ABSTRACT
Virulence has been proposed to be an emergent property, which by definition implies that it is not reducible to its components, but this application of a philosophical concept to the host-microbe interaction has not been experimentally tested. The goals of our study were to analyze the correlation of the phenotype with the ability to cause disease and to determine the dynamics of an experimental cryptococcal infection in Galleria mellonella and Acanthamoeba castellanii. By studying the outcome of infection as host death, we showed that the dynamics of virulence in the G. mellonella/Cryptococcus neoformans interaction follow a predictable pattern. We also found that the experimental temperature and not the presence of virulence factors was a critical parameter defining the pathogenic potential of cryptococcal species. Our results established that cryptococcal species not considered pathogenic could be pathogens given suitable conditions. Our results support the idea that virulence is an emergent property that cannot be easily predicted by a reductionist approach and yet it behaves as a deterministic system in a lepidopteran cryptococcal infection. These findings provide a road map for evaluating whether host-microbe interactions in other systems are chaotic, deterministic, or stochastic, including those with public health importance.
IMPORTANCE
Virulence is a complex phenotype that cannot be easily studied by analyzing its individual components in isolation. By studying the outcome of infection as the death of the host, we found that a given microbial phenotype does not necessarily correlate with its ability to cause disease and that the presence of so-called virulence factors does not predict pathogenicity, consistent with the notion that virulence is an emergent property. This paper reports that the dynamics of virulence in Galleria mellonella larvae infected with the fungus Cryptococcus neoformans follows a predictable pattern. Establishing that virulence is an emergent property is important because it implies that it is not reducible to its components, and consequently, this phenomenon needs to be studied by a holistic approach.
Introduction
Virulence is one outcome of the interaction of a microbe and a host and is a complex phenotype that depends on factors displayed both by the microbe and the host. Virulence is a dependent variable that is “contingent on the availability of a susceptible host and the context and nature of the host-microbe interaction” (54). In this regard, virulent organisms can be defined as such only in the presence of susceptible hosts, and the phenomenon of virulence has been proposed to be an emergent property (1, 2). Emergent properties are properties of a system that cannot be fully explained by the addition of the individual system components (3).
In evolutionary terms, virulence has been mostly regarded as the outcome of the coevolution of a pathogen and its host, and such has been the acquisition of virulence factors (4); however, in accidental pathogens acquired from the environment, the majority of encounters with a host are dead-end events. The presence of virulence traits and the ability to survive within the host might be seen as the side products of the survival adaptations to their natural environment (5). Plasticity and nonspecificity with regard to susceptible hosts are common features of human environmental fungal pathogens such that most can infect phylogenetically distant hosts.
Among the accidental pathogens are the pathogenic cryptococci (PC), which are defined as such because they do not have a known need for a host and are environmentally acquired (6). The PC comprise the Cryptococcus neoformans-Cryptococcus grubii group and their sister species Cryptococcus gattii (7, 8). The closest relatives to the PC are Cryptococcus amylolentus-Tsuchiyaea wingfieldii and Filobasidiella depauperata (9, 10). Outside the PC group, the other members of the Filobasidiella clade are saprobes mostly associated with insect frass. Even without the need for a mammalian host to complete their life cycle, the PC are responsible for a considerable burden of mortality and morbidity in humans, with an estimated 1 million cases of cryptococcal meningitis/year, leading to about 600,000 deaths (11). The PC have a battery of well-described virulence factors, including heat tolerance, the presence of a polysaccharide capsule, melanin production via a phenoloxidase enzyme (laccase), and extracellular secretion of urease, type B phospholipase (12–15), etc. However, the known virulence factors other than heat tolerance contribute less than half of the total virulent phenotype of the PC (16), and other attributes might be important in host colonization and disease progression.
In this study, we investigated the dynamics of cryptococcal infection with the goal of determining whether the virulence of an environmental microbe followed a predictable pattern. Unpredictability of microbial virulence may be due to stochastic (nondeterministic) or deterministic chaotic systems. Even though chaos is intrinsically deterministic, it is difficult to make predictions in chaotic systems due to their nonlinear dynamics and the high sensitivity to initial conditions (17–19). We have developed two methods to study virulence. First, we analyzed the pattern of deaths in a time series in a given host-microbe interaction to determine the dynamics of our system. Second, we formulated a quantitative measure of virulence using death of the host as the observable outcome. Our results suggest that virulence in our experimental system is intrinsically deterministic and most of the variations observed are due to random factors present in the system rather than to deterministic chaotic components. We also evaluated the pathogenic potential of traditionally nonpathogenic cryptococcal species to determine whether phenotypic characters shared with the pathogenic cryptococci can be useful predictors of virulence. Our findings indicate that isolated fungal traits do not predict virulence, thus supporting the notion that virulence is an emergent property.
RESULTS
Virulence in three cryptococcal species.
To determine whether the phenotypic characteristics correlated with the pathogenic potential as a measurement of predictability for virulence, we compared two cryptococcal species, Cryptococcus amylolentus and Cryptococcus podzolicus, to the well-described pathogen C. neoformans. We compared the presence of canonical virulence factors like those found in C. neoformans, such as the capsule, extracellular urease and phospholipase activity, and melanin production, with the capacity to cause death in G. mellonella larvae and the protozoan Acanthamoeba castellanii.
C. amylolentus is the closest known relative to the PC (9), whereas C. podzolicus (20) is more distantly related (21) but was reported to be able to melanize and possess a capsule (7, 22).
We observed positive staining by immunofluorescence assays (IFA) on the surface of C. amylolentus cells using the monoclonal antibody (MAb) 18B7 to glucuronoxylomannan (GXM), which is consistent with the presence of a capsule. The polysaccharide in C. amylolentus was in close proximity to the cell body and formed a very thin capsule (Fig. 1A and C). The India ink exclusion assay revealed a clear area surrounding some of the yeasts (Fig. 1B and D), and transmission electron microscopy (TEM) also suggested the presence of a small capsule (see Fig. S3 in the supplemental material). Capsular growth was observed primarily in aging cultures and was not induced by the conditions that favored capsular growth in C. neoformans. The capsule never reached the dimensions of C. neoformans capsules regardless of the conditions tested (Fig. 1A and B). We did not observe a capsule for any strain of C. podzolicus under the experimental conditions used in this study (Fig. 1E to G and H; also, see Fig. S3A in the supplemental material).
FIG 1 .
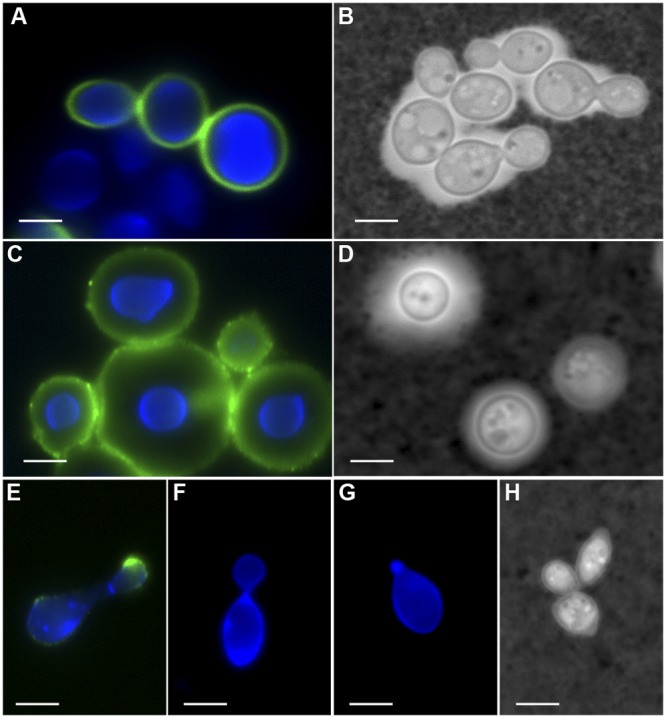
Capsular detection in different cryptococcal species. (A, C, and E to G) IFA for GXM with MAb 18B7 (green); the cell wall is stained with UviTex2B (blue). (B, D, and H) India ink. Cells were grown for 6 days in MM at 25°C. (A and B) C. amylolentus; (C and D) C. neoformans var. grubii; (E to H) C. podzolicus. (A) Some cells showed positive staining for GXM, closely surrounding the cell wall. (B) The India ink exclusion test revealed the presence of a clear halo that is not homogeneous for all cells. (C) Characteristic capsular pattern for GXM stain. (D) Clear halo corresponding to the capsule. (E) Only a small number of cells in strain CBS 6490 stained positive for MAb 18B7, showing a punctate pattern around the cell body and stronger intensity in the budding daughter cell. (F and G) Strains CBS 9357 and CBS 9358 did not show any positive staining for MAb 18B7. (H) C. podzolicus did not exhibit positive India ink exclusion. The deposition of GXM in C. amylolentus and C. podzolicus did not suggest the presence of a capsule similar to that of C. neoformans. Scale bar, 10 µm.
