Abstract
Introduction
Over the past years, temperature and pH-sensitive hydrogels was developed as suitable carriers for drug delivery. In this study temperature and pH-sensitive hydrogels was designed for an oral insulin delivery modeling.
Methods
NIPAAm-MAA -HEM copolymers were synthesized by radical chain reaction with 86:4:10 (5% w/v) ratios respectively. Reaction was carried out in 1,4-Dioxane under Nitrogen gas-flow. The copolymers were characterized with FT-IR, 1H-NMR and DSC. Copolymers were loaded with regular insulin by modified double emulsion method with ratio of 1:10. Release study carried out in two different pH (pH=2 and 7.4 for stomach and intestine simulation respectively) at 37ºC. For each pH, a 5 mL suspension of the insulin containing hydrogel was taken in to a cellulose acetate dialysis membrane, and the dialysis membrane was allowed to float in a beaker containing 15 mL of buffer solution. The beakers were placed in a shaker incubator maintained at 37ºC. Phosphate buffer (0.1 M, pH 3)/ acetonitrile (60/40) was used as the mobile phase in HPLC assay.
Results
Yield of reaction was 86% with an optimum Lower Critical Solution Temperature point (30ºC). In-vitro studies showed a control release behavior via pH changes which the amount of insulin releases was 80% and 20% at pH=2 and 7.4 respectively.
Conclusion
Results showed that by optimizing polymerization and loading method we could achieve a suitable nano system for oral delivery of insulin.
Keywords: Oral Drug Delivery, NIPAAm, Hydrogel, Insulin
Introduction
Diabetes mellitus is a disorder caused by decreased production of insulin or by decreased ability to use insulin, leading to increase glucose levels in the blood (Morçöl et al. 2004). As the disease progresses tissue or vascular damage ensues leading to severe complications such as retinopathy, nephropathy, neuropathy, cardiovascular disease, and foot ulceration (Sweetman et al. 2007).
Diabetes mellitus may be categorized into several types but the two major types are type 1 (insulin-dependent diabetes mellitus; IDDM) and type 2 (non-insulin-dependent diabetes mellitus; NIDDM) (Sweetman et al. 2007).
Patients with type 2 diabetes who cannot be controlled adequately by oral therapy and diet need insulin either in addition to or in place of oral therapy. Second type of diabetes is a progressive disease, and about 30% of those on sulfonylureas will be transferred to insulin treatment within 4 years. Type 2 patients who become pregnant should also be switched from oral therapy to insulin.
The aim of insulin therapy is to achieve the best possible control of blood-glucose concentrations without the risk of the hypoglycemia.
Usually, insulin is injected subcutaneously two to four times a day (Kim et al. 2003). It may cause either lipoatrophy or lipohypertrophy. According to latter information, the subcutaneous injection of insulin has various disadvantages such as hyperinsulinemia, pain, allergic reactions and low patient compliance. Another important issue with insulin is that parenteral administration does not replicate the normal dynamics of endogenous insulin release, resulting in a failure to achieve a lasting glycemic control in patients (Morishita et al. 2006). Despite the advances in development of injectable insulin analogues, the goal of optimal glycemic control has remained elusive (Elsayed et al. 2009). Therefore, there has been significant interest in the development of non-invasive delivery systems for insulin (Kim et al. 2003). Substantial progress has been made recently in non-invasive methods for insulin administration such as pulmonary, nasal, rectal and oral in order to replace parenteral therapy (Elsayed et al. 2009).
Pharmaceutical strategies such as enzyme inhibitors, muco-adhesive polymeric systems, encapsulation or microspheres, micronization and nano sizing for insulin based on particulate carriers, have been proposed to maximize oral insulin bioavailability in insulin delivery systems, to have been investigated as potentially good techniques for targeting the drug directly or close to the site of action (Marschütz et al. 2000).
Pharmaceutical nanotechnology focuses on formulating therapeutically active agents in biocompatible nano forms such as nanoparticles, nanocapsules, micellar systems, and conjugates (Hamidi et al. 2008).
“Smart” hydrogels, or stimuli-sensitive hydrogels, on the other hand, are very different from inert hydrogels in that they can “sense” changes in environmental properties such as pH and temperature and respond by increasing or decreasing their degree of swelling (Lin et al. 2006). PH-sensitive hydrogels are useful in oral drug delivery as they can protect peptide/protein drugs in the digestive track. The pH responsiveness of hydrogels also facilitates lysosomal escape during gene delivery (Lin et al. 2006).
Another important stimulus for causing hydrogel responsiveness is temperature (Lin et al. 2006). Temperature-responsive polymers and hydrogels exhibit a volume phase transition at a certain temperature, which causes a sudden change in the solvation state. Polymers, which become insoluble upon heating, have a so-called lower critical solution temperature (LCST) (Okano et al. 1990). Most commonly used synthetic polymer for fabricating temperature sensitive hydrogels is poly N-isopropyl acrylamide (NIPAAM), which possesses a lower critical solution temperature (LCST) at around 32 °C. The value of the LCST can be increased or decreased by copolymerizing hydrophilic or hydrophobic polymers with poly NIPAAM (Akerman et al. 1998; Sakuma et al. 2001; Wang et al. 2008).
