Abstract
Introduction
Citric acid-polyethylene glycol-citric acid (CPEGC) triblock dendrimers can serve as potential delivery systems.
Methods
In this investigation, CPEGC triblock dendrimers were synthesized and then imidazole groups were conjugated onto the surface of the G1, G2 and G3 of the obtained dendrimers. In order to study the type of the interactions between the functionalized dendrimers and a drug molecule, Naproxen which contains acidic groups, was examined as a hydrophobic drug in which the interactions would be of the electrostatic kind between its acidic groups and the lone pair electrons of nitrogen atom in imidazole groups. The quantity of the trapped drug and also the amount of its release were measured with UV spectrometric method in pH 1, 7.4 and 10. The average diameter of the nanocarriers was measured by Dynamic Light Scattering (DLS) technique
Results
The size range of particles was determined to be 16-50 nm for different generations. The rate of the release increased in pH=10 in all generations due to the increases in Naproxen solubility and the hydrolysis of the esteric bonds in the mentioned pH. The results showed that the amount of the trapped drug increased with the increase in the generation of the dendrimer and pH.
Conclusion
Based on our findings, we suggest CPEGC triblock dendrimers possess great potential to be used as drug/gene delivery system.
Keywords: Dendrimer, Nanocarrier, Encapsulation, Drug Delivery, Naproxen, Citric Acid
Introduction
Dendrimers are a new class of polymeric belongings. Dendrimer chemistry is one of the most attractive and hastily growing areas of new chemistry (Klajnert and Bryszewska, 2001, Tomalia et al., 1985, Spataro et al., 2010). The structure of these resources has a great contact on their physical and chemical possessions (Didehban et al., 2009b, Svenson and Tomalia, 2005, Haririan et al., 2010). Medical application is one of the private behaviors of the dendrimers (Didehban et al., 2009a). A choice of biologically active molecules, such as antibodies (D'Emanuele and Attwood, 2005) and sugar molecules (Ganta et al., 2008, Torchilin, 2001, Paleos et al., 2004) have been conjugated to the chain ends of the dendrimers. For example: Zanini and Roy (Schenning et al., 1998) organized a series of thiosialodendrimers by coupling a-thiosialoside to dissimilar generations of the dendrimers and investigated their binding to the silica acid- specific lectin from Limaxflavus. These structures are highly branched macromolecules with low polydispersity that supply many exciting opportunity to design novel drug-carriers, gene delivery systems and imaging agents (Basavaraj et al., 2009, Crampton and Simanek, 2007). There are attempts to use dendrimers in the targeted delivery of drugs and other beneficial agents (Namazi and Jafarirad, 2011). Drug molecules can be overloaded both inside the dendrimers as well as attached to the exterior groups (Jansen et al., 1995). Sialylated dendrimers, called sialodendrimers, have been shown to be effective inhibitors of the haemagglutination of human erythrocytes by influenza viruses. The first step in the infection of a cell by influenza virus is the attachment of the virus to the cell membrane. The attachment occurs through the interaction of a virus receptor haemagglutininwith sialic-acid groups presented on the surface of the cell (Wang et al., 2003). Sialodendrimersbind to haemagglutinin and thus prevent the attachment of the virus to cells. They can be useful curative agents in the prevention of bacterial and viral infections (Sigal et al., 1996). In addition, water soluble dendrimers are capable of binding and solubilising small acidic hydrophobic molecules with antifungal or antibacterial properties. The bound substrates may be unconfined upon contact with the target organism. Such complexes may be considered as potential drug delivery systems (Boas and Heegaard, 2004, Tomalia et al., 1990). An area that has concerned great notice is the contact between drugs and dendrimers (Liu et al., 2000). Several types of interactions have been explored, which can be broadly subdivided into the trap of drug molecules within the dendritic design (involving electrostatic, hydrophobic and hydrogen bond interactions) and the interaction between a drug and the surface of the dendrimers (electrostatic and covalent interactions) (Namazi and Adeli, 2005, Veronese et al., 1998). The applications of such systems have been several-fold, counting the use of dendrimers to improve drug solubility and bioavailability, and to act as release modifiers and platforms for drug targeting (Ambade et al., 2005, Kurtoglu et al., 2009, Qiu and Bae, 2006). The synthesis of the dendrimers from citric acid, and poly (ethylene glycol) (G1, G2 and G3) was already reported from our laboratory (Namazi and Adeli, 2003). In this work, we conjugated imidazole groups onto the surface of the dendrimers and investigated how the guest molecules get trapped in the dendritic compounds. Here, we report the preparation of the drug/dendrimer complexes containing G1-Imz, G2-Imz and G3-Imz of dendritic citric acid. Also the quantity of loaded drug and the controlled release of naproxen drug molecule from the synthesized dendritic compounds were investigated in pH 1, 7.4 and 10.
