Abstract
Introduction
Among several biosensing approaches, electrochemical-based procedures have been described as one of the most common and useful methods for sensing because of their simplicity, sensitivity, accuracy, and low cost. The electroactive species, which called redox, play a main role in the electrochemical-based approaches. Among several redox molecules used for electrochemical experiments, ferrocene is one of the commonly used redox molecules. However, instability of ferrocenium ion in the chloride containing solutions appeared to be weakness of this redox molecule limiting its utilization.
Methods
In the current study, Juglone was attached (using EDC/NHS coupling method) to the 3'-amino-modified terminus of the immobilized specific aptamer of codeine, which was successfully used in a cyclic electrochemical voltammetry procedure.
Results
The cyclic voltammogram peak of aptamer-attached Juglone was observed in the potential range of +0.4 to +0.9 V and the fabricated aptamer-based sensor was used for detection of different concentrations of codeine in the phosphate buffer 0.1 M solution containing 2 M NaCl.
Conclusion
Based on these findings, it can be suggested that the new aptamer-attached Juglone could be considered as an effective alternative redox molecule in particular with oligonucleotide-based sensing systems.
Keywords: Aptamer, Aptasensor, Biosensor, Cyclic Voltammetry, Redox
Introduction
In recent years, significant progress has been made in the development of biosensors for different applications such as medical diagnosis (Song et al. 2007; Trkarslan et al. 2009), drugs (Alonso Lomillo et al. 2003; Joseph et al. 2003) and toxins (Liu et al. 2004; Pancrazio et al. 1998; Stevens et al. 2007) tracing, and other purposes (Gao et al. 2008; Tombelli et al. 2000). Aptamer-based biosensors, called aptasensors, are a new group of biosensors that have been introduced in recent years and attracted a lot of interests in the field of biosensors.Aptamers are single stranded DNA or RNA sequences with high affinity and specificity to a specific target such as antibodies. These properties make aptamers appropriate tool to be used as the recognition part of biosensors in the optical, electrochemical, or other types of affinity biosensors (Lee et al. 2008; Song et al. 2008; Tombelli et al. 2005). Despite of simultaneous progress in the optical and electrochemical sensing, many scientists prefer electrochemical techniques because of their simplicity, sensitivity, accuracy, and low cost (Velasco-Garcia 2009; Wang 2006). In the electrochemical measurements, the biorecognition element, called redox, is one of the main components of assay procedures and acts as a transducer by following methods: these molecules may be used as the electroactive element of the label free electrochemical procedures (being existed in the solution without any attachment to the sensors or surface of the electrode), used as a biomolecule-attached element (being attached to the biomolecular part of the sensor), or used as a surface-attached redox molecules (being attached to the surface of the electrode) (Odenthal and Gooding 2007). Among the mentioned methods, the biomolecule-attached redox molecules are very popular in biosensor designing and ferrocene is one of the commonly used redox molecules in these types of studies (Baker et al. 2006; Ferapontova et al. 2008; Li et al. 2008; Wang et al. 2009; Xiao et al. 2007). Despite of vast usage of the ferrocene as the redox molecule in the field of biosensors, instability of the ferrocenium ion in the chloride containing buffer solutions or in strong nucleophilic reagents is one of the major disadvantages of using this molecule (Hurvois and Moinet 2005; Prins et al. 1972; Xiao et al. 2007). Moreover, since the redox molecules play an important role as electroactive species, an effective redox molecule could be very important in the improvement and progression of existed techniques in the biosensors fabrication and, therefore, enhance the studies in the field of sensing. The 5-hydroxy- 1,4-naphthoquinone, Juglone, a natural quinone obtained from walnut trees, is an electroactive molecule which has been used as a surface attached redox molecule in recent studies (Baker et al. 2006; March et al. 2008). Although quinine derivatives has been described as biomolecular-attached redox species before (Chatterjee and Rokita 1994), using of Juglone as an attached-redox molecule in aptasensors is a novel approach in the field of oligonucleotide-based biosensors. Based on our findings, it can be suggested that the new aptamer-attached Juglone could be considered as an effective alternative redox molecule in particular with oligonucleotide-based sensing systems.