Our aim in this study is not only preparation hydrogelic system with good smart-release behavior, but also by optimizing loading method and monomers proportion we would achieve higher efficiencies and releasing profiles for insulin loaded smart hydrogels for oral delivery of insulin.
Methods
Materials and instruments
N-iso propyl acryl amide (NIPAAm) obtained from Acros Organics (NJ, USA) and purified by recrystallization from n-hexane-toluene (90:10 v/v).
Hydroxy ethyl methacrylate (HEM) was purchased from Merck chemical co. (Germany) and was vacuum distilled at 65 ˚C/5 mmHg.
Methacrylic acid (MAA) (Merck chemical Co., Germany) was used as supplied.
Tri ethylene glycol di-methacrylate (TEGDM) were purchased from Fluka and used without further purification.
Regular human insulin prepared from drug store, Iran Hormone.
Sodium mono hydrogen phosphate and dihydrogen phosphate was purchased from Sigma-Aldrich Co. (Steinhein, Germany), High performance liquid chromatography (HPLC) (Waters), FT-IR spectrometer instrument (Shimazu FT-IR 8400, Kyoto Japan), 1 H-NMR (Bruker spectra spin 400 MHz, Leipzig, Germany), Scanning electron microscopy (SEM, Leo Model 440i),Differential scanning calorimetry (DSC 7 Perkin Elmer, USA), Universal 320R centrifuge (Beckman, Optima, TLX, USA), Magnetic stirrer (Velp, Italy), Digital balance with accuracy 0.0001 (Shimazu, Japan), Dialysis membrane (Cellu SepH1) with MWCO of 2000 and 0.5 µm membrane (Millex AP, Millipore), Frieze dryer (Christ Alpha 1-4, USA) were used.
Preparation of cross-linked poly (NIPAAm-MAA-HEM) hydrogels
Terpolymers of NIPAAm + MAA + HEM were synthesized by free-radical polymerization method in 1, 4-dioxan with 5%w/w of the polymer (Figure 3). Water-soluble NIPAAm, MAA, and HEM monomers were used in 86:4:10 molar ratios using a standard experimental protocol. For preparation of cross-linked NIPAAm-MAA-HEM hydrogel, TEGDM was used.In this approach, 300μl of TEGDM was used as cross-linker. Benzoyl peroxide (0.3 mol% with respect to the monomers) was added as initiator of polymerization. The mixture was magnetically stirred and degassed with nitrogen gas for 30 minutes. The polymerization was fulfilled at 70°C for 24 h under nitrogen atmosphere. The synthesized polymer was precipitated using Hexane in liquid nitrogen bath. For further purification, the copolymer was precipitated in diethyl ether which was dissolved in tetrahydro furan (THF). The resulted suspension was centrifuged at 14,000 rpm, at 20°C for 20 min using the Universal 320R centrifuge (Beckman, Optima and TLX, USA). Further centrifugation was also conducted to ensure the collection of all the dispersed particles. The aqueous solution of polymeric micelles centrifuged (8000 rev.min-1) and the collected particles washed twice with phosphate buffer, and separated using the previously described centrifugation method. The mixture was purified by dialysis for 5 days using dialysis membrane (Cellu SepH1) and the external aqueous solution was removed two times a day and displaced with fresh distilled water. The polymerization yield of the experiment was 86.5%.
Preparation of Insulin loaded copolymers
Modified Double emulsion method was used for loading insulin in the ter polymer. Poly vinyl alcohol (PVA) was used as an emulsifier with 0.1 % concentration. First 100 mg of the synthesized and freeze dried copolymer was dissolved in 5 ml of chloroform. Then 50 IU (international unit) of insulin as aqueous solution was added in solution and homogenized in 15000 rpm for 30 seconds. Then 50 ml of PVA was added to the mixture and homogenized in 20000 rpm and 50 seconds. The mixture was stirred for 30 minutes for further evaporation of chloroform and loading of insulin and then centrifuged in 13000 rpm and 25°C. Loaded ter polymer lyophilized and stored in 2-8 °C for further use.
Determination of drug content of particles
To determine efficacy of drug loading, 100 mg dried powder of loaded polymer was placed in dialysis membrane (Cellu SepH1) with MWCO of 2000 and stirred in 100 mL phosphate buffer (pH=7.2) at room temperature for 3 hours. After complete dissolution of insulin loaded polymer, the amount of drug in the solution was quantified using HPLC with UV spectrophotometer at λmax=210nm. Na2HPO4 and acetonitrile were used as mobile phase with portion of 60/40. The flow rate and retention time for insulin were 1ml/min and 8.2 minute respectively.
Drug incorporation efficiency was expressed drug entrapment (%w/w).
