Abstract
Introduction
Application of nanoparticles has been extensively increased in last decades. Nanoparticles of noble metals such as gold, platinum and especially silver are widely applied in medical and pharmaceutical applications. Although, variety of physical and chemical methods has been developed for production of metal nanoparticles, because of destructive effects of them on environment, biosynthetic methods have been suggested as a novel alternative. Some bacteria and microalgae have different ranges of potentiality to uptake metal ions and produce nanoparticles during detoxification process. In the present work, we study the potential of three Lactobacilli and three algal species in production of AgNPs in different concentrations of silver nitrate.
Methods
Utilizing AAS, XRD and TEM methods, Nannochloropsis oculata, Dunaliella salina and Chlorella vulgaris as three algal species in addition to three Lactobacilli including L. acidophilus, L. casei, L. reuteri were monitored for production of silver nanoparticles. Three concentrations of AgNO3 (0.001, 0.002, 0.005 M) and two incubation times (24h and 48h) were included in this study.
Results
Our findings demonstrated that C. vulgaris, N. oculata and L. acidophilus have the potential of nanosilver production in a culture medium containing 0.001 M of AgNO3 within 24 hours. Also L. casei and L. reuteri species exhibited their potential for production of silver nanoparticles in 0.002 M concentration of AgNO3 in 24 hours. The size range of particles was approximately less than 15 nm. The uptake rate of silver in the five species was between 1.0 to 2.7 mg/g of dry weight. Nanoparticle production was not detected in other treatments and the algae Dunaliella.
Conclusion
The biosynthesis of silver nanoparticles in all of three Lactobacilli and two algal species including N. oculata and C. vulgaris was confirmed.
Keywords: Silver nanoparticles, Biosynthesis, Lactobacilli, Microalgae
Introduction
Development of technologies for producing nanoparticles, because of their key role in the future world could be one of the most valuable findings of human being. Nanoparticles have a wide range of use in different fields of industry, medicine and basic science. Utilizing nanoparticles especially silver nanoparticles in production of cosmetics, detergents and hygienic goods is widely practiced. The AgNPs (Silver nanoparticles) are reported to be nontoxic to human and most effective against bacteria, viruses, and other eukaryotic microorganisms at very low concentration and without any side effects (Jeong et al. 2005). Only one gram of AgNPs is known to impart antibacterial properties to hundreds of square meters of substrate material (Thakkar et al. 2010).
High demands for nanoparticles have been led to the production of them in large scales. So a wide range of industrial methods have been developed to synthesize metal nanoparticles. Some of these procedures are involved using toxic solvents or high energy consumption. This leads to a growing awareness of the need for developing clean, nontoxic and environmentally friendly procedures.
An attractive possibility is employing microorganisms, like bacteria (Husseiny et al. 2007), fungi (Bhainsa and D'Souza 2006), and alga (Singaravelu et al. 2007) in nanomaterial production. Creation of nanoparticles in suitable size, shape and dispersity is one of the challenges of present nanotechnology. Improvements at biological methods could allow the use of nano-biotechnology in a variety of applications (Bharde et al. 2006; Roh 2006; Bharde 2007), as these characteristics could be controlled in biological production by optimizing cultural and environmental conditions (Narayanan and Sakthivel 2010). There are reports denoting that the morphology of nanoparticles could be altered by different pH and temperature treatments (Gericke and Pinches 2006).
In the present work we study the potential of three Lactobacilli and three algal species in production of AgNPs in different concentrations of silver nitrate.
Materials and methods
Biosynthesis of AgNPs by Lactobacilli
Three species of Lactobacilli consisting L. acidophilus, L. casei and L. reuteri were grown in triple replications inside 1000 ml Erlenmeyer flasks containing 500 ml of MRS broth medium. Cultures were incubated at 37°C in a shaking rate of 150 rpm for 24 h. Then three different concentrations of AgNO3 (0.001, 0.002, 0.005 M) were added into the appropriate bacterial cultures during the log phase. Harvesting of bacterial mass was carried out in 24 and 48 hours by centrifuging at 4000 rpm for 20 min at 4°C. The bacterial pellets were rinsed thrice with sterile distilled water to remove any medium leftovers. Supernatant solutions were used for TEM observation of extracellular biosynthesis. The bacterial biomasses were dried in 70°C for 15 h and grinded to a powdered form and used for X-Ray Diffraction analysis (XRD) and Atomic absorption spectrophotometry (AAS).
