Abstract
Introduction
We have already shown the protective effects of soy protein on rheumatoid arthritis in rats. In this study, the effects of genistein and daidzein, two isoflavones from soy on rheumatoid arthritis prognosis and prevention in rats have been investigated.
Methods
Rheumatoid arthritis was induced in female Sprague-Dawley rats using collagen type II plus adjuvant. Rats were then treated with soy protein (7 g/kg), dexamethasone (1 mg/kg), genistein (20 mg/kg genistein), daidzein (20 mg/kg genistein) and casein (in control groups) by daily gavage feedings for 50 days. Scores of arthritis were recorded every day for each paw of animal. Serum concentrations of TNF-α, IL-6, adiponectin and leptin were characterized. Tibiotarsal tissue was used for histopathologic analyses.
Results
Treatment with genistein and daidzein resulted in not only a reduction in disease symptoms but also a delay in the onset of symptoms. Results from delayed-type hypersensitivity test demonstrated that the ear thickness in treated rats was significantly lower than that in the control group (p<0.05). There was a reduction in TNF-α, IL-6, adiponectin and leptin serum concentrations after treatment with genistein and daidzein. Dexamethasone reduced the serum concentrations of TNF-α, IL-6 and adiponectin but increased leptin serum level. Prevention of the tissue damage and joint inflammation was also observed following treatment with two soy isoflavones.
Conclusion
soy isoflavones, daidzein and especially genistein, could significantly improve rheumatoid arthritis symptoms in rats. The structural similarity of isoflavones to estrogen could be the possible underlying mechanism involved in the function.
Keywords: Genistein, Daidzein, Soy Protein, Dexamethasone, Rheumatoid Arthritis, Adiponectin, Leptin, TNF-α, IL-6
Introduction
Rheumatoid arthritis (RA) is a common chronic and systemic immune-mediated inflammatory disease affecting at least one percent of the world population (Wang et al. 2007). Only in the United States, more than two million people are affected with the disease. The mortality and morbidity of the disease is rising promptly and the average life expectancy is 10-18 years shorter for people with RA than that for general population (Martinez et al. 2001).
RA is usually characterized by persistent synovitis, deformation and degradation of articular cartilages and bones (Wang et al. 2007). It is speculated that TNF-α, IL-6 and IL-1β produced by monocytes, macrophages and synovial finroblasts play key roles in progression of the disease (Tsuji et al. 2006). Adiponectin also has a key role in the pathogenesis of RA and has been characterized as an osteoarthritis preventing factor. According to some studies, decrease in the concentration of adiponectin is associated with increase in the level of inflammatory mediators such as C-reactive protein (CRP) and IL-6 (Senolt et al. 2006). Leptin secreted from adipose tissues is believed to be involved in RA and its function in balancing immune responses has been gaining attention recently (Palmer and Gabay 2003).
Two therapeutic options available for patients with RA are non-steroidal anti-inflammatory drugs (NSAIDs) and disease modifying antirheumatic drugs (DMARDs) (Smolen and Steiner 2003). Drugs are mostly used to alleviate RA symptoms. Besides being highly expensive, these drugs suffer from side effects; therefore, seeking alternative treatment protocols, including the use of alternative medicinal plants (e.g. Bambusa arundinacea, Semecarpus anacardium, etc) in RA management have received considerable attention in the last few years.
Flavonoids can alleviate the inflammation and immune response by inhibition of regulatory enzymes involved in the metabolism of the inflammatory mediators (Manthey 2000). Several trials have evaluated the effect of soy consumption on endothelial function; most of them assessed endothelial function by flow-mediated vasodilatation and few focused on the biochemical markers (Azadbakht et al. 2007). Genistein and daidzein are the most abundant isoflavones of soy which are both structurally similar to the selective modulators of estrogen, tamoxifen, and synthetic isoflavones receptors. It would be possible that isoflavones from soy similarly affect cartilage metabolism and alleviate RA symptoms. Regarding the antioxidant effects of soy isoflavones on annulling many inflammatory processes and down-regulatory activity of genistein on leptin (Szkudelski et al. 2005), it is speculated that isoflavones from soy have beneficial effects on RA status in patients. Although the effects of soy isoflavones have been investigated in several disorders and the in vitro anti-inflammatory effects have partly been proved, until now, no in vivo study has shown the effects of soy isoflavones in RA. More recently, we reported the protective properties of soy protein on RA (Mohammad Shahi et al. 2011). In this study, soy protein was found to be a potent immunomodulatory inhibitor of RA in rats. It could delay the onset of RA, reduced cartilage erosion, and synovitis inflammation. Administration of soy protein significantly suppressed the progression of collagen II-induced arthritis (CIA) and inhibited the production of TNF-α, IL-6, leptin and adiponectin (Mohammad Shahi et al. 2011).
In the present research paper, we intended to determine the effects of soy isoflavones, genistein and daidzein on RA in rats and compare their effects with those of soy isolated protein and dexamethasone. The time for RA onset was evaluated among treated and untreated animals together with the serum concentrations of IL-6, TNF-α, adiponectin and leptin in the studied groups.
Materials and methods
Materials
Bovine Collagen type II and Incomplete Frund's Adjuvant (IFA) were purchased from Chondrex and Sigma, USA, respectively. Genistein and daidzein 100% pure were purchased from LC Labs, USA.
Animals and diet
Female Sprague-Dawley rats (8-10 weeks of age, weighing 180-200 g) were obtained from Laboratory Animal House of Tabriz University of Medical Sciences, Tabriz, Iran and were adapted with the experimental environment for 2 weeks prior to study. Housing conditions and experimental procedures were in accordance with standards (Radzikowski 2006). The cages were placed under pathogen-limiting conditions in a temperature and humidity-controlled colony room (22–24o C and 45 to 50% relative humidity) with a 12 hr day–night cycle. Food and water were provided ad libitum and the body weights were recorded every three days.
Thirty-six rats were selected and randomly divided into six equal groups as described in Table 1. Treatments were carried out for 50 days after CIA induction and casein was used as an inert protein in control groups (Nagasawa et al. 2003).
