Abstract
Introduction
Electrochemical impedance spectroscopy (EIS) is a simple and highly sensitive technique that can be used for evaluation of the aptamer-target interaction even in a label-free approach.
Methods
To pursue the effectiveness of EIS, in the current study, the folding properties of specific aptamer for methamphetamine (METH) (i.e., aptaMETH) were evaluated in the presence of METH and amphetamine (Amph). Folded and unfolded aptaMETH was mounted on the gold electrode surface and the electron charge transfer was measured by EIS.
Results
The Ret of methamphetamine-aptaMETH was significantly increased in comparison with other folding conditions, indicating specific detection of METH by aptaMETH.
Conclusion
Based on these findings, methamphetamine-aptaMETH on the gold electrode surface displayed the most interfacial electrode resistance and thus the most folding situation. This clearly indicates that the aptaMETH can profoundly and specifically pinpoint METH; as a result we suggest utilization of this methodology for fast and cost-effective identification of METH.
Keywords: Aptamer, Electrochemical Impedance, Spectroscopy, Gold Nanoparticles, Biosensor
Introduction
Aptamers are ribonucleic acid (RNA) or single strand deoxyribonucleic acid (ssDNA) sequences. Depending on their three dimensional structure, they are able to bind specifically to various targets such as organic and inorganic molecules, peptides, proteins or even complete cells (Clark and Remcho 2002, Ellington and Szostak 1992, Lupold et al 2002). Aptamers could be obtained through a consecutive process which is called Systematic Evolution of Ligands by EXponential enrichment (SELEX). They can be modified for immobilization and labeled with some reporter molecules, without changing their affinity to the target (Torres-Chavolla and Alocilja 2009).
Aptamer-based biosensors are increasingly exploited for the detection of different kinds of targets even in biological fluids (Hu et al 2012, Meini et al 2012, Zhang et al 2012, Zhao et al 2012, Kashefi-Kheyrabadi and Mehrgardi 2012). Labeling of aptamers is a time consuming and laborious work that drives researches towards label-free detection techniques (Kwon et al 2011, Li et al 2011b, Rahim Ruslinda et al 2011).
Electrochemical impedance spectroscopy (EIS) is considered as a very powerful technique for the analysis of interfacial properties related to biorecognition events such as aptamer-target folding occurring at the modified surfaces. Biosensing with EIS is based on the change of electron transfer resistance using a redox probe couple such as [Fe(CN)6]3−/4−, which has received great attention due to its high sensitivity yet simplicity (Lee et al 2008, Li et al 2008, Fang et al 2008, Li et al 2011a, Meini et al 2011). It should be also evoked that greater sensitive detection could be achieved by amplifying the electrochemical signals using different kinds of nanoparticles (NPs) such as gold NPs (Wang et al 2006).
In our previous study (under review data for publication), we introduced a specific ssDNA aptamer for the detection of methamphetamine, named as “aptaMETH”, which was obtained through SELEX process. AptaMETH displayed markedly high binding affinity with a dissociation constant (Kd) in range of nanomolar concentration. The aim of this study was to revalidate the specificity and sensing potential of the selected aptamer (aptaMETH) against methamphetamine through EIS approach.
Material and methods
Apparatus and reagents
For the electrochemical measurements, we used potentiostat-galvanostat AutoLab (Echo Chemie BV Model PGSTAT-302N, Netherlands). All experiments were performed using a conventional three-electrode system with a fabricated aptasensor or bare gold (diameter, 2 mm) as the working electrode, Ag/AgCl (sat. KCl) as the reference electrode, and platinum counter electrode. All potentials were referred to the reference electrode. All experiments were carried out at room temperature (20 ◦C). UV-VIS spectroscopy was performed by Cecil 7500 UV-VIS spectrophotometer (Cecil instruments, Cambridge, UK).
HAuCl4·3H2O, bovine serum albumin (BSA) and trisodium citrate were obtained from Sigma-Aldrich Co., (Taufkirchen, Germany). All reagents were of analytical grade, and Millipore Milli-Q water (>18 MΩ cm) was used throughout experiment. Tris buffer pH = 7.40 containing 5 mM K3Fe(CN)6/K4Fe(CN)6was chosen as the supporting electrolyte for the electrode characterization.
