Abstract
Introduction
Dorema glabrum is an endangered species that grow in Transcaucasia and North West of Iran. The plant has extensive uses e.g. as an herbal remedy or food additive in these regions. The chemical composition of hydrodistilled oil of D. glabrum growing in Iran was analyzed by GC-MS for the first time.
Methods
The essential oil of air-dried roots was obtained by hydrodistillation using a Clevenger type apparatus. The oil was sub-jected to GC-MS analysis and its free radical scavenging properties were determined by DPPH method.
Results
Thirty four constituents were identified that represented 81.6% of the total oil. The main compounds were delta-Cadinene (12.77%), beta-bisabolene (7.48%), alpha-Fenchyl acetate (6.32%), Copaene (5.68%) and Cubenol (5.42%). The essential oil had weak free radical scavenging properties with the RC50 value of 2.24 mg/mL.
Conclusion
Present work is the first report on chemical composition of the essential oil obtained from D. glabrum roots. GC-MS Analysis showed that the oil was rich in sesquiterpenes. It deems that weak free radical scavenging activity of the oil is due to absence of potent antioxidant compounds.
Keywords: Dorema glabrum, Essential Oil, Sesquiterpenes, DPPH
Introduction
The genus Dorema (Apiaceae) is represented by seven species in Iranian flora, among them Dorema glabrum Fisch. C.A. Mey, D. aucheri Boiss and D. ammonicum D. Don are endemic (Mozaffarian 2003). Dorema glabrum is a perennial herb that grows in loamy or rocky slopes of Nakhichevan, Autonomous Republic-Azerbaijan, Armenia and Iran. Though, according to Rechinger, distribution of Dorema glabrum is restricted to Transcaucasia region (Nakhichevan and Armenia zone) (Rechinger 1987), recent works show that this plant is growing in some locations in North-West of Iran (Mozaffarian 2007, Ajani et al 2008).
Members of this genus possess antispasmodic, expectorant, carminative, diaphoretic, mild diuretic, emmenagogue, stimulant, vasodilator (Ghollassi Mood 2008, Yousefzadi et al 2011a), antimicrobial and antifungal (Shahidi et al 2002, Kumar et al 2006, Yousefzadi et al 2011a) and hepatoprotector (Govind 2011) properties and are intensively used as a green vegetable or as a folk medicine for treatment of many diseases (Ibadullayeva et al 2011). According to the common folk believes of Armenian and Azeri people, D. glabrum can cure many anomalies especially different kinds of cancer. It seems that extensive use of the plant for medicinal and domestic purposes is the major cause of dramatic reduction in the natural resources of D. glabrum (Ibadullayeva et al 2011, Gabrielian 1981).
In a preliminary work, the crude extract of the plant demonstrated antioxidant activity and anti-lipidemic effects (Dehghan et al 2009). To the best of our knowledge, there is no report on the chemical composition and pharmacological properties of D. glabrum and this paper is the first report on GC/MS analyses of the essential oils obtained from the roots of the plant.
Materials and methods
Plant material
Underground parts of Dorema glabrum Fisch. C.A. Mey were collected during the fruiting stage from rocky slopes of Aras River bank; Jolfa, Eastern Azerbaijan (38 30’ 9.2”, 45 27’36.2”; 1590 m, 15 km from Jolfa to St. Stephanus Church), Iran in July 2008. A voucher specimen (TUM-FPh-541) has been deposited at the Herbarium of the Faculty of Pharmacy, Tabriz University of Medical Sciences.
Oil extraction
Air-dried and finely powdered roots were subjected to hydrodistillation for 3 h using a Clevenger type apparatus and the yielded oil was subsequently dried over anhydrous sodium sulphate.
GC-MS analysis
The GC-MS analysis was carried out on a Shimadzu GCMS-QP5050A gas chromatograph- mass spectrometer fitted with a fused silicon capillary DB-1 column (60 m x 0.25 mm i.d., 0.25 µm film thickness). Helium was used as the carrier gas at a flow rate of 1.3 mL/min). The oven temperature was kept at 50° C for 2 min and programmed to 260° C at a rate of 3° C/min. The injector temperature was 230 ºC and split ratio was adjusted at 1:63. The MS was taken at the following condition: ionization potential, 70 ev, ion source temperature 200 ºC; quadrupole 100 ºC; solvent delay 8 min; mass range, em voltage 3000 volts. Identification of compounds was based on direct comparison of the retention times and mass spectral data with those for standard compounds, and computer matching with the NIST 21, NIST 107 and WILEY229 library, as well as by comparison of the fragmentation patterns of the mass spectra with those reported in the literature (Adams 2004).
