Abstract
Introduction
The integrity of the cells/tissues in anterior and/or posterior segments of the eye plays a crucial role in biofunctions of the vision. To maintain ocular homeostasis, selective restrictiveness of the ophthalmic membranes and barriers control must act on shuttling of biomolecules. Thus, not all attempts to apply de novo nanotechnology approaches for ocular pharmacotherapy have met with the same successes as those cited here in this review, and sometimes these novel technologies tools provoke a great deal of challenges and hurdles mainly because of functional presence of these barriers.
Methods
Recent published articles related to applications of ocular nanomedicines were reviewed and highlighted in this review article.
Results
It seems the emergence of nanomedicines have arisen great hopes for ophthalmic pharmacotherapy, in which nanostructured medicines are expected to be able to cross the restrictive barriers of the eye. Although such fast inauguration of ocular nanomedicines will literally convey new challenges in the regulatory and translational processes, it will also grant a prolific platform from which many exciting, and yet unimagined, applications of biomedical nanotechnology will emerge for pharmacotherapy of the eye.
Conclusion
This review provides recent advancements on ocular nanomedicines.
Keywords: Molecular Medicine, Nanotechnology, Nanomedicine, Ocular Drug Delivery, Ophthalmology
Introduction
The unique structural and functional properties of the eye are synchronized by visual cells and transparent tissues. The regulatory mechanism of this organ relies mainly on tight cellular barrier between eye’s anterior and posterior segments which controls fluids and solutes traverse through membranes (the schematic illustration of the eye structure and main ocular barriers are demonstrated in Fig. 1.). Similarly, drug transport via these barriers is also highly controlled and limited; hence, application of novel drug delivery/ targeting strategies for effective pharmacotherapy seems to be crucial.
Fig. 1.
Schematic illustration of the main structure of the eyeand the ocular barriers. The primary physiologic blockage against installed drugs is the tear film. Cornea is the main route for drug transport to the anterior chamber (I). The retinal pigment epithelium and the retinal capillary endothelium are the main barriers for systemically administered drugs (II). Intravitreal injection is an invasive strategy to reach the vitreous (III). The administered drugs can be carried from the anterior chamber away either by venous blood flow after diffusing across the iris surface (1) or by the aqueous humor outflow (2). Drugs can be removed from the vitreous away through diffusion into the anterior chamber (3), or by the blood–retinal barrier (4). The image was adopted with permission from (Barar et al. 2009).
Recent advances in gene based therapeutics and novel nano-sized delivery targeting/delivery agents are generating new insights for the ocular disease therapy. However, efficient delivery and adequate bioavailability of such medications should be verified (Barar et al. 2008; Urtti 2006).
Blood ocular barriers
Normal ocular structure and visual function properties are maintained by blood ocular barriers consisting of two main components (i.e. blood aqueous barrier (BAB) and blood retinal barrier (BRB)). This barrier physically separates blood vessels from internal segment of eye, controlling passage of any particle/chemical into ocular tissues. As illustrated in Fig. 1, ocular medications administered via local or systemic routes must overcome this barrier to achieve adequate concentration and maintenance in retina and vitreous. Furthermore, blood ocular barrier maintains tissue/fluid composition and produces aqueous humor (Cunha-Vaz 1997a). The control of inflow/outflow of aqueous humor provides the sufficient pressure inside the eye (Fischbarg 2006). In the following sections, the location of blood aqueous and blood retinal barriers will be discussed and their functionality to impede drug transport will further be reviewed.
Blood-aqueous barrier
The BAB is formed by tight junctions of iris vascular endothelium and non-pigmented layer of ciliary epithelial cells (Fig. 1). This structure is located in the anterior part of the eye, preventing undesired traverse of exogenous materials into the ocular posterior segment and providing transparency and chemical equilibrium of the ocular fluids (Cunha-Vaz 1997b; Freddo 2001).It is of note that iris blood vessels withstand macromolecule (e.g. horse radish peroxidase with a molecular size of 40 kDa) passage, whilst capillary of ciliary body is less restrictive and allows favored outward traverse of substances toward systemic circulation. Moreover passive transport through BAB, results in rapid elimination of substances with small molecular size and more lipophilicity as compared to large and hydrophilic molecules. For example, the clearance of pilocarpine is13.0 µl/ml in rabbits whereas inulin clearance is close to the rate of aqueous humor turnover (i.e. 3.0–4.7 ml/min) (Conrad and Robinson 1977).
The blood-retinal barrier
The blood-retinal barrier (BRB), which locates in the posterior part of the eye, contains two types of cells, i.e. 1) the retinal capillary endothelial (RCE)cells, and 2) the retinal pigment epithelial (RPE)cells. These cells form the inner andouter BRB, respectively. Basically, the specialized transport processes and the restrictive barrier functions RPE selectively control the transportation of nutrients/compounds, by which functionalities only designated nutrients can be traversed between choroid and retina (Duvvuri et al. 2003; Mitra et al. 2006). The inner BRB covers the lumen of retinal capillaries that are able to selectively protect the retina from circulating molecules of the blood. In fact, the RCE cells possess intercellular tight junctions which are formed upon the intercellular communications of the RCE cells with the glial cells (Gardner et al. 1999), similar tothat in the blood–brain barrier (BBB) by brain microvasculature endothelia (Janzer and Raff 1987). Due to the functional expression of tight junctions and intercommunications with astrocytes and Müller cells, RCE cells display biological characteristics similar to BBB (the brain capillary endothelial cells associated with pericytes and astrocytes) with trans-endothelial electrical resistance (TEER) value of 1500-2000 (W.cm 2 ) (Barar and Omidi 2008; Omidi et al. 2003a; Omidi et al. 2008; Smith et al. 2007).
Given these facts, it appears that the satisfactorily delivery and efficient pharmacological effects of drugs within the vitreous and the retina require systemic or intravitreal drug administration. Nevertheless, systemic application via oral or intravenous administration necessitates very high doses of the drug because the blood flow and restrictive functionality of BRB allow only very trivial fraction of the drug to reach the posterior chamber (typically only 1-2% of the concentration in the plasma). But, it should be evoked that administration of large portion of the drug can be associated with inadvertent adverse consequences (Selvin 1983).
In short, damage of the normal BRBappears to be the common feature to many retinal degenerative diseases such as diabetes. Thus development of novel modalities to prevent loss of barrier properties or restore barrier properties is considered as a highpriority in ophthalmology.
Strategies to overcome blood ocular barriers
In recent years, for treatment of many oculardisorders, there has been a profound shift towards implementation of more efficient treatment paradigms. For example, the neurodegenerative disorder “glaucoma” which is associated with elevatedintraocular pressure has affected many patients’ lives, while its treatment has fortunately moved from the management of intraocular pressure tothe prevention of neurodegeneration and maintenance of retinal function. The artificial tears is no longer the main treatment for the dry eye which is a common cause of patient visits to eye care specialists damaging the ocular surface. It is now being controlled with Restasis® (cyclosporinA ophthalmic emulsion), which targetedthe immune component of the disease (Attar et al. 2005).
In ocular pharmacotherapy, the biggest challenge is achievement of the preferred concentration at the intended ocular tissue. To tackle this issue, a variety of conventional ocular drug delivery systems have been developed for the production of effective ophthalmic drug formulations. Most of these ophthalmic drugs are delivered to the eye via aqueous vehicles. Nonetheless,the aqueous vehicles exhibit poor ocular bioavailability due to rapid drainage, lacrimation and tear turnover, and if penetration occurs, only a short duration of action will be observed (Hillaireau et al. 2006; Lang 1995).
Moreover, application of many potentially active ophthalmic compounds is seriously limited because of their very low water solubility. They, accordingly, needto be administeredeither through alternative routes or by optimized delivery system. Among various approaches for improving ophthalmic delivery of lipophilic drugs, hydrogels, microparticles, nanoparticles and liposomal formulationshave been shown to favor topical targeting and to improve drug bioavailability (Patravale et al. 2004). Of these DDS, nanoformulationshave raised promising potential for efficient ocular delivery (Bucolo et al. 2004). In fact, the colloidal nanoparticle drug carriers emerge to be useful for ocular absorption enhancement through various mechanisms including: prolonged drug residence time in the cornea and conjunctival, sustained drug release from the delivery system, and reduced pre corneal drug loss (Bu et al. 2007). Surprisingly, over the past two decades,nanoformulations of ophthalmic drugs have not yet been undertaken in clinical practice as fast as it was expected to be.
Drug-polymer nanoformulations
Drug delivery systemswith biodegradable/bioerodible polymers can provide asignificant advantage over the non-degradable systems becausethe entirety is eventually absorbed by the body,eliminating the need for subsequent removal. However, these polymers are predisposed and time dependent due to erosion, which can occur through the following mechanisms: a) cleavage ofthe cross-linked or water-soluble backbone in the cross-linkedwater-soluble macromolecules, b) hydrolysis,ionization, or protonation of pendant groups in the water-insoluble macromolecules, and c) hydrolytic cleavage of labile bonds inthe polymer backbonehigh-molecular-weight, water-insolublemacromolecules (Kimura and Ogura 2001).
The pattern of drug release largely depends upon the association of drug with polymers since two approaches can be undertaken for formulation, i.e. a drug core surrounded bya rate-controlling biodegradable membrane, or the drug dispersed within polymer(s). Of the polymer based systems, the nanoparticles are colloidal drug carrier systemswith a size range of 10 to 1000 nm, while nanospheres are solid matricial structures carrying drug molecules within the matrices and/or adsorbed onthe surfaces of the colloidal carriers and finally nanocapsules are smallcapsules with a central core surrounded by a polymeric shell with dissolved/adsorbed drug molecules in core/surface interface.
Upon our literature survey, the most commonlyused polymers in the ophthalmic drug formulationsare: poly(alkyl cyanoacrylates), PCL, andpoly(lactic acid)/poly(lactic-co-glycolic acid) (PLA, PGA, PLGA). Moreover, some others such as chitosan (CS), ERL/ERS, PS, and poly(acrylic acid) (PAA) as well as the bovine serum albumin (BSA) has also been exploited for ocular delivery as drug-loaded nanoformulations. Fig. 2 represents the chemical structures of some important polymers used in the preparation of nanoformulations.
Fig. 2.
