Abstract
Introduction
Streptomyces, gram-positive and aerobic bacteria, are distinguished genus of Actinomycetes. This economically important genus is well studied owing to its capacity in producing more than 70% of antibiotics. In fact, need for novel, safe and more efficient antibiotics is a key challenge to the pharmaceutical industry today, moreover, increase in opportunistic infections in the immune compromised host has influenced this demand. Nowadays, evaluating morphological and biochemical differences as well as studying streptomyces genetic diversity via molecular indicators seem to be the most common method for screening this genus.
Methods
In this research we evaluate the potential of antibiotic production and characterize the UV and FTIR spectroscopy and HPLC (High performance liquid chromatography) analysis pattern of streptomyces from various locations in northwest of Iran. Regarding this, 30 soil samples were collected randomly from different zones of northwest region of Iran. Then, following the extraction of secondary metabolite, the UV and FTIR spectroscopy analysis was carried out for characterization of the various extracts.
Results
Considering the coordinate analysis of UV and FTIR spectroscopy pattern, the isolate G614C1 with substantial antimicrobial activity exhibited absorption at 3411 cm-1 which is indicator of hydroxyl groups, absorption at 2856 and 2915 cm-1 indicating hydrocarbon chassis, and absorption at 1649 cm-1 indicating a double bond of polygenic compound.
Conclusion
These results highlight the importance of streptomyces isolates in antibiotic production. HPLC confirmed the production when compared with standards.
Keywords: UV Spectroscopy, FTIR Spectroscopy, Streptomyces, Antimicrobial Activity
Introduction
Streptomyces as the most important genus of Actinomycetesare the most abundant soil microorganisms under a wide variety of conditions. Actinomycetesstrains are characterized by the production of important extracellular bioactive compounds and majority of those strains belong to species within the genus Streptomyces which produce two–thirds of the clinically important antibiotics. This genus was confirmed to be a promising bacteria against several pathogens and are well known for their potential to produce a large number of inhibitory metabolites used in industry and pharmacy (Baltz 1998; Berdy 1995).
Recently many of studies on the isolation, characterization and genotyping of soil streptomycetes have been conducted (Saadoun et al. 1997; Saadoun et al. 1998; Saadoun et al. 1999a; Saadoun and Al-Momani 1996). Further studies showed various streptomycetes isolates inhibiting the growth of several multi-resistant Gram-positive pathogens (Saadoun et al. 1999b; Saadoun and Gharaibeh 2002; Saadoun and Gharaibeh 2003). Experiments on the nature of the inhibitory metabolite produced by S. violaceusniger showed a maximum absorption in the UV region at 210-260 nm (Saadoun et al. 1999b). In a different study, the various extract of Streptomyces isolates exhibit inhibitory effects against Candida albicans, therefore the properties of the extract determined by UV-spectra absorbance peaks (Saadoun and Al-Momani 2000). Similarly, a study by Ilićet al. (Ili et al. 2005) on 20 different Streptomyces isolates from the soils of Southeastern Serbia indicated that the UV spectra of the culture extracts for the active isolates showed absorbance peaks ranging between 221 and 240 nm. The UV spectra of the active compounds in methanol showed peaks at 217 and 221 nm.
Therefore this study was undertaken to demonstrate the potential of streptomyces, collected from different habitats in Northwest region of Iran. The further evaluation of the promising isolates was carried out using the UV & FTIR spectroscopy.
Materials and methods
Isolation of bacterial strain
Collected soil samples, from 10-15 cm depth, were kept in 4○C until incubation, while noting sampling region’s explicit features such as pH and altitude (Ishii et al. 1983). In order to prepare the soil suspension, 5 g of soil was transferred into a sterile bottle then; 45 ml of distilled water was included and was shaken for 30 min. Five sets of ten-fold serial dilution was prepared from original supernatant, then 100 µl of third concentration was applied for inoculation of Starch Casein Agar (SCA) and the plates incubated for 7 days in 28○C. Morphological features of clones such as color of colonies and secreted pigment were used for preliminary categorization of bacterial population. The incongruent colonies were kept at -80○C and tagged as a discrete isolate based on their sampling location and order of colony isolated from same soil sample (e.g., the code “G614 C1” stand for the first colony that was isolated from Urmiain West Azerbaijan province) (Kreuze et al. 1999; Oskay et al. 2004).