Materials and methods
Materials
Poly (ethylene glycol) 600 diacid (acid number 175, 96–98%, from Fluka) was dehydrated over Na2SO4. Citric Acid and pyridine (purified with refluxing over NaOH for 2 h and succeeding distillation) were obtained from Merck. Thionylchloride (from Merck) was purified by refluxing a mixture of 10 wt% linseed oil in thionylchloride for 2h and subsequent distillation. Dicyclohexylcarbodiimide (DCC) was purchased from Merck. Imidazole and Naproxen were purchased from Sigma Aldrich and Merck, respectively.
Instrumental measurement
FT-IR spectra were measured on a Shimadzu Model FT-IR-8101M spectrometer. 1 H NMR spectra was recorded on FT-NMR (400 MHz) Brucker in DMSO-d6. For the investigation of dendrimer/drug complex compounds UV 2100 Shimadzu spectrophotometer was applied.
Methods
The synthesis of G1- Imz, G2-Imz and G3-Imz
A solution of G1, G2 and G3 (2 g) in 15 mL of dry THF was added to a round-bottom flask equipped with reflux condenser, argon inlet, dropping funnel and magnetic stirrer 0.2 mL of dry pyridine was added to this solution by the dropping funnel in 15 min. The mixture was stirred vigorously for 10 min. Then a solution of DCC in 10 mL of dry THF was added to the mixture at 0 °C by the dropping funnel and was stirred for 20 min. After a drop wise addition of a solution of imidazole in 10 mL of THF, the mixture was stirred at 0 °C for 1 h, then under argon at room temperature for 24 h and for 48 h at 50°C. The solution was filtered off and was placed at 5 °C for 24 h and again it was filtered off. The product was precipitated in diethyl ether which was repeated for several times. The product was dissolved in 5 mL of water and stirred for 24 hat room temperature. The solution was filtered off and poured in 5 mL of water at 25 °C. The mixture was conducted into a cellophane membrane dialysis bag. The bag was closed and transferred into a flask containing 100 mL of water maintained at 25 °C. The external water was removed after 24 h and 100 mL of fresh water was added instead. The product was removed from the dialysis bag and dried under vacuum at 50 °C as an amorphous compound. The structure ofG2-Imz and G3-Imz are shown in Scheme 1A-B.
Scheme 1.
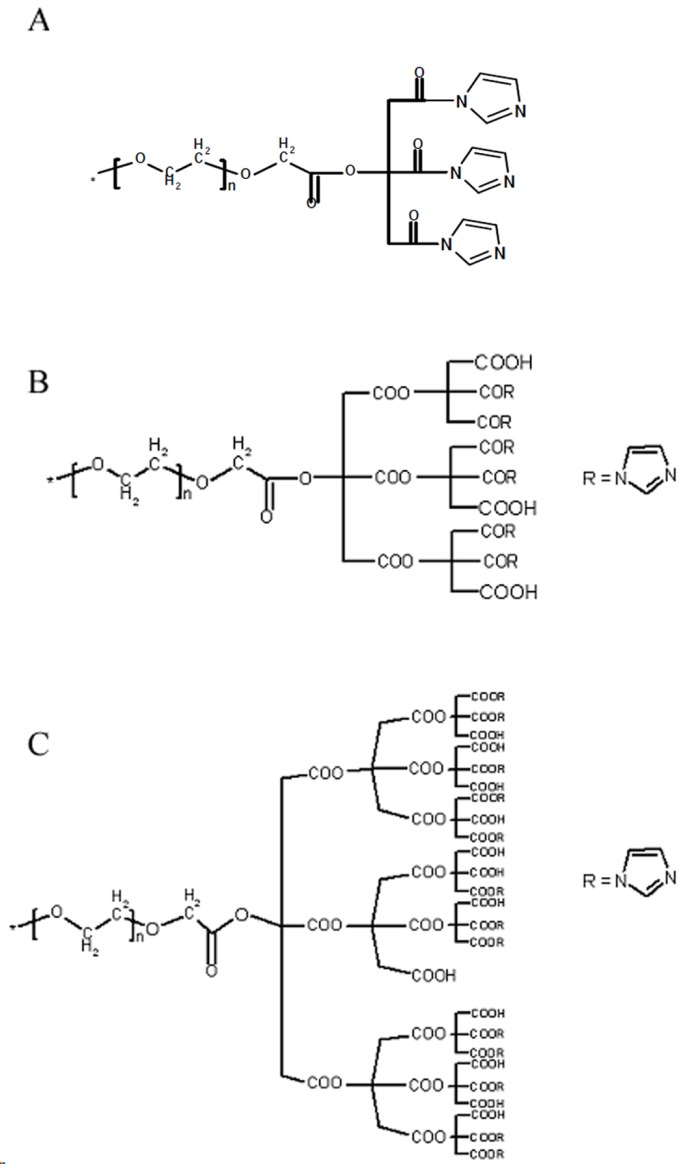
The dendrimer/imidazole conjugates. (A) G1-Imz conjugates. (B) G2-Imz conjugates. (C) G3-Imz conjugates.