Synthesis of gold nanoparticls
90 ml of 3 × 10−4 M aqueous solution of chloroauric acid (HAuCl4) was allowed to boil. at this point, 3.6 ml of 6.8 × 10−2 M sodium citrate was added dropwisely while stirring. Following the addition of sodium citrate, the solution started darkening and finaly turned into bluish-gray or purple in few min. After approximately 10 minutes, the reaction was completed and the final color of the solution was deep wine red. After boiling for one min, the solution was allowed to reach room temprature and then analyzed by UV-VIS spectrophotometry, according to (Qiu et al 2010).
Pretreatment of electrode
Firstly, a gold disk electrode (Herisau, Switzerland) with 2 mm diameter was polished repeatedly with 1.0, 0.3 and 0.05 μm Al2O3 powder on fine abrasive paper and washed with water ultrasonically. Before modification, the bare electrode was scanned in 0.5 M H2SO4 between -0.2 and 1.5 V until a reproducible cyclic voltammogram (CV) was obtained. After that, the electrode was rinsed carefully with double distilled water and allowed to dry at room temperature.
Folding condition of aptaMETH
Pure aptaMETH (1000 nM) in binding buffer [200 mM NaCl, 20 mM Tris–HCl (pH 7.4) and 5 mM MgCl2] were denatured at 80 °C for 10 min and renatured at 4 °C for 15 min, then equilibrated at room temperature for 30 min. Five µl of the pure equilibrated aptaMETH, methamphetamine and amphetamine-incubated aptaMETH were mounted on the electrode surface with 2 mm diameter and allowed to dry at room temperature.
Electrochemical Impedance Spectroscopy (EIS)
Impedance measurements were obtained using a standard three-electrode electrochemical cell that included: bare gold, gold NPs deposited Au electrode, aptamer modified Au NPs/Au electrode, aptamer-methamphetamine and aptamer-amphetamine modified Au NPs/Au electrode as the working electrode, a platinum counter-electrode and an Ag/AgCl/KCl (sat’d) reference electrode in 10 mM Tris (5 mM Fe(CN)64–/3–) and pH was set at 7.40. The dynamic impedance measurements were performed at a single frequency (between 0.1 and 100000 Hz with signal amplitude of 5 mV). All impedance experiments were conducted with Autolab PGSTAT 302 N. All experiments were carried out at 20 °C.
Fig. 1 represents schematic illustration of folding properties of aptaMETH in the presence of amphetamine and methamphetamine.
Fig. 1.
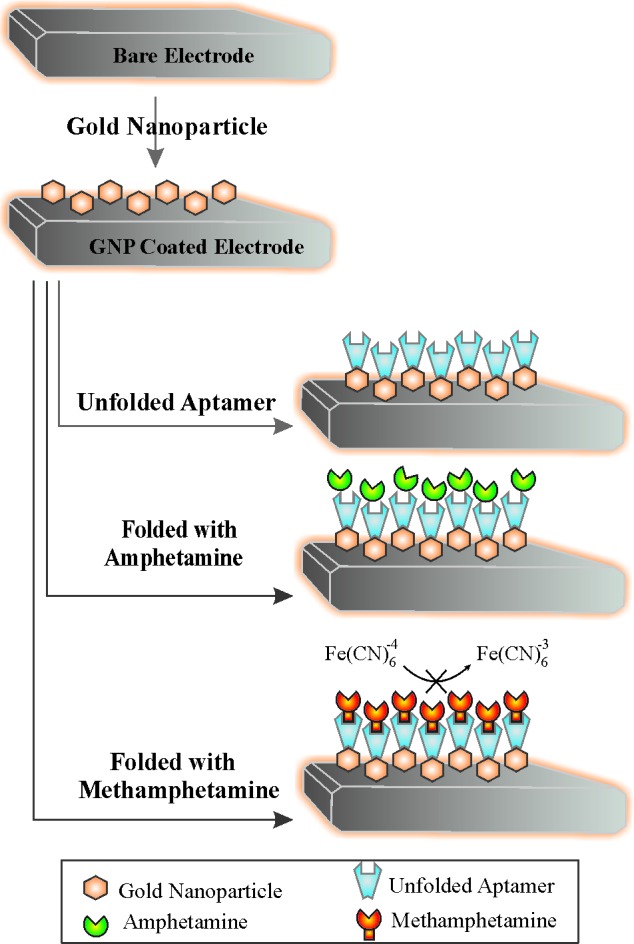
Schematic representation of folding properties of aptaMETH in the presence of amphetamine and methamphetamine.