Table 1. Experimental groups.
| Group No. | Rats | Treatment |
| 1 | Healthy control | 7 g/kg casein |
| 2 | CIA-control | 7 g/kg casein |
| 3 | CIA-soy protein-treated | 7 g/kg soy protein |
| 4 | CIA-genistein-treated | 7 g/kg casein+20 mg/kg genistein |
| 5 | CIA-daidzein-treated | 7 g/kg casein+20 mg/kg daidzein |
| 6 | CIA-dexamethasone-treated | 7 g/kg casein+1 mg/kg dexamethasone |
In order to establish the required serum concentrations of the active compounds, rats were gavaged with test compounds from 1 week prior to the induction of CIA two times a day (8 am and 2 pm) for 50 days (Cai et al. 2007; Gu and Brandwein 1998).
Preparation of CIA rats
Collagen type II plus adjuvant was used to immunize rats according to the protocol recommended by Chondrex Inc. Collagen type II was dissolved in 0.01 M acetic acid (final concentration, 2 mg/ml) by shaking overnight at 4ºC and used as a fresh solution. To make an emulsion of collagen type II and incomplete Freund’s adjuvant (IFA), an electrical ultra-homogenizer (Ultra-Turrax T 25 Basic IKA-WERKE, 24000 rpm with a 5 mm blade) was used. All mixing process was carried out in an ice tank. Then, 2.5 ml IFA was added to an eppendorf and the prepared collagen type II solution was added drop-wise to give a homogenized solution. Mixing continued at 24,000 rpm until a thick emulsion was obtained (2-3 min) and emulsion stability was tested by adding one drop of the emulsion into a beaker of water (A stable emulsion appeared as a solid clump in water without dispersion, while spreading onto the water surface was indicative of an unstable emulsion). After transfer to Hamilton glass syringe, 100 µl of the cold emulsion was subcutaneously injected to the base of the tail 2-3 cm from the body on day 0 and the procedure was repeated at day 7.
Treatments
Genistein and daidzein (20 mg/kg/day) and dexamethasone (1 mg/kg/day) were daily dissolved in water and mixed with casein; then, fed by oral gavage to the rats two times daily. Soy isolated protein (7gr/kg/day) was prepared similarly but without addition of casein.
Rating of clinical symptoms
Severity of rheumatoid arthritis was assessed in rats paw under the supervision of a rheumatologist using a standard double blind scoring system, in which 0=no change, 1=swelling and erythema of the digit, 2=mild swelling and erythema of the limb, 3=gross swelling and erythema of the digit, 4=gross deformity and inability to use the limb. The arthritis score for each mouse was the sum of all paw scores where the highest score per mouse would be 16 (Khalifeh et al. 2008).
Delayed type hypersensitivity method
Twelve days after immunization with collagen type II, cell-mediated immunity assessment was done using delayed type hypersensitivity (DTH) method. Briefly, 50 µg collagen type II in 0.15 M phosphate buffer (pH 7.4) was injected subcutaneously to right ears of rats. Same volume of phosphate buffer was injected to the left ears which were used as controls. Prior to injections, the thickness of both ears was measured using a micrometer and the place for measurement was marked as injection site. Twenty-four hr after injection, rats were anesthetized and ear thickness was measured again. The difference of thickness between right and left ears was used as an indicator of the intensity of DTH reaction (Gu and Brandwein 1998; Wang et al. 2007).
Histopathological assessment
Rats were sacrificed; tarsal and tibiotarsal joints were isolated. Histopathological examination was performed after routine fixation with 10% buffered formalin, decalcification, dehydration, cleaning, impregnation, paraffin embedding, longitudinal sectioning (with rotary microtome) and staining with hematoxylin and eosin. Histopathological change in tarsal joints was scored using the following parameters: 0=normal, 1=mild inflammatory cell infiltration of synovium, 2=moderately severe inflammatory cell infiltration with early cartilage loss, 3=severe inflammation with pannus formation, marked cartilage loss, and/or particular bone erosions. Three histological sections were reviewed for each of the right and left hind paws of each rat and a mean histopathologic severity score derived for the six histological sections in each animal (Gu and Brandwein 1998; Wang et al. 2007).
Measurement of TNF-α, IL-6, leptin and adiponectin
At the end of the study, the mean concentrations of TNF-α, IL-6, adiponectin and leptin were measured using a commercial ELISA kit (Bender MedSystems, Austria). Concentrations were measured using the calibration curves.
Statistical analysis
The results were expressed as the mean±SEM (n = 6). All data were evaluated statistically using ANOVA, and the statistical significance of difference was assessed by the one-way Tukey-Kramer method; Values of P<0.05 were considered statistically significant.
Results
RA onset and symptom intensity
The onset and intensity of symptoms in CIA rats were rated as previously mentioned in methods section. Results have been depicted in Table 2 and Fig. 1.
Table 2. The average intensity of rheumatoid arthritis symptoms in collagen-induced arthritic rats*.
| Group | Symptom intensity | P1 | P2 |
| Healthy control | - | - | - |
| CIA control | 6.83 ± 0.47 | - | 0.000 |
| CIA + soy protein (7 gr/kg) | 2.66 ± 0.88 | 0.020 | 0.156 |
| CIA + daidzein (20 mg/kg) | 2.16 ± 0.74 | 0.000 | 0.262 |
| CIA + genistein (20 mg/kg) | 1.33 ± 0.84 | 0.000 | 0.758 |
| CIA + dexamethasone (1 mg/kg) | 1.00 ± 0.63 | 0.000 | - |
* All data are presented as mean±SD, (n=6 for each group)
P1: In comparison with CIA control, P2: In comparison with dexamethasone group (Independent samples t-test)
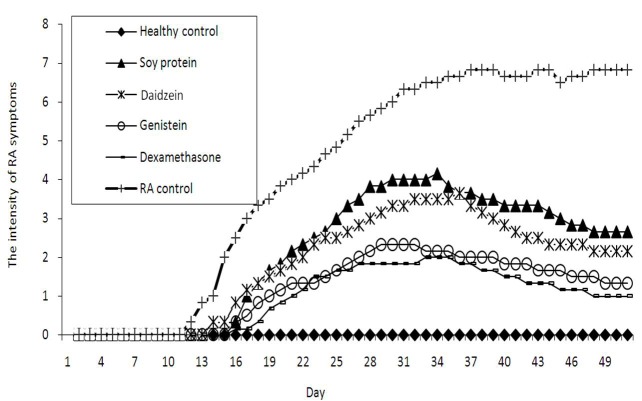
Fig. 1 .
Effects of 20 mg/kg/day genistein and daidzein, 1 mg/kg/day dexamethasone, and 7gr/kg/day soy protein on the collagen-induced arthritis scores in rats in 50 days.