The preparation of Gn (n=1-3) -Imz/Naproxen complexes
For the preparation of Gn(n=1-3)-Imz/Naproxen complexes, the dendrimers were dissolved in 20 mL of THF and were added to a round-bottom flask containing a solution of Naproxen drug molecule in 20 mL of THF and equipped with a reflux condenser and a magnetic stirrer. Then the solutions were stirred for 2h at 30°C. Complexes were precipitated in n-hexane and then dissolved in dichloromethane and again precipitated in diethyl ether to yield yellow compounds. The absorbed bonds in IR spectra for Gn (n=1-3)-Imz/Naproxen complexes are: IR (KBr, cm- 1 01): 1452- 1605(CO2-), 1748 (C=O), (2611) R3NH+, 1065 and 1107 (C-O), 2926 (CH2 citric acid), 3132 (CH Imidazole).
Results
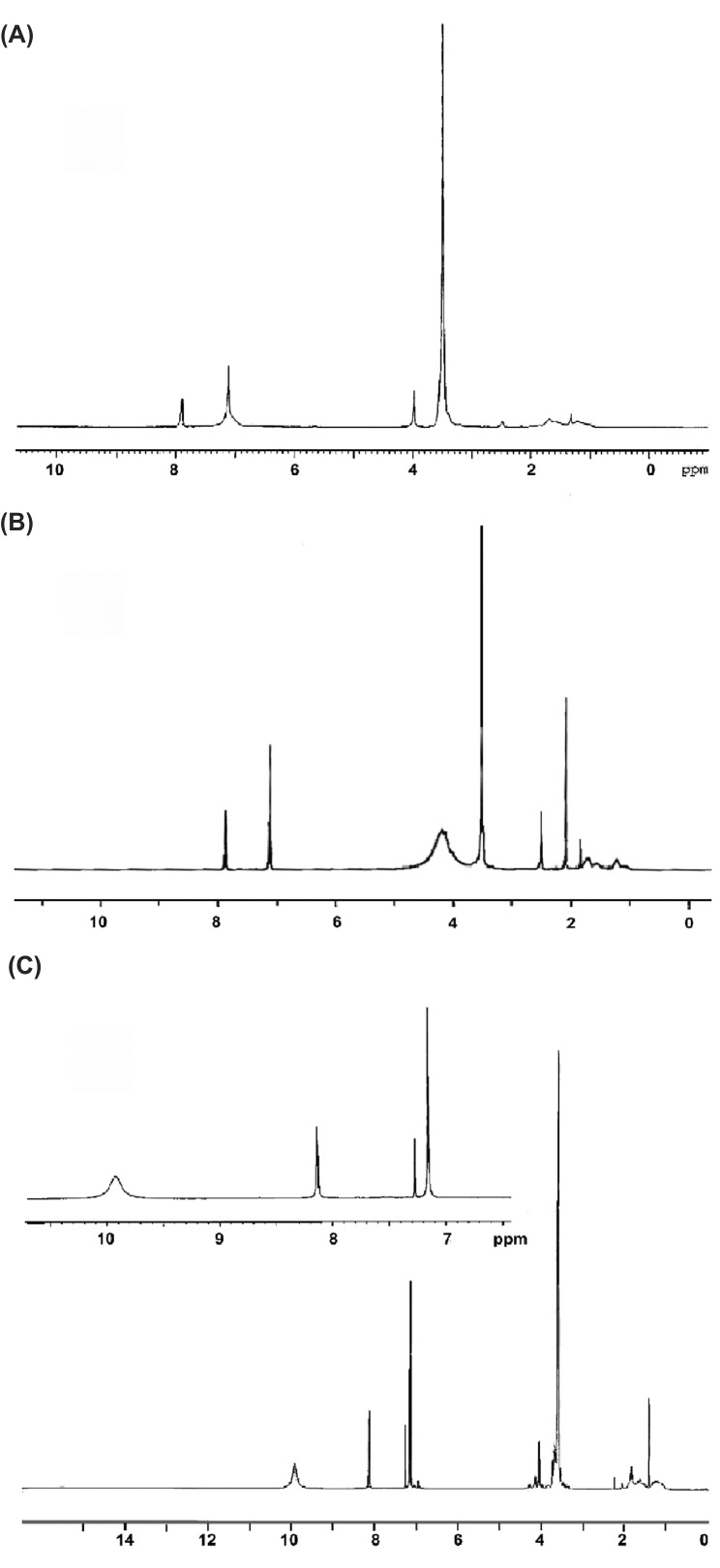
Compound G1 was synthesized through the reaction of ClOC–PEG–COCl with anhydrous citric acid using slightly modified literature method (Namazi and Adeli, 2003).ClOC–PEG–COCl was already produced via the chlorination reaction of diacid poly (ethyleneglycol) using thionylchloride with the yield of 100%. DCC was used for the synthesis of G2. For the preparation of G3 the reaction of G2 with citric acid was carried out using DCC in THF and the product was cut off (Namazi and Adeli, 2003). The compounds G1-Imz, G2-Imz, G3-Imz were synthesized via the reaction of G1, G2 and G3 with imidazole in the presence of dicyclohexylcarbodiimide,as shown in Fig.1A-C representing the 1 H NMR spectra of these compounds. In all spectra the aromatic protons of imidazole groups appear at 7.1-8.1 ppm and the peak at 2.5 ppm is related to DMSO-d6 as the solvent.