Chemical structures of selected polymers used for preparation of ocular nanomedicines. A) Chitosan. B) Polystyrene. C) PLGA( copoly lactic acid/glycolic acid). D) PLA(poly lactic acid). E) Eudragit E. F) Eudragit RL/RS .
Given that the surface of the ocular tissues (e.g., cornea and conjunctiva) is negatively charged, the cationiccolloidal nanoparticles are expected to confer better penetration potential through the ocular membranes and barriers. Of the polymers used for ocular delivery, few polymers (CS, ERL and ERS) grant positively charged nanoparticles (Bu et al. 2007). Of the biodegradable polymers, the poly(lactic-co-glycolic acid) copolymers (PLA, PGA, and PLGA) have been widely utilized as the most promising biodegradable materials, which have also been reported to be the most safe polymers used in vivo successfully with no significant toxicity (Agnihotri and Vavia 2009; Athanasiou et al. 1996; Dong et al. 2006; Kobayashi et al. 1992).
In a study of small pigment epithelium-derived factor (PEDF)neuroprotectiveeffects, peptides injected intravitreally as free peptides or delivered in PLGA nanospheres, were tested in retinal ischemicinjury in C57BL/6 mice. This study presented that injection of PEDF peptide (alone or as PLGA-based nanospheres) showed protective effects. However, the PLGA-PEDF nanospheres resulted in longer-term protection of the retinal ganglion cell layer with no noticeable side effects at 7days, thus conferring higher clinical advantages for longer-term treatments of retinal diseases (Li et al. 2006).
Agnihotri and Vavia (2009) successfully loaded diclofenac sodium in PLGA nanosuspensions, which were applied to rabbit eye and examined with a modified Draize test. These polymeric nanoparticles seemed to be devoid of any irritant effect on cornea, iris, and conjunctiva. Further, higher decrease of the sodium arachidonate induced inflammation was obtained by means of PLGA nanoparticles incorporating flurbiprofenin the rabbit eye after topical instillation, thus indicating its usefulness for inhibition of ocular inflammation (Vega et al. 2006). Similarly, Dong and coworkers (2006) reported that the intravitreal implantation of the cyclosporinAloaded PLGA can effectively reduce the intraocular inflammation in rabbits with no toxicity. Further, the intravitreal injections of a suspension of polylacticacid micro/nanospherescontaining 1% adriamycin/doxorubicin were reported to provide sustained, first-orderrelease for approximately two weeks. Using microarray technology, we have examined the toxicogenomic potential of the PLGA-based nanoformulations using small arrays hosing 200 gene spots, as a result of which no significant gene expression changes were observed (our unpublished data). Fig. 3 shows scanning electron micrographs of PLGA.
Fig. 3.
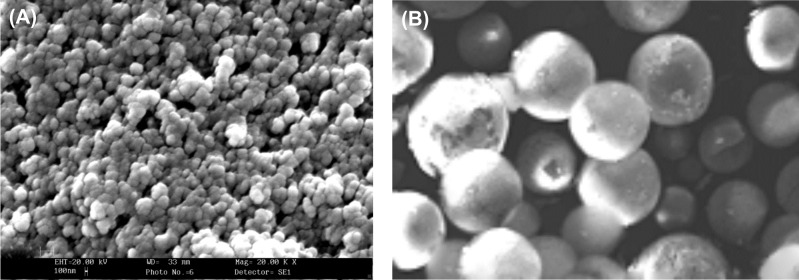
Scanning electron micrographs of packed (A) and particulate single (B) PLGA.
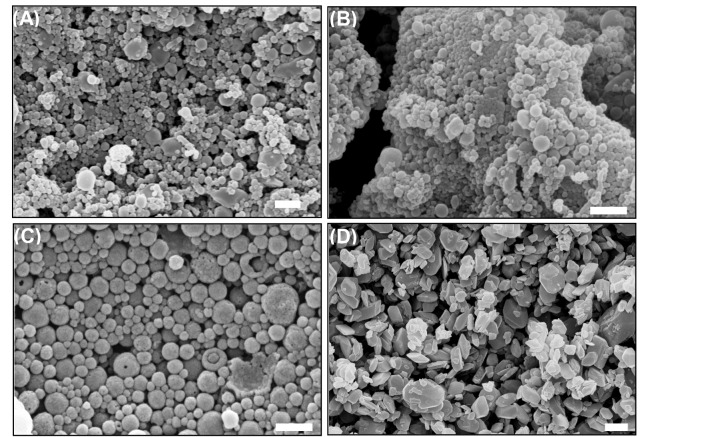
Most of nanoparticles used for ocular investigations appeared to be mucoadhesive and biocompatible, nevertheless polystyrene (PS), Eudragit® RL100 (ERL) and RS100 (ERS) are not biodegradable. Fig.4 shows the scanning electron micrographs of the piroxicam nanoparticles formulated with ERS.
Fig. 4.
Scanning electron micrographs of piroxicam formulations.A) Piroxicam:ERS nanoparticles at the ratios of 1:2.5. B) Piroxicam:ERS nanoparticles at the ratios of 1:10. C) TreatedERS. D) Treated piroxicam. Bar equals to 2 mm. ERS:Eudragit®RS100. The image was adopted with permission from (Adibkia et al. 2007b).
Chitosan, a deacetylated chitin, is biodegradable, biocompatible and nontoxic polymer, whose nanoparticles have been demonstrated to penetrate effectively conjunctival and corneal epithelial cells. It is a promising ophthalmic vehicle because of its probable superior mucoadhesiviness caused by electrostatic interactions with the negative charges of the mucosal layers.
In an interesting investigation, animals were treated with cyclosporine A-loaded chitosan nanoparticles, which resulted in significantly higher corneal and conjunctival drug levels than those treated with a suspension of cyclosporin A in a chitosan aqueous solution or in water (De Campos et al. 2001). It has also been demonstrated that the amounts of fluorescent nanoparticles in cornea and conjunctiva were significantly higher than that of a control solution. These amounts were fairly constant for up to 24 h. A higher retention of chitosan nanoparticles in the conjunctiva compared with in the cornea was observed (De Campos et al. 2004).
Liposomal nanomedicines
The vesicular lipid bilayers are basically defined as “liposomes”, which can contain one or more aqueous compartments. Upon the number of bilayers, these lipid based globular structures can be categorized into multilamellar and unilamellar vesicles. The unilamellar vesicles include small unilamellar vesicles (SUV) and large unilamellar vesicles (LUV). Drugs, based on their solubility characteristics, can be entrapped in the lipid bilayers or the aqueous compartment (Fenwick and Cullis 2008).
Liposomal nanomedicines (LNM) were first developed to encapsulate small conventionaltherapeutic drugs, where the earliest attempts involved passive entrapment of drugs resulted in rapid production of stable, homogeneous populations of LUVs (~100 nm). Owing to the composition of LNMs, they are biodegradable and relatively nontoxic, which makes them interesting as drug-delivery systems. The cationic nanoliposomes have been evaluated for their genotoxicity potential in A431 and A549 cells, which resulted in significant gene expression changes mainly related to apoptosis signaling paths (Omidi et al. 2003b; Omidi et al. 2005). .
Owing to the unique architecture of the nanoliposomes, when they used as an ocular drug delivery systems (DDS), the LNMs can come into intimate contact with corneal and conjunctival epithelial cells, facilitating drug absorption. The main goal of LNMs is to reduce side effects while maintaining or enhancing the efficacy of the administered medication. It should be noticed that the LNMs are not usually taken up by healthy tissue as is the free drug. The normal tissues in corneal/noncorneal routes are continuous, with non-fenestrated endothelium of the vasculature, andtight endothelial junctions (on the order of 5 nm) prevent the extravasation of small liposomal carriers. The basal tissues also inhibit the extravasation of macromolecules. Based upon the disease/drugs used, the LNMs can be used to passively target the designated markers, through which drugs could be accumulated selectively at sites of disease (Fenwick and Cullis 2008).
The impact of a single intravitreal injection of vasoactive intestinal peptide (VIP) loaded in rhodamine-conjugated liposomes (VIP-Rh-Lip) on experimental autoimmune uveoretinitis (EAU) has been investigated in Lewis rats. Clinical and histologic assessments showed that macrophages expressed transforming growth factor-beta2, low levels of major histocompatibility complex class II, and nitric oxide synthase-2 in VIP-Rh-Lip-treated eyes in which the intraocular levels of interleukin (IL)-2, interferon-gamma, IL-17, IL-4, GRO/KC, and CCL5 were reduced with increased IL-13. These findings clearly imply that the encapsulation of VIP within liposomes can effectively deliver VIP into the eye and prevent the EAU (Camelo et al. 2009). Elimination of liposomes from the vitreous occur via a diffusional process through the anterior chamber, where SUVs and LUVs show half-life of 10 and 20 days, respectively (Barza et al. 1987). Drug release from liposomal systems is dependent on the concentration of the drug in the liposome. Thus, in the case of long-term treatment, the high concentration of drugs encapsulated in liposomal carriers may raise problems associated with vitreous clouding; nevertheless these drawbacks may be acceptable in endophthalmitis. Besides, sometimes liposome entrapment can decrease the efficacy of drugs as reported for amphotericin B in a rabbit model with fungal (Candida albicans) endophthalmitis (Barza et al. 1987). Despite huge investigations, at this stage, the liposomal drugs approved by the FDA are: liposomal daunorubicin (DaunoXome®, Gilead Sciences, Inc., approved in 1996); liposomal cytarabine (DepoCyt®, DepoTech Corporation, approved in 1999); liposomal Amphotericin B (AmBisone®, Fujisawa, approved in 1997); liposomal doxorubicin HCl (Doxil®, ALZA Pharmaceuticals, approved in 2007). Still, nano-scaled formulations are under investigations for ocular use.