Free radical scavenging activity: the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
The free radical scavenging effect of the essential oil was assessed using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay (Nazemiyeh et al 2008). DPPH was obtained from Fluka Chemie AG, Bucks and a solution of DPPH (0.08 mg/mL) in chloroform (CHCl3) was used. The essential oil was dissolved in CHCl3 to obtain the stock concentration of 1 mg/mL. Dilutions were made to obtain concentrations of 5×10-1, 2.5×10-1, 1.25×10-1, 6.25×10-2, 3.13×10-2 and 1.56×10-2 mg/mL. Diluted solutions (5 mL each) were mixed with DPPH solution (5 mL) and allowed to stand for 30 min for any reaction to occur. The UV absorbance was recorded at 517 nm. The experiment was done in triplicate and the mean absorption was measured for each concentration. The same manner was followed for the positive control, quercetin.
Results and discussion
Different Dorema species have been in use in orient medicine specially in Middle East countries as folk remedies in asthma, bronchitis, diabetes, infections or mummifying agent (Ghollassi Mood 2008, Yousefzadi et al 2011, Lev et al 2000, Lev et al 2002, Ram et al 2011). Existing documents show that Iranian people were familiar with applications of Dorema ammoniacum and collection of its resin-gum ammoniacum was started nearly 4,000 years ago in Persia (Duthie 1956).
Dorema glabrum is an endangered species (Ibadullayeva et al 2011, Gabrielian 1981) which has limited distribution and was formerly believed that it was restricted to Caucasia and Transcaucasia region. Recent researches carried out by our team and also other groups obviously show that this plant is growing wildly in different areas of Eastern Azerbaijan (Nazemiyeh et al 2008, Aras River bank, Jolfa, TUM-FPh-541; Talebpour et al 2010, Miyaneh, Zanjan Road, 40 21’ 37”, 38 48’ 47”) (unpublished data) and Western Azerbaijan (Mozaffarian 2007) provinces, Iran. In an old literature, the plant was reported from Afghanistan (Aitchison 1887). Considering its collection area, the issue could not be correct and it seems that the discussed plant should be another species, probably Dorema hyrcanum Kos (Syn.: D. glabrum sensu Aitch.) which grows in North East of Iran and Afghanistan (Mozaffarian 2007). Reviewing the published data shows secondary metabolites of D. glabrum was not investigated, yet and the present work is the first report on the chemical composition of the essential oils of Dorema glabrum. The air-dried roots of Dorema glabrum yielded 0.98% (w/w) colorless essential oils. The GC-MS analysis of the essential oils led to the identification of 34 terpenoidal compounds accounting for over 81.6% of the total oils. The majority of components present in the oils (Table 1) were sesquiterpens (non oxygenated 42.60%, oxygenated 14.18%) and monoterpenes (oxygenated 12.59%).
Table 1. Essential oil composition of Dorema glabrum roots.
| Compounds | RI* | Content (%) |
| Alpha.- Fenchyl acetate | 1205 | 06.32 |
| Carvacrol methyl ether | 1226 | 03.13 |
| Bornyl acetate | 1270 | 02.11 |
| Alpha- Cubebene | 1355 | 00.77 |
| Alpha- Copaene | 1383 | 05.70 |
| Trans Caryophyllene | 1432 | 00.52 |
| E- Geranyl acetone | 1447 | 01.03 |
| Alpha- Humulene | 1460 | 00.51 |
| Alloaromadendrene | 1466 | 01.16 |
| Bicyclosesquiphellandrene | 1487 | 00.38 |
| Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-7-methyl-4-methylene-1-(1-methylethyl)-, (1.alpha.,4a.alpha.,8a.alpha.)- | 1496 | 01.29 |
| Alpha.-Muurolene | 1496 | 01.29 |
| Beta.-Bisabolene | 1503 | 07.48 |
| Gamma.-Cadinene | 1507 | 00.77 |
| Calamenene | 1517 | 04.77 |
| Delta.-Cadinene | 1520 | 12.77 |
| Beta.-Cadinene | 1526 | 01.03 |
| Alpha.-Calacorene | 1527 | 01.55 |
| Trans-.psi.-Ionone | 1550 | 02.19 |
| Germacrene B | 1552 | 01.03 |
| Nerolidol E | 1553 | 02.84 |
| Tetradecanal | 1592 | 00.90 |
| Hexadecane | 1600 | 00.65 |
| Cubenol | 1602 | 05.42 |
| Alpha.-Cadinol | 1610 | 00.65 |
| Delta- Cadinol | 1627 | 02.19 |
| Cadalin | 1630 | 00.90 |
| Tetradecanoic acid | 1740 | 00.39 |
| Hexadecanal | 1795 | 01.68 |
| Heptadecanal | 1898 | 04.26 |
| Hexadecanoic acid | 1940 | 00.65 |
| Eicosane | 2000 | 03.74 |
| Docosane | 2200 | 00.90 |
| Tricosane | 2300 | 00.52 |
| Terpenes | ||
| Monoterpenes: oxygenated | 12.69 | |
| Sesquiterpenes: non oxygenated | 42.60 | |
| Sesquiterpenes: oxygenated | 14.18 | |
| Others | 12.14 | |
| Total identified | 81.60 |
*Retention indices relative to C6 –C24 n-alkanes on DB-1 column
Among the monoterpenes, alpha- fenchyl acetate (6.32) and carvacrol methyl ether (3.13) were the major component while delta- Cadinene (12.78 %), beta-bisabolene (7.48 %), Copaene (5.68 %), Cubenol (5.42 %) and Calamenene (4.77 %) assumed as major sesquiterpens (Fig. 1).