As it was expected, in all collagen-injected animals, the symptoms of rheumatoid arthritis were observed, however, treatment with any four agents could delay the onset of the symptoms compared with CIA control group significantly (Fig. 1). Interestingly, genistein was found to be as effective as dexamethasone in delaying the onset of the rheumatoid arthritis symptoms. Both genistein and dexamethasone could delay the onset of the symptoms for 15.7 days compared to 11.5 days in the CIA control group.
Although there was a gradual increase in the severity of the symptoms up to almost 30 days in all study groups but at lower level compared with the CIA control; the severity started to decrease 32-37 days after treatment in the animals receiving soy protein, genistein, daidzein and dexamethasone (Fig. 1 and Table 2).
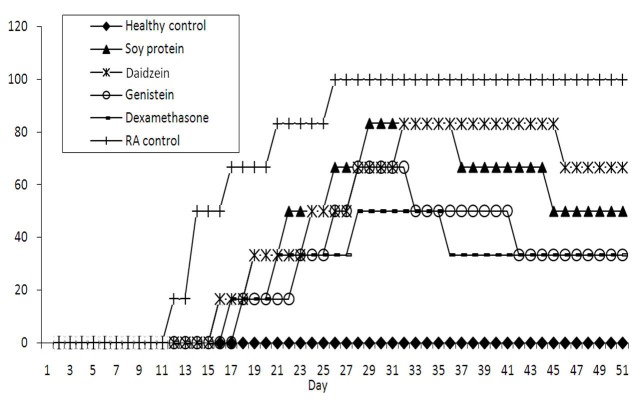
The ability of the soy isoflavones in suppressing immune mediated pathologic process in arthritic rats was also investigated by evaluating the arthritis incidence of CIA in rats. In Fig. 2, the percent incidence of CIA incidence (the relative number of animals with CIA in time) in groups under study has been illustrated. Genistein and dexamethasone had almost similar effects on the incidence of CIA.
Fig. 2 .
Effects of 20 mg/kg/day genistein and daidzein, 1 mg/kg/day dexamethasone, and 7gr/kg/day soy protein on the arthritis incidence in collagen-induced arthritis rats.
As shown in Fig. 2, CIA incidence in CIA control group reaches almost 100 percent, while in treated groups, the incidence of arthritis is lower and the number of diseased animals has decreased.
The intensity of symptoms was also determined through visual examination of the paw; corresponding images can be compared in Fig. 3. Soy isoflavones reduced the CIA inflammation, blush and swelling compared to control.
Fig. 3 .
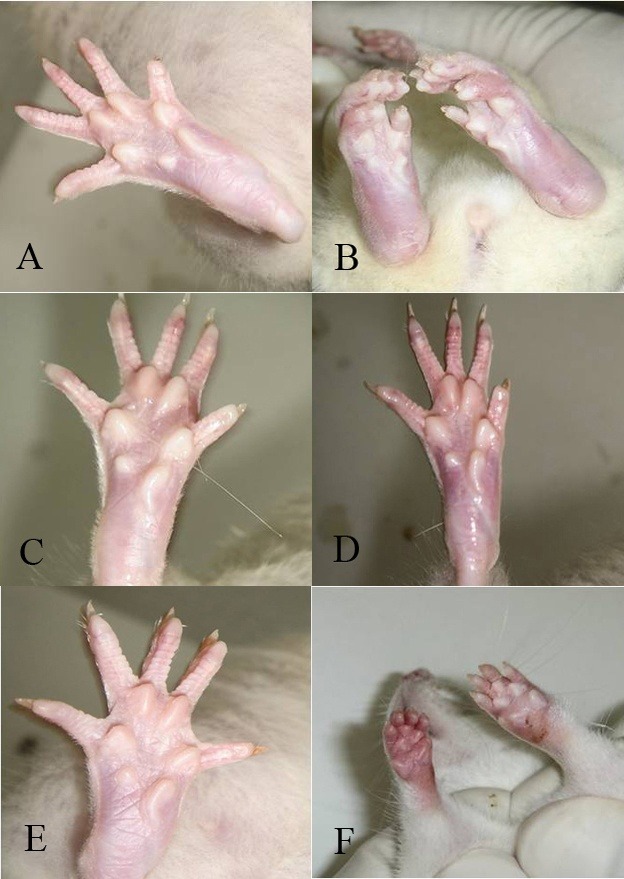
Rheumatoid arthritis was induced by collagen type II and IFA. Paw in healthy controls (A); Paw in CIA control rats (B), Paw in genistein treated CIA rats (C); Paw in daidzein treated CIA rats (D); Paw in dexamethasone treated CIA rats (E); Paw in soy isolated protein treated rats (F), all after 50 days of treatment.
Determination of ear thickness from DTH test
Results obtained from DTH experiment showed that the thickness of ears in collagen-induced arthritic rats increased from 0.50±0.05 mm in the healthy rats to 0.98±0.03 mm in the CIA control group. However, in the treated animals compared with the CIA control, the thickness was significantly lower (p<0.05) with CIA+dexamethasone group having the smallest value (0.61±0.05 mm) followed by CIA+genistein rats (0.64±0.02 mm), CIA+daidzein group (0.68±0.06 mm) and CIA+soy protein group (0.78±0.06 mm). There was no significant difference in the ear thicknesses between dexamethasone and genistein treated animals with the healthy control group (p<0.05).
Histopathologic analysis
Table 3 depicts the intensity of tissue damage after 50 days of treatment with dexamethasone, soy protein and its isoflavones. Results demonstrated a notable decrease in tissue damage after treatment with soy isoflavones and dexamethasone (p<0.05). The curative effect of genistein on the damages induced by collagen was remarkable and comparable to that of dexamethasone.
Table 3. Mean intensity of tissue damage assayed by histopathologic score in two tarsal joints of leg in groups under study after 50 days*.
| Group | Score | P1 | P2 |
| Healthy control | 0 | - | - |
| CIA control | 2.50 ± 0.18 | - | 0.000 |
| CIA + soy protein (7 g/kg) | 0.91 ± 0.30 | 0.001 | 0.091 |
| CIA + daidzein (20 mg/kg) | 0.96 ± 0.34 | 0.003 | 0.100 |
| CIA + genistein (20 mg/kg) | 0.50 ± 0.34 | 0.000 | 0.554 |
| CIA + dexamethasone (1 mg/kg) | 0.26 ± 0.16 | 0.000 | - |
* All data are presented as mean±SD (n=6 for each group)
P1: Comparison with CIA control group, P2: Comparison with dexamethasone group (Independent – samples t- test)
Serum levels of TNF-α and IL-6
Genistein and dexamethasone could approximately reduce TNF-α to the level of healthy control groups after 50 days (p>0.05). Other intervention also lowered TNF-α level respectably. All treatments had significant differences with CIA control rats (p<0.05). Table 4 shows average serum concentration of TNF-α after 50 days.