Fig. 1.
1H NMR (400MHZ) spectra of the dendrimer/imidazole conjugates. (A) G1-Imz in CDCl3. (B) G2-Imz in CDCl3. (C) G3-Imz in CDCl3.
The integral ratio of the aliphatic protons of PEG to the aromatic ones of imidazole was used to account the amount of imidazole groups that were conjugated onto the surface of the dendrimers. These ratios show 33% of functionalization for G1-Imz (see Fig.1A) which is lower in comparison to the integral ratio of G2-Imz in Fig.1B,it indicates higher amount of functionalization for G2-Imz which is 42% and for G3-Imz that was calculated to be 55%. The presence of signals at 8.8-10.1 ppm indicates that some of the acidic groups in the third generation of the dendrimer have not been reacted with imidazole groups also the protons of citric acid were appeared at 1-2.3 ppm.
The size of the nanocarriers
The size of nanocarriers was determined by the means of dynamic light scattering (DLS) technique which are 16, 20 and 45 nm, respectively for G1-Imz, G2- Imz and G3-Imz and are shown in Fig. 2.
Fig. 2.
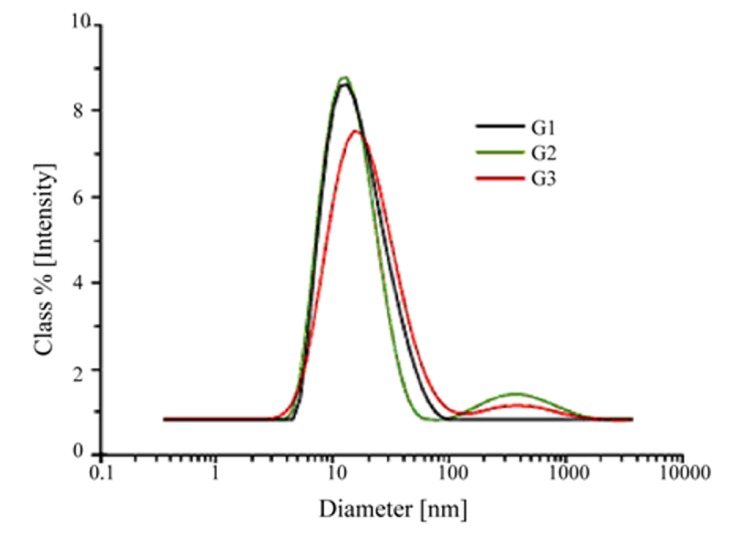
Dynamic Light Scattering diagrams of G1–, G2–, and G3–imidazole conjugates.
Loading Naproxen drug molecule into the dendrimers
The formation of the water-soluble complexes of guest molecules with G1-Imz, G2-Imz and G3-Imz as biocompatible compounds is described here as well. When the guest molecules are drugs, the resulted drug/dendrimer complexes could be used as ideal candidates for carriers and in drug release. Herein, we used Naproxen as the guest molecule that is lipophile and has an acidic group in its structure; its structure is shown in Scheme. 2.
Scheme 2.
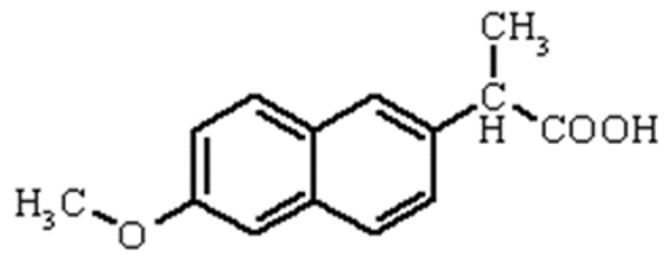
The structure of Naproxen drug molecule.
The electrostatic interaction between the acidic group of Naproxen and the lone pair electrons of nitrogen atom of imidazole in the exterior of the dendrimers was investigated. When Naproxen is loaded, the lone pair electrons of imidazole are protonated with the acidic proton of Naproxen. Therefore, the stretch vibration of carboxylate group in Naproxen is observed at 1400 and 1600 cm- 1 instead of 1700 cm- 1 due to the red shift and the N-H of protonated nitrogen atoms in imidazole appear at 2600-3300 cm- 1 .