Fig. 1.
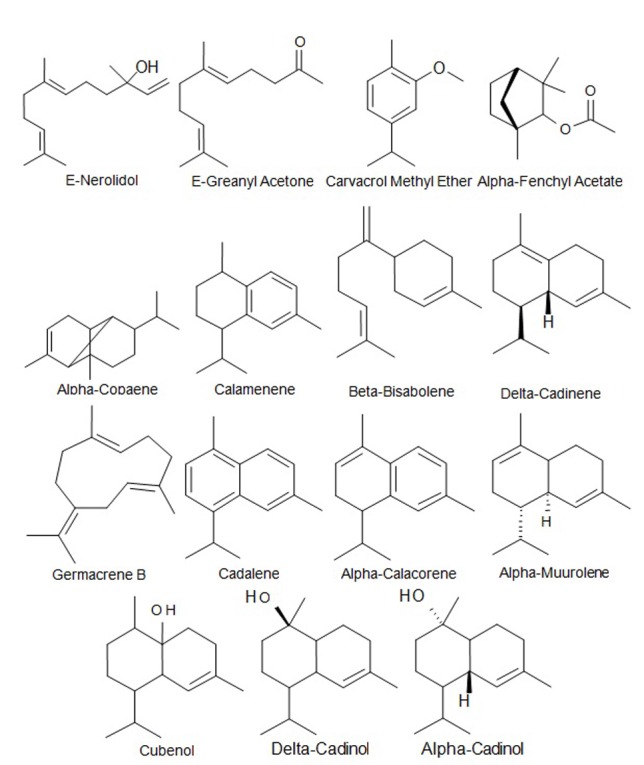
Fig. 1. The major monoterpenes and sesquiterpenes present in the essential oil of D. glabrum roots.
Previous studies on the other Iranian species, Dorema ammoniacum D. Don., revealed the presence of (Z)- and (E)-ocimenone, β-cyclocitral and ar-curcumene in fruits (Yousefzadi et al 2011a, Yousefzadi et al 2011b) and α-gurjunene (49.5 %), β-gurjunene (19.0 %) and α-selinene (4.6 %) in the leaves (Sajjadi et al 2007) as the main components. The findings of the present study are also different from those reported for Dorema aucheri Boiss which mainly comprised of α-Eudesmol (31.2%) and δ-cadinene (10.9%) (Masoudi et al 2006).
The essential oil had weak free radical scavenging properties with the RC50 value of 2.237 mg/mL which was because of the absence of free phenolic or other susceptible scavenging groups in the oil composition.
Conclusion
D. glabrum is a perennial medicinal plant growing commonly in Armenia, Nakhichevan and Iran. The plant is currently used as a remedy for treating cancerous diseases in folk medicine and also as a green vegetable in domestic use. Unfortunately there is no literature and research works on D. glabrum and we believe that extensive works are needed to reveal its medicinal values and protection as well.
Ethical Issues
Not applicable in this research.
Conflict of interests
Authors declared no conflicts of interests.
References
- Adams RP. 2004. Identification of essential oil components by gas chromatography mass spectrometry. Carol Stream, IL, Allured.