Table 4. Average serum concentration of TNF-?*.
| Group | TNF-? (pg/ml) | P1 | P2 | P3 |
| Healthy control | 48.08 ± 1.38 | - | 0.000 | 0.108 |
| CIA control | 103.88 ± 3.75 | 0.000 | - | 0.000 |
| CIA + soy protein | 58.91 ± 2.40 | 0.003 | 0.000 | 0.003 |
| CIA + daidzein | 63.66 ± 1.14 | 0.000 | 0.000 | 0.000 |
| CIA + genistein | 47.83 ± 1.87 | 0.917 | 0.000 | 0.131 |
| CIA + dexamethasone | 40.18 ± 4.24 | 0.108 | 0.000 | - |
P1: Comparison with healthy control group, P2: Comparison with CIA control group, P3: Comparison with dexamethasone group (Independent – samples t-test)
Table 5. shows average serum concentration of IL-6 and corresponding standard errors after 50 days.
Table 5. Average serum concentration of IL-6*.
| Group | IL-6 (pg/ml) | P1 | P2 | P3 |
| Healthy control | 57.25 ± 5.23 | - | 0.000 | 0.316 |
| CIA control | 138.01 ± 10.62 | 0.000 | - | 0.000 |
| CIA + soy protein | 88.81 ± 7.86 | 0.007 | 0.004 | 0.003 |
| CIA + daidzein | 79.05 ± 9.65 | 0.075 | 0.002 | 0.027 |
| CIA + genistein | 50.46 ± 8.81 | 0.523 | 0.000 | 0.822 |
| CIA + dexamethasone | 47.83 ± 7.22 | 0.316 | 0.000 | - |
* All data are presented as mean±SD (n=6 for each group)
P1: Comparison with healthy control group, P2: Comparison with CIA control group, P3: Comparison with dexamethasone group (Independent – samples t-test)
After 50 days all treatments lowered IL-6 concentration significantly compared to CIA controls (p<0.05). Genistein could lower IL-6 concentration as well as dexamethasone (p>0.05).
Serum concentration of adiponectin and leptin
Serum concentration of adiponectin and leptin was also determined as mentioned earlier in methods. Table 6. shows average serum concentration of adiponectin in rats after 50 days.
Table 6. Average serum concentration of adiponectin*.
| Group | Adiponectin (µg/ml) | P1 | P2 | P3 |
| Healthy control | 4.08 ± 0.45 | - | 0.000 | 0.628 |
| CIA control | 7.68 ± 0.25 | 0.000 | - | 0.000 |
| CIA + soy protein | 5.76 ± 0.68 | 0.069 | 0.026 | 0.098 |
| CIA + daidzein | 5.71 ± 0.61 | 0.058 | 0.014 | 0.082 |
| CIA + genistein | 4.65 ± 0.48 | 0.416 | 0.000 | 0.643 |
| CIA+ dexamethasone | 4.36 ± 0.33 | 0.628 | 0.000 | - |
* All data are presented as mean±SD (n=6 for each group)
P1: Comparison with healthy control group, P2: Comparison with CIA control group, P3: Comparison with dexamethasone group (Independent – samples t-test)
All treatments could reduce adiponectin levels in serum compared to CIA controls significantly (p<0.05), and adiponectin level in all treatments was not significantly different from healthy controls (p>0.05). Mean adiponectin concentration differences between dexamethasone and other groups were insignificant (p>0.05). Table 7. shows average serum concentration of leptin after 50 days.
Table 7. Average serum concentration of leptin*.
| Group | Leptin (ng/ml) | P1 | P2 | P3 |
| Healthy control | 1.59 ±0.10 | - | 0.000 | 0.000 |
| CIA control | 2.62 ±0.09 | 0.000 | - | 0.001 |
| CIA + soy protein | 2.59 ±0.07 | 0.000 | 0.819 | 0.000 |
| CIA + daidzein | 2.12 ±0.16 | 0.023 | 0.024 | 0.000 |
| CIA + genistein | 1.83 ±0.08 | 0.110 | 0.000 | 0.000 |
| CIA + dexamethasone | 3.35 ±0.12 | 0.000 | 0.001 | - |
* All data are presented as mean±SD (n=6 for each group)
P1: Comparison with healthy control group, P2: Comparison with CIA control group, P3: Comparison with dexamethasone group (Independent – samples t-test)
Result from Table 7 indicate that while genistein and daidzein significantly (p<0.05) and soy isolated protein insignificantly (p>0.05) lowered leptin concentration, dexamethasone increased leptin concentration in rats serum (p=0.001).
Discussion
In this study, the effects of genistein, daidzein and soy isolated protein on serum concentrations of IL-6, TNF-α, adiponectin and leptin as well as postponing arthritis symptoms in RA rats were investigated and compared with the effects of dexamethasone. The experiments were carried out in two time periods of 30 and 50 days. Results demonstrated that soy isoflavones could reduce arthritis symptoms in all interventions compared to CIA control rats. After treatment, the highest and lowest intensity of symptoms belonged to CIA control and dexamethasone group, respectively. The ability of dexamethasone to reduce the symptoms of RA was comparable to that of genistein and daidzein (p>0.05). Furthermore, the incidence of arthritis decreased at the end of study. Daidzein and genistein could postpone the onset of CIA symptoms for five and six days. Although there are some reports on the effects of isolated soy proteins and isoflavones on some diseases such as vasculitis and osteoarthritis, no study had already been conducted to investigate the effects of genistein and daidzein on progress and treatment of RA. Arjmandi et al have shown the beneficial effects of a 3-month intake of soy protein on osteoarthritis symptoms and quality of life of the patients (Arjmandi et al. 2004). Other studies have also shown therapeutic potential of genistein in menstruated women with metabolic syndrome (Azadbakht et al. 2007) and allergic airway inflammation (Duan et al. 2003).