In Fig. 3A-C FT-IR spectra of Gn (n=1-3)-Imz/Naproxen complexes are shown. Also, other investigations proved that the guest molecules like drug molecule can be loaded in the inner part of the dendrimers.
Fig. 3.
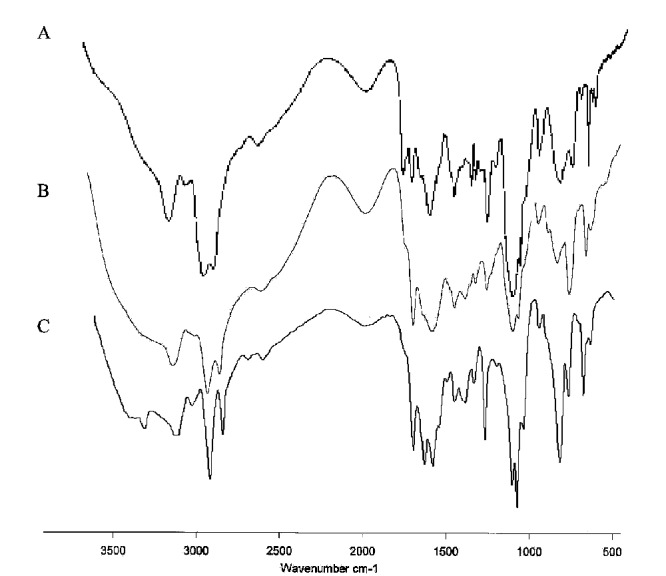
FT- IR spectra of the dendrimer/Naproxen complexes. (A) G1-Imz/Naproxen complex. (B) G2-Imz/ Naproxen complex. (C) G3-Imz/ Naproxen complex.
Calculating the amount of the trapped and released drug molecule
The amounts of the trapped drug for each generation before and after the functionalization with imidazole are displayed in Table 1 which is calculated with two procedures. In the first method, the amount of the trapped drug was measured using the weight of the dendrimers before and after complexation (drug/dendrimer complex).
Table 1. The amount of the trapped drug in Gn (n=1-3) /naproxen complex and Gn (n=1-3) - imz /naproxen complex.
| Gn (n=1-3) /naproxen complex | G1 /drug | G2 /drug | G3 /drug | G1 Imz /drug | G2 Imz /drug | G3 Imz /drug |
| Mol% of drug in complexes | 10.2 | 5.5 | 25 | 38.4 | 39.7 | 45.8 |
The difference between the measured weights gave the quantity of the trapped drug in the dendritic compound. In the second method, UV spectrophotometer was used. For instance, the calculation of the amount of Naproxen in drug/G2-Imz complex is as follows:
Weight of dendrimer after complexation =X
Weight of dendrimer before complexation=Y
X-Y=Weight of trapped drug
X=0.0611 g; Y=0.06 g and equal to 3.013×10-5mol of dendrimer
0.0611-0.06=1.1×10-3 g weight of Naproxen in the complex=4.77×10- 1 mol
4.77×10-6mol drug 3.013×10-5 mol dendrimer
X 100
XMol% of Naproxen=15.5mol %
Discussion
Studying the controlled release of Naproxen from the nanocarriers
The controlled release of Naproxen from the nanocarriers was investigated in pH 1, 7.4, and 10. In order to study the potential application of the first, second and third generations of the linear–dendritic dendrimers, they were functionalized with imidazole groups. Specific concentration of the dendrimer/drug complex was prepared in separate buffer media. Hydrolysis was carried out in a cellophane membrane bags permeable to low molecular weight of compounds and the amount of the drug release was determined in different pH values (pH 1, 7.4, and 10 all at 37 °C).
As it is known, HPLC and UV spectrophotometer are two common methods to study the release of the drug molecules from nanocarriers. In this work we used the latter method and the absorbance of the released drug at its λmaxwas determined. Figures 4 and 5 show the release of the drug from G1, G2 and G3 and G1-Imz, G2-Imz and G3-Imz in different pHs, respectively.
Effective factors on the rate and the amount of the release of the drug molecule
As mentioned before, the release of Naproxen drug molecule was studied in three buffer media with pH 1, 7.4 and 10 which are the pH of body’s biological fluids. The results displayed that both the rate and the amount of the release of the drug from dendrimer/drug complex is influenced by some factors such as the generation of the host molecule, biodegradability of the dendrimers, functional groups on the surface of the dendrimer, solubility of the drug, the interactions between the drug and the dendrimer in the complex and pH and the effects of each factor is considered as the following.