Biosynthesis of AgNPs by Algae
Nannochloropsis oculata, Dunaliella salina and Chlorella vulgaris were the three algal species used in the present study. A similar method was employed for monitoring of nanoparticle biosynthesis by microalgae. Three replicas of each algal culture were considered in 1000ml Erlenmeyer flasks as following:
N. oculata culture was carried out for approximately 120h in basal medium comprising 0.1% (v/v) Walne medium nutrients, 0.1% (v/v) trace metal solution, and 1.7 μM urea as nitrogen source, in artificial seawater (A.S.W).
SK medium (Gladue and Maxey 1994) was utilized for D. salina culture (Fig. 1).
And finally C. vulgaris was grown in M8 medium described by Mandalam and Palsson for 6 days (Mandalam and Palsson 1998).
Fig. 1.

Microalgae culture.
Cultures were exposed to a light level of 1500 lx, in 24±2°C under a photoperiod of 16.8.
AgNO3 solutions were added in the same concentrations as described for bacteria into algal cultures. After 24h and 48h, microalgae were harvested and the whole process was continued as illustrated before.
Characterization of produced AgNPs
The XRD pattern was obtained utilizing Shimadzu XRD- 6000 diffractometer with CuKa (λ = 1.54056 Å) to confirm the biosynthesis of AgNPs. Scans were run from 2Theta = 25º - 80º.X'Pert HighScore software (version 2.0, 2003) was employed to survey the XRD peaks.
Concentration of synthesized AgNPs was determined by means of Shimadzu AA-6300 atomic absorption spectrophotometer using hydrochloric acid extraction method.
The size and dispersity mode of AgNPs was characterized by transmission electron microscopy utilizing Hitachi HT- 7700 microscope.
Results and discussion
Results demonstrate the potential of two algal species consisting N. oculata and C. vulgaris and all three Lactobacilli included in this study for biosynthesis of AgNPs.
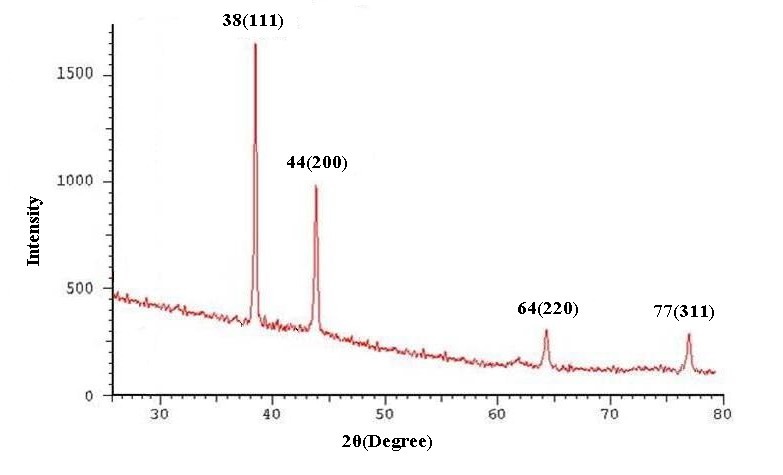
The biosynthesis of AgNPs was confirmed by the trait peaks observed in the XRD image (Fig. 2) as well as by the structural view under the TEM as shown in (Fig. 3).
Fig 2. The XRD Images of silver nanoparticles synthesized by microalgae C. vulgaris (A) and L. acidophilus (B).The diffraction patterns were obtained by measurement of angles at which X-ray beam is diffracted by the crystalline phases in the specimen.
A.