In the presence of phenolic substrates like l-3,4-dihydroxyphenylalanine (l-DOPA) and bird seed agar, C. podzolicus melanized as previously reported (20, 22), although melanization was slower than for C. neoformans var. grubii. There were strain differences in the time of melanization and intensity of the pigmentation (see Fig. S1A and Table S1 in the supplemental material). No melanization was observed for C. amylolentus at any temperature.
Both C. podzolicus and C. amylolentus manifested urease activity similar to that of C. neoformans var. grubii at 25°C and 28°C, only weak activity at 31°C, and no growth at higher temperatures (see Fig. S1B in the supplemental material).
The extracellular phospholipase B of C. neoformans (CNPLB1) contributes to the virulence of this fungus (14). This enzyme has preferential activity for DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine), a major mammalian membrane phospholipid and a component of the lung surfactant, and for Lyso-PC (1-palmitoyl-sn-glycero-phosphocholine) (23), a short-lived derivative of phosphatidylcholine that stimulates neutrophils and is a chemoattractant for macrophages (24–26). To test for phospholipid utilization in the non-heat-tolerant cryptococci, they were plated on media supplemented with either a simple triglyceride, DPPC, or Lyso-PC as the carbon source. All fungi manifested lipase activity as measured by growth on tributyrate (see Fig. S2A in the supplemental material). All fungi grew and consumed Lyso-PC at 25°C and 28°C, but only C. neoformans var. grubii and C. podzolicus strain CBS 9358 grew at 31°C (see Fig. S2B). In addition to C. neoformans, only C. podzolicus strain CBS 9358 grew on DPPC-containing medium and consumed the lipid at 25°C but weakly at 28°C (see Fig. S2C).
C. neoformans and C. amylolentus are pathogenic in amoebae.
The PC can cause disease in distantly related phyla, such as plants, amoebae, and metazoans (27–29). To ascertain whether this pathogenic capacity extended to other non-heat-tolerant cryptococci, we tested the virulence of C. amylolentus and C. podzolicus in two model hosts, the free-living amoeba Acanthamoeba castellanii and the larvae of the lepidopteran Galleria mellonella.
A. castellanii ingested the non-heat-tolerant cryptococcal species more efficiently than C. neoformans. There were more amoebae with ingested fungi (phagocytic percentage [PP]) and more intracellular fungi per amoeba (phagocytic index [PI]) 2 h after coincubation. The greater phagocytic activity against C. amylolentus and C. podzolicus could reflect the absence of a developed capsule, given that the capsule protects against protozoan ingestion (28). However, at 6 h, the PP and the PI diminished and were comparable to those observed with C. neoformans var. grubii (Table 1).
TABLE 1 .
PP and PI for cryptococcia
| Fungal strain | 2 h |
6 h |
||||
|---|---|---|---|---|---|---|
| Mean no. of fungi/cell | PP | PI | Mean no. of fungi/cell | PP | PI | |
| C. neoformans H99 | 0.96 | 16.99 | 16.34 | 1.07 | 32.37 | 34.53 |
| C. amylolentus CBS 6039 | 1.97 | 46.53 | 91.67 | 1.59 | 31.40 | 50.00 |
| C. podzolicus CBS 6490 | 1.85 | 52.15 | 96.32 | 1.57 | 44.77 | 70.35 |
| C. podzolicus CBS 9357 | 1.57 | 33.65 | 52.88 | 1.85 | 38.40 | 71.20 |
A. castellanii ATCC 30234 was incubated 2 and 6 h with the different cryptococci at a 1:1 ratio. The non-heat-tolerant cryptococci have a higher PP (phagocytic percentage), PI (phagocytic index), and mean number of yeast per amoebae than C. neoformans var. grubii (H99).
Next we evaluated the outcome of the interaction of the three cryptococcal species with amoebae after 24 h of coincubation. The amoeba survival was highest in the C. podzolicus CBS 9357 group and lowest for C. neoformans var. grubii (see Table S2 in the supplemental material). Our PP, PI, and amoeba killing results for C. neoformans var. grubii were similar to what was previously reported (28). For the interaction between the non-heat-tolerant cryptococci with A. castellanii, we found that under nutrient-rich conditions, not only was C. amylolentus able to survive in the presence of amoebae, but it also grew better than in rich medium only. However, the outcome of this interaction was very different when the experiment was carried out in starvation. If C. amylolentus was coincubated with A. castellanii in phosphate-buffered saline (PBS), no CFU were recovered after 24 h (Fig. 2). In other words, C. amylolentus was able to survive as well as C. neoformans in the presence of amoebae if there were nutrients in the medium. However, unlike C. neoformans, C. amylolentus was unable to grow or even survive the amoeba interaction under stress conditions and could not efficiently kill the amoebae or use them as a nutrient source.
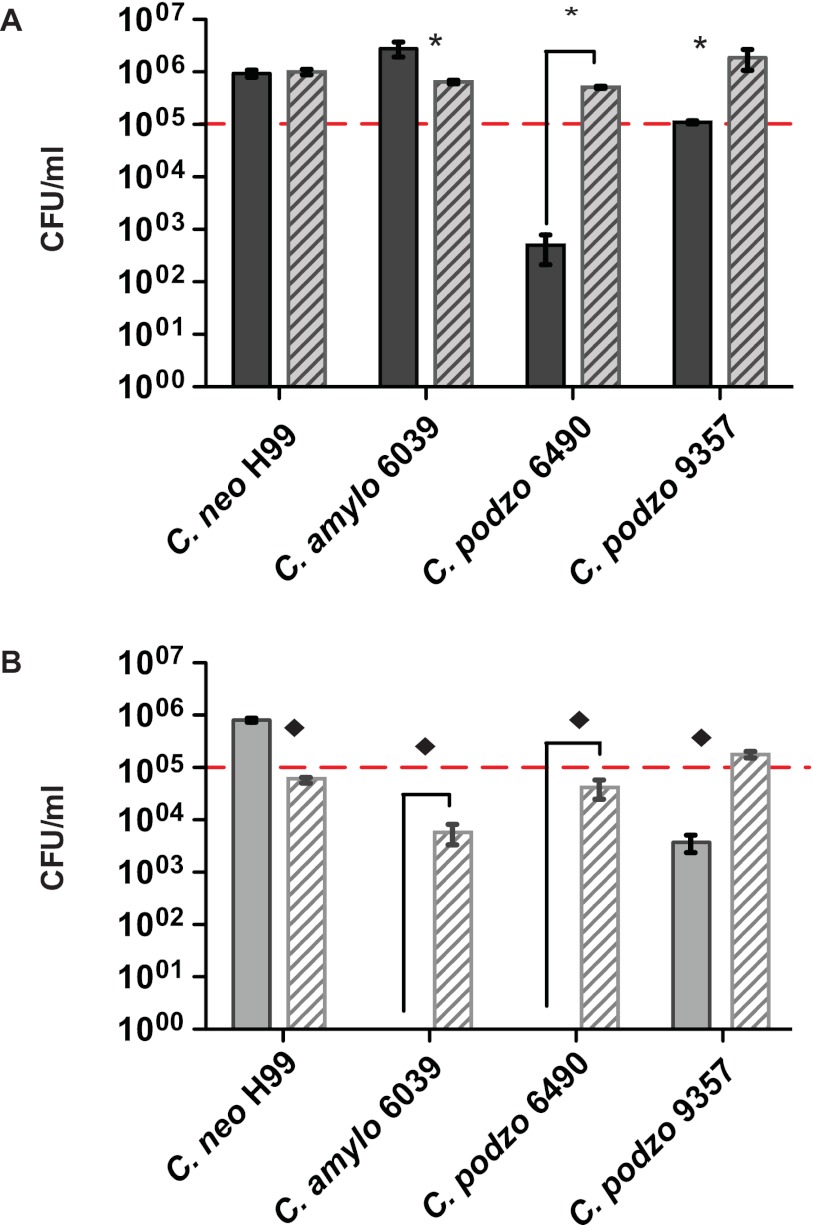
FIG 2 .