- Aitchison IET . 1887 Botanical medicine monographs and sundry: Some plants of Afghanistan, and their medicinal products. American Journal of Pharmacy, 1887, 59(1). [Google Scholar]
- Ajani Y, Ajani A, Cordes JM, Watson MF and Downie SR . 2008 Phylogenetic analysis of nrDNA ITS sequences reveals relationships within five groups of Iranian Apiaceae subfamily Apioideae. Taxon, 57(2), 383-401 [Google Scholar]
- Govind P . 2011 Medicinal plants against liver diseases. International Research Journal of Pharmacy, 2(5), 115-121 [Google Scholar]
- Dehghan G, Fatholahi G, Sheikhzadeh N and Ahmadiasl N . 2009 Hypocholesteremic and antioxidant effects of Dorema glabrum extract in rats fed high cholesterol diet. Journal of the Iranian Chemical Society, 6, 115-143 [Google Scholar]
- Duthie JF . 1956 The umbelliferae group. British Homoeopathic Journal, 45(2), 77-88 [Google Scholar]
- Gabrielian ETS . 1981 The conservation of rare threatened species and types of vegetation in Armenia. Anales Jardin Botanico De Madrid, 37(2), 773-778 [Google Scholar]
- Ghollassi Mood S . 2008 A contribution to some ethnobotanical aspects of Birjand Flora (Iran). Pakistan Journal of Botany, 40(4), 1783-1791 [Google Scholar]
- Ibadullayeva S, Movsumova N, Gasymov H and Mamedli T . 2011 Protection of some rare and endangered vegetable plants in the flora of the Nakhichevan AR. International Journal of Biodiversity and Conservation, 3(6), 224-229 [Google Scholar]
- Kumar VP, Chauhan NS, Padh H and Rajani M . 2006 Search for antibacterial and antifungal agents from selected Indian medicinal plants. Journal of Ethnopharmacology, 107, 182-188 [DOI] [PubMed] [Google Scholar]
- Lev E and Amar Z . 2000 Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. Journal of Ethnopharmacology, 72, 191-205 [DOI] [PubMed] [Google Scholar]
- Lev E and Amar Z . 2002 Ethnopharmacological survey of traditional drugs sold in the Kingdom of Jordan. Journal of Ethnopharmacology, 82, 131-145 [DOI] [PubMed] [Google Scholar]
- Masoudi S, Esmaeili A, Khalilzadeh MA, Rustaiyan A, Moazami N, Akhgar MR, et al. 2006 Volatile constituents of Dorema aucheri Boiss., Seseli libanotis (L.) W.D. Koch var. armeniacum Bordz. and Conium maculatum L. three Umbelliferae herbs growing wild in Iran. Flavour and Fragrance Journal, 21(5), 801-804 [Google Scholar]
- Mozaffarian V. 2003. Dictionary of Iranian plant names. Tehran, Farhang Moaser.
- Mozaffarian V. 2007. Flora of Iran, No.54: Umbelliferae. Tehran, Research Institute of Forests and Rangelands, pp 368-374.
- Nazemiyeh H, Bahadori F, Delazar A, Ay M, Topcu G, Kolak U, et al. 2008 Antioxidant phenolic compounds from the leaves of Erica arborea (Ericaceae). Natural Product Research, 22, 1385-1292 [DOI] [PubMed] [Google Scholar]
- Ram A, Balachandar S, Vijayananth P and Singh VP . 2011 Medicinal plants useful for treating chronic obstructive pulmonary disease (COPD): Current status and future perspectives. Fitoterapia, 82, 141-151 [DOI] [PubMed] [Google Scholar]
- Rechinger KH. 1987. Umbelliferae. In: Rechinger KH. (Ed), Flora Iranica, No.162. Graz, Akademische Druck-und Verlagsanstalt, pp 379-385.
- Sajjadi SE, Ghassemi N and Mohammad Zamani . 2007 Chemical constituents of the essential oil of Dorema ammoniacum D . Don . Leaf, an Iranian resinous plant. Revue des régions Arides, 1, 194 [Google Scholar]
- Shahidi GH, Moein MR, Foroumadi AR and Rokhbakhsh-Zamin F . 2002 Cytotoxic activity of medicinal plants used in Iranian traditional medicine on two strains of Saccharomyces cerevisiae. DARU, 10(4), 162-164 [Google Scholar]
- Ghollassi MoodYousefzadi M, Mirjalili MH, Alnajar N, Zeinali A and Parsa M . 2011. b Composition and in vitro antimicrobial activity of the essential oil of Dorema ammoniacum D . Don . Fruits from Iran. Journal of Serbian Chemical Society, 76(6), 857-863 [Google Scholar]
- Yousefzadi M, Heidari M, Akbarpour M, Mirjalili MH, Zeinali A and Parsa M . 2011. a In vitro cytotoxic activity of the essential oil of Dorema ammoniacum D . Don. Middle-East Journal of Scientific Research, 7(4), 511-514 [Google Scholar]