B.
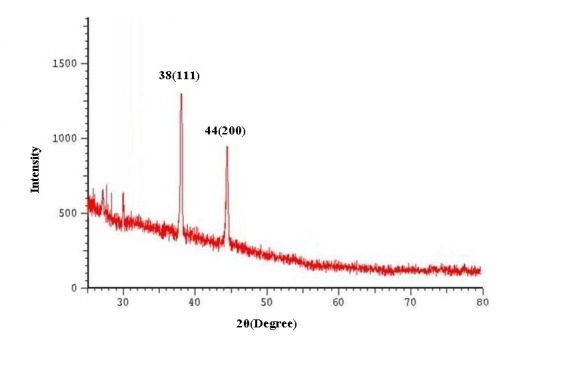
Fig. 3.
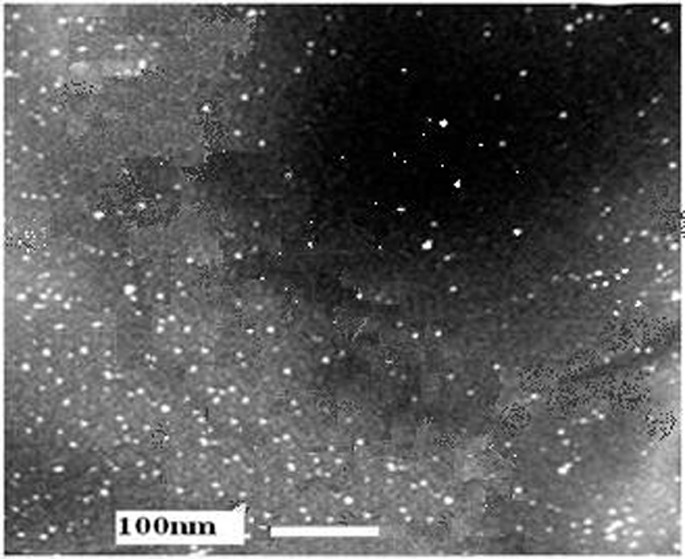
TEM micrograph of Lactobacillus supernatant solution. The scale bar corresponds to 100 nm.
XRD spectra of the samples revealed four distinct peaks for Chlorella vulgaris and two peaks for Lactobacillus species. The observed peaks at the 2θ of 38.18°, 44.3°, 64.55° and 77.54° corresponds to (111), (200), (220) and (311) planes respectively. A comparison of our XRD spectra with the standard spectra of pure crystalline silver structures demonstrates that mentioned peaks best matches with Face-Centered Cubic (FCC) silver crystals (XRD Reference No. 01-087-0718).
Biosynthesis occurred at 0.001 M of AgNO3 for L. acidophilus, N. oculata and C. vulgaris while the optimum concentration for producing AgNPs for L. casei, and L. reuteri was 0.002 M. One possible reason for absence of AgNPs in replica cultures with 0.005 M of AgNO3 could be a toxic or inhibitory effect of higher concentrations on studied microorganisms.
The appropriate time for incubation of all species was 24 h. Incubation of algal and bacterial species for 48h had no significant influence in AgNPs production.
According to measurements derived from atomic absorption spectroscopy, the quantity of Ag+ taken up from solution in the five species was as following. C. vulgaris: 1.0, N. oculata: 2.7, L. acidophilus: 2.548, L. casei: 2.1 and L. reuteri: 1.8 mg/g of dry weight.
TEM micrograph analysis demonstrated proper dispersion of the AgNPs. Nanoparticles were in a size less than 15nm. (Fig. 3) It was clear that even aggregated nanoparticles did not have direct contacts with each other. This could be due to the role of proteins in biosynthesis of AgNPs (Nithya and Ragunathan 2009). The long-term stability of the nanoparticles in the solution is believed to be an influence of proteins which are secreted from microorganisms (Gole et al. 2001; Mukherjee et al. 2002).
The potential of extracellular production of nanoparticles eliminates the need for harvesting AgNPs from the cell enclosure. This makes the present method more cost effective and eco-friendly.