Of the lipid based nanoformulations, the cationic lipids (CLs) have been widely used as gene delivery systems. These structures were initially exploited by Felgneret al. (1987), who used liposomes consisting of N-[1-(2,3-dioleyloxy) propyl]- N,N,N-trimethylammonium chloride (DOTMA) and dioleoylphosphatidylethanolamine (DOPE) for DNA traverse across cell membranes, and showed high level expression of the encoded gene. Numbers of novel cationic lipids have soon after been synthesized and shown to possess similar transfection activity within target cells. Cationic lipids possess either mono- or poly-cationic head groups. DOTMA, dimyristooxypropyl dimethyl hydroxyethyl ammonium bromide (DMRI) and dioleoyloxy-3-(trimethylammonio)propane (DOTAP) are monocationic. While, Dioctadecylamidoglicylspermin (DOGS), N-(1-(2,3-dioleyloxy)propyl)-N-2-(sperminecarboxamido)ethyl)-N,N-dimethyl- ammonium trifluoracetate (DOSPA) and 3beta-(N-(N',N'-dimethylaminoethane)-carbamoyl)cholesterol (DC-Chol) have polycationic head groups. DOGS (transfectam or lipofectin) and DOTMA are examples of mostly used CLs for transfection; reader is directed to see (Liu et al. 2003; Nicolazzi et al. 2003). Cationic lipid-based delivery systems possess positively chargedsurface, at which these lipid based nanosystems can attach the cell surface that normally display negative charges. The cellular toxicity of cationic lipids is deemed to be attributed with the surface charge potential of the cationic lipids. It should be evoked that the lipid-DNA lipoplex is thought to enter cells via adsorptive endocytosis and, by mechanisms not fully understood as yet, release nucleic acids out of the endosomal/lysosomal compartments with the net effect of yielding high uptake and intracellular delivery of genes and oligonucleotides (Pedroso de Lima et al. 2001).
Nanostructured dendrimers
Tomaliaet al. (1984) developed the first dendrimer, which was named the StarburstTMpolyamidoamine (PAMAM) dendrimer due to its dendritic branches and controlled starburst growth. This macromolecule is built on an ammonia core with extending branches of alternating methyl acrylate and ethylene diamine molecules (Tomalia et al. 1984). Fig. 5 represents the chemical structures of PAMAM (generation 3).
Fig. 5.
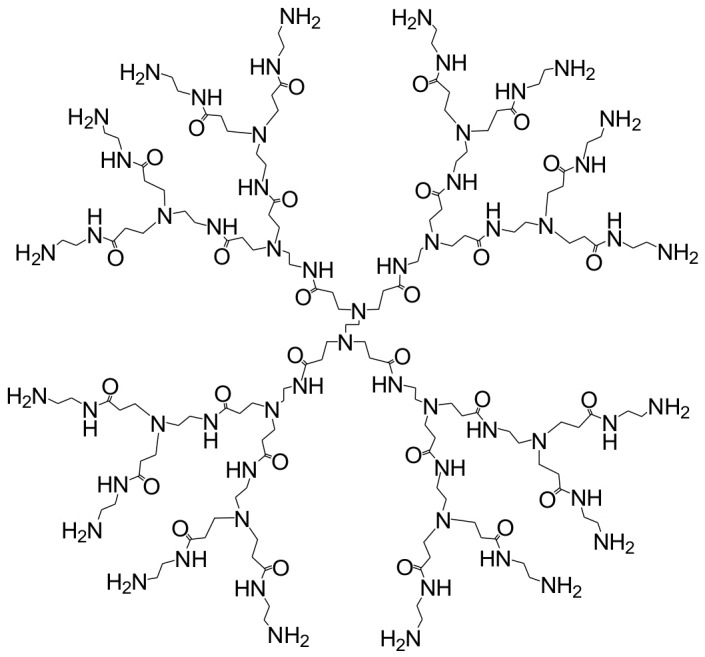
Chemical structures of PAMAM (generation 3).
Dendrimers are composed of concentric, geometrically progressive layers created through radial amplification from a single, central initiator core molecule containing either three or four reactive sites such as ammonia or ethylene diamine. These nano-scale macromolecules are three dimensional and highly branched monodispersed nanostructures that are obtained by an iterative sequence of reaction steps producing a precise, unique branching structure (Loutsch et al. 2003).
Fig. 6 represents the chemical structures of polypropylenimine (PPI)diaminobutane (DAB) dendrimers; i.e., generation 2 (panel A) with 8 protonable surface amine groups and generation 3 (panel B) with 16 protonable surface amine groups.
Fig. 6.
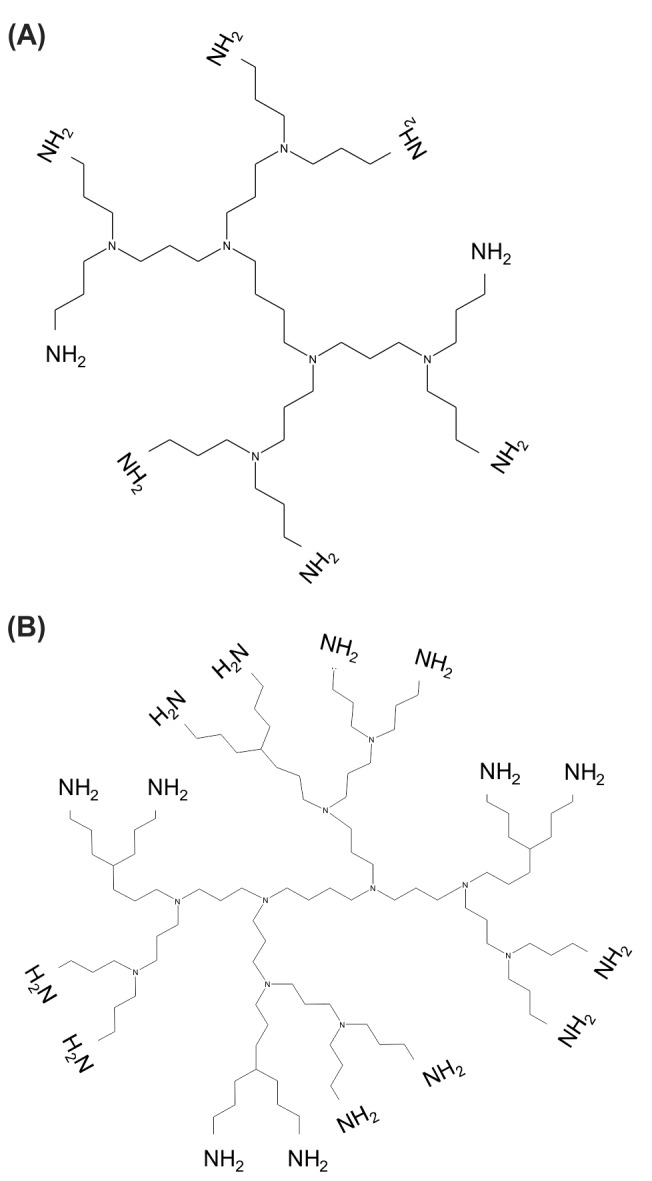
Chemical structures of polypropylenimine (PPI)diaminobutane (DAB) dendrimers. A) DAB generation 2 with 8 protonable surface amine groups. B) DAB generation 3 with 16 protonable surface amine groups.
These nanostructures provide globular nanosystems of 1-100 nm depending on the molecular weight and number of generations. Its surface ultimately determines the structure’s interactions with its environment, as a result of which drugs/genes can be incorporated with and released in a controlled manner (Vandervoort and Ludwig 2007).
Interestingly, the influence of a controlled incremental increase in size, molecular weight and number of amine, carboxylate and hydroxyl surface groups in several series of PAMAM dendrimers for controlled ocular drug delivery were investigated. The duration of residence time for various generations (1.5, 2-3.5 and 4) in the New Zealand albino rabbit resulted in longer residence time for the solutions containing dendrimers with carboxylic and hydroxyl surface groups, which was largely dependent on size and molecular weight (Vandamme and Brobeck 2005).
The modification of the dendrimer surface (e.g., addition of functional groups) is achievable through the addition of either subnanoscopic (e.g., small molecules) or nanoscaled reactants (e.g., DNA, antibodies, and proteins). The latter appears to be the preferred approach. For example, in a study to inhibit the laser-induced choroidal neovascularization (CNV), lipophilic amino-acid dendrimer was exploited to deliver an anti-vascular endothelial growth factor (VEGF) oligonucleotide (ODN) into the eyes of rats. Analysis of fluorescein angiograms of laser photocoagulated eyes revealed that dendrimer plus ODN significantly inhibited the development of CNV for 4-6 months by up to 95% in the initial stages, while ODN alone showed no significant difference (Marano et al. 2005).
Interestingly, generation 2 polypropyleneimineoctaamine dendrimers crosslinked with collagen were reported to support human corneal epithelial cell growth and adhesion, with no cell toxicity. Thus, these nanostructures might be suitable scaffolds for corneal tissue engineering(Duan and Sheardown 2006). In ocular gene therapy, the control of gene transfection within the eye is merely an important issue, in particular when a light-induced delivery of DNA, drugs or other biological factors is the main objective. In a study, Nishiyama et al, (2005) devised a ternary complex composed of a core containing DNA packaged with cationic peptides and enveloped in the anionic dendrimer phthalocyanine (with a photosensitizing action). They showed that the ternary complex was able to profoundly (100-fold) enhance transgene expression in vitro with reduced photocytotoxicity, in which subconjuctival injection of the ternary complex followed by laser irradiation resulted in transgene expression only in the laser-irradiated site. This, surely, is a new biomedical application for dendrimeric nanostructures with successful results in the photochemical-internalization-mediated gene delivery in vivo (Nishiyama et al. 2005).
Nanomedicines paradigms in ocular diseases
In some diseases of the eye such as diabetic retinopathy, central retinal vein occlusion, choroidal neovascularisation (CNV) and intraocular solid tumors, angiogenesis play a key role, thus targeting the biomarkers within the ocular tissue is deemed to be an efficient treatment modality (Sahoo et al. 2008). Further, explicitly, no lymph system is presented in the retina environment. Thus, in retinal diseases attributed with neovascularization (e.g. wet AMD), treatment modes could be similar to the strategies which are recruited against solid tumors, i.e. displaying enhanced permeability and retention (EPR) effects. These facts further highlights the biological impacts on required pharmacotherapy to achieve enhanced drug permeation,controlled release of drugs, and targeted pharmacotherapy through specific targeting markers. The biological characteristics of the eye render this organ exquisitely impervious to the foreign substances, thus, for attainment of an optimal concentration at the intended ocular tissue of action through circumventing the ocular barriers, colloidal nanoparticle drug carriers have been devoted a great deal of attention (Bu et al. 2007).