Results
Characterization of gold NPs
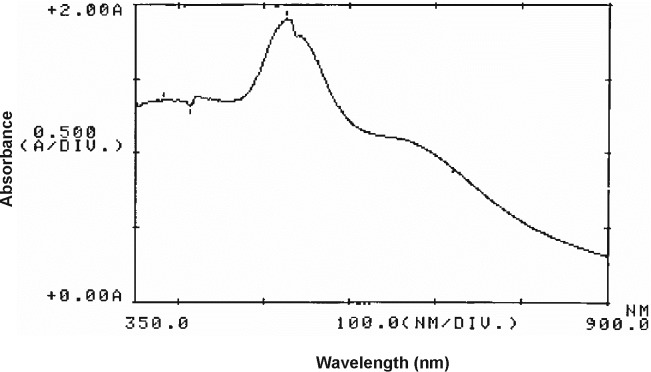
Characterization of synthesized gold NPs were performed by UV-VIS spectroscopy (Amendola and Meneghetti, 2009). Fig. 2 shows UV-VIS spectra of the synthesized gold NPs with average diameter of 16 nm.
Fig. 2.
UV-VIS spectra of gold nanoparticles.
EIS characterization of folded and unfolded aptamers
EIS provides valuable information about the interfacial properties of the modified surface of the electrode.
The circuit includes four elements as follow: 1) the ohmic resistance of the electrolyte solution (Rs); 2) capacitance of double layer, (Cdl); 3) the electron resistance transfer of the aptaMETH-modified layer (Ret) and 4) Warburg impedance (Zw).
Table 1 show electrical parameters’ variations associated with folding effects of aptaMETH at the nano-Au modified surface.
Table 1. Electrical parameters’ variations associated with folding effects of aptaMETH at nano-Au modified surface.
| Analysis | Rs(Ω) | C(F) | Ret(Ω) | Zw(Ω) |
| Bare gold electrode | 358.28 | 5.97×10- 7 | 23766 | 2.56×10- 5 |
| Au NPs/ Au electrode | 359.02 | 8.49×10- 7 | 15774 | 1.76×10- 4 |
| AptaMETH-modified electrode | 355.99 | 8.14×10- 7 | 83473 | 1.06×10- 4 |
| AptaMETH and Amph | 340.354 | 6.89×10- 7 | 170830 | 3.49×10- 5 |
| AptaMETH and METH | 340.13 | 6.32×10- 7 | 367000 | 2.31×10- 5 |
Interferences from the aptamer folding are only possible on Ret and Cdl parameters. An inconsiderable change was observed in Rs and Zw for the faradic impedance record in our experiments during the different surface modifications. Only Cdl and Ret represent significant variation (Table 1).
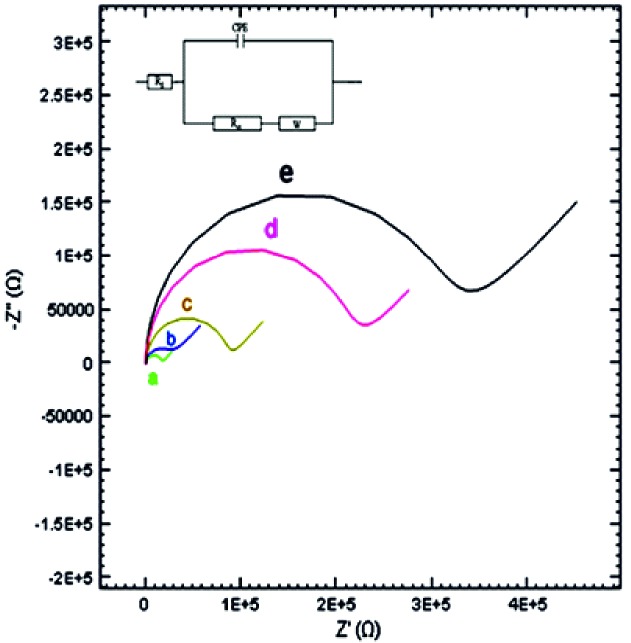
Fig. 3 represents the Nyquist plots from electrochemical impedance spectroscopy after the multiple surface modifications and Randle’s equivalent circuit. Multiple steps of electrode modifications including bare electrode, Au/NPs deposited gold electrode, unfolded aptaMETH-modified electrode, amphetamine-incubated aptaMETH, and methamphetamine-incubated aptaMETH are shown in Fig. 3 from a to e, respectively.
Fig 3.