One of the possible mechanisms of genistein and daidzein action is their estrogen-like effects. Genistein is structurally related to estrogen and binds estrogen receptors. It has already been demonstrated that estrogen-like sexual hormones improve and prevent RA status (McMurray 2001). It is also possible that the isoflavones exert their effects through a direct effect on cartilage metabolism. Both estrogen α and β receptors can be found in the cartilage of human joints, so cartilage may also be a target for modulators of estrogen receptors. Intra-articular injection of estrogen leads to the destruction of matrix collagen by destroying lactate dehydrogenase enzyme in chondrocytes. Estrogen-like isoflavones that bind to estrogen receptors and interfere with local estrogen function can be effective in osteoarthritis (Arjmandi et al. 2004).
Soy isoflavones are also able to inhibit nitric oxide (NO) production (Sheu et al. 2001). It has been reported that genistein can down-regulate NO synthase in chondrocytes by inhibiting tyrosine kinase (Arjmandi et al. 2004). Hamalainen et al tested the anti-inflammatory effects of daidzein and genistein on NO production in LPS-activated macrophages. Isoflavones could decrease the expression of inducible nitric oxide (iNOs), NF-KB and STAT1 (both factors are needed for iNOs translation) (Hamalainen et al. 2007). Cyclo-oxygenase 2 inhibition is another mechanism of action of isoflavones which is regulated by the nuclear factor NF-KB (Manthey 2000). In the current study, dexamethasone could reduce the intensity of symptoms and delay the onset of CIA in rats. The effects of daidzein on symptom intensity and CIA onset time had not already been studied. The current investigation demonstrates the anti-inflammatory properties of daidzein in postponing the onset and inhibiting RA progression.
DTH test was used to evaluate the cellular immunity in rats. The primary subcutaneous injection of antigen in adjuvant leads to antigen uptake by antigen presenting cells (APCs) in skin (Saint-Mezard et al. 2004) which after activation migrate to local lymph nodes where immature T cells are activated (Cumberbatch and Kimber 1992). Secondary injection of the antigen leads to inflammation of the skin which up-regulates P-Selectin and chemokines to recruit macrophages, CD4+ and CD8+ cells. These events eventually lead to delayed hypersensitivity, inflammation, swelling and erythema of the skin that can be measured quantitatively (Yoshimoto et al. 2000). Current study showed that the highest and lowest thickness of ear in rats was related to CIA control and healthy control, respectively. After 50 days, genistein and daidzein had significantly different means from CIA controls (p<0.05). The inhibition of cell-mediated immunity reaction by genistein had already been shown (Verdrengh et al. 2003).
Estrogen has been proved to decrease the cell-mediated immunity in rats (Holmdahl and Jansson 1988), thus similar function of estrogen and genistein can be a possible mechanism of action of genistein (Yellayi et al. 2003). Recently, in vivo immunosuppressive properties of genistein have been reported (O'Connor et al. 2002). Genistein is now known as an immune disrupting agent, acting as a protein kinase and eventually blocking the immune process by B cells which returns to the fact that genistein decreases the production of TNF-α and IL-6 (Verdrengh et al. 2003). Studies indicate that IL-6 administration increases DTH and deteriorates CIA (Verdrengh et al. 2003). Genistein also inhibits the secretion of granule enzyme from activated macrophages (Kim et al. 2001) and neutrophils (Naucler et al. 2002). Adhesion of leukocytes to the endothelial cells is affected and reduced by genistein which eventually leads to a lower rate of migration of inflammatory cells (Weber et al. 1995). Lymphocytes treated with genistein do not turn into active migrating cells (Hauzenberger et al. 1997). T cell number and activity reduction may be the most important mechanism of genistein in suppressing inflammation and delayed hypersensitivity.
Results of the current study demonstrated that CIA leads to a significant increase in serum concentration of proinflammatory cytokines (IL-6 and TNF-α) compared to healthy control rats, a result which is in line with the result of other studies (Cai et al. 2007; Kim et al. 2005). Results demonstrated a statistically significant decrease in TNF-α and IL-6 after treatment with isoflavones and soy isolated protein with genistein being the most powerful (Tables 4 and 5). Other in vitro studies have also proved that genistein decreases the level of TNF-α and IL-6 after induction of inflammation by LPS (Sadowska-Krowicka et al. 1998). Circulatory TNF-α level also decreased after soy milk intake by menstruated women (Huang et al. 2005). TNF-α secreting cells are largely found in arthritic joints indicating that TNF-α is involved in the pathogenesis of disease. IL-6 is an important cytokine involved in RA and many RA symptoms are associated with IL-6 deregulation. Thus, reducing IL-6 has long been considered a therapeutic target in RA management. Our results showed that dexamethasone could reduce TNF-α and IL-6 levels significantly after 50 days of treatment.
Adiponectin level increased in CIA control rats significantly in our study. Similar results have been obtained in different studies which show that adiponectin level in RA patients rises in serum and synovial fluid. Chen et al reported that adiponectin has a protective role in arthritis (Chen et al. 2006). According to Chen et al., the continuous destruction of cartilage matrix in osteoarthritis is the result of the local imbalance of proteinases and their inhibitors in the cartilage (Chen et al. 2006). Adiponectin has been shown to possess a diversity of anti-inflammatory properties which include inhibition of leukocyte colony formation, reducing phagocytosis and reducing TNF-α and IL-6 from macrophages. Researchers believe that adiponectin anti-inflammatory properties rise from the inhibition of NF-KB and reducing the production of IL-1β (Kumada et al. 2004; Wulster-Radcliffe et al. 2004). The results of current study demonstrated that genistein, daidzein, soy isolated protein and dexamethasone lowered the serum concentration of adiponectin and the results from genistein and daidzein were significant (p<0.05). In 2008, Park et al found that genistein inhibits lipid aggregation in human preadipocytes which was attributed to the decrease in division and differentiation of the cells (Park et al. 2008). Thus, the lower concentration of adiponectin in serum of rats can be ascribed to the effect of genistein in reducing lipid aggregation and adipocytokine secretion from adipocytes. Degawa-Yamauchi et al. showed that dexamethasone suppresses the secretion and release of adiponectin from isolated human adipocytes after 24 hr (Degawa-Yamauchi et al. 2005).