Materials and methods
Materials
5-Hydroxy- 1,4-naphthoquinone 97% Acros (Juglone), 3-mercaptopropionic acid ≥98% Merck (3-MPA), N-hydroxysuccinimide ≥99% Merck (NHS), synthesis grade of Merck's N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC), Codeine phosphate 99% Iran Temad (CP), and 6-Mercaptohexanol97% Sigma-Aldrichwere purchased and used without further purification. The previously reported specific RNA-aptamer sequence for codeine (Win et al. 2006) was synthesized by Microsynth with the 5'-terminus, a C6 aliphatic thiol, and 3'-tarminus, a C7 primary aliphatic amin, modifications (5'-SHC6-GGG ACA GGG CUA GCU UAG UGC UAU GUG AGA AAA GGG UGU GGG GG-C7NH2-3').
Instrumentation and procedures
Melting points (obtained by an electrothermal 9100 apparatus), infrared spectra (recorded by a shimadzu (8400) FT-IR spectrometer), and 400 MHz 1 H NMRs (recorded on a Bruker Avanceinstrument, AC 80) had been performed for tracing the synthesized compounds. Column chromatography was performed for further purification by using silica gel 60 (230-400 mesh) and DCM-methaol as stationary and mobile phases respectively.
Electrochemical measurements were performed by a Metrohm Autolab 302N Potentiostats-Galvanostats with the three customary electrodes, an Ag/AgCl/KCl 3 M reference electrode; a platinum wire auxiliary electrode; and a gold disk electrode as working electrode (with 2 mm diameter, purchased from Azar Electrode Co.). Total controlling of the electrochemical procedures was carried out by NOVA software (version 1.5, Eco Chemie BV).
All the electrochemical experiments were performed atroom temperature and in the 50 mL of different concentrations of CP in phosphate buffer solution (PBS) 0.1 M containing 2 M sodium chloride (NaCl), pH 7.0.
Synthesis of N-Hydroxysuccinimide ester of β(( 5-hydroxy– 1,4-naphthoquinonyl) thio) propionic acid (Jug-PE)
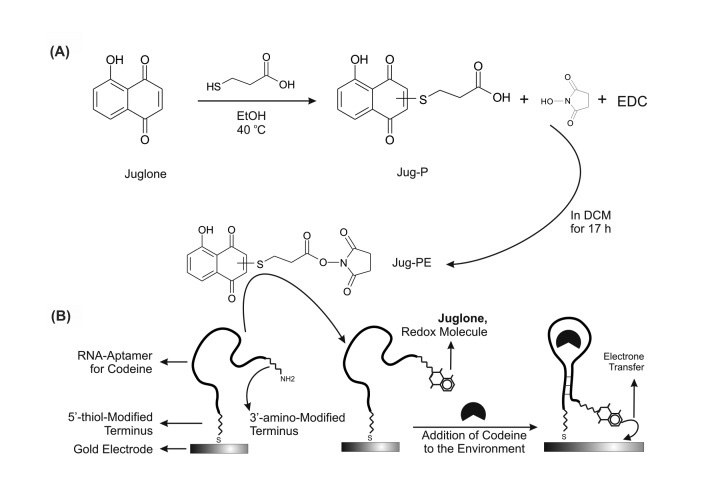
Jug-PE was prepared by the coupling reaction of β(( 5-hydroxy– 1,4-naphthoquinonyl) thio) propionic acid (Jug-P) and NHS in presence of EDC (Fig. 1A) (Teh et al. 2005; Wu et al. 2007). First, the Jug-P was synthesized by the one-step reaction of Juglone and 3-MPA. The synthesis of this intermediate is based on the substitution of thiols on the quinone's rings under a mild condition (Piro et al. 2005; Villalba et al. 2008). A mixture of Juglone (210 mg, 1.2 mmol) and 3-MPA (123 mg, 100 µL, 1.2mmol) in 6 mL ethanol were stirred at 40 oC for 1 hour. The mixture was then cooled to room temperature and remained stirringly for 2-3 hours to complete the reaction. After filtering the precipitate and performing a washing step by cold ethanol, the precipitate was purified by silica gel column (10:1 DCM/methanol) and the product was obtained by evaporating the solvent (Chatterjee and Rokita 1994; Mital et al. 2008; Piro et al. 2005). The purity of the light orange product, Jug-P, (40% yields, M.p. 216oC) was confirmed by TLC. FT-IR νmax (KBr) 3450(O-H Aromatic), 3100 (O-H Carboxylic acid), 2990, 1650 (C=O), 1600 (C=O) 1453, 1376, 1210, 1100 cm-.
Fig. 1.