Emergence of nano-scaled pharmaceuticals like nanosuspensions, solid lipid nanoparticles and liposomes appear to resolve the solubility-related problems of poorly soluble drugs such as piroxicam, dexamethasone, methylprednisolone, budenoside, gancyclovir (Kayser et al. 2005). Based upon the biological architecture of the eye together with the physicochemical characteristics of the nanostructured medicines (i.e., particle charge, surface properties and relative hydrophobicity), these medications can be designed to successfully circumvent the blood-eye barriers. Since encapsulation of drugs can grant further protection as well as prolonged/controlled release, thus they confer better controlling tools for some chronic ocular diseases like chronic CMV retinitis, in which the intravitreal delivery of ganciclovir (GCV) seems to be the preferred strategy. Given its 13 h half-life, frequent injections of GCV is necessary to maintain therapeutic levels,however its use may be limited due to the consequential side effects such as cataract development, retinal detachment and endophthalmitis (Jabs 1995). Thus, to avoid repeated injections, intravitreal implants can be used to provide prolonged drug release even though some drawbacks like astigmatism and vitreous hemorrhage as well as couple of surgery requirements may limit its use, too (Muccioli and Belfort, Jr. 2000). These difficulties can be overcomed by using nanomedicines made up of various natural/biodegradable polymers like albumin and PLGA, because of their smaller size and controlled release properties (Sahoo et al. 2008).
Piloplex®, consisting of endophthalmitispoly (methyl) methacrylate–co-acrylic acid, is a nano-scaled colloidal carrier system effectively used in glaucoma patients as twice-daily instillations. Multidimensional mechanisms appear to be involved for the pharmacologic action of ocular nanosystems including extending the time of drug residency in the cornea/conjunctiva, sustaining drug release from its carrier, reducing the precorneal drug loss and targeting the desired biomarker (Bu et al. 2007; Sahoo et al. 2008; Vandervoort and Ludwig 2007). Thus, it is highly desirable to exploit bioadhesive materials for formulation of nanosystems to be retained in the cul-de-sac after topical administration.
Various biodegradable and non-biodegradable carriers have been used, e.g. poly(lactic acid), PLGA, chitosan, poly(isobutyl cyanoacrylate) and Eudragit RS100 or RL100 (Bu et al. 2007). Erodible nanosystems are superior because the self-eroding process of the hydrolyzable polymer exert less harm on tissue (Herrero-Vanrell and Refojo 2001; Jose Alonso 2004). For example, PLGA colloidal nanoparticles were exploited to deliver gene-based therapeutics to the retinal pigment epithelial cells (Bejjani et al. 2005). The sustained-release nanosuspension of piroxicam and methylprednisolone acetate were formulated using Eudragit to control the endotoxin-induced uveitis in rabbits (Adibkia et al. 2007a; Adibkia et al. 2007b). For treatment of chronic ocular diseases (e.g. CMV retinitis), localized prolonged nanomedicines can be effectively used as a safer alternative of the frequent injections that may cause cataract development, retinal detachment, endophthalmitis and vitreous hemorrhage(Sahoo et al. 2008).
Nanosuspensions in ocular inflammation
The steroidal and non-steroidal anti-inflammatory drugs (NSAIDs) are routinely used in ocular surgeries, even though they often impose some adverse reactions. These medications are the most studied drugs to be exploited as ocular nanomedicines. Accordingly, localized therapy of ocular inflammation by these pharmaceuticals need to be optimized since most ocular diseases are classically treated with topicaleye-drops which usually require frequent utilization of highlyconcentrated solutions. Enormous efforts, thus, have so far been devoted to maximize thelocalized delivery and targeting of desired pharmaceuticals using hydrogels, micro- and/or nanoparticles andliposomal formulations. We have previously reported that nanosuspension of piroxicam can control the endotoxin-induced uveitis (EU) in rabbits (Adibkia et al. 2007b), where cationic polymer (i.e. Eudragit®RS100) was used to formulate nanosuspensions of piroxicam by means of solvent evaporation/extraction technique (the also called single emulsion technique).
Given that the Eudragit®RS100 possesses an appropriate stabilityandsize distribution characteristics together with its positive surface charge of about 30 mV, it is considered as a suitable ocular DDS (Pignatello et al. 2002a). The positively charged nanoformulations may interact with anionic mucins presented in the tear film, and cause consequential prolongation of drug residency time on the corneal surface (Dillen et al. 2006). Besides, the nanosuspensionsmay also confer more comfortableness for and better acceptance by patients in comparison with the routine ophthalmic suspensions that are basically formulated in micrometer ranges and show poor characteristics (Zimmer and Kreuter 1995). The ERL nanoparticles containing cloricromene (acoumarine derivative with antithrombotic and anti-ischemic activities) with positive zetapotential values (+27.3 mV) and a particle size of 80 nm were topically applied to rabbit eyes and showed no sign of toxicity or irritation to ocular tissues. A sustained release was observed in vitro as well as in vivo, resulting in a doubled AUC compared with an aqueous solution (Bucolo et al. 2004).
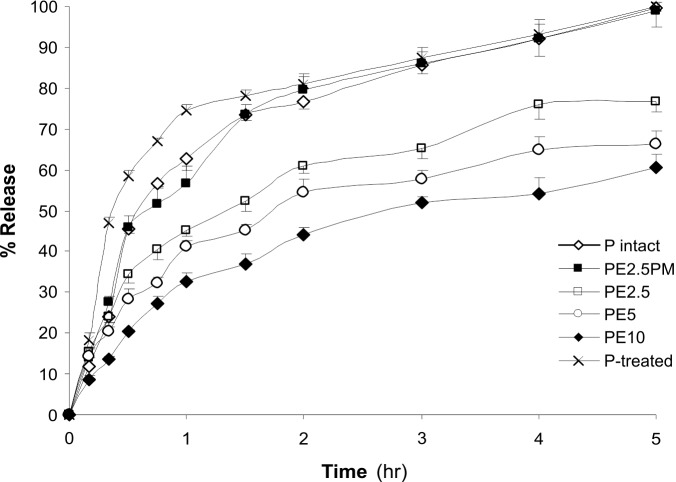
Fig. 7 represents in vitro release profiles of piroxicam (P) Eudragit®RS100 nanoparticles.
Fig. 7.
In vitro drug release profiles.P-intact andP-treated represent the intact and treated piroxicam, respectively. PE2.5indicatesthe piroxicam:Eudragit®RS100 nanoparticles at the ratios of 1:2.5.PM stands for physical mixture. Data represent mean value of 3-4 replications ± SE.The image was adopted with permission from (Adibkia et al. 2007b).
Overall, all nanoparticles showed a prolongedrelease profile without burst effect (Fig.4), in which the complete release of drug after 24 hr (obeyed from Higuchi diffusioncontrolled modelkinetics) explicitly indicate that there exists a structuralhomogeneity of the polymeric matrix, and also a moreuniform distribution of the drug. Modeling of drug release from nanoparticles of ciprofloxacin:Eudragit®has also been described by Dillen (2006),whose work showed that the release rate data fitted tothe Higuchi’s kinetic model. Based on our findings, treatment with piroxicamnanosuspensions significantly reduced observational symptoms of uveitis (based on Hogan's classification method) such as redness, presence of fibrin, photophobia, and lacrimation. We assume that the prolonged impacts of piroxicamnanosuspensions may be due to its interaction with local cellular components because of the positive surface charge of the nanoparticles in addition to the greater penetration and cellular uptake (Pignatello et al. 2002a).
Given the cellular responses to the lipopolysaccharide (component of gram-negative bacterial cell wall) induced uveitis (Koizumi et al. 2003; Marie et al. 1999), it can be assumed that the piroxicamnanosuspensions perhaps favor thecellular recovery from EU by conferring a better therapeutic effect because of increased cellular uptake and enhanced inhibitory mechanism on the expression of the inflammatory mediators. Similarly, we formulated nanosuspensions of methylprednisolone acetate (MPA) using ERS to pursue their impacts on the inhibition of inflammatory symptoms in rabbits with EU. We found that the utilization of MPA-ERS nanosuspensions confers a controlled ocular delivery of MPA (Adibkia et al. 2007a).
Although molecular biology aspects of such therapies for uveitis is yet to be mechanistically investigated, it appears that the application of these types of nanosuspensions as a non-invasive approach seems to be safer controlled ocular delivery of anti-inflammation agents for inhibition of the uveitis symptoms. Similar results have been reported for ibuprofen and flurbiprofen (Pignatello et al. 2002a; Pignatello et al. 2002b).
Artificial vesicles such as liposomes, niosomes and discomes have been successfully utilized as vehicle for the ophthalmic drugs (e.g. oligonucleotides, acetazolamide, pilocarpine HCI, cyclopentolate and timolol maleate) resulting in improved ocular bioavailability. Of these, positively charged nanostructures seem to be preferentially captured at the negatively charged corneal surface and slow down drug elimination by lacrimal flow (Kaur et al. 2004; Sahoo et al. 2008). Using the laser-targeted delivery (LTD), it is likely now to release and activate the encapsulated drug within the heat-sensitive liposomes injected intravenously (Asrani et al. 2006). By virtue of being encapsulated, the drug is confined into the liposomes and shielded from general metabolism, by which efficient pharmacological effects with minimal adverse reactions are expected.