In current study, leptin serum concentration in CIA control rats increased after 50 days. In the past few years, leptin has been proved to be an inflammatory mediator by interaction with its receptors in blood, endothelial vascular cells, smooth muscle cells and osteoblasts. According to Bokarewa et al (Bokarewa et al. 2003), leptin level is higher in plasma than the synovial fluid of RA patients. They also found a higher level of leptin in plasma and synovial fluid of RA patients than that in healthy people. Leptin has a dual action in inflammation. On one hand, leptin activates monocytes/macrophages and stimulates the production of proinflammatory molecules like TNF-α, IL-6, INF-γ and IL-2; and on the other hand, it presents anti-inflammatory properties due to stimulation of secretion of IL-1 (Bokarewa et al. 2003) and thus is thought to have a key role in the pathogenesis of RA. Stimulation of the hypothalamus by direct injection of leptin to brain suppresses the response caused by LPS and TNF-α (Bokarewa et al. 2003). Leptin-deficient mice (ob/ob) are relatively tolerant to the antigen-induced arthritis (Busso et al. 2002). On the contrary, leptin injection to the joint diminishes the severity of joint manifestations in septic arthritis (Hultgren and Tarkowski 2001). Chondrocytes and fibroblasts are both sensitive to leptin and their proliferation is enhanced by leptin stimulation. Meanwhile, incubating these cells with TNF-α and IL-1β stimulates leptin production. TNF-α has long been known as a regulator of ob gene (Finck et al. 1998). Leptin and INF-γ have a synergistic function on NOs stimulation. Results from our experiments revealed that leptin levels in groups with genistein and daidzein intake was significantly different from both healthy and CIA control groups and was lower than CIA control group. Szkudelski et al proved the inhibitory effects of genistein on leptin secretion in male rats, but daidzein had not a significant effect (Szkudelski et al. 2005). Genistein could also reduce leptin levels in rat isolated adipocytes in vitro, but had no effect on leptin gene expression, while daidzein could not reduce leptin in this study. Phipps et al administered low and high doses of soy isoflavones to menopause and non-menopause women for 100 days. Although it had also been proved that estrogens increase leptin levels, even high doses of isoflavones could not increase leptin levels in plasma (Phipps et al. 2001). It has been demonstrated that genistein and daidzein inhibit lipogenesis and at the same time stimulate lipolysis (Szkudelski et al. 2005). Genistein, but not daidzein, reduces glucose transport by insulin in rats isolated adipocytes. Furthermore, genistein can block glucose transport by GLUT1 (Szkudelski et al. 2005). Inhibition of glucose transport in adipocytes leads to inhibition of leptin secretion. Another explanation for lower leptin secretion is the ability of genistein in inhibition of division and differentiation of human pre-adipocytes (Park et al. 2008). In the present study, genistein and daidzein could lower leptin concentration in the plasma relative to CIA control group which is likely due to suppressing inflammation. After 50 days, dexamethasone caused a significant increase in leptin concentration compared to other groups. Other studies have also shown that dexamethasone can lead to increase in leptin secretion (Cartmill et al. 2006; Papaspyrou-Rao et al. 1997). Corticosteroids have a questionable role in physiologic regulation of leptin, but probably hypercortisolemia and hyperinsulinemia caused by dexamethasone can be the reason for increased production of leptin (Laferrere et al. 2002). In addition, dexamethasone as a lipolysis enhancer may lead to increased production of leptin (Laferrere et al. 2002). Dexamethasone may directly or indirectly influence ob gene expression in adipose tissue (Larsson and Ahren 1996).
Induction of CIA elicited a variety of clinical symptoms in rats which included penetration of inflammatory cells to the synovial space, cartilage destruction, pannus formation and bone loss (Hida et al. 2005; Liu et al. 2005). After treatment with dexamethasone, genistein, daidzein and soy isolated protein for 50 days, there was a significant relief from symptoms in rats (p<0.05). These results confirm the favorable effects of treatment in prevention and treatment of RA. Until now, no study had evaluated the effects of soy isoflavones on RA symptoms and most studies were related to other traditionally used herbs in Asian countries (Hida et al. 2005; Liu et al. 2005).
Genistein and daidzein possess structural similarities with selective estrogen receptor modulators such as tamoxifen and synthetic isoflavones and, thus, could be considered as new agents in treatment of inflammatory diseases. Genistein and daidzein prevent RA symptoms through multiple mechanisms which include decrease in the production of TNF-α and IL-6, modulation of leptin and adiponectin concentration and reduction of leukocyte adhesion to vascular endothelial cells in the site of inflammation.
Ethical Issues
None to be declared.
Conflict of interests
The authors declare no conflict of interests.
