SUMMARY
Clinical applications of advanced nanomedicines such as PEGylated liposomal doxorubicin and paclitaxel-albumin bioconjugates have significantly improved the cancer treatment strategies. However, these pharmaceuticals lack early detection and single cell tracking capabilities. Thus, engineering of smart multifunctional theranostics appear to be our next step for simultaneous diagnosis and therapy of cancer. Clinical translation of multifunctional theranostics appears to be dependent upon specificity of cancer biomarkers, biocompatibility of components used for formulation, and advancement of bioconjugation techniques. While many cancer biomarker candidates often fail to be used for clinical diagnosis/therapy because of their nonspecific functional expression in normal tissues, biocompatibility of materials used for bioconjugation also needs to be approved. All these issues need to be fully addressed prior to the translation of smart multifunctional cancer theranostics.
Keywords: Cancer Nanomedicines, Cancer Targeting, Cancer Theranostics, Cancer Therapy
Cancer is fundamentally characterized by the irregular wild proliferation of abnormal cells that are capable of invasion and metastasize. Because of the widespread incidence of cancer, it is considered as a one of the threatening diseases worldwide leading causes of death, its early detection is an important challenge. An early detection of cancer is a crucial process, at which point the treatment modality can successfully administered. However, cancer is a complex disease that affects a variety of cells/tissues.
To date, cancer chemotherapy has been accepted as an effective modality after surgery and/or radiation even though such medicaments are always associated with inadvertent side effects because of cytotoxic nature of anticancer agents. Thus, to resolve this problem, many researchers tend to engineer controlled release drug delivery systems (DDS) to: 1) maintain the concentration of anticancer drugs at safe levels, and 2) engineer long circulating to achieve longer half-life and for better patient compliance. Figure 1A represents plasma concentration profile for traditional and controlled release drug delivery systems.
Fig. 1 .
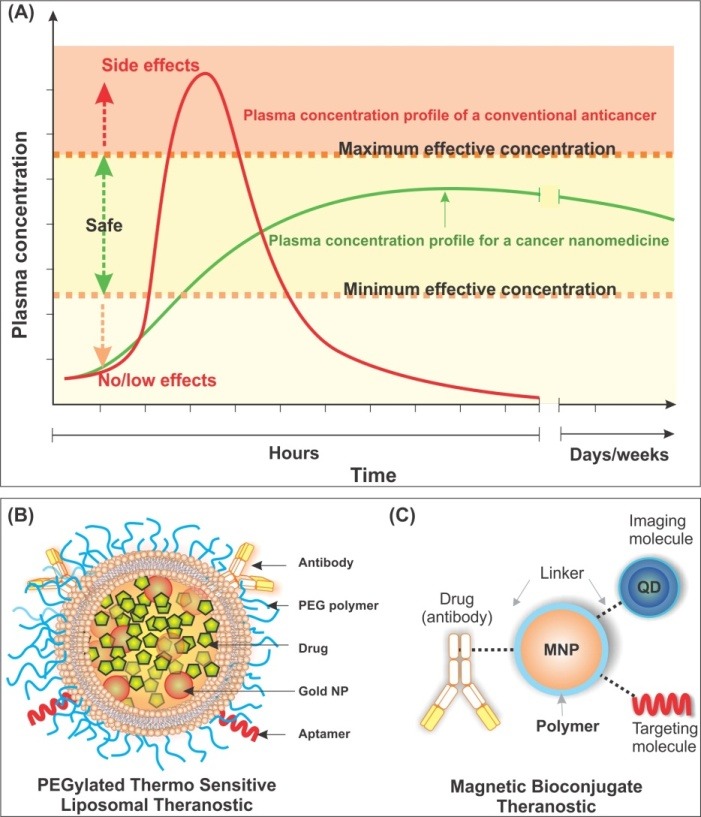
Schematic representation of plasma concentration profile of anticancer drugs (A) and cancer theranostics (B and C).A) Plasma concentration profile of traditional and controlled release drug delivery systems. B) A PEGylated thermo sensitive liposomal theransotic showing entrapped drugs (green) and imaging gold nanoparticles (red) with targeting biomolecules (antibody/aptamer). C) A magnetic bioconjugate theranostics linked with antibody (drug) and targeting molecules (aptamer). Images are from our unpublished works (produced by Omidi and Coukos). QD: Quantum dot. MNP: Magnetic nanoparticle. NP: Nanoparticle.
For example, doxorubicin is widely commonly used in the treatment of a wide variety of cancers, PEGylated liposome-encapsulated form of doxorubicin (Doxil™) is a FDA approved medicament for treatment of ovarian cancer and multiple myeloma. The half-life of Doxil™ is greater than 48hr compare to significantly shorter half-life of doxorubicin. Besides, the PEGylated liposomal doxorubicin is a stealth nanosystem that can circumvent the opsonization and consequently skipping from immune system clearance.