Target organisms
A series of indicative bacteria have been used in this experiment including: E. coli, K. pneumoniae, S. flexneri, L. monocytogenes, B .cereus, Y. enterocolitica and S. aureus.
UV-Spectra of Streptomyces extracts
Streptomycetes isolates were cultured in 250 ml flasks containing 50 ml liquid medium (containing, beef extract 3.0 g; peptone 5.0 g; glucose 2.0 g; pH 7.5). Flasks were inoculated with 1 ml of Streptomyces spores suspension (10 7 CFU/ml) and incubated at 28 °C for 7 days with shaking at 100 rpm. Control flasks were not inoculated with the Streptomyces spores and were treated as above. After reaching the bacteria to the special concentration, the content of each flask was centrifuged at 2000 g for 10 min. Approximately 20 ml of each of the centrifuged fermented broth was extracted with 15 ml of n-Butanol after which absorption spectra in UV region (200-450 nm) were determined using a Jenway or Unicam UV-visible spectrophotometer. The organic solvent for extraction of the culture broth and UV spectrophotometer used in different studies are mentioned in Table 1.
Table 1. The UV spectroscopy data for the ethyl acetate extract of selective isolates.
| Strain | S.aureus | E. coli | Y.enterocolitica | B.cereus |
| G614 C1 | 17 | 15 | 13 | 17 |
| K36 C5 | 0 | 28 | 17 | 29 |
| M00 C1 | 25 | 0 | 17 | 22 |
| G151 C1 | 27 | 18 | 0 | 12 |
| K47 C1 | 14 | 11 | 0 | 16 |
| G1111 1 | 14 | 0 | 21 | 22 |
Ultraviolet (UV) and Fourier transform infrared (FTIR) spectral analysis
UV-spectra of various Streptomycetes isolates obtained from this study were subjected to comparison of general pattern, maximum absorbance peaks and range of wave length. Each active extract was determined in the UV region (200-400nm) by using a Perkin-Elmer Lambda 30 UV/VIS spectrophotometer (AH and Aysel 2003). Then FTIR spectrum of each active extract was detected using Shimadzu IR-470 plus. The spectra were also scanned in the 400 to 4000 cm- 1 range and plotted as intensity versus wave number(Augustine et al. 2005).
High performance liquid chromatography (HPLC) chromatography
Qualitative analysis was performed by silica gel thin-layer chromatography with a solvent mixture of petroleum ether: acetone (19:1, v/v) as mobile phase and the development was observed under ultraviolet lamp. Separation of carotenoids was also carried out by HPLC on a C18, 3µm column with acetonitrile: methanol: propanol (40:50:10). The flow rate was 0.8 ml/min (Dharmaraj et al. 2009).
Results
This study demonstrates the potential of streptomyces in production of antibiotic and further evaluates the antimicrobial activity of the various isolates through the UV & FTIR spectroscopy. Various 35 soil samples of northwest region of Iran were isolated and identified to be the disparate colonies. Among them 12 isolates distinguished due to their antibacterial activity against E. coli, K. pneumonie, S. flexneri, L. monocytogenes, B .cereus, Y. enterocolitica, and S. aurous. Surprisingly, some of the Streptomyces isolates including G614 C1, K36 C5, M00 C1, G151 C1, K47C1and G1111 revealed a significant antibacterial activity against indicator microorganisms. The following percentage of streptomyces isolates exhibited inhibitory effect against the indicator bacteria including: Y.enterocolitica (58%), L.monocytogenes (42%), K.pneumoniae (37%), B.cereus, S.aureus, and E.coli (33%), S. flexneri (17%and 24%) table 1.
According to the result (Fig.1, 2 and Table 2) absorbance peak ranges (215-270nm), as well as the characteristics of absorption peaks signifies a highly polygene nature of the extract.
Fig.1.
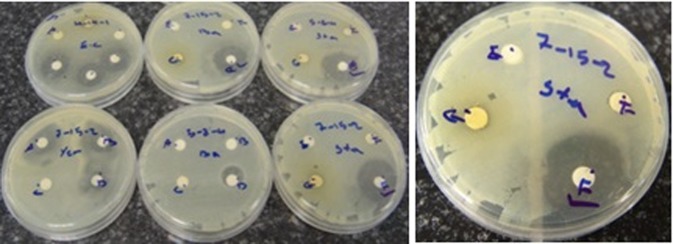
Zone of inhibition exhibited by the various extract of the different Streptomyces isolates.