References
- Arjmandi BH, Khalil DA, Lucas EA, Smith BJ, Sinichi N, Hodges SB, et al. 2004 Soy Protein May Alleviate Osteoarthritis Symptoms. Phytomedicine, 11(7-8), 567-575 [DOI] [PubMed] [Google Scholar]
- Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Hu FB and Willett WC . 2007 Soy Consumption, Markers of Inflammation, and Endothelial Function: a Cross-Over Study in Postmenopausal Women With the Metabolic Syndrome. Diabetes Care, 30(4), 967-973 [DOI] [PubMed] [Google Scholar]
- Bokarewa M, Bokarew D, Hultgren O and Tarkowski A . 2003 Leptin Consumption in the Inflamed Joints of Patients With Rheumatoid Arthritis. Ann Rheum Dis, 62(10), 952-956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso N, So A, Chobaz-Peclat V, Morard C, Martinez-Soria E, Talabot-Ayer D, et al. 2002 Leptin Signaling Deficiency Impairs Humoral and Cellular Immune Responses and Attenuates Experimental Arthritis. J Immunol, 168(2), 875-882 [DOI] [PubMed] [Google Scholar]
- Cai X, Zhou H, Wong YF, Xie Y, Liu ZQ, Jiang ZH, et al. 2007 Suppression of the Onset and Progression of Collagen-Induced Arthritis in Rats by QFGJS, a Preparation From an Anti-Arthritic Chinese Herbal Formula. J Ethnopharmacol, 110(1), 39-48 [DOI] [PubMed] [Google Scholar]
- Cartmill JA, Thompson DL Jr, Del Vecchio RP, Storer WA and Crowley JC . 2006Leptin Secretion in Horses: Effects of Dexamethasone, Gender, and Testosterone. Domest Anim Endocrinol, 31(2), 197-210 [DOI] [PubMed] [Google Scholar]
- Chen TH, Chen L, Hsieh MS, Chang CP, Chou DT and Tsai SH . 2006 Evidence for a Protective Role for Adiponectin in Osteoarthritis. Biochim Biophys Acta, 1762(8), 711-718 [DOI] [PubMed] [Google Scholar]
- Cumberbatch M and Kimber I . 1992 Dermal Tumour Necrosis Factor-Alpha Induces Dendritic Cell Migration to Draining Lymph Nodes, and Possibly Provides One Stimulus for Langerhans' Cell Migration. Immunology, 75(2), 257-263 [PMC free article] [PubMed] [Google Scholar]
- Degawa-Yamauchi M, Moss KA, Bovenkerk JE, Shankar SS, Morrison CL, Lelliott CJ, et al. 2005 Regulation of Adiponectin Expression in Human Adipocytes: Effects of Adiposity, Glucocorticoids, and Tumor Necrosis Factor Alpha. Obes Res, 13(4), 662-669 [DOI] [PubMed] [Google Scholar]
- Duan W, Kuo IC, Selvarajan S, Chua KY, Bay BH and Wong WS . 2003 Antiinflammatory Effects of Genistein, a Tyrosine Kinase Inhibitor, on a Guinea Pig Model of Asthma. Am J Respir Crit Care Med, 167(2), 185-192 [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelley KW, Dantzer R and Johnson RW . 1998 In vivo and in vitro Evidence for the Involvement of Tumor Necrosis Factor-Alpha in the Induction of Leptin by Lipopolysaccharide. Endocrinology, 139(5), 2278-2283 [DOI] [PubMed] [Google Scholar]
- Gu WZ and Brandwein SR . 1998 Inhibition of Type II Collagen-Induced Arthritis in Rats by Triptolide. Int J Immunopharmacol, 20(8), 389-400 [DOI] [PubMed] [Google Scholar]
- Hamalainen M, Nieminen R, Vuorela P, Heinonen M and Moilanen E . 2007 Anti-Inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-KappaB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit Only NF-KappaB Activation Along With Their Inhibitory Effect on INOS Expression and NO Production in Activated Macrophages. Mediators Inflamm, 2007), 45673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauzenberger D, Klominek J, Holgersson J, Bergstrom SE and Sundqvist KG . 1997 Triggering of Motile Behavior in T Lymphocytes Via Cross-Linking of Alpha 4 Beta 1 and Alpha L Beta 2. J Immunol, 158(1), 76-84 [PubMed] [Google Scholar]
- Hida S, Miura NN, Adachi Y and Ohno N . 2005 Effect of Candida Albicans Cell Wall Glucan As Adjuvant for Induction of Autoimmune Arthritis in Mice. J Autoimmun, 25(2), 93-101 [DOI] [PubMed] [Google Scholar]
- Holmdahl R and Jansson L . 1988 Estrogen-Induced Suppression of Collagen Arthritis . III . Adult Thymectomy Does Not Affect the Course of Arthritis or the Estrogen-Mediated Suppression of T-Cell Immunity. Brain Behav Immun, 2(2), 123-132 [DOI] [PubMed] [Google Scholar]
- Huang Y, Cao S, Nagamani M, Anderson KE, Grady JJ and Lu LJ . 2005 Decreased Circulating Levels of Tumor Necrosis Factor-Alpha in Postmenopausal Women During Consumption of Soy-Containing Isoflavones. J Clin Endocrinol Metab, 90(7), 3956-3962 [DOI] [PubMed] [Google Scholar]
- Hultgren OH and Tarkowski A . 2001 Leptin in Septic Arthritis: Decreased Levels During Infection and Amelioration of Disease Activity Upon Its Administration. Arthritis Res, 3(6), 389-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifeh MS, Hananeh W, Al-Rukibat R, Okour O and Boumezrag A . 2008 Clinical and Histopathological Evaluation of MDP/Collagen Induced Arthritis Rat Model (MCIA) After Treatment With Urtica Dioica, Plantago Major and Hypericum Perforatum L Herbal Mixture. Exp Anim, 57(2), 101-110 [DOI] [PubMed] [Google Scholar]
- Kim KS, Lee SD, Kim KH, Kil SY, Chung KH and Kim CH . 2005 Suppressive Effects of a Water Extract of Ulmus Davidiana Planch (Ulmaceae) on Collagen-Induced Arthritis in Mice. J Ethnopharmacol, 97(1), 65-71 [DOI] [PubMed] [Google Scholar]
- Kim YK, Jang YY, Kim DH, Ko HH, Han ES and Lee CS . 2001 Differential Regulation of Protein Tyrosine Kinase on Free Radical Production, Granule Enzyme Release, and Cytokine Synthesis by Activated Murine Peritoneal Macrophages. Biochem Pharmacol, 61(1), 87-96 [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, et al. 2004 Adiponectin Specifically Increased Tissue Inhibitor of Metalloproteinase-1 Through Interleukin-10 Expression in Human Macrophages. Circulation, 109(17), 2046-2049 [DOI] [PubMed] [Google Scholar]