The generation of the host molecule
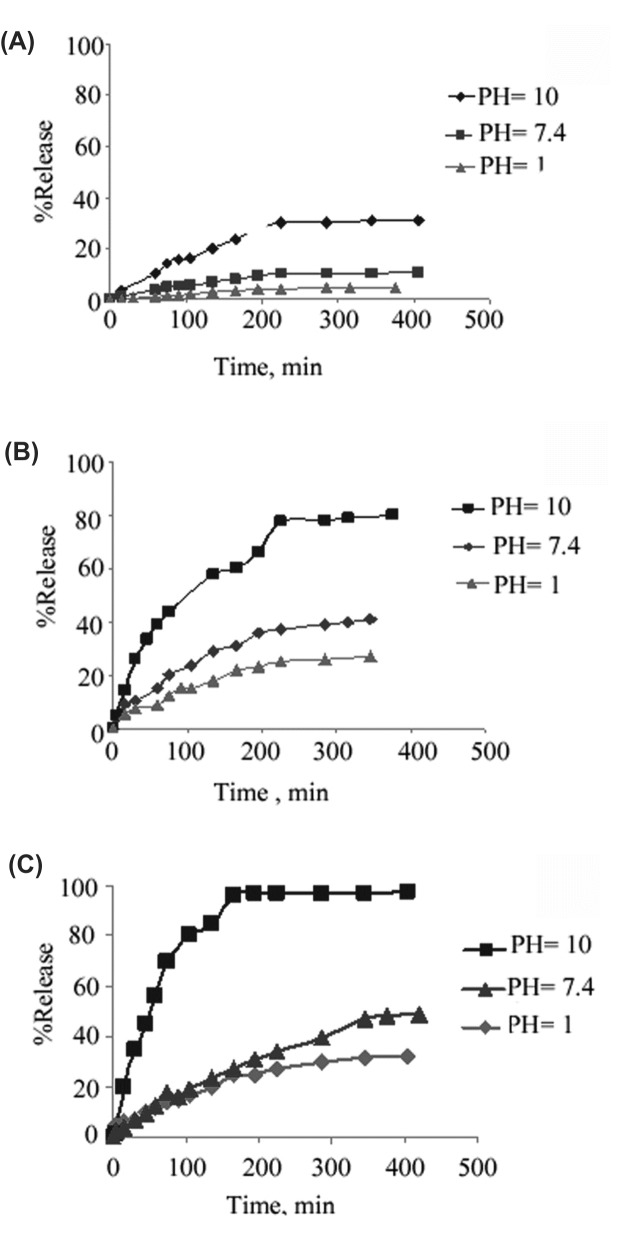
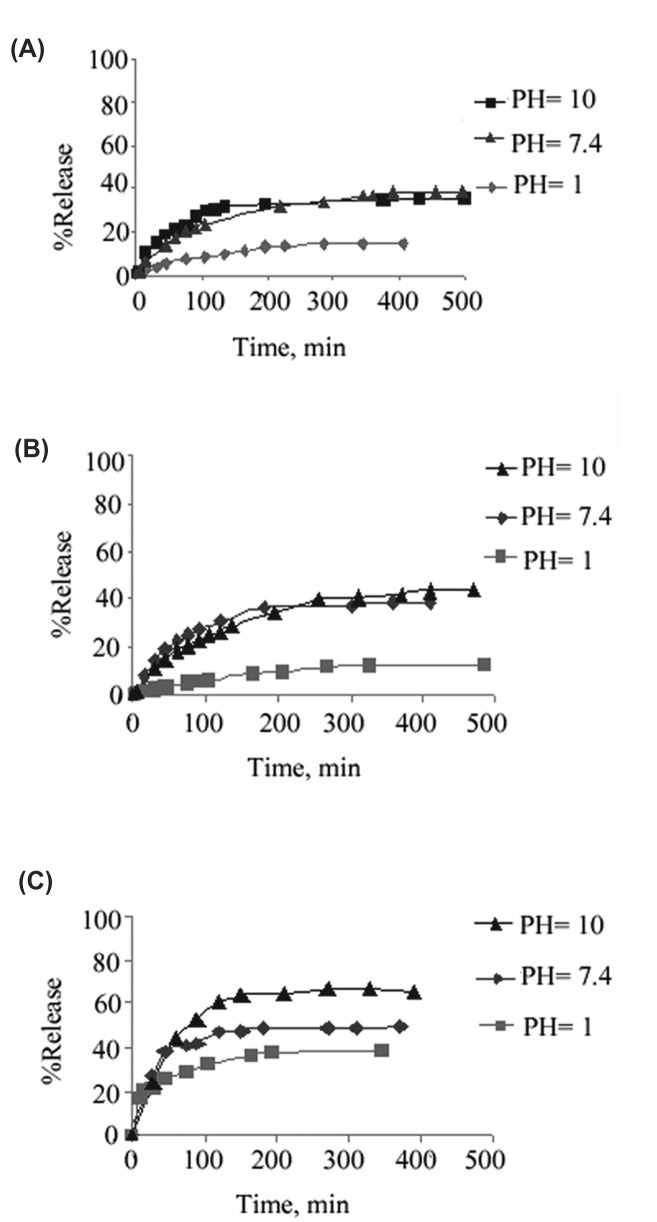
The release profiles show (Fig. 4. and Fig. 5.) that in both imidazole-functionalized dendrimers and unfunctionalized ones, since higher generations possess more cavities, the higher amounts of the loaded drug as well as the higher amounts of the release are observed. Thus, the amount of the release diminishes through the order of G3>G2>G1 and G3-Imz>G2-Imz>G1-Imz.