Fungal survival after the interaction with A. castellanii. Solid bars correspond to fungi coincubated with amoebae. Striped bars correspond to fungi alone. Dotted lines indicate the initial number of fungal CFUs used to inoculate each well. (A) Fungi and amoebae incubated in rich amoeba medium (PYG). In the presence of amoebae but without other stress conditions in the system, C. neoformans var. grubii (H99) and C. amylolentus (CBS 6039) were able to grow; C. podzolicus, on the other hand, was not able to grow and in the case of strain CBS 6490 was efficiently killed by the amoebae. (B) Fungi and amoebae incubated in PBS. C. neoformans var. grubii was able to grow feeding on amoebae. C. amylolentus was highly susceptible to nutrient deprivation and in the presence of amoebae was completely cleared. C. podzolicus CBS 6490 was cleared by amoebae, whereas strain CBS 9357 diminished but survived. * and ◆, P < 0.05.
In summary, C. amylolentus was readily phagocytized by amoebae, but the drop in the PI and PP after 6 h, which was accompanied with an increase in CFU counting at 24 h, indicated that this fungus was able to survive the interaction either by avoiding intracellular killing or by rapid replication in the presence of amoebae. In contrast, C. podzolicus CBS 6490 was highly susceptible to amoeba killing under both nutrient-rich and under nutrient-depleted conditions, and C. podzolicus strain CBS 9357 persisted in the presence of amoebae, suggesting that this strain had significant resistance to amoeba predation (Fig. 2).
Pathogenicity for G. mellonella larvae.
To ascertain the pathogenic potential of C. amylolentus and C. podzolicus, we used the G. mellonella larva model (30). We studied the virulence of the three different cryptococcal species as a function of inoculum size and temperature. Since G. mellonella is an ectothermic organism, we controlled its body temperature by controlling the ambient experimental temperature and compared the effect of temperature changes in the survival of infected larvae. In this series of experiments, we tested three different inocula and five different temperatures. The lowest was the optimal growing temperature for non-heat-tolerant cryptococci, and the maximum was not permissive for growth of non-heat-tolerant fungi (data not shown).
To measure the relative virulence of the different fungi studied and to better analyze the large amount of data generated, we developed a parameter named “virulence rate as death outcome” (ViRaDO). Our function measures the interaction of a host-microorganism pair resulting in death of the host, extracting the data from the survival proportions in survival curves. To develop the ViRaDO concept, we used the population biology definition of virulence, namely, increased host mortality due to pathogen infection (31). The ViRaDO allowed us to evaluate the pathogenic capability of the different cryptococci against G. mellonella larvae and to compile the data obtained in different G. mellonella survival assays to analyze the outcome of a given interaction under different conditions.
We observed that for C. neoformans var. grubii, the virulence increased as a function of the temperature. For C. neoformans var. grubii, the effect of inoculum size on the outcome of infection was less dramatic than that of temperature (Table 2; Fig. 3A). Faster replication could account for greater virulence of C. neoformans var. grubii at higher temperatures, and this could explain the higher virulence at 32°C than at 25 to 30°C but not at 35°C and above, since replication slows above 32°C. The increased virulence of C. neoformans var. grubii at temperatures higher than the optimal growing temperature could be a consequence of an increased stress response in the fungus, making it more fitted to survive in hostile conditions (32).
TABLE 2 .
Mean virulence rate as death outcome
| Temp (°C) | Mean (SD) ViRaDO at indicated inoculuma |
|||||
|---|---|---|---|---|---|---|
| 5 × 104 | 5 × 105 | 5 × 106 | 5 × 104 | 5 × 105 | 5 × 106 | |
|
C. neoformans H99 |
C. amylolentus CBS 6039 |
|||||
| 25 | 17.65 (4.24) | 39.71 (15.32) | 78.68 (6.02) | 2.33 (1.53) | 30.76 (7.45) | 84.51 (10.04) |
| 28 | 34.81 (3.86) | 40.43 (11.52) | 71.57 (8.09) | 2.47 (2.16) | 10.99 (4.07) | 77.06 (8.38) |
| 30 | 68.28 (6.29) | 73.90 (4.09) | 83.21 (1.39) | 5.51 (4.78) | 6.86 (5.86) | 41.93 (19.29) |
| 32 | 63.11 (7.47) | 70.47 (3.59) | 82.05 (2.05) | 0.20 (0.28) | 14.37 (0.99) | 25.55 (8.06) |
| 35 | 74.62 (5.90) | 82.72 (6.18) | 86.07 (7.23) | 21.25 (10.11) | 28.72 (13.58) | 38.06 (13.76) |
|
C. podzolicus CBS 6490 |
C. podzolicus CBS 9357 |
|||||
| 25 | 5.34 (3.66) | 1.72 (1.49) | 5.64 (2.45) | 1.72 (1.49) | 1.10 (1.33) | 7.72 (2.21) |
| 28 | 3.05 (2.77) | 1.70 (2.94) | 27.75 (7.13) | 1.11 (1.92) | 11.36 (2.73) | 36.95 (4.70) |
| 30 | 10.05 (14.24) | 4.44 (6.39) | 34.30 (19.01) | 16.05 (1.56) | 5.08 (2.06) | 22.79 (12.28) |
| 32 | 6.89 (1.94) | 8.97 (4.47) | 6.41 (1.82) | 15.44 (13.52) | 7.24 (1.92) | 14.72 (2.58) |
| 35 | 35.82 (7.99) | 33.06 (4.61) | 45.97 (4.81) | 38.06 (20.09) | 38.33 (8.55) | 31.81 (7.57) |
|
C. podzolicus CBS 9358 |
NI |
PBS |
||||
| 25 | 0.49 (0.42) | 5.15 (2.94) | 9.80 (11.54) | 6.50 (6.43) | 6.50 (8.14) | |
| 28 | 11.19 (9.76) | 8.11 (5.99) | 33.93 (9.90) | 1.43 (1.43) | 1.60 (2.37) | |
| 30 | 10.05 (7.71) | 6.13 (6.46) | 17.72 (6.98) | 3.98 (1.05) | 6.37 (2.09) | |
| 32 | 6.86 (4.94) | 18.19 (0.24) | 24.92 (5.09) | 3.14 (2.84) | 2.52 (2.20) | |
| 35 | 20.42 (25.32) | 20.69 (2.65) | 19.31 (10.34) | 21.45 (13.56) | 26.94 (12.51) | |
The mean virulence rate as death outcome (ViRaDO) and SD were obtained from three independent experiments for each condition (inoculum and temperature).
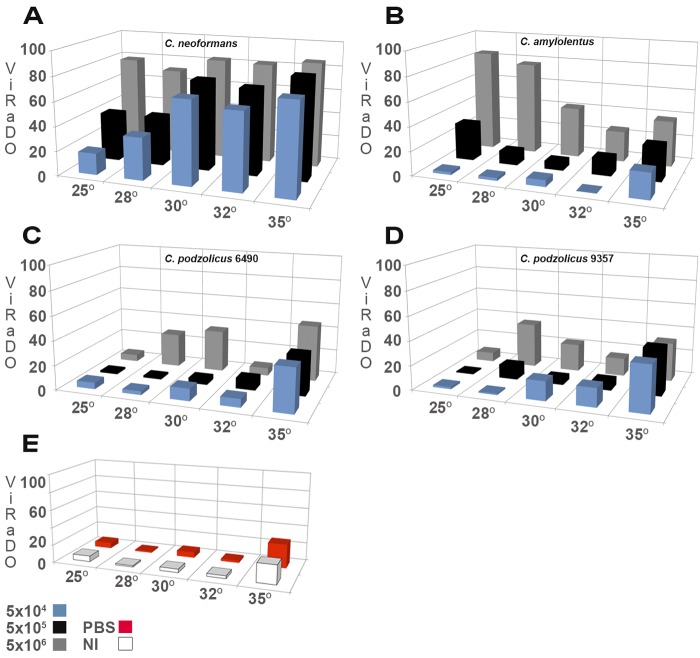
FIG 3 .