- Laferrere B, Caixas A, Fried SK, Bashore C, Kim J and Pi-Sunyer FX . 2002 A Pulse of Insulin and Dexamethasone Stimulates Serum Leptin in Fasting Human Subjects. Eur J Endocrinol, 146(6), 839-845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H and Ahren B . 1996 Short-Term Dexamethasone Treatment Increases Plasma Leptin Independently of Changes in Insulin Sensitivity in Healthy Women. J Clin Endocrinol Metab, 81(12), 4428-4432 [DOI] [PubMed] [Google Scholar]
- Liu M, Dong J, Yang Y, Yang X and Xu H . 2005 Anti-Inflammatory Effects of Triptolide Loaded Poly(D,L-Lactic Acid) Nanoparticles on Adjuvant-Induced Arthritis in Rats. J Ethnopharmacol, 97(2), 219-225 [DOI] [PubMed] [Google Scholar]
- Manthey JA . 2000 Biological Properties of Flavonoids Pertaining to Inflammation. Microcirculation, 7(6 Pt 2), S29-S34 [PubMed] [Google Scholar]
- Martinez MS, Garcia-Monforte A and Rivera J . 2001 Survival Study of Rheumatoid Arthritis Patients in Madrid (Spain) . A 9-Year Prospective Follow-Up. Scand J Rheumatol, 30(4), 195-198 [DOI] [PubMed] [Google Scholar]
- McMurray RW . 2001 Estrogen, Prolactin, and Autoimmunity: Actions and Interactions. Int Immunopharmacol, 1(6), 995-1008 [DOI] [PubMed] [Google Scholar]
- Mohammad Shahi M, Rashidi MR, Mahboob S, Haidari F, Rashidi B and Hanaee J. 2011. Protective Effect of Soy Protein on Collagen-Induced Arthritis in Rat. Rheumatology International, ), 1-8. [DOI] [PubMed]
- Nagasawa A, Fukui K, Kojima M, Kishida K, Maeda N, Nagaretani H, et al. 2003 Divergent Effects of Soy Protein Diet on the Expression of Adipocytokines. Biochem Biophys Res Commun, 311(4), 909-914 [DOI] [PubMed] [Google Scholar]
- Naucler C, Grinstein S, Sundler R and Tapper H . 2002 Signaling to Localized Degranulation in Neutrophils Adherent to Immune Complexes. J Leukoc Biol, 71(4), 701-710 [PubMed] [Google Scholar]
- O'Connor TP, Liesen DA, Mann PC, Rolando L and Banz WJ . 2002 A High Isoflavone Soy Protein Diet and Intravenous Genistein Delay Rejection of Rat Cardiac Allografts. J Nutr, 132(8), 2283-2287 [DOI] [PubMed] [Google Scholar]
- Palmer G and Gabay C . 2003 A Role for Leptin in Rheumatic Diseases? . Ann Rheum Dis, 62(10), 913-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaspyrou-Rao S, Schneider SH, Petersen RN and Fried SK . 1997 Dexamethasone Increases Leptin Expression in Humans in vivo. J Clin Endocrinol Metab, 82(5), 1635-1637 [DOI] [PubMed] [Google Scholar]
- Park HJ, Yang JY, Ambati S, Della-Fera MA, Hausman DB, Rayalam S, et al. 2008 Combined Effects of Genistein, Quercetin, and Resveratrol in Human and 3T3-L1 Adipocytes. J Med Food, 11(4), 773-783 [DOI] [PubMed] [Google Scholar]
- Phipps WR, Wangen KE, Duncan AM, Merz-Demlow BE, Xu X and Kurzer MS . 2001 Lack of Effect of Isoflavonic Phytoestrogen Intake on Leptin Concentrations in Premenopausal and Postmenopausal Women. Fertil Steril, 75(6), 1059-1064 [DOI] [PubMed] [Google Scholar]
- Radzikowski C . 2006 Protection of Animal Research Subjects. Sci Eng Ethics, 12(1), 103-110 [DOI] [PubMed] [Google Scholar]
- Sadowska-Krowicka H, Mannick EE, Oliver PD, Sandoval M, Zhang XJ, Eloby-Childess S, et al. 1998 Genistein and Gut Inflammation: Role of Nitric Oxide. Proc Soc Exp Biol Med, 217(3), 351-357 [DOI] [PubMed] [Google Scholar]
- Saint-Mezard P, Berard F, Dubois B, Kaiserlian D and Nicolas JF . 2004 The Role of CD4+ and CD8+ T Cells in Contact Hypersensitivity and Allergic Contact Dermatitis. Eur J Dermatol, 14(3), 131-138 [PubMed] [Google Scholar]
- Senolt L, Pavelka K, Housa D and Haluzik M . 2006 Increased Adiponectin Is Negatively Linked to the Local Inflammatory Process in Patients With Rheumatoid Arthritis. Cytokine, 35(5-6), 247-252 [DOI] [PubMed] [Google Scholar]
- Sheu F, Lai HH and Yen GC . 2001 Suppression Effect of Soy Isoflavones on Nitric Oxide Production in RAW 264 .7 Macrophages. J Agric Food Chem, 49(4), 1767-1772 [DOI] [PubMed] [Google Scholar]
- Smolen JS and Steiner G . 2003 Therapeutic Strategies for Rheumatoid Arthritis. Nat Rev Drug Discov, 2(6), 473-488 [DOI] [PubMed] [Google Scholar]
- Szkudelski T, Nogowski L, Pruszynska-Oszmalek E, Kaczmarek P and Szkudelska K . 2005 Genistein Restricts Leptin Secretion From Rat Adipocytes. J Steroid Biochem Mol Biol, 96(3-4), 301-307 [DOI] [PubMed] [Google Scholar]
- Tsuji G, Koshiba M, Nakamura H, Kosaka H, Hatachi S, Kurimoto C, et al. 2006 Thioredoxin Protects Against Joint Destruction in a Murine Arthritis Model. Free Radic Biol Med, 40(10), 1721-1731 [DOI] [PubMed] [Google Scholar]
- Verdrengh M, Jonsson IM, Holmdahl R and Tarkowski A . 2003 Genistein As an Anti-Inflammatory Agent. Inflamm Res, 52(8), 341-346 [DOI] [PubMed] [Google Scholar]
- Wang C, Dai Y, Yang J, Chou G, Wang C and Wang Z . 2007 Treatment With Total Alkaloids From Radix Linderae Reduces Inflammation and Joint Destruction in Type II Collagen-Induced Model for Rheumatoid Arthritis. J Ethnopharmacol, 111(2), 322-328 [DOI] [PubMed] [Google Scholar]
- Weber C, Negrescu E, Erl W, Pietsch A, Frankenberger M, Ziegler-Heitbrock HW, et al. 1995 Inhibitors of Protein Tyrosine Kinase Suppress TNF-Stimulated Induction of Endothelial Cell Adhesion Molecules. J Immunol, 155(1), 445-451 [PubMed] [Google Scholar]
- Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA and Spurlock ME . 2004 Adiponectin Differentially Regulates Cytokines in Porcine Macrophages. Biochem Biophys Res Commun, 316(3), 924-929 [DOI] [PubMed] [Google Scholar]
- Yellayi S, Zakroczymski MA, Selvaraj V, Valli VE, Ghanta V, Helferich WG, et al. 2003 The Phytoestrogen Genistein Suppresses Cell-Mediated Immunity in Mice. J Endocrinol, 176(2), 267-274 [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Wang CR, Yoneto T, Matsuzawa A, Cruikshank WW and Nariuchi H . 2000 Role of IL-16 in Delayed-Type Hypersensitivity Reaction. Blood, 95(9), 2869-2874 [PubMed] [Google Scholar]