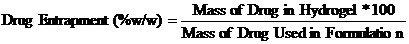
In vitro release study
The in vitro release of insulin from hydrogel was carried out at 37ºC and in two different pH (pH 2 and 7.4 for simulation of stomach and intestine pH, respectively). For each pH, a 5 mL suspension of the insulin containing hydrogels (40 mg hydrogels in 5 mL buffer solution) was taken in to a cellulose acetate dialysis membrane (Sigma, D-9652), and the dialysis membrane was allowed to float in a beaker containing 15 mL of buffer solution. The beakers were placed in a shaker incubator maintained at 37ºC. 200 µl samples were removed from the external buffer solution and were replaced with fresh buffer solution. The insulin released in to the medium was analyzed using an HPLC assay. Chromatographic separation was achieved using phosphate buffer (0.1 M, pH 3)/ acetonitrile (60/40) as the mobile phase at a flow rate of 1.0 mL/min. The UV detection was at 210 nm. The run time for the assay was 11 min and the retention time for insulin was 8.2 min.
Thermal studies
Two methods used to evaluate thermal behavior. In first method, thermal behavior or LCST behavior of the PNIPAAm hydrogel samples was determined using a differential scanning calorimeter. All hydrogel samples were immersed in distilled water at room temperature and allowed to swell to equilibrium before the DSC measurement. For each DSC measurement, about 10 mg of equilibrium swollen hydrogel sample (along with its water) was placed inside a hermetic aluminum pan, and then sealed tightly by a hermitic aluminum lid. The thermal analysis was performed from 25ºC to 50ºC on the swollen hydrogel sample at a heating rate of 3 ºC/min under dry nitrogen. The onset point of the endothermal peak, determined by the intersecting point of two tangent lines from the baseline and slope of the endothermic peak was used to determine LCST.
At second approach, the cloud point (CP) measurement (turbidimetry) method was employed. Optical transmittance of aqueous polymer solution at various temperatures was measured at 500 nm wavelength using UV-Vis spectrometer (UV- 160 Shimadzu) with increasing solution temperatures (18-50 °C). Heating rate was 0.5 °C /min. At each step, the samples were stabilized for 10 min before measurements. Values for the LCST of polymeric solutions were determined as the temperature at the inflexion point in the normalized absorbance versus temperature curve.
Swelling studies
For the study of temperature-dependent equilibrium swelling ratio the hydrogel samples were equilibrated in deionized water (pH 7.0) for at least 24h at 20˚C and 37˚C. For the pH-dependence study the hydrogel samples were equilibrated in the aqueous media in pH 1.3 and pH 7.4 buffered solutions.
The swelling ratio of samples was measured gravimetrically. After the excess water on the sample surface was removed by filter papers, the samples were weighted (Ww). The dry weight (Wd) of each sample was determined after drying to constant weight under vacuum overnight at 50 ˚C The swelling ratio, Q, was calculated from the equation:
Q = Ws/Wd; where Ws is the weight of water in the swollen sample (Ws= Ww- Wd).
Fourier transform infrared spectroscopy study
FT-IR spectra obtained by FT-IR spectrophotometer for blank polymer and drug loaded nanoparticles using KBr discs.
Morphological study
The morphology of the particles was also investigated for blank copolymer and drug and drug loaded copolymer using scanning electron microscopy (SEM, Leo model 440i). Dried hydrogel were analyzed at 15–20 kV by scanning electron microscopy after metallization by gold coating (Edwards Sputter coating S 150).
1 H- NMR study
The chemical structures of the copolymers were determined by 1 H- NMR (Bruker spectra spin 400 MHz, Leipzig, Germany).
Statistical analysis
Data was represented as mean values ± SEM. Statistical assessment of difference between mean values was performed by least significance difference (LSD) test at p<0.05 using SPSS software.
Results
Synthesis of cross-linked NIPMAAM-MAA-HEM copolymer
Cross-linked copolymer of NIPAAm-MAA-HEM was prepared by free radical copolymerization of the monomers in 86:4:10 molar ratios in presence of TEGDM as cross-linking agent and benzoyl peroxide (BPO) as the initiator (Figure 1).
Fig. 1 .
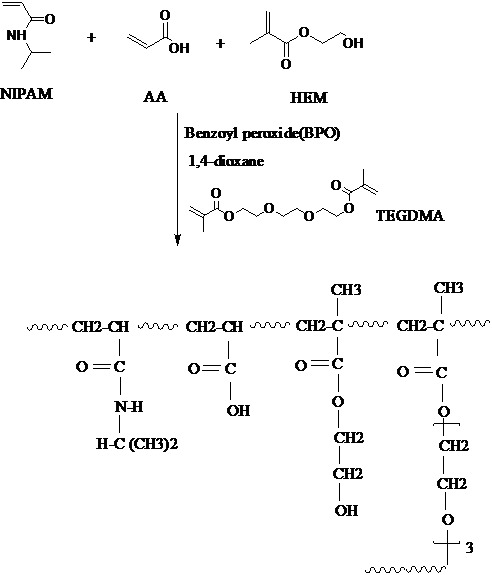
Synthesis and chemical structure of cross-linked P (NIPAAm -MAA-HEM).
Chemical characteristics of P (NIPAAm-MAA-HEM)
Chemical structure of the cross-linked P (NIPAAm-MAA-HEM) was confirmed by FT-IR and 1 H-NMR spectroscopy.