Virulence as death outcome. 3D graphic comparison of three inocula and 5 temperatures for the fungi used. (A) The C. neoformans var. grubii curve shows that the ViRaDO increased as a function of inoculum and temperature. (B) C. amylolentus had a bimodal pattern. At 25°C and 28°C, C. amylolentus was highly pathogenic for G. mellonella larvae but only at the highest inoculum, 5 × 106 CFU/larva. At 30°C and 32°C, the virulence decreased for the three different tested inocula. At 35°C there is an increase in larval mortality in the three inoculation groups. (C) C. podzolicus CBS 6490 showed an increased virulence as a function of the temperature up to 30°C, and at 32°C, the fungus virulence decreased, followed by an increase at 35°C. (D) C. podzolicus CBS 9357 manifested its greatest virulence at 28°C. (E) Noninjected (NI) and PBS-injected larva controls.
Virulence in the non-heat-tolerant cryptococci was dependent of the number of inoculated organisms and the experimental temperature. C. amylolentus was the most virulent for G. mellonella larvae, manifesting a bimodal pattern. At 25°C and 28°C, C. amylolentus was highly pathogenic for G. mellonella larvae, but only at the highest inoculum, 5 × 106 CFU/larva (Fig. 3B; Table 2). At 30°C and 32°C, the virulence decreased. At 35°C, there was an increase in larval mortality in the three inoculation groups (Fig. 3B; Table 2). At 25°C, the killing curves of C. neoformans var. grubii and C. amylolentus were not statistically different for 5 × 106 CFU/larva (Fig. 4).
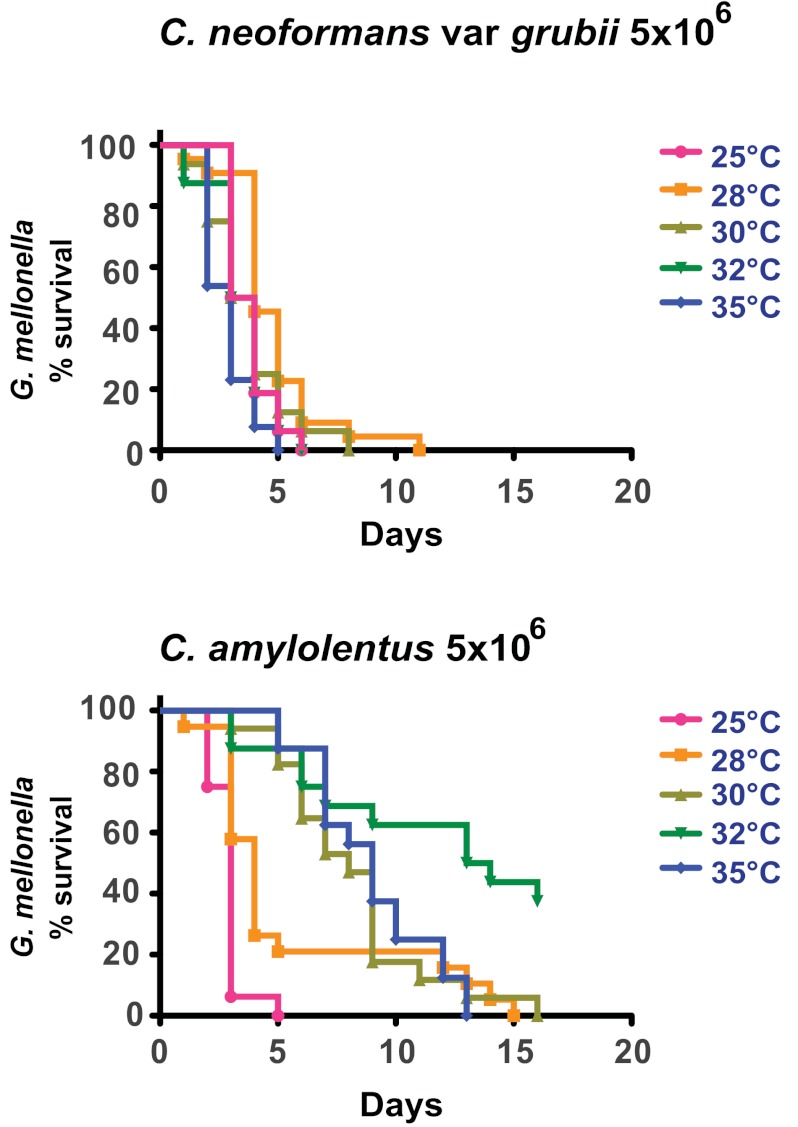
FIG 4 .
G. mellonella survival after infection with cryptococcal spp. Larvae were infected with 5 × 106 CFU of C. neoformans var. grubii (H99) (top) or C. amylolentus (bottom). Survival experiments were performed at a range of temperatures from 25 to 35°C. The killing curve at 25°C showed no statistical difference (P = 0.8507) for larvae infected with C. neoformans var. grubii or C. amylolentus.
In the case of C. podzolicus, we observed that its capability to kill larvae was strain dependent, showing peaks of virulence at 28°C and 35°C (Fig. 3C and D; Table 2). The overall virulence of C. podzolicus was low compared to that of the other cryptococci. At 25°C larval mortality was no higher than in the controls. The slight increase in larval mortality infected with C. podzolicus at 35°C can be partially attributed to a basal increase in mortality in untreated larvae and PBS-injected larvae (Fig. 3E). The results suggested that heat stress contributed to increased host susceptibility for infection. To rule out the possibility that larval deaths were caused by the large amount of yeast inoculated into the hemocoel, we tested the effect of injecting heat-killed yeast on larva survival for all strains and found no evidence of reduced survival even when 5 × 106 yeast cells/larva were given (data not shown).
Virulence displays a deterministic behavior.
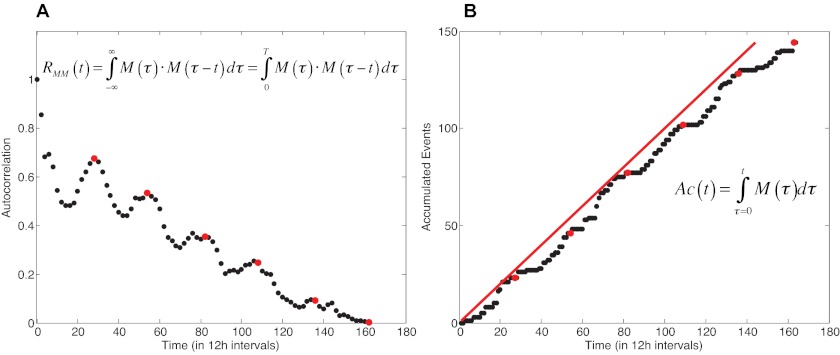
G. mellonella larvae are a well-validated metazoan host model to study C. neoformans infection (30). In the present study, we used this model to analyze whether virulence followed a predictable or a nonpredictable pattern. For this analysis, we infected the larvae as described above and followed the survival of 240 individuals at 12-h intervals, in a time series over ca. 100 concatenated days. To determine the dynamic temporal behavior of our virulence model, we calculated the autocorrelation, RMM(t), and the rate of accumulated events (deaths), Ac(t), of the concatenated time series (18, 33). We found that under the tested conditions, virulence followed a predictable pattern, suggesting that virulence may be a deterministic process explained by the periodic pattern of both the autocorrelation function, RMM(t), and the accumulated events, Ac(t) (Fig. 5). Our analysis showed no indication of chaos.
FIG 5 .
Virulence has a predictable periodicity. (A) The autocorrelation function plot, RMM(t), shows a periodic pattern over time (red dots). (B) The accumulated-events function, Ac(t), also shows a periodic shape (red dots) and runs parallel to the identity equation (y = x; red line), demonstrating its predictability. These results suggest the deterministic and predictable nature of virulence in our host-pathogen model.
