, it is accepted that a change in conductivity at a given aqueous volume fraction is consistent with phase transformation from reverse (w/o) to normal-type systems (o/w) through the emergence of bicontinuous structures.[10] The conductivity of samples without phytosphingosine increased slowly up to 17% of water, which is consistent with the existence of w/o systems. Between 17 and 26% water, a sharp rise in values was observed, suggesting an increase in aqueous droplets' interlinking process and the emergence of bicontinuous structures.[10] Above 28%, conductivity values reached a plateau until the system lost stability and became turbid. Similar results were obtained for phytosphingosine-containing formulations, indicating that at 1% the lipid does not affect the organization of the system. The percolation threshold was further studied from the plot of dconductivity/dwater as a function of the water content.[28] Both plots (Figures 1c and 1f) exhibited maxima at 26–27% water, confirming the percolative behaviour of the systems. Above 30%, dconductivity/dwater dropped, displaying a trend towards more constant values and suggesting transformation into water-continuous systems.[28] Based on these results, the structure of ME-O (which contains 10% water and is located below the maximum) is consistent with w/o, while the structures of ME-W with and without phytosphingosine (31% of water) are more consistent with o/w.
The pHs of ME-W and ME-Wphy were 8.7 and 9.5, respectively. These values fall within a similar range compared to other polyglucoside-based formulations containing monoglycerides.[29] The pH of ME-O was not determined since it contained only 10% water. All microemulsions displayed slightly negative zeta potential values at their original pH, internal phase diameters ranging from 43 to 51 nm and polydispersity indices up to 0.21 (Table 1), a value which is generally associated with narrow size distribution.[12] The pHs of ME-W and ME-Wphy were adjusted to 5.0 for further characterization and evaluation. This was performed for three reasons: (i) as a free base with a pK ∼ 9, phytosphingosine is expected to be ionized at physiological pH and below, conferring a positive charge to ME-Wphy at pH = 5.0, (ii) the skin surface pH ranges between 5 and 5.9, and formulations displaying pH closer to this range are generally considered safe, and (iii) since lipoic acid is a weak acid with a pKa of 4.7, reducing the formulation pH from over 8.0 to 5.0 should increase the unionized/ionized drug ratio, increasing delivery as penetration of unionized species is often favoured.[30,31] This pH adjustment had no effect on the internal phase diameter or on ME-W zeta potential, but increased the zeta potential of ME-Wphy from −4.3 to +29.1 mV, providing it with a positive charge. These results agree with previous reports demonstrating an increase in zeta potential as the pH of formulations containing phytosphingosine decreased.[12,13] Addition of the drugs did not largely affect the size of ME-W and ME-Wphy, but reduced the zeta potential of ME-Wphy (although still keeping it positive). ME-W and ME-Wphy with pH adjusted to 5.0 were used in skin penetration and antioxidant activity studies.
Table 1.
Characteristics of selected microemulsions
| Formulation | Original | pH = 5 | With druga | |||
|---|---|---|---|---|---|---|
| Size(nm)/P.I. | Zeta potential (mV) | Size(nm)/P.I. | Zeta potential (mV) | Size(nm)/P.I. | Zeta potential (mV) | |
| ME-O | 50.3/0.21 | −1.1 | — | — | 53.5/0.22 | −1.9 |
| ME-W | 43.4/0.20 | −2.5 | 42.0/0.18 | −2.7 | 47.8/0.22 | −2.5 |
| ME-Wphy | 50.7/0.18 | −4.3 | 53.2/0.21 | +29.1 | 54.8/0.18 | +20.1 |
P.I., polidispersity index. aSize and zeta potential for ME-O containing drug were determined at the original pH, whereas these parameters for ME-W and ME-Wphy were determined after pH adjustment to 5.
The short-term stability of ME-Wphy was assessed because it delivered the largest amounts of tocopherol into the skin (see results in ‘In-vitro skin penetration assays’ section). Formulation characteristics remained fairly unchanged (Table 2), while lipoic acid and α-tocopherol content varied within 5.5–6.5% during this time. Considering that the stability of vitamins in conventional formulations is often considered poor,[32] and that no formulation stabilizer was included in ME-Wphy, our results indicate that ME-Wphy is able to incorporate the antioxidants without compromising formulation characteristics and drug content after short-term storage.