Juglone (Jug) based nanosensors. A) Schematic representation for synthesis of Jug-PE: The Jug-PE was synthesized from Jug-P (a product of Juglone and 3-MPA reaction) by EDC/NHS coupling reaction. The synthesized Jug-PE would have used as a attached redox molecule for the biosensor designing.B) The codeine's aptasensor: The specific aptamer of codeine was immobilized on a gold surface via its 5'-thiolated terminus. Then, the redox molecule, Jug-PE, was attached to the 3'-amino modified terminus of the aptamer's sequence and use for target sensing.
A 0.25 mmol of synthesized Jug-P (70 mg) was dissolved in 5 mL dry dichloromethane (DCM) and added NHS (0.3 mmol, 35 mg) and EDC (0.3 mmol, 57 mg). The mixture was stirred at room temperature for 17 hours to complete the reaction. Added water (5 mL), and the mixture was extracted by dichloromethane (3 × 10 mL). The organic layers were combined, dried over MgSO4, and evaporation of the solvent afforded green residue. Purification of the residue by silica gel chromatography afforded the desired product, 75% yield, as a mixture of keto and enol forms (Ferapontova et al. 2008; Teh et al. 2005; Wu et al. 2007). FT-IR νmax (KBr) 3470(O-H Aromatic), 2990, 2850, 1750 (C=O), 1650 (C=O) 1453, 1350, 1210, 1120 cm- 1 . 1 HNMR (CDCl3, 400 MHz): δ 12.1 (s, 1H), 11.9 (s, 1H), 11.6 (s, 1H), 7.70-7.55 (m, 2H), 7.30-7.18 (m, 2H), 6.94 (s, 1H, enol form), 6.62 (s, 1H, enol form), 6.58 (s, 1H, enol form), 3.22-2.80 (m, 8H).
Preparation of the electrode
For efficient immobilization of aptamer on the surface of the electrode, the impurities have been removed from the electrode's surface by physical and electrochemical procedures which had been described in literature (Baker et al. 2006; El-Deab and Ohsaka 2004; Merrill et al. 2005; Xiao et al. 2007). After complete steps of polishing, the 5'-thiol, 3'-amino-modified RNA-aptamer was immobilized via its 5'-thiolated terminus on the surface of gold electrode by self-assemble monolayer procedure (Wink et al. 1997). The self-assemble monolayer has been formed by placing a 50 µL volume of 5 µM modified RNA-aptamer on the polished surface of electrode for 18 hours. The unbound aptamers have been removed from the surface by rinsing with the phosphate buffer (pH 7.0) for several times and the Jug-PE, redox molecule, was attached to the 3'-terminus of aptamer as previously described (Fig. 1B), followed by two-hours treating with 2 mM 1-mercaptohexanol (Ferapontova et al. 2008; March et al. 2008; Xiao et al. 2007). The electrode gets ready in this stage and immediately would being used in experimental sections.
Results
Electrochemical characterization
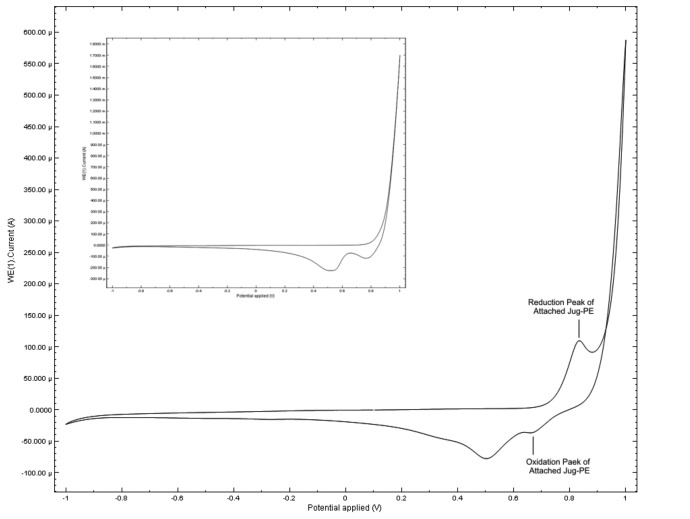
For investigating the exact place of aptamer-attached Juglone's oxidation and reduction peaks on the voltammogram, at first, a cyclic voltammetry scan was taken in the potential range of -1 to +1 V and the scan rate of 0.15 V/s in PBS 0.1 M containing 2 M NaCl. Then, the second cyclic voltammetry scan was taken in the same condition as well as a 10 µM concentration of CP. By comparing the obtained scan in the presence of CP with the background scan, it has been investigated that the reduction oxidation and peaks of aptamer-attached Juglone are appeared in the potential range of +0.4 to +0.9 V. The reduction peak appears at 8.5 V and the oxidation peak appears at 6.5 V on the voltammogram (Fig. 2).