Figure 2 shows the FT-IR spectrum of poly (NIPMAAm-MAA-HEM) cross-linked with TEGDM.
Fig. 2.
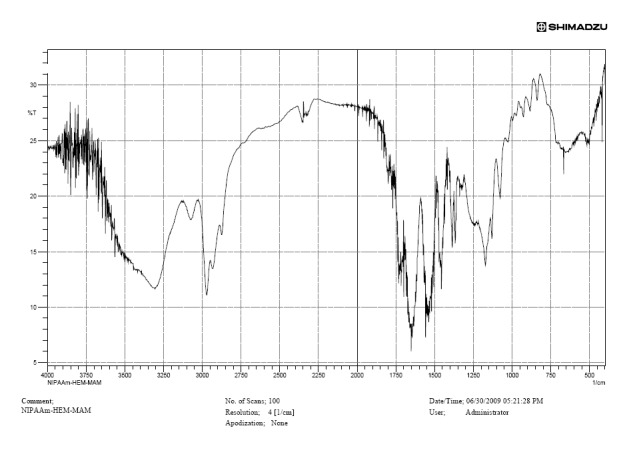
FT-IR spectrum of poly (NIPMAAM-MAA-HEM) cross-linked with TEGDM.
The main peaks are (λ cm- 1 ): 1725, 1650, 3430 and 2928.
Figure 3 shows the 1 H NMR spectrum of poly (NIPMAAM-MAA-HEM).
Fig. 3.
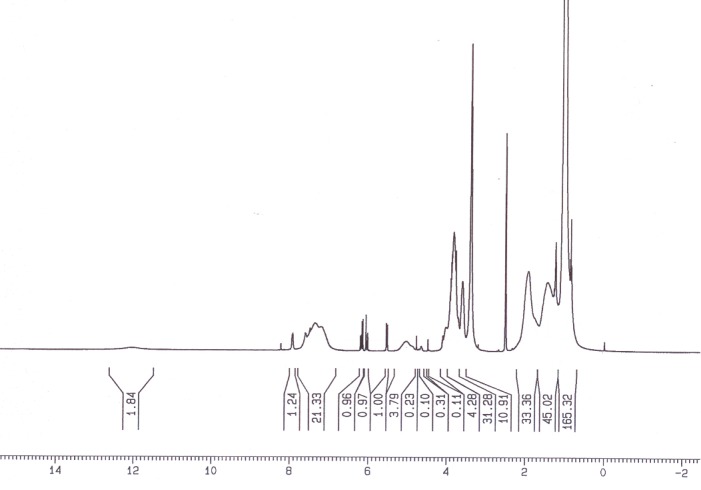
1H NMR spectrum of poly (NIPMAAM-MAA-HEM).
Morphological study
Figure 4 shows scanning electron microscopic (SEM) morphology of poly (NIPMAAM-MAA-HEM) cross-linked with TEGDM.
Fig. 4.
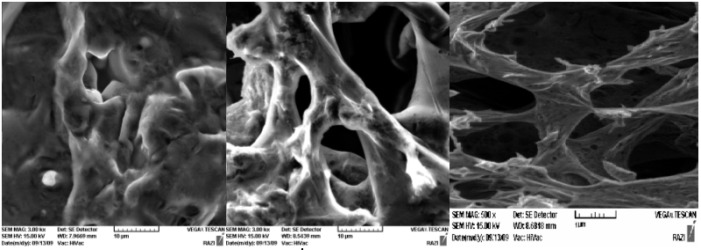
SEM examinations of poly (NIPMAAM-MAA-HEM) cross-linked with TEGDM (Magnification= 10000) (right: unload hydrogel, middle and left: insulin loaded hydrogel).
SEM picture shows network form of the hydrogel polymer, which is required for capturing of the medicine.
In vitro release study
Figure 5 shows calibration curve for insulin in mentioned condition.
Fig. 5.
Calibration curve for insulin.
Figure 6 and 7 represent polymer release behavior in two different pH, which was selected as a simulation for stomach, and intestine pH respectively.
Fig. 6.
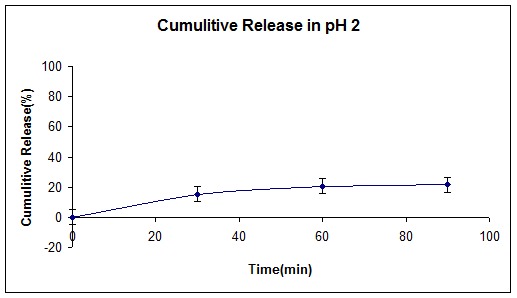
Cumulative release curve of insulin loaded (NIPMAAM-MAA-HEM) cross-linked with TEGDM in pH= 2.
Fig. 7.
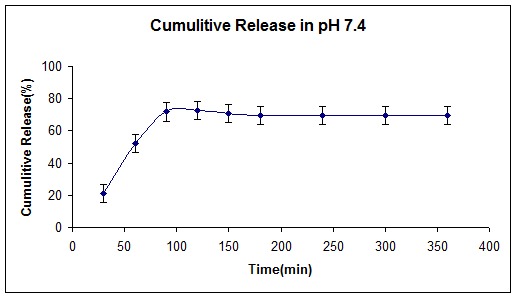
Cumulative release curve of insulin loaded (NIPMAAM-MAA-HEM) cross-linked with TEGDM in pH= 7.4.