Photodynamic therapy: implementation of nanosystems
Photodynamic therapy (PDT) with verteporfin for choroidal neovascularization associated with retinal pigment epithelium detachment AMD (Pece et al. 2007), and combination of PDT with aforementioned nanomedicines (Ju et al. 2008; Lazic and Gabric 2007) have revealed promising results. These medications are unable to completely cure AMD, but they significantly decelerate the progression of the lesion growth in a proportion of patients. Ocular gene therapy has reached clinical trials (e.g., for inherited retinal degeneration), which possibly mark the culmination of decades of investigations (Bainbridge and Ali 2008). The eye, as a valuable model system for gene therapy, is a unique highly compartmentalized organ for efficient delivery of small volumes of viral (e.g., adeno/lenti-viral vectors) (Auricchio et al. 2002; Auricchio 2003; Hamilton et al. 2006) or non-viral (e.g. PEGylatednanoliposomes and niosomes) (Bloquel et al. 2006; ndrieu-Soler et al. 2006; Peeters et al. 2005; Sanders et al. 2007) vectors. Among them, the PEGylated non-viral nucleic acid nanostructures prevent their interaction with undesired biomolecules and providepromising results (Sanders et al. 2007). Besides, recent significant progresses in the mapping and cloning of retinal disease genes have provided great potential for gene therapy in the eye, e.g., gene replacement in the inherited retinal degenerations (Leber's congenital amaurosis due to defects in the gene encoding the enzyme RPE65) (Bainbridge et al. 2006; Le et al. 2007). In 2005, Kataoka and his coworkers reported light-induced gene transfer frompackaged DNA enveloped in a dendrimericphotosensitizer. For efficienttransfection, the endosomalescape of the polyplexes is the main obstacle. This can be resolved by use of polycationic systems that possessbuffering capacity (the so-called proton sponge effect). Thus, to obtain efficient photochemical internalization (PCI), these researchers assumed that the control of subcellularlocalization of photosensitizers may be a key to the PCI-mediatedgene delivery with reduced cytotoxicity. At which, they developed a light-responsive gene carrier basedon a ternary complex of pDNA, cationic peptides and anionicdendrimer-based photosensitizers, “dendrimer phthalocyanine” (DPc). In their work, the core polyplex was formed froma quadruplicated cationicpeptide (CP4), where a peptide (CP2: C(YGRKKRRQRRRG)2)was dimerized through a disulphide linkage, and pDNA wasmixed with the CP4 peptide at a molar ratio of cationic aminoacids to a phosphate anion in DNA (i.e., N/P ratio of 2).
Using a luciferase (Luc) reporter gene assay in HeLa cells, they showed the transfection efficiency and cytotoxicity ofthe pDNA/CP4 polyplex and pDNA/CP4/DPc ternary complexeswith varying charge ratios of DPc after irradiation of thelight with increasing fluence. For in vivo PCI-mediatedgene delivery, they pursued the transfection of areporter gene (a variant of yellow fluorescent proteins, Venus)to the conjunctival tissue in rat eyes on laser irradiation after subconjunctival injectionof the ternary complex. The pDNA/CP4/DPc ternary complex with a charge ratio of1:2:1 achieved significant gene expression only at the laserirradiatedsite in the conjunctiva 2 daysafter irradiation. This is a clear example of emergence of nanosystems towards futuristic use in PDT.
Genonanomedicines, monoclonal antibodies and nanobodies
In Sept. 2006, the global bio-nanotech company pSivida announced the initiation of a phase II clinical trial of Mifepristone as an eye drop treatment for steroid associated elevated intraocular pressure (see http://www.psivida.com/default.asp), for formulation of which a nanocarrier has possibly been used. More recently, a branched PEGylated anti VEGF aptamer (pegaptanib sodium marketed as Macugen®) was approved by the FDA for the treatment of neovascular AMD, which demonstrated the first oligonucleotide aptamer nanomedicine. It suppresses the pathological angiogenesis in the neovascular AMD by specifically targeting the extracellular VEGF resulting in inhibition of angiogenesis, reduction of permeability of the vascular bed and diminution of inflammation (Bakri and Kaiser 2006).
Further, ranibizumab is arecombinant humanized monoclonal antibody fragment (marketed as Lucentis®) that targets VEGF-A, an important mediator in the development of choroidal neovascularization, and reduces neovascularization and leakage in the wet AMD (Bakri and Kaiser 2006). Ranibizumab (48 kDa) is a markedly smaller molecule than RhuMAbVEGF (bevacizumab, Avastin®, 148 kDa) that is in early clinical testing for treatment of the choroidal neovascularization via intravitreal route (Bakri and Kaiser 2006). Unlike RhuMAbVEGF, the ranibizumab is able to penetrate the retina and enter the subretinal space after intravitreal injection because of its notable size difference.
The heavy-chain-antibodies (HCAbs) have recently been discovered in the blood of camelids. Because of their nano-scaled size (diameter of ~2.5 nm and height of ~4 nm), the antigen-binding units of these HCAbscomprising only a single Ig fold (see Fig. 8). Thus, they are called “Nanobodies®”, whose several remarkable characteristics (i.e. being small, non-immunogenic, very stable, highly soluble, and easy to produce in large quantities) make them ideal candidates as next-generation immunotherapies. Antigen-specific Nanobodies® can easily be derived from the VHH of HCAbs that are circulating in the serum of immunized llamas or camels.
Fig. 8.
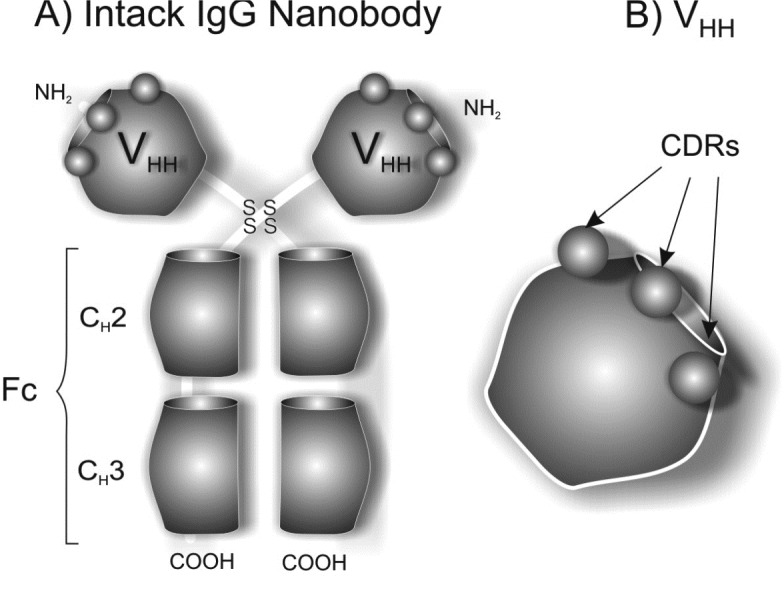
Schematic structure of heavy-chain-antibodies (HCAbs). The image was adopted with permission from (Majidi et al. 2009).
Nanobodies® appear to be inherently soluble and stable, which usually do not aggregate and possess high homology with human VH frameworks. Besides, they can be further humanized for use as therapeutics since these humanized nanostructured HCAbs are able to retain their characteristics and were shown to induce minimum immunogenicity (Muyldermans et al. 2009).
It should be evoked that the Nanobodies® can also be derived from the VH domains of conventional antibodies, at which humanized Nanobodies® (the process that also calledcamelization) can be achieved through substitutions of specific amino-acid to improve these unstable VH domains to become more stable with higher solubility. In fact, the single-domain nature of HCAbs confer several unique features in comparison with conventional Abs, although the conventional Abs show various beneficial characteristics including higher affinity and selectivity for a target, Nanobodies®display additional characteristics that make them superior as potential drug molecules. To our best knowledge, surprisingly, no studies have been conducted to use these unique structures for ocular targeting, but it is anticipated that they are not far from putting in practice.
Bioavailability of ocular nanomedicines
A variety of nanoparticle carriers undergo cellular uptake into ocular tissues via endocytosis. However, their characterization is limited to a qualitative basis only. The uptake percentage of the total dose nanoparticles and its contribution to overall ocular drug bioavailability remain unknown. In addition, an ophthalmic drug applied to the eye is subjected to metabolism when the drug penetrates across BEB into the site of action. In fact, there exist many researches demonstrating the functional expression of various enzymes involved in a variety of stages of drug metabolism and detoxification (Duvvuri et al. 2004; Rose and Bode 1991). Of these drug-metabolizing enzymes, oxidoreductases (e.g., aldehyde oxidase, ketone reductase, cyclooxygenase, monoamine oxidase and P450), hydrolases (e.g., aminopeptidase, acetylcholinesterase, carboxylesterase, aryl sulfatase, P-glucuronidase), and conjugating enzymes (e.g., arylamineacetyltransferase and glutathione S-transferase); for review see (Attar et al. 2005; Bu et al. 2007; Duvvuri et al. 2004). These metabolizing machieries of the eye are primarily expressed in various tissues (e.g., the retina-choroid), which appear to play an important role in ocular homeostasis by preventing entry of xenobiotics into, and/or eliminating xenobiotics from, the ocular tissues. Various cytochrome P450 (CYP) enzymes have been identified in ocular tissues including CYPs lA, 1B1, 2B, 2C, 2J, 3A, 4B1, 39A1, and NADPHreductase (Attar et al. 2005). Ocular nanomedicines loom to optimize the ocular bioavailability, for example a single topical instillation of acyclovir-PLA nanospheresin rabbits following resulted in significantly higher drug levels compared to the freedrug formulation and exhibited a sustained acyclovir releasefor up to 6 h in aqueous humor (Giannavola et al. 2003). Kassemet al. (2007) evaluated the effect of particle size in the micron and nano-size ranges as well as the effect of viscosity of the nanosuspension on the ocular bioavailability of glucocorticoid drugs (hydrocortisone, prednisolone and dexamethasone) by measuring the intraocular pressure of normotensive Albinorabbits. They showed the nanosuspensions always enhance the rate and extent of ophthalmic drug absorption as well as the intensity of drug action. This clearly highlights higher bioavailability of nanosuspensionsin comparison with micro-crystalline (Kassem et al. 2007). Recently, to provide long-term extraocular drug delivery using CS polymer, cyclosporin A (CyA) was formulated as nanoparticle with CS using an ionic gelation technique. The CyA-CS nanoparticles yielded mean size of 293 nm with zeta potential of +37 mV. In vitro release studiesrevealed prolonged drug release for a 24h period. In vivo tests showed that, following topical instillation of CyA-CS nanoparticles to rabbits, therapeutic concentration was obtained in cornea and conjunctivaduring at least 48 h, where the levels were significantly higher than those obtained following instillation of a CS solution containing CyA and an aqueous CyA suspension (De Campos et al. 2001). Very recently, to improve the precorneal residence time and the ocular bioavailability of indomethacin (IM), Badawi et al. (2008) deveoloped chitosan based nanoparticles (280 nm) and nanoemulsion (220-690 nm) using ionic gelation and spontaneous emulsification techniques, respectively. In vivo studies on eyes of rabbits displayed clearer healing of corneal by nanoemulsion, while CS nanocarriers were able to contact intimately with the corneaproviding slow gradual IM release with long-term drug level thereby increasing delivery to both external and internal ocular tissues (Badawi et al. 2008). These findings support similar previous results (Calvo et al. 1996), in which suspensions of nanoparticles andnanocapsules made of poly-epsilon-caprolactone (PECL) yielded profound increased ocular bioavailability of indomethacin in rabbits eyes. Similarly, enhanced bioavailability was reported for topical use of nanoparticles of amikacin-poly(butyl cyanoacrylate) (PBCA), acyclovir-poly(ethyl cyanoacrylate), betaxolol-poly(isobutyl cyanoacrylate), cloricromene-ERL, cyclophosphamide-PBCA, hydrocortisone-BSA, ibuprofen-ERS/ERL, metipranolol-PIBCA/PCL, progesterone-PBCA; for more details reader is directed to see (Bu et al. 2007). These all animal models based works are clear evidences for impacts of nano-scaled medicaments in ocular therapy despite their medical practices requires clinical trials.