Table 2.
Stability of ME-Wphy containing α-tocopherol and lipoic acid
| Time (days) | Size (nm)/P.I. | Zeta potential (mV) | Microscopic characteristics | Drug remaining (%) | |
|---|---|---|---|---|---|
| Lipoic acid | Tocopherol | ||||
| 0 | 54.8/0.18 | +20.1 | Isotropic | 97.4 ± 0.8 | 97.9 ± 1.2 |
| 1 | 52.9/0.17 | +18.9 | Isotropic | 96.4 ± 1.9 | 95.6 ± 1.0 |
| 7 | 56.7/0.20 | +22.6 | Isotropic | 91.0 ± 4.5 | 92.4 ± 2.9 |
In-vitro skin penetration assays
The skin penetration and transdermal delivery of lipoic acid and α-tocopherol are depicted in Figure 2. Compared to the control formulation, lipoic acid delivery into and across the skin was not significantly (P > 0.05) increased by the microemulsions, even though there was a trend towards increased penetration into viable layers by ME-W.
Figure 2.
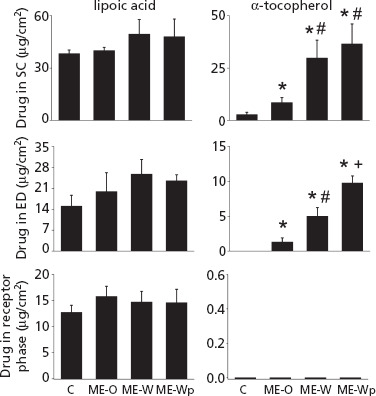
Penetration of α-tocopherol and lipoic acid in the stratum corneum (SC), viable layers of the skin (ED) and permeation in the receptor phase after 12 h, comparing the microemulsions and the control solution in propylene glycol. *P < 0.01 compared to control, #P < 0.05 compared to ME-O, +P < 0.05 compared to ME-O and ME-W. Each point represents mean ± standard deviation of five to six replicates.
Compared to lipoic acid, the amounts of α-tocopherol delivered into the whole skin (SC + ED) were much smaller when the control formulation was used, and increased significantly with the microemulsions. Accordingly, α-tocopherol delivery into the SC by the microemulsions was 3–13-fold higher (P < 0.01) compared to the control formulation. Delivery into viable layers increased from undetected to 1.3 ± 0.6, 5.0 ± 1.2 and 9.8 ± 0.9 μg/cm2 with ME-O, ME-W and ME-Wphy, respectively (P < 0.01). The composition of the formulation played an important role in α-tocopherol delivery: increasing water content (with a consequent internal structure change to o/w) to obtain ME-W increased delivery into the SC and ED by 3.5- and 3.7-fold, respectively, compared to ME-O. This is consistent with previous reports that water-continuous formulations increase the skin penetration of other lipophilic compounds.[23,33] Addition of phytosphingosine and generation of the positive charge (ME-Wphy) did not affect α-tocopherol delivery into the SC, but promoted a 2-fold increase in its penetration into viable skin layers compared to ME-W. This is also consistent with previous observations that cationic formulations containing phytosphingosine are effective in increasing the skin penetration of lipophilic compounds (by 1.3–1.5-fold), such as prednicarbate and flumethasone pivalate.[13,34] No α-tocopherol was detected in the receptor phase, suggesting that the amounts of drug delivered transdermally may be smaller than 150 ng/cm2 (since the method detection limit was 50 ng/ml).