Figure 8 and 9 represent two chromatograms for intact insulin and loaded hydrogel respectively.
Fig. 8.
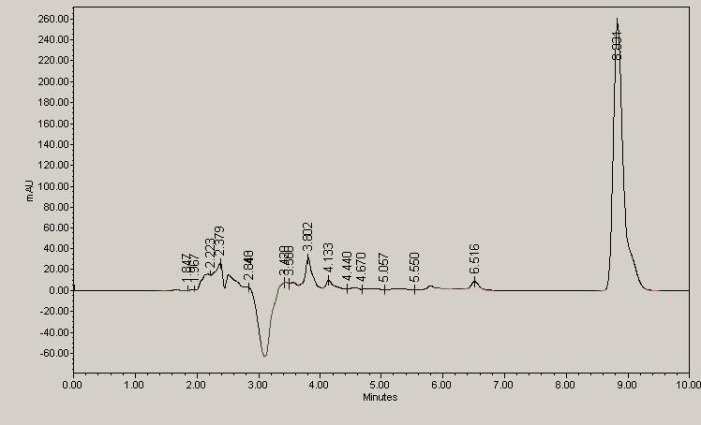
10IU Regular insulin HPLC chromatogram.
Fig. 9.
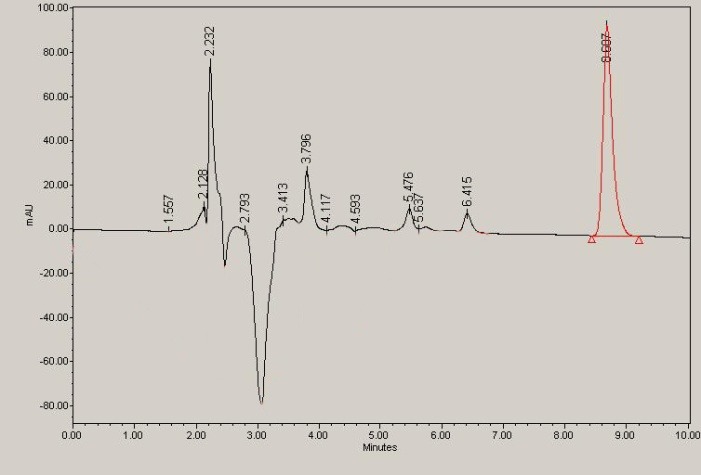
Loaded copolymer at time 80 minute of release at pH 7.4.
Thermal studies
Figure 10 shows DSC thermogram of cross-linked polymer.
Fig. 10.
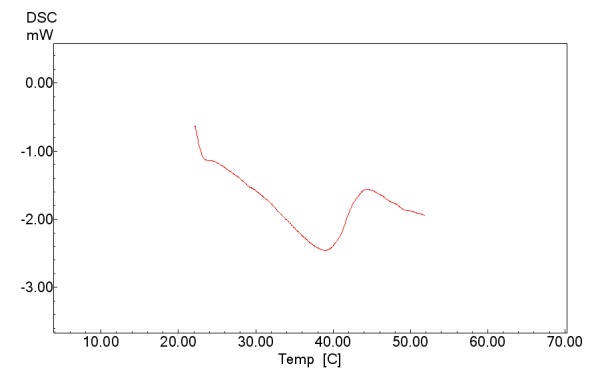
DSC thermogram of (NIPMAAM-MAA-HEM) cross-linked with TEGDM.
Figure 11 shows cloud point measurements of cross-linked P (NIPAAm -MAA-HEM) hydrogel in distilled water.
Fig. 11.
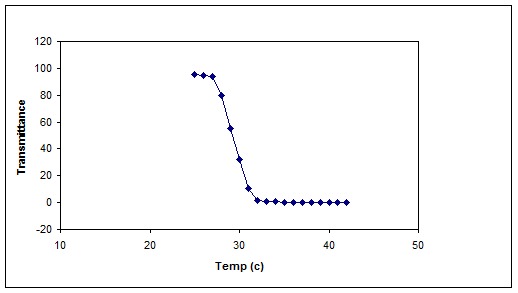
ypical cloud point measurement of cross-linked P (NIPAAm -MAA-HEM) hydrogel in distilled water.
Swelling studies of cross-linked P (NIPAAm-MAA-HEM) hydrogels
Figure 12 illustrates the dependence of the Q of the hydrogels as a function of temperature over the range from 22 to 45 °C.
Fig. 12.
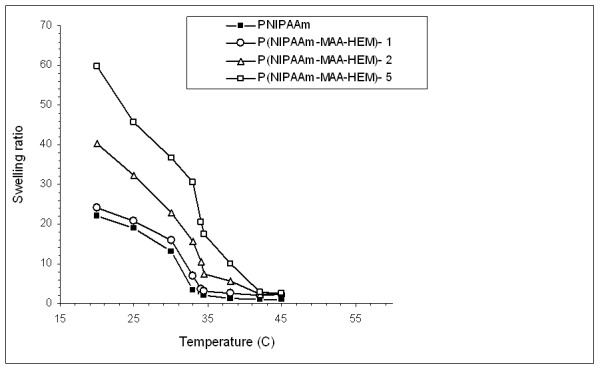
Equilibrium swelling ratios of the conventional PNIPAAm and cross-linked hydrogels over the temperature range of 21 to 45 °C.