Future prospective of ocular therapies
In ocular drug therapy, the need for safe application of medications to the posterior segment is deemed to be even more important than the surface delivery. Treatment of intricate posterior segment diseases crucially necessitates safe drug delivery to the retina, the choroid, or the ciliary body. Systemic delivery and devices inserted into the vitreous are valuable strategies, so are the biodegradable/nonbiodegradable controlled-release implants inserted into both aqueous and vitreous. Moreover, in recent years, there has been a dramatic increase in understanding of the pathobiology of ocular diseases at cellular/molecular level. There exists now a large number of drugs under/in development (Frank 2003). For ocular drug therapy, this state of high flux resulted in few advanced therapeutics such as Visudyne®, Macugen® and the angiostaticanecortave acetate (Retaane®) which is administered as periocular injection every six months (Bakri and Kaiser 2006; Hayek et al. 2007).
In close proximity, it is also predictable to perceive nano-scaled technologies in practice, providing promising platform for improved non-invasive ocular drug delivery. However, further developments need to be accomplished to render the nanosystems more effective. The primary practical approach to provide nanomedicines with the necessary site adherence and site retention to achieve carrier and drug targeting in topical ocular therapy is to endow them with the ability to be a bioadhesive system, perhaps by utilization the natural biopolymers such as hyaluronic acid. The mutual use of penetration enhancers along with nanomedicines without compromising the stability of the system could also provide higher ocular bioavailability. The bioadhesive nanosystems can maximize ocular drug absorption by prolonging drug residence time in the cornea and conjunctiva and minimize precorneal drug loss, resulting in increased patient compliance. For development of the ocular bioadhesive systems, as localized sustained released medications, nonbiodegradable systems appear to be adequate to treat perforations and ulcerations. Ideally for long-term use, however, these systems should be nontoxic biodegradable adhesives with site specificity and minimal immunogenicity, yet improving bioavailability by enhancing absorption (particularly for protein/peptide based macromolecules) or inhibiting the metabolizing enzymes.
Based on the unique bioarchitecture of the eye, it is considered as a perfect organ for gene therapy because the delivery vector can rarely escape to the systemic sites. To date, the ocular pathologies have been tackled with 17 trials (phase I/II) focused on different conditions including retinitis pigmentosa, glaucoma, diabetic macular edema and AMD, while totally 1537 gene therapy clinical trials are in development; for more details see the following website (http://www.wiley.co.uk/genmed/clinical/). This highlights the growing interests in gene therapy of the ocular diseases, for which futuristic genomedicines are deemed to become more effective therapeutics by exploiting molecular Trojan delivery systems for safe shuttling of genomedicines (e.g. antisense, ribozyme and siRNA) and targeting the desired biomarkers (Janoria et al. 2007; Maguire and Bennett 2006). There is much excitement about the potential of the short interfering RNA (siRNA), which has remarkably rapidly moved towards applications. At this stage, 9 clinical trials are being developed for its implementation and most of these trials are involved in the ocular disease: 1) a phase I trial on “Cand5 anti-VEGF RNAievaluation”, which was started in 2004 by Acuity Pharmaceuticals, 2) a phase II trial on “Cand5 anti-VEGF RNAievaluation (CARE) trial” which was started in 2005 by Acuity Pharmaceuticals, 3) a phase II trial on “RNAiassessment of cand5 in diabetic macular edema (RACE) trial” which was started in 2006 by Acuity Pharmaceuticals, 4) a phase I trail on “Open-label, dose-escalation single dose trial with Ssrna-027 in patients with AMD”, which was in 2005 by Allergan Inc., 5)a phase II trial on “intravitreal injections of a siRNA in patients with AMD” targeting the vascular endothelial growth factor receptor-1 (Sirna-27) which was started in 2006 by Allergan Inc.
The LTD and PDT seem to be promising methodologies to deliver and to activate therapeutic and diagnostic agents to the retina and choroid. However, their successful applications largely depend on the appropriateness of the agent. Perhaps, combination of these techniques with gene therapy could benefit the ocular diseases. The encapsulated cell technology (ECT) and cell therapy appear to grant treatment potentials for the ocular diseases. ECT implants consist of living cells encapsulated within a semipermeable polymer membrane and supportive matrices, which are genetically engineered to produce a specific therapeutic substance to target a specific disease or condition. Once implanted, it allows the outward passage of the therapeutic product (Tao et al. 2006). It is anticipated that the biological properties of the eye would undergo the desired alterations through application of these technologies. However, for implementation of the cell therapy technology in human eyes, the validation of the technique will be a critical step. Besides, the cellular and subcellular/molecular aspects of the target tissues should be fully addressed and the ocular disease related biomarkers should be exclusively clarified. Possibly, high throughput screening technologies (e.g., DNA/protein array and phage display screening methodologies) would facilitate investigations towards specific targeting.
Finally, it should be stated that not all attempts to apply de novo nanotechnology approaches in biomedical sciences have met with the same success as those cited herein this review, and sometimes these novel technologies tools provoke a great deal of challenges and hurdles. In fact, the nanostructures appear not to function in thesame predictive ways that routinely used smallmolecules act, although this field is experiencing a rapidgrowth period with major advancesin numerous diverse ways. Current preclinical investigations seem to provide new approaches to diagnose disease,to deliver specific therapy, and to monitor the biological impacts deeply. Although such fast inauguration of methodological alterations may eventually literally convey new challenges in theregulatory processes, it may grant a prolific platform from which will emerge many exciting, and yet unimagined, applicationsof biomedical nanotechnology.
Ethical Issues
None to be declared.
Conflict of interests
Authors declare no conflict of interests.
Acknowledgments
Authors like to express their gratitude to the Tabriz University of Medical Sciences for the financial support and Dr Y Omidi for his kind advice and the editorial support.
References
- Adibkia K, Omidi Y, Siahi MR, Javadzadeh AR, Barzegar-Jalali M, Barar J, et al. 2007. aInhibition of Endotoxin-Induced Uveitis by Methylprednisolone Acetate Nanosuspension in Rabbits. J Ocul Pharmacol Ther, 23(5), 421-432 [DOI] [PubMed] [Google Scholar]
- Adibkia K, Siahi Shadbad MR, Nokhodchi A, Javadzedeh A, Barzegar-Jalali M, Barar J, et al. 2007. bPiroxicam Nanoparticles for Ocular Delivery: Physicochemical Characterization and Implementation in Endotoxin-Induced Uveitis. J Drug Target, 15(6), 407-416 [DOI] [PubMed] [Google Scholar]
- Agnihotri SM and Vavia PR . 2009Diclofenac-Loaded Biopolymeric Nanosuspensions for Ophthalmic Application. Nanomedicine, 5(1), 90-95 [DOI] [PubMed] [Google Scholar]
- Asrani S, Goldberg MF and Zeimer R. 2006. Thermal-Sensitive Liposomes, In: Intraocular Drug Delivery, Jaffe GJ, Ashton P, Pearson PA, Eds. Taylor & Francis Group, LLC, New York, pp 143-156.
- Athanasiou KA, Niederauer GG and Agrawal CM . 1996Sterilization, Toxicity, Biocompatibility and Clinical Applications of Polylactic Acid/Polyglycolic Acid Copolymers. Biomaterials, 17(2), 93-102 [DOI] [PubMed] [Google Scholar]
- Attar M, Shen J, Ling KH and Tang-Liu D . 2005Ophthalmic Drug Delivery Considerations at the Cellular Level: Drug-Metabolising Enzymes and Transporters. Expert Opin Drug Deliv, 2(5), 891-908 [DOI] [PubMed] [Google Scholar]
- Auricchio A . 2003Pseudotyped AAV Vectors for Constitutive and Regulated Gene Expression in the Eye. Vision Res, 43(8), 913-918 [DOI] [PubMed] [Google Scholar]
- Auricchio A, Rivera VM, Clackson T, O'Connor EE, Maguire AM, Tolentino MJ, et al. 2002Pharmacological Regulation of Protein Expression From Adeno-Associated Viral Vectors in the Eye. Mol Ther, 6(2), 238-242 [DOI] [PubMed] [Google Scholar]
- Badawi AA, El-Laithy HM, Siahi ShadbadEl Qidra RK, El MH and El dM . 2008Chitosan Based Nanocarriers for Indomethacin Ocular Delivery. Arch Pharm Res, 31(8), 1040-1049 [DOI] [PubMed] [Google Scholar]
- Bainbridge, J. W.; Ali, R. R. Ocular gene therapy trials due to report this year; Keeping an eye on clinical trials in 2008. Gene Ther. 2008. Ref Type: In Press.
- Bainbridge JW, Tan MH and Ali RR . 2006Gene Therapy Progress and Prospects: the Eye. Gene Ther, 13(16), 1191-1197 [DOI] [PubMed] [Google Scholar]
- Bakri SJ and Kaiser PK. 2006. Antiangiogenic Agents: Intravitreal Injection, In: Intraocular Drug Delivery, Jaffe GJ, Ashton P, Pearson PA, Eds. Taylor & Francis Group, LLC, New York, pp 71-84.