Microemulsion effect on the barrier function of the skin
Compared to control, all microemulsions decreased skin electrical resistance and increased transepidermal water loss (Figure 3). Microemulsion-induced changes in skin electrical resistance and in transepidermal water loss were 2.7–3-fold and 2.3–2.7-fold larger, respectively, than control-induced changes, demonstrating that results from both methods are in agreement, and that the microemulsions are able to disrupt the barrier function of the tissue, which is in accordance with other studies using MCG.[9] No significant difference (P > 0.05) between ME-O and ME-W was observed, even though ME-O contained 7% more MCG. This suggests that larger differences in the penetration enhancer concentration might be necessary to promote a measureable reduction in the barrier function of the skin. Moreover, no significant (P > 0.05) difference between ME-W and ME-Wphy was observed, suggesting that addition of phytosphingosine in ME-Wphy did not affect the barrier-disrupting effect of the formulation despite the fact that it is involved in ceramide production and in maintaining the barrier property of the skin.[16] Larger concentrations of phytosphingosine or longer treatment periods may reduce the microemulsion-induced barrier disruption as it was previously reported that reversal of sodium lauryl sulfate-induced increases in transepidermal water loss occurred only after 72 h of treatment with phytosphingosine.[35] Taken together, these results suggest that changes in the composition, structure and charge of the microemulsions studied here have no significant effect on their ability to modulate the barrier function of the skin.
Figure 3.
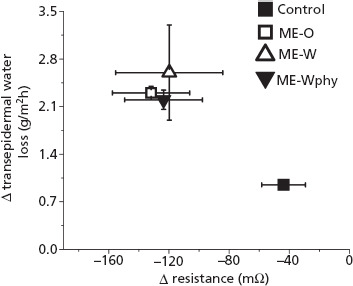
Effect of treatment with propylene glycol (control), ME-O, ME-W and ME-Wphy on skin electrical resistance and transepidermal water loss. The changes in resistance and transepidermal water loss were calculated based on initial values. Each point represents means ± standard deviation of four replicates. All microemulsions induced significantly (P < 0.05) larger changes in electrical resistance and transepidermal water loss compared to control.
In-vitro drug release
Having demonstrated that differences in α-tocopherol and lipoic acid cutaneous delivery among the microemulsions cannot be attributed to variations in formulations' ability to disrupt the skin barrier (as evidenced by similar decreases in electrical resistance of the skin), we next investigated whether differences in drug release could parallel some of the differences observed in penetration. The amounts of lipoic acid released from the microemulsions (Figure 4) were similar (P > 0.05), ranging from 27.0 ± 4.3 to 34.1 ± 4.2% at 8 h, and resulting in similar release rates (3.63 to 4.45%/h, Table 3). The cumulative amount of α-tocopherol released from ME-W and ME-Wphy was also similar at all time-points, but significantly higher (P < 0.05) compared to ME-O after 6 h (∼2-fold, Figure 4). Consequently, ME-O provided a significantly smaller (∼1.8, P < 0.05, Table 3) release rate than ME-W. Linear relationships between cumulative release versus time with coefficient of determination superior to 0.98 were obtained for all microemulsions studied and both drugs, suggesting that release of lipoic acid and α-tocopherol during the studied period follows zero-order kinetics, and that, even though cumulative drug release may differ, the release kinetics are not affected by the microemulsion composition.
Figure 4.
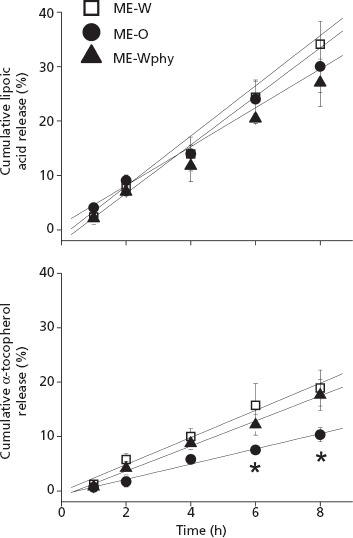
In-vitro drug release of α-tocopherol and lipoic acid from the microemulsions. *P < 0.05 compared to ME-W.
Table 3.