Discussion
Synthesis and chemical structure of cross linked NIPMAAM-MAA-HEM copolymer
Cross-linked poly (NIPAM-MAA-HEM) hydrogel was synthesized by free radical cross-linking copolymerization of monomers in mentioned conditions. Chemical structure of the hydrogel was confirmed by FT-IR and 1 H-NMR spectroscopy.
FT-IR spectrum of polymer is shown in Figure 4. Absorbance of amide carbonyl group in NIPAAm occurs at 1650 cm- 1 , bending frequency of amide N-H appears at 1550 cm- 1 , stretch vibration for C=O in ester bondsof HEM and MAA appears at 1730 cm- 1 . Taking into account the overlap in TEGDM and HEM, the most important bonds from HEM are at: 3400 cm- 1 [ν (OH)], 2990 cm- 1 [ν (CH3)as, ν (CH2)as], 1074 cm- 1 [ν (O-C), alcohol], 1021 cm- 1 [ν (C-O), ester], 749 cm- 1 [bending (O=C-O)].
Figure 5 shows a typical 1 H-NMR spectrum of the chemical composition of synthesized novel copolymer. 1 H NMR of copolymer exhibits the two broad peaks (–CH–CH2–) at 1.4–1.9 ppm and a peak (–NH–CH–) at 3.8 ppm which belongs to the hydrogen proton of NIPAAm units of the chain. What is more, the intensity of methyl group enhances significantly due to the introduction of methyl group of NIPAAm (chemical shift at 1.2 ppm).The AA units of the copolymer showed three peaks; the chemical shift at 1.1 ppm due to the –CH, a signal at 1.9 ppm for the methine group, and a weak signal at 12 ppm representing the carboxylic proton.
The SEM examinations resulted in network shape of the copolymer, which is required for better capturing of the medicine (Figure 6).
Thermal studies
An acknowledged and acceptable mechanism of the phase separation of PNIPAAm is described as resulting from the breakdown of hydrogen bonds and the enhancement of hydrophobic interactions. Generally, a hydrophilic/hydrophobic balance exists in PNIPAAm side chains. At room temperature favorable hydrogen bonding interactions between hydrophilic amide groups in the polymers and water molecules result in the good solubility of PNIPAAm. With further increasing temperature, the hydrophilic/hydrophobic balance shifts to a more hydrophobic nature, the hydrogen bonds are broken and the aggregation process takes place. This results in the phase transition of the PNIPAAm chains at around LCST. In addition copolymerization of hydrophilic monomers with NIPAAm may increase the LCST value, which caused by the changes of the hydrophilic/hydrophobic ratio. Figure 13 shows visual illustration of aqueous hydrogel solution below (left) and above (right) LCST. As illustrated in Figure 15 below LCST the solution of hydrogel is clear but upon heating (at about 30 ˚C) the solution became turbid because of the aggregation of the polymer.
Fig. 13.

Visual observation of macroscopic phase separation of aqueous solution of cross-linked P (NIPAAm -MAA-HEM)-1 hydrogel.
DSC thermogram indicates a thermal changing in 30ºC that shows LCST point for the copolymer (Figure 10). Figure 11 shows the typical cloud point measurement of cross-linked P (NIPAAm-MAA-HEM) hydrogel. As shown in Figure 11 the LCST of the cross-linked hydrogel is about 30 ˚C, which is lower than the LCST of the pure PNIPAAm homopolymer (~ 32 ˚C). Incorporation of hydrophobic monomers shift the hydrogel towards a more hydrophobic nature, and the LCST shifts to a lower temperature (Table 1).
Table 1. Lower critical solution temperature (LCST) of cross-linked P (NIPAAm-MAA-HEM) hydrogels.
| Sample | TEGDM (wt %) | LCST (°C) |
| P (NIPAAm-MAA-HEM)-1 | 1 | 34 |
| P (NIPAAm-MAA-HEM)-2 | 2 | 33.5 |
| P (NIPAAm-MAA-HEM)-5 | 5 | 30 |
Swelling studies of cross-linked P (NIPAAm-MAA-HEM) hydrogels
Figure 12 illustrates the dependence of the Q of the hydrogels as a function of temperature over the range from 22 to 45 °C. The Q of all samples is higher at low temperature due to the fact that PNIPAAm is swellable and hydrophilic at temperatures below the LCST. The Q of the gels decreased quickly as the temperature increased to above 30 °C. Generally, based on the swelling ratio experiments, the phase transition temperature or LCST is regarded as the temperature point at which the Q decreases dramatically and the phase separation degree is the greatest. Compared to conventional PNIPAAm, the Q of the cross-linked hydrogels is higher (Figure 12). MAA and HEM are highly hydrophilic monomers, which increase the hydrophilicity of the gel. As shown in Figure 12, increasing the amount of cross-linker (TEGDA) from 1% to 5% increases the swelling ratio of the gel. The concentration of TEGDMA decreased from P (NIPAAm-MAA)-1 to P (NIPAAm-MAA)-3, which led to a more cross-linked network in the prepared gel and more water molecules absorbed within the gel.