Conclusion
Three algal species including Nannochloropsis oculata, Dunaliella salina, and Chlorella vulgaris and three lactobacilli including L. acidophilus, L. casei, L. reuteri were subjected to an experiment to be assessed for their capability in biosynthesis of silver nanoparticles. In addition to N. oculata and C. vulgaris, all three Lactobacilli were found to be promising in production of mentioned nanomaterials.
Ethical Issues
None to be declared.
Conflict of interests
The authors declare no conflict of interests.
Acknowledgments
We would like to thank Artemia and Aquatic Animals Research Institute of Urmia University for their constant support and Dr. Khalil Azizpoor for providing bacteria.
References
- Bhainsa KC and D'Souza SF . 2006 Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids and Surfaces B: Biointerfaces, 47(2), 160-164 [DOI] [PubMed] [Google Scholar]
- Bharde A, Rautaray D, Bansal V, Ahmad A, Sarkar I, Yusuf SM, et al. 2006 Extracellular biosynthesis of magnetite using fungi. Small, 2(1), 135-141 [DOI] [PubMed] [Google Scholar]
- Bharde A, Rao M, Prabhune A and Sastry M . 2007 Bacterial enzyme mediated biosynthesis of gold nanoparticles. Journal of Nanoscience and Nanotechnology, 7(12), 4369-4377 [DOI] [PubMed] [Google Scholar]
- Gericke M and Pinches A . 2006 Biological synthesis of metal nanoparticles. Hydrometallurgy, 83(1-4), 132-140 [Google Scholar]
- Gladue R and Maxey J . 1994 Microalgal feeds for aquaculture. Journal of Applied Phycology, 6(2), 131-141 [Google Scholar]
- Gole A, Dash C, Ramakrishnan V, Sainkar SR, Mandale AB, Rao M, et al. 2001 Pepsin−gold colloid conjugates: preparation, characterization, and enzymatic activity. Langmuir, 17(5), 1674-1679 [Google Scholar]
- Husseiny MI, El-Aziz MA, Badr Y and Mahmoud MA . 2007 Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 67(3-4), 1003-1006 [DOI] [PubMed] [Google Scholar]
- Jeong S, Yeo S and YI S . 2005 The effect of filler particle size on the antibacterial properties of compounded colymer/silver fibers. Journal of Materials Science, 40(20), 5407-5411 [Google Scholar]
- Mandalam RK and Palsson B . 1998 Elemental balancing of biomass and medium composition enhances growth capacity in high-density Chlorella vulgaris cultures. Biotechnology and Bioengineering, 59(5), 605-611 [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Senapati S, Mandal D, Ahmad A, Khan MI, Kumar R, et al. 2002 Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. ChemBioChem, 3(5), 461-463 [DOI] [PubMed] [Google Scholar]
- Narayanan KB and Sakthivel N . 2010 Biological synthesis of metal nanoparticles by microbes. Advances in Colloid and Interface Science, 156(1-2), 1-13 [DOI] [PubMed] [Google Scholar]
- Nithya R and Ragunathan R . 2009 Synthesis of silver nanoparticle using Pleurotus sajor caju and its antimicrobial study. Digest Journal of Nanomaterials and Biostructures, 4(4), 623-629 [Google Scholar]
- Roh Y, Vali H, Phelps TJ and Moon JW . 2006 Extracellular synthesis of magnetite and metal-substituted magnetite nanoparticles. Journal of Nanoscience and Nanotechnology, 6(11), 3517-3520 [PubMed] [Google Scholar]
- Singaravelu G, Arockiamary JS, Kumar VG and Govindaraju K . 2007 A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids and Surfaces B: Biointerfaces, 57(1), 97-101 [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Mhatre SS and Parikh RY . 2010 Biological synthesis of metallic nanoparticles. Nanomedicine: Nanotechnology, Biology, and Medicine, 6(2), 257-262 [DOI] [PubMed] [Google Scholar]