- Barar J, Asadi M, Mortazavi-Tabatabaei SA and Omidi Y . 2009Ocular Drug Delivery: Impact of in Vitro Cell Culture Models. J Ophth Vision Res, 4(4), 238-252 [PMC free article] [PubMed] [Google Scholar]
- Barar J, Javadzadeh AR and Omidi Y . 2008Ocular Novel Drug Delivery: Impacts of Membranes and Barriers. Expert Opin Drug Deliv, 5(5), 567-581 [DOI] [PubMed] [Google Scholar]
- Barar J and Omidi Y . 2008Bioelectrical and Permeability Properties of Brain Microvasculature Endothelial Cells: Effects of Tight Junction Modulators. Journal of Biological Sciences, 8(3), 556-562 [Google Scholar]
- Barza M, Stuart M and Szoka F Jr . 1987Effect of Size and Lipid Composition on the Pharmacokinetics of Intravitreal Liposomes. Invest Ophthalmol Vis Sci, 28(5), 893-900 [PubMed] [Google Scholar]
- Bejjani RA, BenEzra D, Cohen H, Rieger J, Andrieu C, Jeanny JC, et al. 2005Nanoparticles for Gene Delivery to Retinal Pigment Epithelial Cells. Mol Vis, 11), 124-132 [PubMed] [Google Scholar]
- Bloquel C, Bourges JL, Touchard E, Berdugo M, BenEzra D and Behar-Cohen F . 2006Non-Viral Ocular Gene Therapy: Potential Ocular Therapeutic Avenues. Adv Drug Deliv Rev, 58(11), 1224-1242 [DOI] [PubMed] [Google Scholar]
- Bu HZ, Gukasyan HJ, Goulet L, Lou XJ, Xiang C and Koudriakova T . 2007Ocular Disposition, Pharmacokinetics, Efficacy and Safety of Nanoparticle-Formulated Ophthalmic Drugs. Curr Drug Metab, 8(2), 91-107 [DOI] [PubMed] [Google Scholar]
- Bucolo C, Maltese A, Maugeri F, Busa B, Puglisi G and Pignatello R . 2004Eudragit RL100 Nanoparticle System for the Ophthalmic Delivery of Cloricromene. J Pharm Pharmacol, 56(7), 841-846 [DOI] [PubMed] [Google Scholar]
- Calvo P, Alonso MJ, Vila-Jato JL and Robinson JR . 1996Improved Ocular Bioavailability of Indomethacin by Novel Ocular Drug Carriers. J Pharm Pharmacol, 48(11), 1147-1152 [DOI] [PubMed] [Google Scholar]
- Camelo S, Lajavardi L, Bochot A, Goldenberg B, Naud MC, Brunel N, et al. 2009Protective Effect of Intravitreal Injection of Vasoactive Intestinal Peptide-Loaded Liposomes on Experimental Autoimmune Uveoretinitis. J Ocul Pharmacol Ther, 25(1), 9-21 [DOI] [PubMed] [Google Scholar]
- Conrad JM and Robinson JR . 1977Aqueous Chamber Drug Distribution Volume Measurement in Rabbits. J Pharm Sci, 66(2), 219-224 [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz JG . 1997. aThe Blood-Ocular Barriers: Past, Present, and Future. Doc Ophthalmol, 93(1-2), 149-157 [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz JG . 1997. bThe Blood-Ocular Barriers: Past, Present, and Future. Doc Ophthalmol, 93(1-2), 149-157 [DOI] [PubMed] [Google Scholar]
- Siahi ShadbadEl QidraDe Campos AM, Diebold Y, Carvalho EL, Sanchez A and Alonso MJ . 2004Chitosan Nanoparticles As New Ocular Drug Delivery Systems: in Vitro Stability, in Vivo Fate, and Cellular Toxicity. Pharm Res, 21(5), 803-810 [DOI] [PubMed] [Google Scholar]
- Siahi ShadbadEl QidraDe CamposDe Campos AM, Sanchez A and Alonso MJ . 2001Chitosan Nanoparticles: a New Vehicle for the Improvement of the Delivery of Drugs to the Ocular Surface . Application to Cyclosporin A. Int J Pharm, 224(1-2), 159-168 [DOI] [PubMed] [Google Scholar]
- Dillen K, Vandervoort J, Van den and Ludwig A . 2006Evaluation of Ciprofloxacin-Loaded Eudragit RS100 or RL100/PLGA Nanoparticles. Int J Pharm, 314(1), 72-82 [DOI] [PubMed] [Google Scholar]
- Dong X, Shi W, Yuan G, Xie L, Wang S and Lin P . 2006Intravitreal Implantation of the Biodegradable Cyclosporin A Drug Delivery System for Experimental Chronic Uveitis. Graefes Arch Clin Exp Ophthalmol, 244(4), 492-497 [DOI] [PubMed] [Google Scholar]
- Duan X and Sheardown H . 2006Dendrimer Crosslinked Collagen As a Corneal Tissue Engineering Scaffold: Mechanical Properties and Corneal Epithelial Cell Interactions. Biomaterials, 27(26), 4608-4617 [DOI] [PubMed] [Google Scholar]
- Duvvuri S, Majumdar S and Mitra AK . 2003Drug Delivery to the Retina: Challenges and Opportunities. Expert Opin Biol Ther, 3(1), 45-56 [DOI] [PubMed] [Google Scholar]
- Duvvuri S, Majumdar S and Mitra AK . 2004Role of Metabolism in Ocular Drug Delivery. Curr Drug Metab, 5(6), 507-515 [DOI] [PubMed] [Google Scholar]
- Fenwick BW and Cullis PR . 2008Liposomal Nanomedicines. Expert Opin Drug Deliv, 5(1), 25-44 [DOI] [PubMed] [Google Scholar]
- Fischbarg J. 2006. The Biology of the Eye. Elsevier Science, Amsterdam.
- Frank RG . 2003New Estimates of Drug Development Costs. J Health Econ, 22(2), 325-330 [DOI] [PubMed] [Google Scholar]
- Freddo TF . 2001Shifting the Paradigm of the Blood-Aqueous Barrier. Exp Eye Res, 73(5), 581-592 [DOI] [PubMed] [Google Scholar]
- Gardner TW, Antonetti DA, Barber AJ, Lieth E and Tarbell JA . 1999The Molecular Structure and Function of the Inner Blood-Retinal Barrier . Penn State Retina Research Group. Doc Ophthalmol, 97(3-4), 229-237 [DOI] [PubMed] [Google Scholar]
- Giannavola C, Bucolo C, Maltese A, Paolino D, Vandelli MA, Puglisi G, et al. 2003Influence of Preparation Conditions on Acyclovir-Loaded Poly-d,l-Lactic Acid Nanospheres and Effect of PEG Coating on Ocular Drug Bioavailability. Pharm Res, 20(4), 584-590 [DOI] [PubMed] [Google Scholar]
- Hamilton MM, Brough DE, McVey D, Bruder JT, King CR and Wei LL . 2006Repeated Administration of Adenovector in the Eye Results in Efficient Gene Delivery. Invest Ophthalmol Vis Sci, 47(1), 299-305 [DOI] [PubMed] [Google Scholar]
- Hayek S, Scherrer M, Barthelmes D, Fleischhauer JC, Kurz-Levin MM, Menghini M, et al. 2007First Clinical Experience With Anecortave Acetate (Retaane). Klin Monatsbl Augenheilkd, 224(4), 279-281 [DOI] [PubMed] [Google Scholar]
- Herrero-Vanrell R and Refojo MF . 2001Biodegradable Microspheres for Vitreoretinal Drug Delivery. Adv Drug Deliv Rev, 52(1), 5-16 [DOI] [PubMed] [Google Scholar]
- Hillaireau H, Le DT and Couvreur P . 2006Polymer-Based Nanoparticles for the Delivery of Nucleoside Analogues. J Nanosci Nanotechnol, 6(9-10), 2608-2617 [DOI] [PubMed] [Google Scholar]
- Jabs DA . 1995Controversies in the Treatment of Cytomegalovirus Retinitis: Foscarnet Versus Ganciclovir. Infect Agents Dis, 4(3), 131-142 [PubMed] [Google Scholar]
- Janoria KG, Gunda S, Boddu SH and Mitra AK . 2007Novel Approaches to Retinal Drug Delivery. Expert Opin Drug Deliv, 4(4), 371-388 [DOI] [PubMed] [Google Scholar]
- Janzer RC and Raff MC . 1987Astrocytes Induce Blood-Brain Barrier Properties in Endothelial Cells. Nature, 325(6101), 253-257 [DOI] [PubMed] [Google Scholar]
- Siahi ShadbadEl QidraDe CamposDe CamposJose Alonso M . 2004Nanomedicines for Overcoming Biological Barriers. Biomedecine & Pharmacotherapy, 58(3), 168-172 [DOI] [PubMed] [Google Scholar]
- Ju M, Mailhos C, Bradley J, Dowie T, Ganley M, Cook G, et al. 2008Simultaneous but Not Prior Inhibition of VEGF165 Enhances the Efficacy of Photodynamic Therapy in Multiple Models of Ocular Neovascularization. Invest Ophthalmol Vis Sci, 49(2), 662-670 [DOI] [PubMed] [Google Scholar]
- Kassem MA, Siahi ShadbadEl QidraDe CamposDe CamposJose Alonsobdel Rahman AA, Ghorab MM, Ahmed MB and Khalil RM . 2007Nanosuspension As an Ophthalmic Delivery System for Certain Glucocorticoid Drugs. Int J Pharm, 340(1-2), 126-133 [DOI] [PubMed] [Google Scholar]
- Kaur IP, Garg A, Singla AK and Aggarwal D . 2004Vesicular Systems in Ocular Drug Delivery: an Overview. Int J Pharm, 269(1), 1-14 [DOI] [PubMed] [Google Scholar]
- Kayser O, Lemke A and Hernandez-Trejo N . 2005The Impact of Nanobiotechnology on the Development of New Drug Delivery Systems. Curr Pharm Biotechnol, 6(1), 3-5 [DOI] [PubMed] [Google Scholar]
- Kimura H and Ogura Y . 2001Biodegradable Polymers for Ocular Drug Delivery. Ophthalmologica, 215(3), 143-155 [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Shiraki K and Ikada Y . 1992Toxicity Test of Biodegradable Polymers by Implantation in Rabbit Cornea. J Biomed Mater Res, 26(11), 1463-1476 [DOI] [PubMed] [Google Scholar]
- Koizumi K, Poulaki V, Doehmen S, Welsandt G, Radetzky S, Lappas A, et al. 2003Contribution of TNF-Alpha to Leukocyte Adhesion, Vascular Leakage, and Apoptotic Cell Death in Endotoxin-Induced Uveitis in Vivo. Invest Ophthalmol Vis Sci, 44(5), 2184-2191 [DOI] [PubMed] [Google Scholar]
- Lang JC . 1995 Ocular Drug Delivery Conventional Ocular Formulation. Adv Drug Deliv Rev, 16), 39-43 [Google Scholar]
- Lazic R and Gabric N . 2007Verteporfin Therapy and Intravitreal Bevacizumab Combined and Alone in Choroidal Neovascularization Due to Age-Related Macular Degeneration. Ophthalmology, 114(6), 1179-1185 [DOI] [PubMed] [Google Scholar]
- Le MG, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, et al. 2007Restoration of Vision in RPE65-Deficient Briard Dogs Using an AAV Serotype 4 Vector That Specifically Targets the Retinal Pigmented Epithelium. Gene Ther, 14(4), 292-303 [DOI] [PubMed] [Google Scholar]
- Li H, Tran VV, Hu Y, Mark SW, Barnstable CJ and Tombran-Tink J . 2006A PEDF N-Terminal Peptide Protects the Retina From Ischemic Injury When Delivered in PLGA Nanospheres. Exp Eye Res, 83(4), 824-833 [DOI] [PubMed] [Google Scholar]
- Liu D, Ren T and Gao X . 2003Cationic Transfection Lipids. Curr Med Chem, 10(14), 1307-1315 [DOI] [PubMed] [Google Scholar]
- Loutsch JM, Ong D and Hill JM. 2003. Dendrimers: An Innovative and Enhanced Ocular Drug Delivery System, In: Ophthalmic Drug Delivery Systems, Mitra AK, Ed. Marcel Dekker, Inc., New York, pp 467-492.