Nyquist plot of different electrodes measured in the presence of 5 mM K3[Fe(CN)6]/ K4[Fe(CN)6] in Tris buffer 10 mM (pH 7.4) at 25 ˚C: (a) bare gold electrode;(b) nano-Au modified electrode; (c) unfolded aptaMETH/ nano-Au modified electrode; (d) amphetamine incubated aptaMETH/ nano-Au modified electrode; (e) methamphetamine incubated aptaMETH/ nano-Au modified electrode.
Discussion
Using SELEX technology, we have previously introduced a specific ssDNA aptamer for the detection of methamphetamine (aptaMETH), which was able to detect METH with high binding affinity and Kd value in nM range (under review for publication). Here, to pursue aptaMETH potential to be used as a biosensor, we exploited EIS for specific detection of methamphetamine. Because of specific and unique conformation of each aptamer for specific target, their three dimensional structure could be altered in the presence of the molecular target. Hence, we hypothesized a logical concept as the conformation displacement of aptaMETH could influence the electron transfer on the electrode interface which could be detected by EIS. To pursue this hypothesis, we used EIS approach.
The folding and unfolding of aptaMETH, which was changed in the presence of methamphetamine and amphetamine, led to the change of impedance. The high affinity of aptaMETH to its target was proved by the folding capability after binding with methamphetamine (Fig. 3).
When the target was incorporated into the specific position of aptamer, three dimensional structure and dynamics of aptamer were changed, which are in agreement with previous reports (Cekan et al 2009). As a result of changing the shape of aptamer, the charge transfer resistance (Ret) of double layer increases apparently as reported previously (Li et al 2008). In order to study the folding, EIS technique was employed as a highly sensitive approach for assessing surface properties. It is noticed that the Ret variation depends on the amount of the folding of aptaMETH (Table 1).
Based upon our previous study, we showed that the aptaMETH can specifically detect the methamphetamine with high binding affinity. Thus, these results revalidate our previous findings. This clearly indicates that methamphetamine-incubated aptaMETH has the most folding state in comparison with amphetamine-incubated aptaMETH that results in an increased level of electron charge transfer resistance because of electrical and steric hindrance.
Conclusion
In the present investigation, for the first time, we recruited the impedance spectroscopy approach for evaluation of the aptaMETH folding in the presence of amphetamine and methamphetamine. The alteration of interfacial properties of electrode upon target-aptaMETH interaction was determined by EIS method. The charge transfer resistance of the methamphetamine-incubated/ nano-Au modified electrode, amphetamine-incubated/ nano-Au modified electrode and unfolded aptaMETH showed to be significantly different from each other. These data revalidate our previous report claiming aptaMETH as a detection probe for methamphetamine with high binding affinity and specificity. The EIS developed here also showed an advantage over standard techniques of folding characterization since it is a simple, fast, cost-effective and highly sensitive label-free approach. This methodology provides a platform for real time monitoring of target-aptamer interaction.
Ethical Issues
No ethical issues to be promulgated.
Conflict of interests
No conflict of interests to be declared.
Acknowledgments
Authors would like to thank Research Center for Pharmaceutical Nanotechnology (RCPN) for supporting this project (grant No: 5/87/89001, which was a part of PhD thesis No: 48). We also express our deep blessing as a memorial to Ms Maryam Harasi, who was the main key scientist (PhD student) for nanobiosensing section at RCPN, but unfortunately she passed away recently.