Release rates of lipoic acid and α-tocopherol from formulations
| Formulation | Release rates (%/h) | |
|---|---|---|
| Lipoic acid | α-tocopherol | |
| ME-O | 3.70 ± 0.63 | 1.38 ± 0.28 |
| ME-W | 4.45 ± 0.63 | 2.48 ± 0.52 |
| ME-Wphy | 3.63 ± 0.63 | 2.30 ± 0.56 |
Antioxidant potential
Having demonstrated that ME-Wphy promotes the highest delivery of α-tocopherol into viable skin layers, we next assessed the levels of TBARS in skin samples treated with plain ME-Wphy (not containing drug, control) and ME-Wphy containing only α-tocopherol, only lipoic acid or both antioxidants. By measuring TBARS levels, we have an indirect estimate of reactive oxygen species generated in the tissue.[26] Our goal was to assess whether the antioxidants delivered were active in the skin, and whether they acted synergistically to decrease oxygen species generation in the tissue.
After treatment with the plain, unloaded ME-Wphy, 0.082 ± 0.002 μmol/cm2 of TBARS was detected in skin tissues (Figure 5). This is approximately 1.6 times more than levels found in blank skin not subjected to lipid peroxidation or other oxidative insults,[36] indicating that treatment with the plain microemulsion cannot inhibit peroxidation induced by tissue incubation with ferrous sulfate. Skin treatment with the microemulsions containing only α-tocopherol or only lipoic acid decreased TBARS levels by 31.9 ± 4.9 and 19.2 ± 4.9%, respectively. These results support previous observations that treatment with α-tocopherol is more effective in preventing oxidation in the skin,[37] especially if we consider that the amount of α-tocopherol delivered in the whole skin (SC + ED) by ME-Wphy is smaller than that of lipoic acid. Treatment with both antioxidants further reduced TBARS levels in the skin by 56.3 ± 7.5% (P < 0.001), which is stronger than what would be expected for an additive effect and suggests synergism between the antioxidants.
Figure 5.
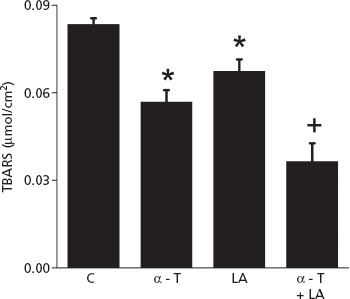
Levels of TBARS in skin treated with the plain ME-Wphy (control), or with ME-Wphy containing only α-tocopherol (α-T), only lipoic acid (LA) or both antioxidants. *P < 0.05 compared to control, +P < 0.01 compared to control.
Discussion
In this study, decylglucoside-based microemulsions were used to promote the co-localization of lipoic acid and α-tocopherol into viable skin layers. Adjusting the pH of formulations to 5.0 affected formulation characteristics only when phytosphingosine was included, with a positive charge being displayed. The characteristics of ME-Wphy remained fairly unchanged during short-term storage, indicating the ability of this formulation to incorporate the antioxidants without compromising their integrity or formulation characteristics. Nevertheless long-term stability studies will be necessary to assess the impact of time on formulation characteristics.
Given its characteristics, lipoic acid skin penetration seems to be less problematic than that of tocopherol, and more of the former was found in viable skin in the absence of microemulsions (even considering that lipoic acid may be partly ionized, as its pKa is 4.7 and the pH of the skin and the formulations is between 5 and 5.9).[31] A trend towards a smaller release and penetration of lipoic acid into viable skin from ME-Wphy compared to ME-W was observed, but no significant difference was detected, suggesting that the presence of the positively-charged phytosphingosine in ME-Wphy did not cause a significant entrapment of the ionized lipoic acid in the formulation. Although the delivery of lipoic acid was not affected by the microemulsions, tocopherol penetration was largely improved. In the absence of the microemulsions, the delivery of tocopherol into viable skin layers was negligible (below the limit of quantification of the method), but its incorporation in the microemulsions allowed tocopherol to reach the viable skin and promoted its co-localization with lipoic acid, which is crucial for the desired synergistic activity.