DISCUSSION
In this study, we tested the idea of virulence as an emergent property by evaluating the virulence of related species that differed in virulence factors. Consistent with the notion that virulence is an emergent property (1), measures of virulence such as mortality were not predictable, since microbes that do not possess any obvious attributes of virulence were highly virulent in a suitable host, while those that express known virulence factors were avirulent. In contrast to unpredictability of a virulent phenotype, our results showed that virulence in an infected population follows a deterministic pattern. These results showed no evidence of the chaotic behavior found in other biological systems, such as prey-predator interactions (34), and the transmission of human diseases like measles (35), suggesting that the outcome of the host-pathogen interaction in our system was not very sensitive to the initial conditions. Our results implied that most of the variation could be attributed to noise present in the system due to host genetic heterogeneity, small variations in inoculum preparation, and differences in the general health of the larvae but that this variation does not affect the determinism of the system. To our knowledge, this is the first study that specifically attempts to distinguish between noisy periodicity and chaos in the outcome of a host-pathogen interaction, to gain a better understanding of virulence dynamics.
The mechanism by which environmental fungi evolve the necessary traits to become facultative human pathogens is a fundamental question of microbial pathogenesis. The traits involved in mammalian virulence have been proposed to have emerged from complex selective pressures encountered by the PC in their natural habitat (36). Due to the similar interactions between phagocytic soil amoebae and mammalian macrophages, it was proposed that one of the selective forces that have shaped the pathogenic phenotype of these organisms is the predation pressure exerted by soil dwellers such as amoebae or small metazoans like nematodes (28, 29, 36, 37).
This hypothesis has found support in other systems. For example, for the bacterium Escherichia coli O175:H7, the Shiga toxin VF improves its survival in the presence of grazing Tetrahymena (38), demonstrating that a trait that may have been selected for bacterial survival in its natural ecological niche can function as a virulence factor in mammals. Both C. neoformans and C. gattii can also be pathogenic to plants (27), and considering that fungus-plant interactions for the PC clade seem to be ancestral, the adaptation for plant colonization might also have played a role in the acquisition of the virulence composite.
Our results with C. amylolentus suggest that certain members of the Filobasidiella clade already had the potential for metazoan pathogenicity before the split of the PC species complex about 24.5 million years ago (9, 39). In fact, the similarities in pathogenicity between C. amylolentus and C. neoformans at ambient temperatures suggest considerable virulence potential for the former, such that this species may be avirulent for mammals by virtue of its thermal intolerance (40). Consequently, one might infer that the key event in the mammalian pathogenicity of the PC species is its ability for heat tolerance, a trait that is not present in the other members of the Filobasidiella clade. Although it is not clear how heat tolerance has evolved, it has been proposed that extended exposure to high environmental temperature is important for the evolution and maintenance of this capacity in C. neoformans (41). Heat adaptation occurs as a trade-off between good performance of an organism across a range of different temperatures and fitness loss (42). If the ecological niche of the PC involved an environment with sustained high mean yearly temperatures then one can imagine a scenario whereby a gain in heat resistance compared with related species inhabiting cooler niches could have driven the early evolution of the PC clade. Recent studies suggesting that C. neoformans var. grubii originated from southern Africa (43, 44) in tropical/subtropical regions are consistent with this notion.
Our work demonstrated that the experimental temperature was a critical parameter for defining the pathogenicity of two members of the Filobasidiella clade, C. amylolentus and the human pathogen C. neoformans var. grubii. Previously, it was reported that the virulence of C. neoformans in the G. mellonella larva model was increased at 37°C relative to 30°C (30). C. amylolentus was highly pathogenic for G. mellonella larvae despite the absence of clearly identified virulence factors. At its optimal growing temperature, its pathogenicity was comparable to that of C. neoformans. The pathogenic potential of C. amylolentus was manifested in a narrow window between optimal growing conditions versus thermal stress. In a previous study using an intermediate inoculum, it was reported that at 24°C C. amylolentus had intermediate virulence against G. mellonella, but this observation was inconsistent between experiments (9). In addition to the findings obtained with the G. mellonella infection model, challenging C. amylolentus with the soil amoeba A. castellanii showed that this fungus was highly susceptible to nutrient deprivation and stress. Under nutrient-rich conditions, C. amylolentus was able to grow regardless of the presence of the amoebae. However, under nutrient deprivation, it was not possible to recover the fungus, suggesting that the amoebae consumed the ingested fungi. These results indicate that even if C. amylolentus and C. neoformans share similar virulent capacities for the moth system, the former is more susceptible to environmental conditions and to stress, perhaps indicating the different lifestyle choices of the two fungi.
For C. podzolicus the presence of factors associated with virulence shared with C. neoformans, like melanization and phospholipid utilization, did not correlate with virulence. The PC capsule is known to function in avoiding phagocytosis by organisms of distantly related phyla, such as amoebae, invertebrates, and mammals (28, 45). However, capsule-independent mechanisms to avoid phagocytosis have recently been described (46). The presence of other strategies to avoid phagocytosis and the finding that clinical isolates of virulent C. neoformans can be capsule deficient (47) suggest that the capsule is important, even if not absolutely required, for pathogenesis. We found that C. amylolentus bears a small capsule but that this fungus does not respond to the capsule-inducing conditions that are effective for the PC.
In this study, we developed a new parameter to quantify virulence as a function of host death, ViRaDO, which allowed easier comparison of large amounts of data from survival studies. This parameter could be useful in studies where comparison of several survival experiments is necessary. Virulence is a complex phenotype, and for the purposes of our study, we used host death as the quantifiable outcome while also considering time of infection. Given that many systems, from weather to prey-predator interactions, exhibit chaotic behavior, we considered whether the same quality was present for virulence in our studied system. The study of the dynamics of host-microbe interactions poses significant challenges because each host-microbe interaction is unique, and it is difficult if not impossible to determine that a system exhibits chaotic dynamics by designing experiments where initial conditions are systematically varied. Here, we took a simple yet powerful approach to search for chaotic signatures in our experimental host-pathogen system. We found no evidence of chaotic behavior in the virulence outcome of the C. neoformans-G. mellonella interaction. Instead, we found that the system was deterministic and the observed variation can be attributed to random events such as differences in experimental infection and/or genetic heterogeneity of the insect host. The finding that in certain systems virulence has deterministic features that lack chaotic signatures, i.e., that the system is not highly sensitive to small variations in the initial conditions, could help explain humanity’s success in controlling various infectious diseases through vaccines, sanitation, and therapeutic drugs. Whether determinism is a general feature of virulence in host-pathogen interactions is a question whose answer must await the completion of other studies addressing similar questions with other host-microbe systems. The method used here to study the dynamics of virulence should be applicable to other host-microbe interactions.
In conclusion, our results show that species that are not traditionally considered pathogenic can be pathogens given suitable conditions and suitable hosts. Furthermore, our findings are consistent with the notion that human activities, such as climate disruption, in particular global warming (48), and its consequent selection for greater thermal tolerance (49) in conjunction with fungal adaptability can favor the emergence of new fungal pathogens. Our work shows that the presence of so-called “canonical” virulence factors such as a capsule, melanization, urease activity, and phospholipid consumption is important to explain virulence in some hosts but not predictive of pathogenicity of an organism. These results are consistent with the notion that virulence is an emergent property that cannot be easily predicted or fully explained by studying its component parts (1). The results suggest the existence of epistemological limits on our ability to explain and predict the emergence of virulence and new pathogenic microbes.
MATERIALS AND METHODS
Species, strains, and media used in this work.
C. neoformans var. grubii strain H99 was obtained from John Perfect (Durham, NC); C. neoformans var. neoformans Cap67 (acapsular strain) ATCC 52817 was obtained from the American Type Culture Collection (ATCC) (Manassas, VA), C. podzolicus strain CBS 6490 was obtained from K. J. Kwon-Chung (Bethesda, MD); C. amylolentus CBS 6039 and C. podzolicus strains CBS 9357 and CBS 9358 were obtained from the CBS-KNAW Fungal Biodiversity Centre (Utrecht, Netherlands). Fungi were grown on YPD broth or agar (1% yeast extract, 2% peptone, 2% dextrose; Becton Dickinson) at 25°C, 28°C, or 31°C depending on the experiment. A. castellanii strain ATCC 30234 was acquired from the ATCC. The amoebae were cultured at 28°C in peptone-yeast extract-glucose broth (PYG ATCC medium 712) as previously described (50).