Fig. 2.
The cyclic voltammogram of attached Jug-PE: A cyclic voltammetry was taken for a 10 µM concentration of CP in PBS 0.1 M, containing 2 M NaCl (Potential range: –1.0 to +1.0, scan rate: 0.15 V/s). The obtained data show that the oxidation and reduction peaks for this redox molecule appear in the +0.4 to +0.9 V range of potential axis on the voltammogram. The inset shows the background cyclic voltammetry scan which was taken in PBS 0.1 M containing 2 M NaCl in absence of CP.
Attached- Juglone as a redox molecule for aptasensor designing
The ability of the attached-Juglone to be used as a redox molecule for aptasensor designing was investigated by codeine addition to the environment. Firstly, a cyclic voltammetry background scan was taken in the potential range of +0.4 to +0.9 V and the scan rate of 0.15 V/s in PBS 0.1 M containing 2 M NaCl. Then, the different amounts of CP were added to the PBS 0.1 M containing 2 M NaCl to prepare different solutions of CP (100 nM, 200 nM, 500 nM, and 1 µM). Comparison of these cyclic voltammograms, taken scans in presence of CP and background scan, shows a significant relation between the CP concentration and the maximum faradic current change in the voltammogram (Fig. 2).
The obtained data show that the maximum faradic current of working electrode change to 61.6 µA, 73.8 µA, 110 µA, and 211 µA in presence of 100 nM, 200 nM, 500 nM, and 1µM CP respectively. The obtained data show that the Juglone-tagged aptasensor presents a linear response to the presence of increasing concentrations of CP.
Discussion
According to the obtained data, the place of oxidation and reduction peaks of aptamer-attached Juglone has being observed in the potential range of +0.4 to +0.9 V. This range is different from the previously reported potential range of the oxidation and reduction peaks of surface-attached Juglone (March et al. 2008). This significant shift of oxidation and reduction peaks to the right position of the potential axis of voltammogram (upper potential range) is probably because of changes in the electrochemical properties of Juglone because of its attachment to the 3'-terminus of codeine's specific RNA-aptamer.
Faradic current change of working electrode in presence of CP could be described by the previously reported mechanism (Fig. 1B) (Ferapontova et al. 2008). In the absence of CP, because, the structure of aptamer is open, the Juglone stay away from the surface of electrode. In this situation, the background scan has been taken. In presence of CP, the structure of aptamer changed to closed format and this phenomenon causes the vicinity of the Juglone to the electrode's surface. The significant change in the spatial structure of aptamer in presence of CP causes to change in faradic current. Since, the more concentrations of CP affect on the more numbers of aptamers, by increasing the concentration of CP the faradic current of working electrode would being increased. So, the faradic current of working electrode could be described as an index for the involved aptamers and it could be introduced as an index for the codeine's concentration in the environment indirectly.
Conclusion
The new redox molecule was used in the PBS buffer containing 2 M NaCl successfully. According to the literature, the faradic current of working electrode is related to the ionic strength of the medium (Xiao et al. 2007), so the ability of this redox molecule to be used in the environments with relatively high concentration of chloride salt could be considered as an important advantage of this molecule. The voltammetric results show that the new aptamer-attached Juglone has a significant oxidation and reduction peak in the µA limit on the voltammogram. Thus,based on these findings, it can be suggested that the new aptamer-attached Juglone could have been considered as an effective alternative redox molecule in particular with oligonucleotide-based sensing, for example oligonucleotide-basedbiosensors.
Ethical Issues
None to be declared.
Conflict of interests
Authors declare no conflict of interests.
Acknowledgments
Authors would like to thankResearch Center for Pharmaceutical Nanotechnology (RCPN) for supporting this project that is a part of number 46 PhD thesis. Also we would like to thank Exir Pharmaceutical Co. for providing the codeine phosphate. Writers wish to thanks Abolfazl Barzegari who counterpart in this project as assistant in carrying out the molecular procedures.