The penetration-enhancing effect of microemulsions may result from an increased drug solubilization capacity, small droplet size associated with a high surface area (which improves interactions and drug transfer into the skin), ability to increase drug release compared to other formulations and ability to modulate the SC organization and decrease its barrier function.[11,33] When comparing various microemulsions, distinct abilities to modulate the skin barrier function and the drug-release rate may have a significant impact on drug penetration in the skin.[9,33] The increase in α-tocopherol penetration by ME-W and ME-Wphy compared to ME-O cannot be attributed to stronger barrier disruption, as evidenced by similar changes in electrical resistance, but may have resulted from the higher release rate provided by ME-W and ME-Wphy. Given the lipophilicity of α-tocopherol (logP = 7.8) and the higher content of oil in ME-O, it is reasonable to expect a more pronounced retention of α-tocopherol in this formulation, resulting in the lower release rate observed and less pronounced skin penetration. These results support previous studies, which indicated that increases in the content of oil promoted retention of lipophilic drugs within formulations, while increases in water provided higher release and penetration of drugs with log P >3.[15,33,38] On the other hand, compared to ME-W, ME-Wphy provided neither stronger barrier disruption nor higher release rate, and therefore its superiority in delivering tocopherol into viable skin layers cannot be attributed to any of these effects. Previous studies have attributed similar results to an increased interaction between, and adsorption of, the positively charged internal phase and the negatively charged corneocytes of the SC.[13,34]
The amounts of tocopherol and lipoic acid delivered into viable skin layers by ME-Wphy were approximately 9.8 and 23.5 μg/cm2, respectively (see Figure 2). Converting these amounts into micromolar concentrations, approximately 11.3 and 57.1 μm of α-tocopherol and lipoic acid were found in viable skin layers, which is approximately equivalent to a 1 : 5 tocopherol : lipoic acid ratio. Assuming that a 1 : 1 ratio is necessary for synergism, our results suggest that even though lipoic acid penetration is not increased by the microemulsions, there is still an excess of this antioxidant compared to tocopherol in viable skin layers, which should be sufficient for recycling the increased amount of α-tocopherol that penetrates in the presence of the microemulsions. Combinations of lipoic acid and vitamin E have been suggested as reducing oxidative damage in brain and cardiac ischemia, and preventing oxidation of low-density lipoproteins,[39,40] and this study now suggests a potential benefit of their combination for the skin.
Conclusions
Decylglucoside-based microemulsions with internal phase diameter below 60 nm were developed as cutaneous delivery systems for α-tocopherol and lipoic acid. Addition of phytosphingosine had little or no effect on size or type of internal structure (o/w versus w/o), but generated cationic microemulsions with suitable short-term stability. The composition and structure of the microemulsion affected the cutaneous delivery of α-tocopherol but not of lipoic acid. More specifically, increases in the content of water and addition of phytosphingosine to obtain ME-Wphy increased α-tocopherol penetration in the skin (SC + ED) 17-fold compared to a drug control solution. Even though lipoic acid delivery was not increased, treatment with ME-Wphy containing both antioxidants promoted a further decrease in TBARS cutaneous levels compared to treatment with each individual antioxidant, suggesting that their combination might provide a potential benefit in terms of protection against damage associated with the generation of reactive oxygen species.
Declarations
Conflict of interest
The Author(s) declare(s) that they have no conflicts of interest to disclose.
Funding
This work was supported by awards from NIH (1R15AR060008-01A1 to Hass), PhRMA foundation (to Lopes) and Albany College of Pharmacy and Health Sciences (to Lopes).