Drug loading efficiency
Table 2 represents drug entrapment of the hydrogel in different TEGDM concentrations. According to the table, by increasing of the cross-linker’s concentration, insulin loading efficiency increases. It comes back to the hydrogel three-dimensional network that in high links, more insulin captures in the network.
Table 2. Drug entrapment in different cross-linker concentrations.
| Sample | TEGDM (wt %) | Polymer (w/w%) | Drug Entrapment (%) |
| P (NIPAAm-MAA-HEM)-1 | 1 | 64.5 | 28.5±4.5 |
| P (NIPAAm-MAA-HEM)-2 | 2 | 64.5 | 34.5±3.4 |
| P (NIPAAm-MAA-HEM)-5 | 5 | 64.5 | 54.3±4.2 |
Drug loading efficiency was high (54%) comparing with other methods which used like changing pH (3%) or changing temperature (2%). These results show that double emulsion method was a good method for loading insulin comparing with other methods.
In vitro release study of insulin loaded cross-linked hydrogel
Figures 8 and 9 show cumulative release (%) of insulin at two different pH according to stomach and intestine pH. Since the resident time in stomach is about 1.5 hours, and the whole time was required to pass the GI tract is about 15 hours, we selected these periods for our study. As shown in Figure 8, only 20% of insulin has been released at pH 2, indicating that the hydrogel had shown pH-responsive behavior due to MAA and HEM monomers in the smart polymer. On the other hand, this pH- responsiveness made for higher release profile (80%) at high pH (7.4) similar to intestine pH. NIPAAm monomer as a thermo-sensitive monomer and according to the LCST point, gives a better and fast release behavior for the copolymer.
At pH 2.0 and 37ºC, the polymers did not swell or dissolve, under these release conditions, surface bound drug is mostly released. At pH 7.4 and 37ºC, the hydrogel network degraded or swelled, depending on the MW of the polymer, and accordingly, drug release was observed from all the polymers. According to the results and recent works (Chaaya et al. 1999), MW plays crucial role not only in drug release mechanism but also in release time. By increasing the hydrophobicity of the hydrogel, MW of the polymer increases and as a result releasing time of the loaded polymer increases.
Conclusion
Poly NIPAAm has the sharpest swelling transition of the class of thermosensitive N-alkyl acrylamide polymers. This feature makes it the most suitable for studies and practical applications. However, poly (NIPAAm) homopolymer shows limited ever, poly (NIPAAm) homopolymer shows limited swelling behavior and poor mechanical properties, which limit its application. These obstacles can nevertheless be overcome by copolymerization of NIPAAm with hydrophilic, hydrophobic, or charged moieties. A tailored, delicate balance between hydrophilic and hydrophobic components on NIPAAm polymers provides desired thermosensitive swelling behavior in aqueous solution and improved mechanical properties. NIPAAm-based polymers can also be tailored to exhibit pH-sensitivity by the addition of acidic or basic groups. The mutual influence of pH and temperature on the swelling of ionizable and thermosensitive hydrogels has been investigated. It was concluded that hydrophilic and charged co monomers increased the LCST due to the increase in hydrophilicity of the polymer, while hydrophobic co monomers decreased the LCST.
In this study, loaded copolymer represented a good smart-release behavior in two different pH, which were selected as a simuli for stomach, and intestine pH. The loading efficiency was 54%. By optimizing the loading method, we can reach to higher loading efficiencies. NIPAAm monomer gives hydrophilic characteristic to the polymer. By optimizing the monomers proportion in the copolymer one can reach to better efficiencies and releasing profiles. Polymers show usually an improved pharmacokinetics compared to small molecule drugs with longer circulation time and the potential for tissue targeting (Schmaljohann et al. 2006). If the polymer is not a drug itself, it often provides a passive function as a drug carrier, reducing immunogenicity, toxicity or degradation, whilst improving circulation time and potentially a passive targeting function. In this case the polymer has to be water-soluble, non-toxic, non-immunogenic and it needs to be safe at all stages of the drug delivery process (e.g. before and after the drug has been released) including a safe excretion.