References
- Amendola V and Meneghetti M . 2009 Size Evaluation of Gold Nanoparticles by UV−vis Spectroscopy. The Journal of Physical Chemistry C, 113(11), 4277-4285 [Google Scholar]
- Cekan P, Jonsson EÖ and Sigurdsson ST . 2009 Folding of the cocaine aptamer studied by EPR and fluorescence spectroscopies using the bifunctional spectroscopic probe Ç. Nucleic Acids Research, 37(12), 3990-3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SL and Remcho VT . 2002 Aptamers as analytical reagents. Electrophoresis, 23(9), 1335-1340 [DOI] [PubMed] [Google Scholar]
- Ellington AD and Szostak JW . 1992 Selection in vitro of single-stranded DNA molecules that fold into specific ligand-binding structures. Nature, 355(6363), 850-852 [DOI] [PubMed] [Google Scholar]
- Fang L, Lü Z, Wei H and Wang E . 2008 A electrochemiluminescence aptasensor for detection of thrombin incorporating the capture aptamer labeled with gold nanoparticles immobilized onto the thio-silanized ITO electrode. Analytica Chimica Acta, 628(1), 80-86 [Google Scholar]
- Hu P, Zhu C, Jin L and Dong S . 2012 An ultrasensitive fluorescent aptasensor for adenosine detection based on exonuclease III assisted signal amplification. Biosensors and Bioelectronics, 34(1), 83-87 [DOI] [PubMed] [Google Scholar]
- Kashefi-Kheyrabadi L and Mehrgardi MA . 2012 Design and construction of a label free aptasensor for electrochemical detection of sodium diclofenac. Biosensors and Bioelectronics, 33(1), 184-189 [DOI] [PubMed] [Google Scholar]
- Kwon D, Jeong H and Chung BH . 2011 Label-free electrochemical detection of human α-thrombin in blood serum using ferrocene-coated gold nanoparticles. Biosensors and Bioelectronics, 28(1), 454-458 [DOI] [PubMed] [Google Scholar]
- Lee JA, Hwang S, Kwak J, Park SI, Lee SS and Lee K-C . 2008 An electrochemical impedance biosensor with aptamer-modified pyrolyzed carbon electrode for label-free protein detection. Sensors and Actuators B: Chemical, 129(1), 372-379 [Google Scholar]
- Li L-D, Zhao H-T, Chen Z-B, Mu X-J and Guo L . 2011. a Aptamer biosensor for label-free impedance spectroscopy detection of thrombin based on gold nanoparticles. Sensors and Actuators B: Chemical, 157(1), 189-194 [Google Scholar]
- Li LD, Zhao HT, Chen ZB, Mu XJ and Guo L . 2011. b Aptamer biosensor for label-free impedance spectroscopy detection of thrombin based on gold nanoparticles. Sensors and Actuators, B: Chemical, 189-194 [Google Scholar]
- Li X, Shen L, Zhang D, Qi H, Gao Q, Ma F, et al. 2008 Electrochemical impedance spectroscopy for study of aptamer–thrombin interfacial interactions. Biosensors and Bioelectronics, 23(11), 1624-1630 [DOI] [PubMed] [Google Scholar]
- Lupold SE, Hicke BJ, Lin Y and Coffey DS . 2002 Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Research, 62(14), 4029-4033 [PubMed] [Google Scholar]
- Meini N, Farre C, Chaix C, Kherrat K, Dzyadevych S and Jaffrezic-Renault N . 2011 Impedimetric Aptasensor for Thrombin Detection. Procedia Engineering, 25(0), 1461-1464 [Google Scholar]
- Meini N, Farre C, Chaix C, Kherrat R, Dzyadevych S and Jaffrezic-Renault N . 2012 A sensitive and selective thrombin impedimetric aptasensor based on tailored aptamers obtained by solid-phase synthesis. Sensors and Actuators, B: Chemical166-167(0), 715-720 [Google Scholar]
- Qiu H, Sun Y, Huang X and Qu Y . 2010 A sensitive nanoporous gold-based electrochemical aptasensor for thrombin detection. Colloids and Surfaces B: Biointerfaces, 79(1), 304-308 [DOI] [PubMed] [Google Scholar]
- Rahim Ruslinda A, Wang X, Ishii Y, Ishiyama Y, Tanabe K and Kawarada H . 2011 Human immunodeficiency virus trans-activator of transcription peptide detection via ribonucleic acid aptamer on aminated diamond biosensor. Applied Physics Letters, 99(12). [Google Scholar]
- Torres-Chavolla E and Alocilja EC . 2009 Aptasensors for detection of microbial and viral pathogens. Biosensors and Bioelectronics, 24(11), 3175-3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Profitt JA, Pugia MJ and Suni II . 2006 Au nanoparticle conjugation for impedance and capacitance signal amplification in biosensors. Anal Chem, 78(6), 1769-73 [DOI] [PubMed] [Google Scholar]
- Zhang DW, Zhang FT, Cui YR, Deng QP, Krause S, Zhou YL, et al. 2012 A label-free aptasensor for the sensitive and specific detection of cocaine using supramolecular aptamer fragments/target complex by electrochemical impedance spectroscopy. Talanta, 92, 65-71 [DOI] [PubMed] [Google Scholar]
- Zhao J, He X, Bo B, Liu X, Yin Y and Li G . 2012 A "signal-on" electrochemical aptasensor for simultaneous detection of two tumor markers. Biosensors and Bioelectronics, 34(1), 249-252 [DOI] [PubMed] [Google Scholar]