References
- Poljsak B, Dahmane R. Free radicals and extrinsic skin aging. Dermatol Res Pract; 135206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozman B et al. Simultaneous absorption of vitamins C and E from topical microemulsions using reconstructed human epidermis as a skin model. Eur J Pharm Biopharm 2009; 72: 69–75. [DOI] [PubMed] [Google Scholar]
- Thiele JJ et al. Depletion of human stratum corneum vitamin E: an early and sensitive in vivo marker of UV induced photo-oxidation. J Invest Dermatol 1998; 110: 756–761. [DOI] [PubMed] [Google Scholar]
- Lexis LA et al. Alpha-tocopherol and alpha-lipoic acid enhance the erythrocyte antioxidant defence in cyclosporine A-treated rats. Basic Clin Pharmacol Toxicol 2006; 98: 68–73. [DOI] [PubMed] [Google Scholar]
- Goraca A et al. Lipoic acid – biological activity and therapeutic potential. Pharmacol Rep 2012; 63: 849–858. [DOI] [PubMed] [Google Scholar]
- Melagraki G et al. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. Eur J Med Chem 2009; 44: 3020–3026. [DOI] [PubMed] [Google Scholar]
- Mukai K et al. Structure-activity relationship of the free-radical-scavenging reaction by vitamin E (alpha-, beta-, gamma-, delta-tocopherols) and ubiquinol-10: pH dependence of the reaction rates. J Phys Chem B 2007; 111: 652–662. [DOI] [PubMed] [Google Scholar]
- Krishnan CV, Garnett M. Electrochemical behavior of the super antioxidant, α-lipoic acid. Int J Electrochem Sci 2011; 6: 3607–3630. [Google Scholar]
- Lopes LB et al. Topical delivery of lycopene using microemulsions: enhanced skin penetration and tissue antioxidant activity. J Pharm Sci 2010; 99: 1346–1357. [DOI] [PubMed] [Google Scholar]
- Hathout RM et al. Microemulsion formulations for the transdermal delivery of testosterone. Eur J Pharm Sci 2010; 40: 188–196. [DOI] [PubMed] [Google Scholar]
- El Maghraby GM. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: effects of cosurfactants. Int J Pharm 2008; 355: 285–292. [DOI] [PubMed] [Google Scholar]
- Yilmaz E, Borchert HH. Design of a phytosphingosine-containing, positively-charged nanoemulsion as a colloidal carrier system for dermal application of ceramides. Eur J Pharm Biopharm 2005; 60: 91–98. [DOI] [PubMed] [Google Scholar]
- Hoeller S et al. Lecithin based nanoemulsions: a comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int J Pharm 2009; 370: 181–186. [DOI] [PubMed] [Google Scholar]
- Savic S et al. Natural surfactant-based topical vehicles for two model drugs: influence of different lipophilic excipients on in vitro/in vivo skin performance. Int J Pharm 2009; 381: 220–230. [DOI] [PubMed] [Google Scholar]
- Lopes LB et al. Enhancement of transdermal delivery of progesterone using medium-chain mono and diglycerides as skin penetration enhancers. Pharm Dev Technol 2009; 14: 524–529. [DOI] [PubMed] [Google Scholar]
- Wartewig S, Neubert RH. Properties of ceramides and their impact on the stratum corneum structure: a review. Part 1: ceramides. Skin Pharmacol Physiol 2007; 20: 220–229. [DOI] [PubMed] [Google Scholar]
- Nielsen PB et al. The effect of alpha-tocopherol on the in vitro solubilisation of lipophilic drugs. Int J Pharm 2001; 222: 217–224. [DOI] [PubMed] [Google Scholar]
- Takahashi H et al. The aqueous solubility and thermal stability of alpha-lipoic acid are enhanced by cyclodextrin. Biosci Biotechnol Biochem 2011; 75: 633–637. [DOI] [PubMed] [Google Scholar]
- Panchagnula R et al. Feasibility studies of dermal delivery of paclitaxel with binary combinations of ethanol and isopropyl myristate: role of solubility, partitioning and lipid bilayer perturbation. Farmaco 2005; 60: 894–899. [DOI] [PubMed] [Google Scholar]
- Rachakonda VK et al. Screening of chemical penetration enhancers for transdermal drug delivery using electrical resistance of skin. Pharm Res 2008; 25: 2697–2704. [DOI] [PubMed] [Google Scholar]