By definition, hydrogels are polymeric networks with three-dimensional configuration capable of imbibing high amounts of water or biological fluids (Zhang et al. 2004a,b). Their affinity to absorb water is attributed to the presence of hydrophilic groups such as –OH, –CONH–, –CONH2–, and –SO3H in polymers forming hydrogel structures. Hydrogels can be prepared from natural or synthetic polymers. Although hydrogels made from natural polymers may not provide sufficient mechanical properties and may contain pathogens or evoke immune/ inflammatory responses, they do offer several advantageous properties such as inherent biocompatibility, biodegradability, and biologically recognizable moieties that support cellular activities. Synthetic hydrogels, on the other hand, do not possess these inherent bioactive properties (Lin et al. 2006). Our goal was merging advantages of two methods (nano-encapsulation and temperature sensitive property of smart hydrogels) together, to obtain a nano system for passing stomach and release insulin in intestine for oral delivery of insulin. As mentioned above, to prepare thermosensitive nanoparticles, specially designed PNIPAAmter polymer was used. The monomers were chosen because (i) PNIPAAm based hydrogel is a typical temperature-sensitive hydrogel that exhibits a volume phase transition in response to temperature changes at around 33°C. (ii) MAA is a versatile hydrophilic co monomer, but its homopolymer does not show a volume phase transition temperature in water. Introduction of MAA component improves the mechanical strength of PNIPAAm hydrogels. (iii) Hydroxy ethyl methacrylate (HEM) is a MAA derivative that shows pH-sensitive behavior.
Ethical Issues
None to be declared.
Conflict of interests
The authors declare no conflict of interests.
Acknowledgments
The authors thank Drug Applied Research Center of Tabriz University of Medical Sciences and Research Vice-Chancellor of Tabriz University oh Medical Sciences for financial support.
References
- Morçöl T, Nagappan P, Nerenbaum L, Mitchell A . 2004Calcium phosphate-PEG-insulin-casein (CAPIC) particles as oral delivery systems for insulin . International Journal of Pharmaceutics.277(1-2): p. 91-97 [DOI] [PubMed] [Google Scholar]
- Sweetman S (Ed), Martindale. 2007. The complete drug reference. London: Pharmaceutical Press. Electronic version.
- Kim B, Peppas NA . 2003 In vitro release behavior and stability of insulin in complexation hydrogels as oral drug delivery carriers . International Journal of Pharmaceutics, 266(1-2): p. 29-37 [DOI] [PubMed] [Google Scholar]
- Morishita M, Goto T, Nakamura K, Lowman AM, Takayama K, Peppas NA . 2006 “Novel Oral Insulin Delivery Systems Based on Complexation Polymer Hydrogels: Single and Multiple Administration Studies in Type 1 and 2 Diabetic Rats”, J . Controlled Release,.110: p. 587-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed A, Remawi MA, Qinna N, Farouk A, Badwan A . 2009 Formulation and characterization of an oily-based system for oral delivery of insulin. European Journal of Pharmaceutics and Biopharmaceutics, 73(2): p. 269-279 [DOI] [PubMed] [Google Scholar]
- Marschütz MK, Caliceti P, Bernkop-Schnürch A . 2000 Oral peptide drug delivery: polymer-inhibitor conjugates protecting insulin from enzymatic degradation in vitro. Biomaterials, 21(14): p. 1499-1507 [DOI] [PubMed] [Google Scholar]
- Hamidi M, Azadi A, Rafiei P . 2008 Hydrogel nanoparticles in drug delivery. Advanced Drug Delivery Reviews, 60(15): p. 1638-1649 [DOI] [PubMed] [Google Scholar]
- Lin CC, Metters AT . 2006 Hydrogels in controlled release formulations: Network design and mathematical modeling . Advanced Drug Delivery Reviews, 58(12-13): p. 1379-1408 [DOI] [PubMed] [Google Scholar]
- Okano T . 1990Thermally on-off switching polymers for drug permeation and release. Journal of Controlled Release, 11(1-3): p. 255-265 [Google Scholar]
- Akerman S, Viinikka P, Svarfvar B, et al. 1998 Drug permeation through a temperature-sensitive poly(N isopropylacrylamide) grafted poly(vinylidene fluoride) membrane. Int J Pharm , 164:29-36: p. 164:29-36 [Google Scholar]
- Sakuma S, Hayashi M, Akashi M . 2001 Design of nanoparticles composed of graft copolymers for oral peptide delivery. Advanced Drug Delivery Reviews, 47(1): p. 21-37 [DOI] [PubMed] [Google Scholar]
- Wang ZC . 2008 Study on novel hydrogels based on thermosensitive PNIPAAm with pH sensitive PDMAEMA grafts. Colloids and Surfaces B: Biointerfaces, 67(2): p. 245-252 [DOI] [PubMed] [Google Scholar]
- Chaaya RG, Miroslav B . 1999Modulating insulin-release profile from pH/ thermosensitive polymeric beads through polymer molecular weight . Journal of Controlled Release, 59: p. 287-298 [DOI] [PubMed] [Google Scholar]
- Schmaljohann D . 2006 Thermoand pHresponsive polymers in drug delivery. Advanced Drug Delivery Reviews, 58(15): p. 1655-1670 [DOI] [PubMed] [Google Scholar]
- Zhang K, Wu XY, Chu CC . 2004. a Synthesis and characterization of partially biodegradable, temperature and pH sensitive Dex-MA/PNIPAAm hydrogels . Biomaterials, 25(19): p. 4719-4730 [DOI] [PubMed] [Google Scholar]
- Zhang K, Wu XY . 2004. b Temperature and pH-responsive polymeric composite membranes for controlled delivery of proteins and peptides . Biomaterials, 25(22): p. 5281-5291 [DOI] [PubMed] [Google Scholar]