References
- Alonso Lomillo MA, Kauffmann JM and Arcos Martinez . 2003HRP-Based Biosensor for Monitoring Rifampicin. Biosensors and Bioelectronics, 18(9), 1165-1171 [DOI] [PubMed] [Google Scholar]
- Baker BR, Lai RY, Wood MS, Doctor EH, Heeger AJ and Plaxco KW . 2006An Electronic, Aptamer-Based Small-Molecule Sensor for the Rapid, Label-Free Detection of Cocaine in Adulterated Samples and Biological Fluids. Journal of the American Chemical Society, 128(10), 3138-3139 [DOI] [PubMed] [Google Scholar]
- Chatterjee M and Rokita SE . 1994The Role of a Quinone Methide in the Sequence Specific Alkylation of DNA. Journal of the American Chemical Society, 116(5), 1690-1697 [Google Scholar]
- El-Deab MS and Ohsaka T . 2004Molecular-Level Design of Binary Self-Assembled Monolayers on Polycrystalline Gold Electrodes. Electrochimica Acta, 49(13), 2189-2194 [Google Scholar]
- Ferapontova EE, Olsen EM and Gothelf KV . 2008An RNA Aptamer-Based Electrochemical Biosensor for Detection of Theophylline in Serum. Journal of the American Chemical Society, 130(13), 4256-4258 [DOI] [PubMed] [Google Scholar]
- Gao Y, Guo F, Gokavi S, Chow A, Sheng Q and Guo M . 2008Quantification of Water-Soluble Vitamins in Milk-Based Infant Formulae Using Biosensor-Based Assays. Food Chemistry, 110(3), 769-776 [Google Scholar]
- Hurvois JP and Moinet C . 2005Reactivity of Ferrocenium Cations With Molecular Oxygen in Polar Organic Solvents: Decomposition, Redox Reactions and Stabilization. Journal of Organometallic Chemistry, 690(7), 1829-1839 [Google Scholar]
- Joseph S, Rusling JF, Lvov YM, Friedberg T and Fuhr U . 2003An Amperometric Biosensor With Human CYP3A4 As a Novel Drug Screening Tool. Biochemical Pharmacology, 65(11), 1817-1826 [DOI] [PubMed] [Google Scholar]
- Lee JO, So HM, Jeon EK, Chang H, Won K and Kim YH . 2008Aptamers As Molecular Recognition Elements for Electrical Nanobiosensors. Analytical and Bioanalytical Chemistry, 390(4), 1023-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Qi H, Peng Y, Gao Q and Zhang C . 2008Electrogenerated Chemiluminescence Aptamer-Based Method for the Determination of Thrombin Incorporating Quenching of Tris(2,2'-Bipyridine)Ruthenium by Ferrocene. Electrochemistry Communications, 10(9), 1322-1325 [Google Scholar]
- Liu X, Song D, Zhang Q, Tian Y and Zhang H . 2004An Optical Surface Plasmon Resonance Biosensor for Determination of Tetanus Toxin. Talanta, 62(4), 773-779 [DOI] [PubMed] [Google Scholar]
- March G, No_Ól V, Piro B, Reisberg S and Pham MC . 2008Nanometric Layers for Direct, Signal-on, Selective, and Sensitive Electrochemical Detection of Oligonucleotides Hybridization. Journal of the American Chemical Society, 130(47), 15752-15753 [DOI] [PubMed] [Google Scholar]
- Merrill DR, Stefan IC, Scherson DA and Mortimer JT . 2005Electrochemistry of Gold in Aqueous Sulfuric Acid Solutions Under Neural Stimulation Conditions. Journal of the Electrochemical Society, 152(7). [Google Scholar]
- Mital A, Negi VS and Ramachandran U . 2008Synthesis and Biological Evaluation of Substituted Naphthoquinone Derivatives As Potent Antimycobacterial Agents. Arkivoc, 2008(15), 176-192 [DOI] [PubMed] [Google Scholar]
- Odenthal KJ and Gooding JJ . 2007An Introduction to Electrochemical DNA Biosensors. Analyst, 132(7), 603-610 [DOI] [PubMed] [Google Scholar]
- Pancrazio JJ, Alonso LomilloBey Jr PP, Cuttino DS, Kusel JK, Borkholder DA, Shaffer KM, et al. 1998Portable Cell-Based Biosensor System for Toxin Detection. Sensors and Actuators B: Chemical, 53(3), 179-185 [Google Scholar]