Fig.2.
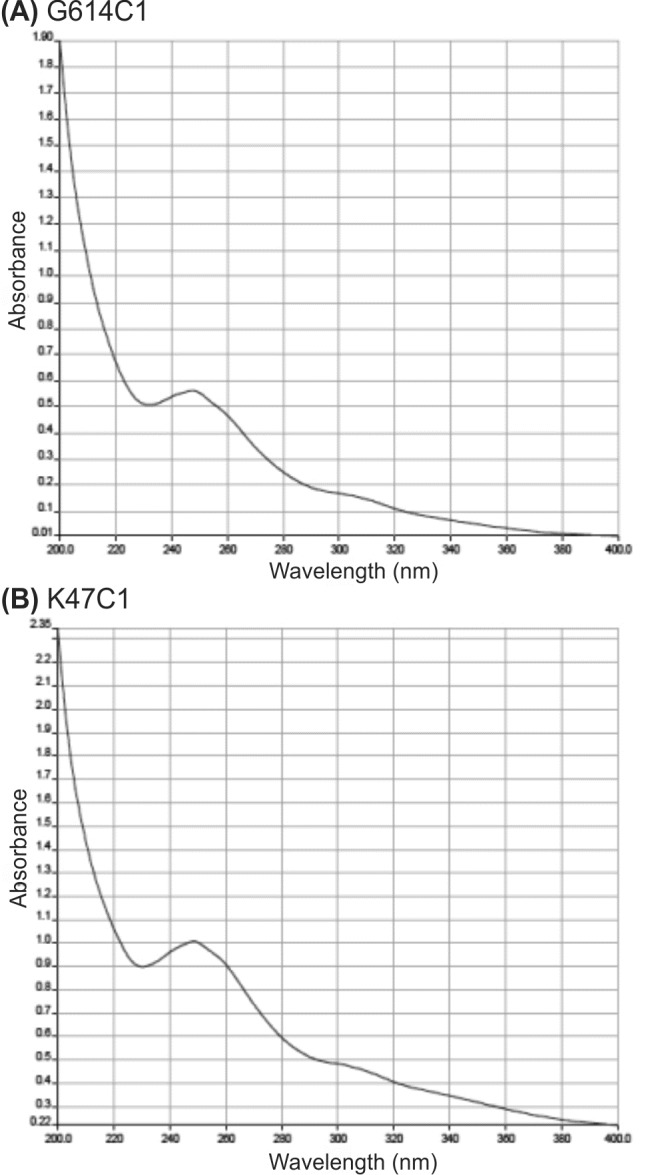
Result of UV spectroscopy for the ethyl acetate extracts of G614 C1 (A) and K47 C1 (B).
Table 2. Maximum absorbance peak for the putative isolates in the UV spectroscopy.
| Strain | λmax (nm) |
| G614 C1 | 251 |
| K36 C5 | 249 |
| M00 C1 | 243 |
| G151 C1 | 247 |
| K47 C1 | 250 |
| G1111 1 | 245 |
Furthermore the pointed spectral data are consistent with Saadounet al. The bioactive compound exhibited a maximum UV absorption at 217–221nm in ethyl acetate extract. Therefore these strains produced a broad-spectrum of anti-microbial compound or several compounds with different activities (Fig. 2). Maximum absorbance peaks observed at 240 nm and again the characteristics of absorption peaks showed a high polygene nature. The spectral data are consistent with those obtained by Swaadounet al. (1999). Slavic et al. (2005) reported that the maximum absorbance peaks of UV spectral data ranged between 215 and 270 nm of streptomyces isolates from the soil samples of South-eastern Serbia.
Accordingly, the FTIR spectrum of ethyl acetate extracts of G614 C1exhibited absorption at 3411 cm- 1 , which indicates hydroxyl groups, the absorption at 2856 and 2915 cm- 1 indicating hydrocarbon chassis and the absorption at 1649 cm- 1 indicating a double bond of polygenic compound (Fig.3). More or less similar trend was observed by Augustine et al. (2005), when they tested the FTIR spectrum of ethyl acetate extract of S.albidoflavus PU23 that exhibited absorption bands at 3296 and 1031.8 cm- 1 , which indicated hydroxyl groups and absorption at 1639 cm-1 indicating double bonding.