Phenotypic analysis of known virulence traits in C. neoformans.
The optimal growth temperature for each of the used strains was determined using a BioScreen analyzer (Growth Curves USA) at 25°C, 28°C, 30°C, 32°C, and 35°C; absorbance was measured every 30 min at 600 nm.
To search for the presence of known cryptococcal virulence traits in C. podzolicus and C. amylolentus we evaluated melanization on L-DOPA minimal medium (MM) and bird seed plates (Becton Dickinson), urease activity on Christensen’s medium (Becton Dickinson); extracellular lipase activity using simple triglycerides (tributyrin) or phospholipids (Lyso-PC [1-palmitoyl-sn-glycero-phosphocholine] [200 µM] and DPPC [1,2-dipalmitoyl-sn-glycero-3-phosphocholine] [800 µM; Sigma-Aldrich]) on spirit blue agar (Becton Dickinson), and capsule production by growing the cells in capsule induction medium (9), MM, or YPD, followed by India ink staining, electron microscopy, and immunofluorescence assays.
Immunofluorescence assays were performed on 4-day-old cells grown at either 25°C or 31°C using the monoclonal antibody (MAb) 18B7, which binds to C. neoformans glucuronoxylomannan (GXM), following published protocols (51, 52).
Electron microscopy.
Samples were fixed with 2.5% glutaraldehyde, 2% paraformaldehyde in 0.1 M sodium cacodylate buffer, postfixed with 1% osmium tetroxide followed by 2% uranyl acetate, dehydrated through a graded series of ethanol, and embedded in LX112 resin (Ladd Research Industries, Burlington, VT). Ultrathin sections were cut on a Reichert Ultracut UCT microtome, stained with uranyl acetate followed by lead citrate, and viewed on a JEOL 1200EX transmission electron microscope at 80 kV.
Acanthamoeba castellanii phagocytosis assay.
A. castellanii cells were plated in 96-well plates at 1 × 104 cells/well and incubated for 24 h at 28°C. After 24 h, inocula were prepared from fresh fungal cultures washed three times in phosphate-buffered saline (PBS), resuspended in PBS, and added to the amoeba culture at a multiplicity of infection (MOI) of 10. Fungal cells were then incubated with A. castellanii at 28°C for various times. Following incubation, the plates were stained with Giemsa, and 100 cells per condition were counted to determine the amount of internalized fungi. Phagocytic index and phagocytic percentage were calculated for each condition. Phagocytic percentage (PP) is the percentage of cells containing fungi; phagocytic index (PI) is the percentage of cells containing at least one yeast multiplied by the mean number of yeasts per positive cell.
Fungal survival assay after A. castellanii-fungus coincubation.
Amoebae and fungi were prepared as described for the phagocytosis assay, with the following differences. Fungi were plated at a MOI of 1. The amoebae and fungi were cocultured in either PYG or PBS for 24 h. After 24 h, the entire contents of each well was passed 6 times through a 27-gauge needle to disrupt the amoebae, and the viability of extracellular and internalized fungi was evaluated by plating serial dilutions in duplicate YPD plates which were incubated at 28°C for 48 h and counting the CFU in each plate. Each experimental group was tested in triplicate.
Amoeba survival.
The percentage of viable A. castellanii cells from the plate used in the fungal survival assay was determined after 24 h interaction with the different fungi at a MOI of 1. Amoeba viability was determined by an exclusion assay using 1:10 trypan blue.
Galleria mellonella survival assays.
To analyze the virulence of the different fungi in G. mellonella, we followed a previously described protocol (30, 53). Briefly, fungal inocula were prepared by growing the different fungi at 25°C, 28°C, or 30°C overnight for C. neoformans H99 or for 48 h for the other species (growing above 28°C); for the 32°C and 35°C experiments, fungi were grown at 30°C. Yeasts were collected by centrifugation, washed twice, and resuspended with PBS. Last-instar G. mellonella larvae (Vanderhorst Wholesale, OH) were injected with a 10-µl Hamilton syringe in one of the prolegs of the fifth abdominal segment with either 5 × 104, 5 × 105, or 5 × 106 CFU/larva or PBS. Each group contained 16 randomly allocated larvae weighing between 200 and 400 mg. Larvae were kept at either 25, 28, 30, 32, or 35°C. Larval survival was monitored and recorded daily. Survival curves were plotted using Prism software to create Kaplan-Meier survival curves (version 5.0d; Prism Computational Sciences, Inc., Madison, WI). For curve comparison, we used the Mantel-Cox test; a P value of ≤0.05 was considered significant. Each experimental condition was tested at least three independent times.
Virulence quantification.
For a quantitative value of the virulence suitable for comparisons between experiments, we developed a normalized method to quantify the data obtained from survival curves. The ViRaDO (virulence rate as death outcome) is measured as the rate of death as the observed outcome. It is a weighted function of the daily death rate of the experiment:
where wi = (D − i + 1) is the weight at day i, defined as the number of days remaining from day i until the end of experiment, D = length of the experiment, and τi = number of deaths on day i/total number of larvae, the daily death rate. The ViRaDO varies between 0 and 1, where 0 corresponds to no deaths during the length of the experiment and 1 corresponds to 100% mortality by day 1. Therefore, the ViRaDO is inversely proportional to the mean survival.
Determination of virulence pattern.
Larvae were infected as described above using 5 × 104 CFU/larva and 40 larvae per group, with 6 repetitions. Larvae were kept at 25°C for 17 days, and mortality was measured every 12 h. To determine the correlation pattern of our virulence model throughout the time series, we performed an autocorrelation analysis on M(t), where M(t) is the concatenated time series of the six survival experiments, defined as death events per period of time (t). Let RMM(t) be the autocorrelation of M(t):
Let Ac(t) be the total accumulated number of deaths at time t:
Both RMM(t) and Ac(t) characterized the behavior of the distribution pattern of the survival series over time. An in-house program in Matlab (MathWorks, Natick, MA) was used to calculate both RMM(t) and Ac(t) functions.
SUPPLEMENTAL MATERIAL
Melanin production and urea consumption. (A) Melanization on bird seed agar. In each plate, the first column contains (from to bottom) C. neoformans var. grubii, C. amylolentus, and C. podzolicus CBS 6490; the second column contains C. podzolicus CBS 9357 and C. podzolicus CBS 9358. By day 3, only C. neoformans var. grubii and C. podzolicus CBS 9357 were melanized, and by day 6, C. podzolicus CBS 6490 and C. podzolicus CBS 9358 were also melanizing. Of all C. podzolicus strains, CBS 6490 produced darker pigmentation on bird seed agar but failed to grow above 28°C. All non-heat-tolerant cryptococci failed to grow at 37°C. (B) Urea utilization on Christensen’s medium at 25°C (indicated by color change from orange to bright pink). All strains were able to use urea; however, their capacity was lower than that of C. neoformans and was delayed. (a and f) C. neoformans var. grubii H99; (b and g) C. amylolentus CBS 6039; (c and h) C. podzolicus CBS 6490;. (d and i) C. podzolicus CBS 9357; (e and j) C. podzolicus CBS 9358. C. podzolicus CBS 6490 and CBS 9357 had higher urea utilization capacity than C. amylolentus or C. podzolicus CBS 9358. Download
Lipid utilization. (A) Simple triglyceride consumption (indicated by color change from blue to white) in different cryptococcal species at different temperatures after 3 days after plating. All tested fungi were able to use butyrate at 25°C and 28°C. At 31°C, C. podzolicus strains started failing to grow and C. amylolentus grew but did not degrade the lipid. Strains include C. neoformans var. grubii H99, C. amylolentus CBS 6039, and C. podzolicus CBS 6490, CBS 9357, and CBS 9358. (B) Lyso-PC consumption at different temperatures after 3 days after plating. At 25°C, all tested fungi were able to grow. As temperature increased, fewer strains could grow on the phospholipid-containing medium. At 31°C, only C. neoformans var. grubii H99 and C. podzolicus CBS 9358 were able to grow. H99, CBS 9357, and CBS 9358 but not CBS 6039 or CBS 6490 seemed to be able to consume Lyso-PC. (C) In medium containing DPPC, only C. neoformans var. grubii H99 was able to grow at all tested temperatures. C. podzolicus CBS 9358 was the only non-heat-tolerant fungus that was able to grow at 25°C and 28°C. Strains include C. neoformans var. grubii H99; C. amylolentus CBS 6039; and C. podzolicus CBS 6490, CBS 9357, and CBS 9358. Download
Ultrastructural characteristics of cryptococcal cells, as determined by transmission electron microscopy. Cells were grown for 6 days in minimal medium at 25°C. (A) C. neoformans H99. The characteristic halo surrounds the cell wall that corresponds to the capsule (arrowhead). (B) The acapsular strain C. neoformans var. neoformans Cap67 was used for comparison. Only the cell wall was visualized, but there was no capsule. (C) Diffuse halo surrounding the cell wall that could correspond to a small capsule (arrowhead). (D) No signs of capsule were observed for any C. podzolicus strain under TEM. Scale bar, 500 nm. Download
Summary of phenotypic traits of the three species used in this study.