Fig. 4.
Release of Naproxen from G1 /drug complex (A), G2 /drug complex (B) and G3 /drug complex(C).
Fig. 5.
Release of Naproxen from G1-Imz/complex(A), G2-Imz/complex(B) and G3-Imz/complex (C).
Biodegradability of the dendrimers
Esteric bonds are formed in the synthesis of CPEGC triblock dendrimers which are easily hydrolyzed in basic solutions and lead to the release of the loaded drug molecules. Hence the rate and the amount of the release diminishes through the order of pH=10>pH=7.4>pH=1.
Functional groups on the surface of the dendrimer
The lone pair electrons of nitrogen atom in imidazole has electrostatic interaction with the acidic group of Naproxen drug molecule which results in higher amount of loading and consequently in more release in comparison with the unfunctionalized dendrimers.
Solubility of the drug molecule
The solubility factor has a significant effect in the rate of the release. As mentioned, Naproxen is a lipophile molecule with acidic group which is less soluble in acidic media so, it shows fewer tendencies to release in acidic solutions while it is highly soluble in basic solutions which leads to higher amount of release. It is obvious that this factor along with the mentioned ones influence the amount and the rate of the release
Conclusion
In this research citric acid dendrimers were used since they are water soluble and biocompatible and low toxic. Imidazole groups were conjugated onto the surface of the dendrimers and the size of the nanocarriers in the distilled water was investigated using DLS technique which was 16, 20 and 45 nm for G1-Imz, G2- Imz and G3-Imz, respectively. Naproxen, a lipophile drug molecule with an acidic group, was loaded into both functionalized and unfunctionalized dendrimers. The electrostatic interaction between the drug molecule and dendrimer in drug/dendrimer complexes was demonstrated with IR spectra. In-vitro release of Naproxen as the guest molecule was investigated in pH 1, 7.4 and 10. It was observed that the rate of the release increased in pH=10 in all generations which was related to the increase in Naproxen solubility and the hydrolysis of the esteric bonds in the mentioned PH. The effect of other factors such as the generation of the host molecule, biodegradability of the dendrimers, functional groups on the surface of the dendrimer and the interactions between the drug and the dendrimer in the complex were studied as well. The amount of the trapped drug in these nanocarriers was measured with two methods. The results showed that the amount of the trapped drug increased with the increase in the generation of the dendrimer and pH.
Ethical Issue
None to be declared.
Conflict of interest
Authors declare no conflict of interests.
Acknowledgments
Authors are pleased to acknowledge the University of Tabriz for financial supports of this work.
References
- Ambade AV, Savariar EN and Thayumanavan S . 2005 Dendrimeric micelles for controlled drug release and targeted delivery. Molecular pharmaceutics, 2(4), 264-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavaraj BV, Hiren MB and Ganesh KB . 2009 Dendrimers: emerging polymers for drug-delivery systems . Eur .J . Pharm . Sci ., 38, 185-196 [DOI] [PubMed] [Google Scholar]
- Boas U and Heegaard PMH . 2004 Dendrimers in drug research. Chemical Society Reviews, 33(1), 43-63 [DOI] [PubMed] [Google Scholar]
- Crampton HL and Simanek EE . 2007 Dendrimers as drug delivery vehicles: non-covalent interactions of bioactive compounds with dendrimers. Polymer International, 56(4), 489-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Emanuele A and Attwood D . 2005 Dendrimer-drug interactions. Advanced Drug Delivery Reviews, 57(15), 2147-62 [DOI] [PubMed] [Google Scholar]
- Didehban K, Namazi H and Entezami AA . 2009. a Dendrimer-based hydrogen-bonded liquid crystalline complexes: Synthesis and characterization. European Polymer Journal, 45(6), 1836-1844 [Google Scholar]
- Didehban K, Namazi H and Entezami AA . 2009. b Synthesis and characterization of liquid crystalline diethanolamine-based dendrimers. Polymers for Advanced Technologies, 20(12), 1127-1135 [Google Scholar]
- Ganta S, Devalapally H, Shahiwala A and Amiji M . 2008 A review of stimuli-responsive nanocarriers for drug and gene delivery. Journal of Controlled Release, 126(3), 187-204 [DOI] [PubMed] [Google Scholar]