- Maguire AM and Bennett J. 2006. Gene Therapy for Retinal Disease, In: Intraocular Drug Delivery, Jaffe GJ, Ashton P, Pearson PA, Eds. Taylor & Francis Group, LLC, New York, pp 157-173.
- Majidi J, Barar J, Baradaran B, Abdolalizadeh J and Omidi Y . 2009Target Therapy of Cancer: Implementation of Monoclonal Antibodies and Nanobodies. Hum Antibodies, 18(3), 81-100 [DOI] [PubMed] [Google Scholar]
- Marano RJ, Toth I, Wimmer N, Brankov M and Rakoczy PE . 2005Dendrimer Delivery of an Anti-VEGF Oligonucleotide into the Eye: a Long-Term Study into Inhibition of Laser-Induced CNV, Distribution, Uptake and Toxicity. Gene Ther, 12(21), 1544-1550 [DOI] [PubMed] [Google Scholar]
- Marie O, Thillaye-Goldenberg B, Naud MC and de Kozak . 1999Inhibition of Endotoxin-Induced Uveitis and Potentiation of Local TNF-Alpha and Interleukin-6 MRNA Expression by Interleukin-13. Invest Ophthalmol Vis Sci, 40(10), 2275-82 [PubMed] [Google Scholar]
- Mitra AK, Anand BS and Duvvuri S. 2006. Drug Delivery to the Eye, In: The Biology of Eye, Fischbarg J, Ed. Academic Press, New York, pp 307-351.
- Muccioli C and Belfort R Jr . 2000Treatment of Cytomegalovirus Retinitis With an Intraocular Sustained-Release Ganciclovir Implant. Braz J Med Biol Res, 33(7), 779-789 [DOI] [PubMed] [Google Scholar]
- Muyldermans S, Baral TN, Retamozzo VC, De BP, De GE, Kinne J, et al. 2009Camelid Immunoglobulins and Nanobody Technology. Vet Immunol Immunopathol, 128(1-3), 178-183 [DOI] [PubMed] [Google Scholar]
- ndrieu-Soler C, Bejjani RA, de BT, Normand N, BenEzra D and Behar-Cohen F . 2006Ocular Gene Therapy: a Review of Nonviral Strategies. Mol Vis, 12), 1334-1347 [PubMed] [Google Scholar]
- Nicolazzi C, Garinot M, Mignet N, Scherman D and Bessodes M . 2003Cationic Lipids for Transfection. Curr Med Chem, 10(14), 1263-1277 [DOI] [PubMed] [Google Scholar]
- Nishiyama N, Iriyama A, Jang WD, Miyata K, Itaka K, Inoue Y, et al. 2005Light-Induced Gene Transfer From Packaged DNA Enveloped in a Dendrimeric Photosensitizer. Nat Mater, 4(12), 934-941 [DOI] [PubMed] [Google Scholar]
- Omidi Y, Barar J, Ahmadian S, Heidari HR and Gumbleton M . 2008Characterization and Astrocytic Modulation of System L Transporters in Brain Microvasculature Endothelial Cells. Cell Biochem Funct, 26(3), 381-391 [DOI] [PubMed] [Google Scholar]
- Omidi Y, Barar J and Akhtar S . 2005Toxicogenomics of Cationic Lipid-Based Vectors for Gene Therapy: Impact of Microarray Technology. Curr Drug Deliv, 2(4), 429-441 [DOI] [PubMed] [Google Scholar]
- Omidi Y, Campbell L, Barar J, Connell D, Akhtar S and Gumbleton M . 2003. aEvaluation of the Immortalised Mouse Brain Capillary Endothelial Cell Line, B .End3, As an in Vitro Blood-Brain Barrier Model for Drug Uptake and Transport Studies. Brain Res, 990(1-2), 95-112 [DOI] [PubMed] [Google Scholar]
- Omidi Y, Hollins AJ, Benboubetra M, Drayton R, Benter IF and Akhtar S . 2003. bToxicogenomics of Non-Viral Vectors for Gene Therapy: a Microarray Study of Lipofectin- and Oligofectamine-Induced Gene Expression Changes in Human Epithelial Cells. J Drug Target, 11(6), 311-323 [DOI] [PubMed] [Google Scholar]
- Patravale VB, Date AA and Kulkarni RM . 2004Nanosuspensions: a Promising Drug Delivery Strategy. J Pharm Pharmacol, 56(7), 827-840 [DOI] [PubMed] [Google Scholar]
- Pece A, Isola V, Vadala M and Calori G . 2007Photodynamic Therapy With Verteporfin for Choroidal Neovascularization Associated With Retinal Pigment Epithelial Detachment in Age-Related Macular Degeneration. Retina, 27(3), 342-348 [DOI] [PubMed] [Google Scholar]
- Pedroso de Lima MC,Simoes S Lima MC,Pires P Lima MC,Faneca H and Duzgunes N . 2001Cationic Lipid-DNA Complexes in Gene Delivery: From Biophysics to Biological Applications. Adv Drug Deliv Rev, 47(2-3), 277-94 [DOI] [PubMed] [Google Scholar]
- Peeters L, Sanders NN, Braeckmans K, Boussery K, Van d V, De Smedt SC, et al. 2005Vitreous: a Barrier to Nonviral Ocular Gene Therapy. Invest Ophthalmol Vis Sci, 46(10), 3553-3561 [DOI] [PubMed] [Google Scholar]
- Pignatello R, Bucolo C, Ferrara P, Maltese A, Puleo A and Puglisi G . 2002. aEudragit RS100 Nanosuspensions for the Ophthalmic Controlled Delivery of Ibuprofen. Eur J Pharm Sci, 16(1-2), 53-61 [DOI] [PubMed] [Google Scholar]
- Pignatello R, Bucolo C, Spedalieri G, Maltese A and Puglisi G . 2002. bFlurbiprofen-Loaded Acrylate Polymer Nanosuspensions for Ophthalmic Application. Biomaterials, 23(15), 3247-3255 [DOI] [PubMed] [Google Scholar]
- Rose RC and Bode AM . 1991Ocular Ascorbate Transport and Metabolism. Comparative Biochemistry and Physiology Part A: Physiology, 100(2), 273-285 [DOI] [PubMed] [Google Scholar]
- Sahoo SK, Dilnawaz F and Krishnakumar S . 2008Nanotechnology in Ocular Drug Delivery. Drug Discov Today, 13(3-4), 144-151 [DOI] [PubMed] [Google Scholar]
- Sanders NN, Peeters L, Lentacker I, Demeester J and De Smedt . 2007Wanted and Unwanted Properties of Surface PEGylated Nucleic Acid Nanoparticles in Ocular Gene Transfer. J Control Release, 122(3), 226-235 [DOI] [PubMed] [Google Scholar]
- Selvin BL . 1983Systemic Effects of Topical Ophthalmic Medications. South Med J, 76(3), 349-358 [DOI] [PubMed] [Google Scholar]
- Smith M, Omidi Y and Gumbleton M . 2007Primary Porcine Brain Microvascular Endothelial Cells: Biochemical and Functional Characterisation As a Model for Drug Transport and Targeting. J Drug Target, 15(4), 253-268 [DOI] [PubMed] [Google Scholar]
- Tao W, Wen R, Laties A and Aguirre GD. 2006. Cell-Based Delivery Systems:Development of Encapsulated Cell Technology for Ophthalmic Applications, In: Intraocular Drug Delivery, Jaffe GJ, Ashton P, Pearson PA, Eds. Taylor & Francis Group, LLC, New York, pp 111-128.
- Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, et al. 1984New Class of Polymers: Starburst-Dendritic Macromolecules. Polymer Journal, 17(1), 117-132 [Google Scholar]
- Urtti A . 2006Challenges and Obstacles of Ocular Pharmacokinetics and Drug Delivery. Adv Drug Deliv Rev, 58(11), 1131-1135 [DOI] [PubMed] [Google Scholar]
- Vandamme TF and Brobeck L . 2005Poly(Amidoamine) Dendrimers As Ophthalmic Vehicles for Ocular Delivery of Pilocarpine Nitrate and Tropicamide. J Control Release, 102(1), 23-38 [DOI] [PubMed] [Google Scholar]
- Vandervoort J and Ludwig A . 2007Ocular Drug Delivery: Nanomedicine Applications. Nanomed, 2(1), 11-21 [DOI] [PubMed] [Google Scholar]
- Vega E, Egea MA, Valls O, Espina M and Garcia ML . 2006Flurbiprofen Loaded Biodegradable Nanoparticles for Ophtalmic Administration. J Pharm Sci, 95(11), 2393-2405 [DOI] [PubMed] [Google Scholar]
- Zimmer A and Kreuter J . 1995Microspheres and Nanoparticles Used in Ocular Delivery Systems. Adv Drug Deliv Rev, 16), 61-73 [Google Scholar]