Amoeba survival after 24 h interaction with the different fungi. The lower survival rate was observed in the group incubated with C. neoformans var. grubii and the highest with C. podzolicus strain CBS 9357. The amoeba survival experiment was done under nutrient-rich conditions.
ACKNOWLEDGMENTS
This work was supported by NIH grants HL059842-3, A1033774, A1052733, and AI033142 to A.C. M.A.G.-S. was partially supported by a postdoctoral fellowship (19900-147229) from CONACYT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Juan Jimenez and Geoffrey Perumal for assistance with TEM.
M.A.G.-S., A.C., and A.B conceived and designed the experiments. M.A.G.-S. performed the experiments. M.A.G.-S., D.I.G., A.B., and C.S. analyzed the data. M.A.G.-S., D.I.G., and A.C. wrote the paper.
Footnotes
Citation Garcia-Solache MA, Izquierdo-Garcia D, Smith C, Bergman A, Casadevall A. 2013. Fungal virulence in a lepidopteran model is an emergent property with deterministic features. mBio 4(3):e00100-13. doi:10.1128/mBio.00100-13.
REFERENCES
- 1. Casadevall A, Fang FC, Pirofski LA. 2011. Microbial virulence as an emergent property: consequences and opportunities. PLoS Pathog. 7:e1002136 http://dx.doi.org/:10.1371/journal.ppat.1002136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ponge JF. 2005. Emergent properties from organisms to ecosystems: towards a realistic approach. Biol. Rev. Camb. Philos. Soc. 80:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayr E. 1982. The growth of biological thought: divergence, evolution, and inheritance. Belknap Press of Harvard University, Cambridge, MA [Google Scholar]
- 4. Alizon S, Hurford A, Mideo N, Van Baalen M. 2009. Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J. Evol. Biol. 22:245–259 [DOI] [PubMed] [Google Scholar]
- 5. Brown SP, Cornforth DM, Mideo N. 2012. Evolution of virulence in opportunistic pathogens: generalism, plasticity, and control. Trends Microbiol. 20:336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casadevall A, Pirofski LA. 2007. Accidental virulence, cryptic pathogenesis, Martians, lost hosts, and the pathogenicity of environmental microbes. Eukaryot. Cell 6:2169–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bovers M, Hagen F, Kuramae EE, Boekhout T. 2008. Six monophyletic lineages identified within Cryptococcus neoformans and Cryptococcus gattii by multi-locus sequence typing. Fungal Genet. Biol. 45:400–421 [DOI] [PubMed] [Google Scholar]
- 8. Kavanaugh LA, Fraser JA, Dietrich FS. 2006. Recent evolution of the human pathogen Cryptococcus neoformans by intervarietal transfer of a 14-gene fragment. Mol. Biol. Evol. 23:1879–1890 [DOI] [PubMed] [Google Scholar]
- 9. Findley K, Rodriguez-Carres M, Metin B, Kroiss J, Fonseca A, Vilgalys R, Heitman J. 2009. Phylogeny and phenotypic characterization of pathogenic Cryptococcus species and closely related saprobic taxa in the Tremellales. Eukaryot. Cell 8:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodriguez-Carres M, Findley K, Sun S, Dietrich FS, Heitman J. 2010. Morphological and genomic characterization of Filobasidiella depauperata: a homothallic sibling species of the pathogenic Cryptococcus species complex. PLoS One 5:e9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530 [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Aisen P, Casadevall A. 1995. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect. Immun. 63:3131–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang YC, Kwon-Chung KJ. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 14:4912–4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, Wright LC, Sorrell TC, Leidich SD, Casadevall A, Ghannoum MA, Perfect JR. 2001. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 39:166–175 [DOI] [PubMed] [Google Scholar]
- 15. Olszewski MA, Noverr MC, Chen GH, Toews GB, Cox GM, Perfect JR, Huffnagle GB. 2004. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am. J. Pathol. 164:1761–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McClelland EE, Bernhardt P, Casadevall A. 2006. Estimating the relative contributions of virulence factors for pathogenic microbes. Infect. Immun. 74:1500–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sobottka M, de Oliveira LPL. 2006. Periodicity and predictability in chaotic systems. Am. Math. Mon. 113:415–424 [Google Scholar]
- 18. Kostelich EJ. 1997. The analysis of chaotic time-series data. Syst. Contr. Lett. 31:313–319 [Google Scholar]
- 19. Casdagli M. 1991. Chaos and deterministic versus stochastic nonlinear modeling. J. R. Stat. Soc. B Stat. Methodol. 54:303–328 [Google Scholar]
- 20. Golubev SWI, Staib F. 2000. Green and brown colour effects in tremellaceous yeast fungi on Staib agar. Mycoses 43:1–5 [DOI] [PubMed] [Google Scholar]
- 21. Fell JW, Boekhout T, Fonseca A, Scorzetti G, Statzell-Tallman A. 2000. Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int. J. Syst. Evol. Microbiol. 50:1351–1371 [DOI] [PubMed] [Google Scholar]
- 22. Petter R, Kang BS, Boekhout T, Davis BJ, Kwon-Chung KJ. 2001. A survey of heterobasidiomycetous yeasts for the presence of the genes homologous to virulence factors of Filobasidiella neoformans, CNLAC1 and CAP59. Microbiology 147:2029–2036 [DOI] [PubMed] [Google Scholar]
- 23. Djordjevic J. 2010. Role of phospholipases in fungal fitness, pathogenicity and drug development-lessons from Cryptococcus neoformans. Front. Microbiol. 1:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quinn MT, Parthasarathy S, Steinberg D. 1988. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc. Natl. Acad. Sci. U. S. A. 85:2805–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. 2004. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat. Med. 10:161–167 [DOI] [PubMed] [Google Scholar]
- 26. Liu-Wu Y, Hurt-Camejo E, Wiklund O. 1998. Lysophosphatidylcholine induces the production of IL-1β by human monocytes. Atherosclerosis 137:351–357 [DOI] [PubMed] [Google Scholar]
- 27. Xue C, Tada Y, Dong X, Heitman J. 2007. The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host Microbe 1:263–273 [DOI] [PubMed] [Google Scholar]
- 28. Steenbergen JN, Shuman HA, Casadevall A. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. U. S. A. 98:15245–15250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mylonakis E, Ausubel FM, Perfect JR, Heitman J, Calderwood SB. 2002. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 99:15675–15680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, Diener A. 2005. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 73:3842–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anderson RM, May RM. 1982. Coevolution of hosts and parasites. Parasitology 85:411–426 [DOI] [PubMed] [Google Scholar]
- 32. Brown SM, Campbell LT, Lodge JK. 2007. Cryptococcus neoformans, a fungus under stress. Curr. Opin. Microbiol. 10:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaplan DT, Glass L. 1992. Direct test for determinism in a time series. Phys. Rev. Lett. 68:427–430 [DOI] [PubMed] [Google Scholar]
- 34. Benincà E, Huisman J, Heerkloss R, Jöhnk KD, Branco P, Van Nes EH, Scheffer M, Ellner SP. 2008. Chaos in a long-term experiment with a plankton community. Nature 451:822–825 [DOI] [PubMed] [Google Scholar]
- 35. Olsen LF, Schaffer WM. 1990. Chaos versus noisy periodicity: alternative hypotheses for childhood epidemics. Science 249:499–504 [DOI] [PubMed] [Google Scholar]