Fig.3.
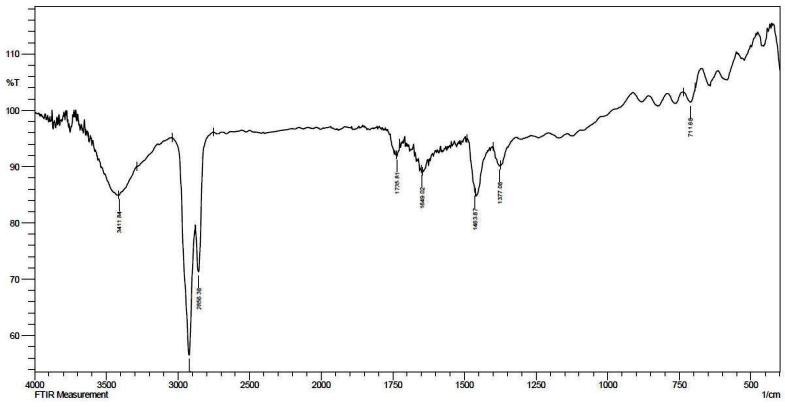
FTIR spectrum of the secondary metabolite isolated from G614 C1.
Despite the fact that, HPLC results of antimicrobial agents at conditions of pressure 71.96% with a flow rate of 1.5 where the mobile phase 0.1% phosphoric acid and pH 4, gave a peak at 7.567 retention time (Fig.4).
Fig.4.
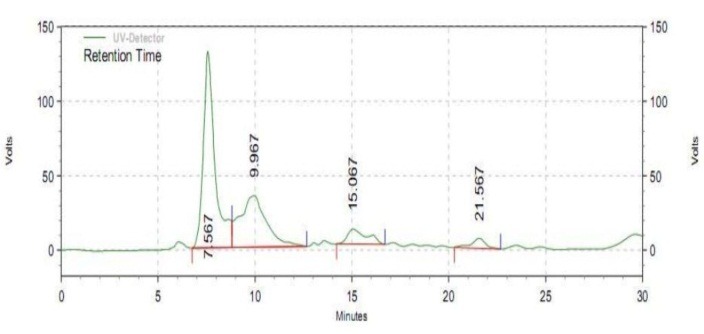
HPLC chromatography of ethyl acetate extraction of G614C1.
Discussion
Considering UV & FTIR spectroscopy and according to the result (Figs.1, 2 and Table 2) of absorbance peak ranges (215-270nm), as well as the characteristics of absorption peaks which signifies a highly polygene nature of the extract it is besides similarities in the general UV spectra and maximum absorbance peaks.The result presented in this investigation could explain the ability of the Streptomyces sp. to produce antibiotics. Furthermore the pointed spectral data are consistent with Saadounet al (Saadounet al. 1999c).
Slavicaet al. (2005) reported that the maximum absorbance peaks of UV spectral data ranged between 215 and 270 nm of streptomyces isolates from the soil samples of Southeastern Serbia which is identical to data presented by this study(Slavicaet al. 2005). Accordingly there is a demanding need for new and more effective antibacterial for use in more economical uses through industries. Considering the results on antibiotic production potential of G614C1isolate it might be cited that streptomyces potential in antibacterial production could meet this demand.
HPLC results confirmed the production of colorless carotene and phytoene. The results of thepresent finding correlate with previous findings of light-induced carotenogenesis inStreptomyces coelicolor.
Conclusion
Streptomyces, gram-positive and aerobic bacteria, are distinguished genus of Actinomycetes that are economically important genus because of their potential in producing more than 70% of antibiotics. Since production of novel and more efficient antibiotics needs detection of high yielding bacteria, in the current study, we evaluated 30 soil samples (collected randomly from different zones of northwest region of Iran) towards their antibiotic production potentialusing UV and FTIR spectroscopy and HPLC methods. Based upon UV, FTIR and HPLC analyses, the isolate G614C1 and K47C1 displayed promising results, inhibiting some important pathogenic bacteria.
Ethical Issue
None to be declared.
Conflict of interests
Authors declare no conflict of interests.
Acknowledgments
Authors would like to thank the Research Centre for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences (Tabriz, Iran) for financial support and the Agriculture Biotechnology Research Institute of Iran (Tabriz, Iran) for the administrative and some technical support.