- Herwadkar A et al. Low frequency sonophoresis mediated transdermal and intradermal delivery of ketoprofen. Int J Pharm 2012; 423: 289–296. [DOI] [PubMed] [Google Scholar]
- Pollack GH. Cells, Gels and the Engines of Life: A New, Unifying Approach to Cell Biology. Seattle: Ebner and Sons, 2001. [Google Scholar]
- Rozman B et al. Temperature-sensitive microemulsion gel: an effective topical delivery system for simultaneous delivery of vitamins C and E. AAPS PharmSciTech 2009; 10: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert S et al. Transdermal delivery of two antioxidants from different cosmetic formulations. Int J Cosmet Sci 2003; 25: 5–13. [DOI] [PubMed] [Google Scholar]
- Rangarajan M, Zatz JL. Effect of formulation on the delivery and metabolism of alpha-tocopheryl acetate. J Cosmet Sci 2001; 52: 225–236. [PubMed] [Google Scholar]
- Crovara Pescia A et al. On the assessment of photostability of sunscreens exposed to UVA irradiation: from glass plates to pig/human skin, which is best? Int J Pharm 2012; 427: 217–223. [DOI] [PubMed] [Google Scholar]
- Pepe D et al. Decylglucoside-based microemulsions for cutaneous localization of lycopene and ascorbic acid. Int J Pharm 2012; 434: 420–428. [DOI] [PubMed] [Google Scholar]
- Podlogar F et al. Structural characterisation of water-Tween 40/Imwitor 308-isopropyl myristate microemulsions using different experimental methods. Int J Pharm 2004; 276: 115–128. [DOI] [PubMed] [Google Scholar]
- Savic S et al. Topical vehicles based on natural surfactant/fatty alcohols mixed emulsifier: the influence of two polyols on the colloidal structure and in vitro/in vivo skin performance. J Pharm Sci 2009; 98: 2073–2090. [DOI] [PubMed] [Google Scholar]
- Baspinar Y et al. Development of a positively charged prednicarbate nanoemulsion. Int J Pharm 2010; 383: 201–208. [DOI] [PubMed] [Google Scholar]
- Schmid-Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol 2006; 19: 296–302. [DOI] [PubMed] [Google Scholar]
- Ostacolo C et al. Alpha-tocopherol pro-vitamins: synthesis, hydrolysis and accumulation in rabbit ear skin. J Control Release 2004; 99: 403–413. [DOI] [PubMed] [Google Scholar]
- Zhang J, Michniak-Kohn B. Investigation of microemulsion microstructures and their relationship to transdermal permeation of model drugs: ketoprofen, lidocaine, and caffeine. Int J Pharm 2011; 421: 34–44. [DOI] [PubMed] [Google Scholar]
- Baspinar Y, Borchert HH. Penetration and release studies of positively and negatively charged nanoemulsions-Is there a benefit of the positive charge? Int J Pharm 2012; 430: 247–252. [DOI] [PubMed] [Google Scholar]
- Ideta R et al. Orally administered glucosylceramide improves the skin barrier function by upregulating genes associated with the tight junction and cornified envelope formation. Biosci Biotechnol Biochem 2011; 75: 1516–1523. [DOI] [PubMed] [Google Scholar]
- Urikura I et al. Protective effect of fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci Biotechnol Biochem 2011; 75: 757–760. [DOI] [PubMed] [Google Scholar]
- Tsai FJ et al. Evaluation of the antioxidative capability of commonly used antioxidants in dermocosmetics by in vivo detection of protein carbonylation in human stratum corneum. J Photochem Photobiol B 2012; 112: 7–15. [DOI] [PubMed] [Google Scholar]
- Kantarci G et al. Comparison of different water/oil microemulsions containing diclofenac sodium: preparation, characterization, release rate, and skin irritation studies. AAPS PharmSciTech 2007; 8: E91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE et al. Recycling of vitamin E in human low density lipoproteins. J Lipid Res 1992; 33: 385–397. [PubMed] [Google Scholar]
- Stoyanovsky DA et al. Endogenous ascorbate regenerates vitamin E in the retina directly and in combination with exogenous dihydrolipoic acid. Curr Eye Res 1995; 14: 181–189. [DOI] [PubMed] [Google Scholar]