- 36. Casadevall A. 2012. Amoeba provide insight into the origin of virulence in pathogenic fungi, p 1–10 In Mylonakis E, Ausubel FM, Gilmore M, Casadevall A, Recent advances on model hosts, vol 710. Springer Verlag, New York, NY. [DOI] [PubMed] [Google Scholar]
- 37. Steenbergen JN, Casadevall A. 2003. The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans. Microbes Infect. 5:667–675 [DOI] [PubMed] [Google Scholar]
- 38. Meltz Steinberg K, Levin BR. 2007. Grazing protozoa and the evolution of the Escherichia coli O157:H7 Shiga toxin-encoding prophage. Proc. R. Soc. Lond. B 274:1921–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ngamskulrungroj P, Gilgado F, Faganello J, Litvintseva AP, Leal AL, Tsui KM, Mitchell TG, Vainstein MH, Meyer W. 2009. Genetic diversity of the cryptococcus species complex suggests that Cryptococcus gattii deserves to have varieties. PLoS One 4:e5862 http://dx.doi.org/:10.1371/journal.pone.0005862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robert VA, Casadevall A. 2009. Vertebrate endothermy restricts most fungi as potential pathogens. J. Infect. Dis. 200:1623–1626 [DOI] [PubMed] [Google Scholar]
- 41. Xu J. 2004. Genotype-environment interactions of spontaneous mutations for vegetative fitness in the human pathogenic fungus Cryptococcus neoformans. Genetics 168:1177–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhan J, McDonald BA. 2011. Thermal adaptation in the fungal pathogen Mycosphaerella graminicola. Mol. Ecol. 20:1689–1701 [DOI] [PubMed] [Google Scholar]
- 43. Litvintseva AP, Carbone I, Rossouw J, Thakur R, Govender NP, Mitchell TG. 2011. Evidence that the human pathogenic fungus Cryptococcus neoformans var. grubii may have evolved in Africa. PLoS One 6:e19688 http://dx.doi.org/:10.1371/journal.pone.0019688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Litvintseva AP, Mitchell TG. 2012. Population genetic analyses reveal the African origin and strain variation of Cryptococcus neoformans var. grubii. PLoS Pathog. 8:e1002495 http://dx.doi.org/:10.1371/journal.ppat.1002495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilder JA, Olson GK, Chang YC, Kwon-Chung KJ, Lipscomb MF. 2002. Complementation of a capsule deficient Cryptococcus neoformans with Cap64 restores virulence in a murine lung infection. Am. J. Respir. Cell Mol. Biol. 26:306–314 [DOI] [PubMed] [Google Scholar]
- 46. Chun CD, Brown JC, Madhani HD. 2011. A major role for capsule-independent phagocytosis-inhibitory mechanisms in mammalian infection by Cryptococcus neoformans. Cell Host Microbe 9:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Torres HA, Prieto VG, Raad II, Kontoyiannis DP. 2005. Proven pulmonary cryptococcosis due to capsule-deficient Cryptococcus neoformans does not differ clinically from proven pulmonary cryptococcosis due to capsule-intact Cr. neoformans. Mycoses 48:21–24 [DOI] [PubMed] [Google Scholar]
- 48. Keller CF. 2007. Global warming 2007. An update to global warming: the balance of evidence and its policy implications. Sci. World J. 7:381–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garcia-Solache MA, Casadevall A. 2010. Global warming will bring new fungal diseases for mammals. mBio 1:e00061-10 http://dx.doi.org/10.1128/mBio.00061-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moffat JF, Tompkins LS. 1992. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect. Immun. 60:296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cleare W, Casadevall A. 1998. The different binding patterns of two immunoglobulin M monoclonal antibodies to Cryptococcus neoformans serotype A and D strains correlate with serotype classification and differences in functional assays. Clin. Diagn. Lab. Immunol. 5:125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Casadevall A, Perfect JR. 1998. Cryptococcus neoformans. ASM Press, Washington, DC [Google Scholar]
- 53. Cotter G, Doyle S, Kavanagh K. 2000. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol. Med. Microbiol. 27:163–169 [DOI] [PubMed] [Google Scholar]
- 54. Casadevall A, Pirofski L. 2001. Host-pathogen interactions: the attributes of virulence. J. Infect. Dis. 184:337–344 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Melanin production and urea consumption. (A) Melanization on bird seed agar. In each plate, the first column contains (from to bottom) C. neoformans var. grubii, C. amylolentus, and C. podzolicus CBS 6490; the second column contains C. podzolicus CBS 9357 and C. podzolicus CBS 9358. By day 3, only C. neoformans var. grubii and C. podzolicus CBS 9357 were melanized, and by day 6, C. podzolicus CBS 6490 and C. podzolicus CBS 9358 were also melanizing. Of all C. podzolicus strains, CBS 6490 produced darker pigmentation on bird seed agar but failed to grow above 28°C. All non-heat-tolerant cryptococci failed to grow at 37°C. (B) Urea utilization on Christensen’s medium at 25°C (indicated by color change from orange to bright pink). All strains were able to use urea; however, their capacity was lower than that of C. neoformans and was delayed. (a and f) C. neoformans var. grubii H99; (b and g) C. amylolentus CBS 6039; (c and h) C. podzolicus CBS 6490;. (d and i) C. podzolicus CBS 9357; (e and j) C. podzolicus CBS 9358. C. podzolicus CBS 6490 and CBS 9357 had higher urea utilization capacity than C. amylolentus or C. podzolicus CBS 9358. Download
Lipid utilization. (A) Simple triglyceride consumption (indicated by color change from blue to white) in different cryptococcal species at different temperatures after 3 days after plating. All tested fungi were able to use butyrate at 25°C and 28°C. At 31°C, C. podzolicus strains started failing to grow and C. amylolentus grew but did not degrade the lipid. Strains include C. neoformans var. grubii H99, C. amylolentus CBS 6039, and C. podzolicus CBS 6490, CBS 9357, and CBS 9358. (B) Lyso-PC consumption at different temperatures after 3 days after plating. At 25°C, all tested fungi were able to grow. As temperature increased, fewer strains could grow on the phospholipid-containing medium. At 31°C, only C. neoformans var. grubii H99 and C. podzolicus CBS 9358 were able to grow. H99, CBS 9357, and CBS 9358 but not CBS 6039 or CBS 6490 seemed to be able to consume Lyso-PC. (C) In medium containing DPPC, only C. neoformans var. grubii H99 was able to grow at all tested temperatures. C. podzolicus CBS 9358 was the only non-heat-tolerant fungus that was able to grow at 25°C and 28°C. Strains include C. neoformans var. grubii H99; C. amylolentus CBS 6039; and C. podzolicus CBS 6490, CBS 9357, and CBS 9358. Download
Ultrastructural characteristics of cryptococcal cells, as determined by transmission electron microscopy. Cells were grown for 6 days in minimal medium at 25°C. (A) C. neoformans H99. The characteristic halo surrounds the cell wall that corresponds to the capsule (arrowhead). (B) The acapsular strain C. neoformans var. neoformans Cap67 was used for comparison. Only the cell wall was visualized, but there was no capsule. (C) Diffuse halo surrounding the cell wall that could correspond to a small capsule (arrowhead). (D) No signs of capsule were observed for any C. podzolicus strain under TEM. Scale bar, 500 nm. Download
Summary of phenotypic traits of the three species used in this study.
Amoeba survival after 24 h interaction with the different fungi. The lower survival rate was observed in the group incubated with C. neoformans var. grubii and the highest with C. podzolicus strain CBS 9357. The amoeba survival experiment was done under nutrient-rich conditions.