- Piro B, Haccoun J, Pham MC, Tran LD, rubin A, Perrot H, et al. 2005Study of the DNA Hybridization Transduction Behavior of a Quinone-Containing Electroactive Polymer by Cyclic Voltammetry and Electrochemical Impedance Spectroscopy. Journal of Electroanalytical Chemistry, 577(1), 155-165 [Google Scholar]
- Prins R, Korswagen AR and Kortbeek AGTG . 1972Decomposition of the Ferricenium Cation by Nucleophilic Reagents. Journal of Organometallic Chemistry, 39(2), 335-344 [Google Scholar]
- Song MJ, Yun DH, Min NK and Hong SI . 2007Electrochemical Biosensor Array for Liver Diagnosis Using Silanization Technique on Nanoporous Silicon Electrode. Journal of Bioscience and Bioengineering, 103(1), 32-37 [DOI] [PubMed] [Google Scholar]
- Song S, Wang L, Li J, Fan C and Zhao J . 2008Aptamer-Based Biosensors. TrAC Trends in Analytical Chemistry, 27(2), 108-117 [Google Scholar]
- Stevens RC, Soelberg SD, Eberhart BT, Spencer S, Wekell JC, Chinowsky TM, et al. 2007Detection of the Toxin Domoic Acid From Clam Extracts Using a Portable Surface Plasmon Resonance Biosensor. Harmful Algae, 6(2), 166-174 [Google Scholar]
- Teh HF, Gong H, Dong XD, Zeng X, Lai Kuan, Yang X, et al. 2005Electrochemical Biosensing of DNA With Capture Probe Covalently Immobilized Onto Glassy Carbon Surface. Analytica Chimica Acta, 551(1-2), 23-29 [Google Scholar]
- Tombelli S, Minunni M and Mascini M . 2005Analytical Applications of Aptamers. Biosensors and Bioelectronics, 20(12), 2424-2434 [DOI] [PubMed] [Google Scholar]
- Tombelli S, Mascini M, Braccini L, Anichini M and Turner APF . 2000Coupling of a DNA Piezoelectric Biosensor and Polymerase Chain Reaction to Detect Apolipoprotein E Polymorphisms. Biosensors and Bioelectronics, 15(7-8), 363-370 [DOI] [PubMed] [Google Scholar]
- Trkarslan z, Kayahan SK and Toppare L . 2009A New Amperometric Cholesterol Biosensor Based on Poly(3,4-Ethylenedioxypyrrole). Sensors and Actuators B: Chemical, 136(2), 484-488 [Google Scholar]
- Velasco-Garcia MN . 2009Optical Biosensors for Probing at the Cellular Level: A Review of Recent Progress and Future Prospects. Seminars in Cell & Developmental Biology, 20(1), 27-33 [DOI] [PubMed] [Google Scholar]
- Villalba MM, Litchfield VJ, Smith RB, Franklin AM, Lawrence NS and Davis J . 2008Rapid Assessment of the Latent Hazard Posed by Dissolved Mercaptans Within Aqueous Effluent. Journal of Hazardous Materials, 154(1-3), 444-450 [DOI] [PubMed] [Google Scholar]
- Wang J . 2006Electrochemical Biosensors: Towards Point-of-Care Cancer Diagnostics. Biosensors and Bioelectronics, 21(10), 1887-1892 [DOI] [PubMed] [Google Scholar]
- Wang X, Dong P, Yun W, Xu Y, He P and Fang Y . 2009A Solid-State Electrochemiluminescence Biosensing Switch for Detection of Thrombin Based on Ferrocene-Labeled Molecular Beacon Aptamer. Biosensors and Bioelectronics, 24(11), 3288-3292 [DOI] [PubMed] [Google Scholar]
- Win MN, Klein JS and Smolke CD . 2006Codeine-Binding RNA Aptamers and Rapid Determination of Their Binding Constants Using a Direct Coupling Surface Plasmon Resonance Assay. Nucleic Acids Research, 34(19), 5670-5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink T, Alonso LomilloBey JrVan Zuilen SJ, Bult A and Van Bennekom . 1997Self-Assembled Monolayers for Biosensors. Analyst, 122(4), 122(4) [DOI] [PubMed] [Google Scholar]
- Wu L, Yan F and Ju H . 2007An Amperometric Immunosensor for Separation-Free Immunoassay of CA125 Based on Its Covalent Immobilization Coupled With Thionine on Carbon Nanofiber. Journal of Immunological Methods, 322(1-2), 12-19 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Lai RY and Plaxco KW . 2007Preparation of Electrode-Immobilized, Redox-Modified Oligonucleotides for Electrochemical DNA and Aptamer-Based Sensing. Nature protocols, 2(11), 2875-2880 [DOI] [PubMed] [Google Scholar]