For effective clinical implementation of smart multifunctional theranostics, we need to: 1) advance technologies for specific targeting of cancer cells, 2) improve imaging/sensing methods, 3) develop biocompatible long circulating bioshuttles for simultaneous delivery of targeting moiety, imaging agent and therapy, and 4) track and control cancerous single cells/bioconvoysto avoid distribution of oncogenic messages (the so called metastasis). Of various advancements holding great promise for improving the sensing/imagining cancerous cells appears to be quantum dots (QDs) conjugated nanoparticles (Wang and Chen 2011). Conjugation of such nanosystems with targeting moieties (e.g., aptamer or monoclonal antibody) and cytotoxic agents can lead to all-in-one theranostics modalities (McCarron and Faheem 2010; Shapira et al. 2011). Besides these macromolecular nanosystems can be further conjugated to magnetic nanoparticles to reinforce their accumulation in the desired sites (Cole et al. 2011; Singh 2011). Fig. 1 (panels B and C) shows schematic representation of different types of smart multifunctional theranostics. Having implemented these smart theranostics, such “bioshuttles” can track the target cells (via antibody/aptamer), drug release can be activated (e.g., thermo-sensitive polymers), and its profile can be controlled too. Once attached to cancer cells, photo-thermal therapy can be also applied through gold nanoparticles (NPs) using near infra-red (NIR) lasers (Fig. 1B). Besides, magnetic nanoparticle (MNP) bioconjugates can be directed to desired targets (Fig. 1C).
Simultaneous pinpointing of cancerous cells by multifunctional theranostics can even impart existence of a single cancerous cell at the course period of treatment. Such real time monitoring at molecular cellular levels can significantly favor the targeted drug therapy, which is largely dependent upon the exquisite sensitivity and versatility of optical technologies. The optical emission of traditional organic fluorophores is normally used to visualize the activities of biomolecules even though their use may be contributed with photobleaching‚ sensitivity to biological conditions‚ and inability to excite multiple fluorophores. The optical and electronic properties of inorganic fluorophores (e.g., QDs and PhosphorDots™) can be tuned during the synthesis process by changing their size‚ shape‚ or composition. They have been widely used for in vitro cell biomolecule/cell tracking purposes as well as in DNA nanobiosensors (Dolatabadi et al. 2011a).
In most of cases, there is a need for alteration of the native structure of a macromolecule to provide functional targets for surface modification or conjugation since the required chemical groups are not present on one of the target molecules and must be created (Hermanson 2008). Engineering and bioconjugation of various components of multifunctional theranostics appear to be our big future tasks since we need to test a wide range of nanobiomaterials from biopolymers to carbon nanotubes (Dolatabadi et al. 2011b). Much greater challenges seem to be ahead upon their clinical translation as the cost is huge for development of such modalities (Omidi 2011). This clearly means that we need to implement the molecular Trojan horses in a smarter manner.
Ethical issues
No ethical issues to be declared.
Conflict of interests
No conflict of interest to be declared.
Acknowledgments
Author expresses his sincere gratitude to Prof. George Coukos (University of Pennsylvania, Philadelphia, USA) for his visionary advice upon impacts of theranostics in cancer diagnosis and therapy.
References
- Cole AJ, Yang VC and David AE . 2011 Cancer theranostics: the rise of targeted magnetic nanoparticles. Trends Biotechnol, 29(7), 323-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolatabadi JEN, Mashinchian O, Ayoubi B, Jamali AA, Mobed A, Losic D, et al. 2011. a Optical and electrochemical DNA nanobiosensors. TrAC - Trends in Analytical Chemistry, 30(3), 459-472 [Google Scholar]
- Dolatabadi JEN, Omidi Y and Losic D . 2011. b Carbon nanotubes as an advanced drug and gene delivery nanosystem. Current Nanoscience, 7(3), 297-314 [Google Scholar]
- Hermanson GT. 2008. Bioconjugate techniques. Amesterdam, Elsevier Inc.
- Mccarron PA and Faheem AM . 2010 Nanomedicine-based cancer targeting: a new weapon in an old war. Nanomedicine (Lond), 5(1), 3-5 [DOI] [PubMed] [Google Scholar]
- Omidi Y . 2011 Translational researches require effective protocols for knowledge and technology transfer and integration. BioImpacts, 1(2), 71-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira A, Livney YD, Broxterman HJ and Assaraf YG . 2011 Nanomedicine for targeted cancer therapy: Towards the overcoming of drug resistance. Drug Resist Updat, 14(3), 150-163 [DOI] [PubMed] [Google Scholar]
- Singh SP . 2011 Multifunctional magnetic quantum dots for cancer theranostics. J Biomed Nanotechnol, 7(1), 95-97 [DOI] [PubMed] [Google Scholar]
- Wang Y and Chen L. 2011. Quantum dots, lighting up the research and development of nanomedicine. Nanomedicine. [DOI] [PubMed]