- Haririan I, Alavidjeh MS, Khorramizadeh MR, Ardestani MS, Ghane ZZ and Namazi H . 2010 Anionic linear-globular dendrimer-cis-platinum (II) conjugates promote cytotoxicity in vitro against different cancer cell lines. International journal of nanomedicine, 563-75 [DOI] [PMC free article] [PubMed]
- Jansen JFGA, Meijer EW and Debrabandervandenberg EMM . 1995 The Dendritic Box - Shape-Selective Liberation of Encapsulated Guests. Journal of the American Chemical Society, 117(15), 4417-4418 [Google Scholar]
- Klajnert B and Bryszewska M . 2001 Dendrimers: properties and applications. Acta Biochimica Polonica, 48(1), 199-208 [PubMed] [Google Scholar]
- Kurtoglu YE, Navath RS, Wang B, Kannan S, Romero R and Kannan RM . 2009 Poly(amidoamine) dendrimer-drug conjugates with disulfide linkages for intracellular drug delivery. Biomaterials, 30(11), 2112-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MJ, Kono K and Frechet JMJ . 2000 Water-soluble dendritic unimolecular micelles: Their potential as drug delivery agents. Journal of Controlled Release, 65(1-2), 121-131 [DOI] [PubMed] [Google Scholar]
- Namazi H and Adeli M . 2003 Novel linear-globular thermoreversible hydrogel ABA type copolymers from dendritic citric acid as the A blocks and poly(ethyleneglycol) as the B block. European Polymer Journal, 39(7), 1491-1500 [Google Scholar]
- Namazi H and Adeli M . 2005 Dendrimers of citric acid and poly (ethylene glycol) as the new drug-delivery agents. Biomaterials, 26(10), 1175-83 [DOI] [PubMed] [Google Scholar]
- Namazi H and Jafarirad S . 2011 Controlled release of linear-dendritic hybrids of carbosiloxane dendrimer: The effect of hybrid's amphiphilicity on drug-incorporation; hybrid-drug interactions and hydrolytic behavior of nanocarriers. International journal of pharmaceutics, 407(1-2), 167-173 [DOI] [PubMed] [Google Scholar]
- Paleos CM, Tsiourvas D, Sideratou Z and Tziveleka L . 2004 Acid- and salt-triggered multifunctional poly(propylene imine) dendrimer as a prospective drug delivery system. Biomacromolecules, 5(2), 524-529 [DOI] [PubMed] [Google Scholar]
- Qiu LY and Bae YH . 2006 Polymer architecture and drug delivery. Pharmaceutical Research, 23(1), 1-30 [DOI] [PubMed] [Google Scholar]
- Schenning APHJ, Elissen-Roman C, Weener JW, Baars MWPL, van der and Meijer EW . 1998 Amphiphilic dendrimers as building blocks in supramolecular assemblies. Journal of the American Chemical Society, 120(32), 8199-8208 [Google Scholar]
- Sigal GB, Mammen M, Dahmann G and Whitesides GM . 1996 Polyacrylamides bearing pendant alpha-sialoside groups strongly inhibit agglutination of erythrocytes by influenza virus: The strong inhibition reflects enhanced binding through cooperative polyvalent interactions. Journal of the American Chemical Society, 118(16), 3789-3800 [Google Scholar]
- Spataro G, Malecaze F, Turrin CO, Soler V, Duhayon C, Elena PP, et al. 2010 Designing dendrimers for ocular drug delivery. European Journal of Medicinal Chemistry, 45(1), 326-334 [DOI] [PubMed] [Google Scholar]
- Svenson S and Tomalia DA . 2005 Dendrimers in biomedical applications--reflections on the field. Advanced Drug Delivery Reviews, 57(15), 2106-29 [DOI] [PubMed] [Google Scholar]
- Tomalia DA, Baker H, Dewald JR, Hall M, Kallos G, Martin S, et al. 1985 A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym J, 17(1), 117-132 [Google Scholar]
- Tomalia DA, Naylor AM and Goddard WA . 1990 Starburst dendrimers: molecular level control of size, shape, suface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed Engl, 29138-175
- Torchilin VP . 2001 Structure and design of polymeric surfactant-based drug delivery systems. Journal of Controlled Release, 73(2-3), 137-172 [DOI] [PubMed] [Google Scholar]
- Veronese FM, Marsilio F, Caliceti P, De Filippis P, Giunchedi P and Lora S . 1998 Polyorganophosphazene microspheres for drug release: polymer synthesis, microsphere preparation, in vitro and in vivo naproxen release. Journal of Controlled Release, 52(3), 227-237 [DOI] [PubMed] [Google Scholar]
- Wang Z, Itoh Y, Hosaka Y, Kobayashi I, Nakano Y, Maeda I, et al. 2003 Mechanism of enhancement effect of dendrimer on transdermal drug permeation through polyhydroxyalkanoate matrix. Journal of bioscience and bioengineering, 96(6), 537-40 [DOI] [PubMed] [Google Scholar]