References
- AH N and Aysel U . 2003Investigation of the Antimicrobial Activity of Some Streptomyces Isolates. Turk J Biol, 27, 79-84 [Google Scholar]
- Augustine SK, Bhavsar SP and Kapadnis BP . 2005A Non-Polyene Antifungal Antibiotic FromStreptomycesAlbidoflavus PU 23. Journal of Biosciences, 30(2), 201-211 [DOI] [PubMed] [Google Scholar]
- Baltz RH . 1998Genetic Manipulation of Antibioticproducing Streptomyces. Trends in Microbiology, 6(2), 76-83 [DOI] [PubMed] [Google Scholar]
- Berdy J . 1995Are Actinomycetes Exhausted As a Source of Secondary Metabolites? RUSSIAN BIOTECHNOLOGY C/C OF BIOTEKHNOLOGIIA, 7, 3-23 [Google Scholar]
- Dharmaraj S, Ashokkumar B and Dhevendaran K . 2009Fermentative Production of Carotenoids From Marine Actinomycetes. Iranian Journal of Microbiology, 1(4). [Google Scholar]
- Ili SB, Konstantinovi SS and Todorovi ZB . 2005UV/VIS Analysis and Antimicrobial Activity of Streptomyces Isolates. Facta universitatis-series: Medicine and Biology, 12(1), 44-46 [Google Scholar]
- Ishii K, Kondo S and Nishimura Y . 1983Decilorubicin, a New Anthracycline Antibiotic. Journal of Antibiotics, 36(4), 451-453 [DOI] [PubMed] [Google Scholar]
- Kreuze JF, Suomalainen S, Paulin L and Valkonen JPT . 1999Phylogenetic Analysis of 16S RRNA Genes and PCR Analysis of the Nec1 Gene From Streptomyces Spp . Causing Common Scab, Pitted Scab, and Netted Scab in Finland. Phytopathology, 89(6), 462-469 [DOI] [PubMed] [Google Scholar]
- Oskay M, Tamer AU and Azeri C . 2004Antibacterial Activity of Some Actinomycetes Isolated From Farming Soils of Turkey. African Journal of Biotechnology, 3(9), 441-446 [Google Scholar]
- Saadoun I and Al-Momani F . 1996Bacterial and Streptomyces Flora of Some Jordan Valley Soils. Actinomycetes, 7, 95-99 [Google Scholar]
- Saadoun I and Al-Momani F . 2000Activity of North Jordan Soil Streptomycete Isolates Against Candida Albicans. World Journal of Microbiology and Biotechnology, 16(2), 139-142 [Google Scholar]
- Saadoun I, Al-Momani F, Malkawi HI and Mohammad MJ . 1999. aIsolation, Identification and Analysis of Antibacterial Activity of Soil Streptomycetes Isolates From North Jordan. Microbios, 100(395), 41 [PubMed] [Google Scholar]
- Saadoun I and Gharaibeh R . 2002The Streptomyces Flora of Jordan and Its' Potential As a Source of Antibiotics Active Against Antibiotic-Resistant Gram-Negative Bacteria. World Journal of Microbiology and Biotechnology, 18(5), 465-470 [Google Scholar]
- Saadoun I and Gharaibeh R . 2003The Streptomyces Flora of Badia Region of Jordan and Its Potential As a Source of Antibiotics Active Against Antibiotic-Resistant Bacteria. Journal of arid environments, 53(3), 365-371 [Google Scholar]
- Saadoun I, Hameed KM and Moussauui A . 1999. bCharacterization and Analysis of Antibiotic Activity of Some Aquatic Actinomycetes. Microbios, 99(394), 173 [PubMed] [Google Scholar]
- Saadoun I, Mohammad MJ, Malkawi HI, Al-Momami F and Meqdam M . 1998Diversity of Soil Streptomycetes in Northern Jordan. Actinomycetes, 9, 52-60 [Google Scholar]
- Saadoun I, Schrader KK and Blevins WT . 1997Identification of 2-Methylisoborneol (MIB) and Geosmin As Volatile Metabolites of Streptomyces Violaceusniger. Actinomycetes, 8, 37-41 [Google Scholar]
- Slavica BI, Sandra SK and Zoran BT . 2005UV/VIS Analysis and Antimicrobial Activity of Streptomyces Isolates. Medicine and Biology, 12, 44-46 [Google Scholar]


