SUMMARY
Whole extermination of cancerous cells/tissue seems no longer to be a dream. Exploiting advanced photoactive nanomaterials such as functionalized fullerenes and carbon nano-tubes (CNTs) can act as CNT nanobombs (CNT-NBs) when exposed to the near infrared (NIR) radiation. PEGylated CNTs tagged with an antibody/aptamer can target cancer cells. Once attached to cancer cells, the NIR emission (700-1100 nm), in which body tissues are mostly transparent, can be applied to CNT-NBs which can absorb the light and get heated up. The resultant enhanced temperature can abolish the cancer. Once stealth CNT-NBs are tagged with imaging moieties, it would be a matter of computer gaming for physician who can inject it for real time visualization and destruction of cancer by activation of the NIR laser. While, many nanosystems (NSs) are still in waiting list for clinical translation, our dreams may come true by applying stealth CNT-NBs against cancer.
Keywords: Carbon Nanotubes, Photothermal Therapy, Cancer Nanomedicines, Cancer Theranostics, Targeted-Therapy of Cancer
Ideally, efficient cancer treatment depends on accuracy of diagnosis and effectiveness of the specific treatment modalities. Cancer patients normally undergo diagnosis prior to treatment, but the currently used techniques fail to show early detection potential. This problem, associated with the traditional chemotherapy/immunotherapy protocols that often fail mainly because of lack of differentiation between aberrant and normal cells, results in systemic toxicity. Development of multifunctional nanomedicines emerged the new concept “theranostics” as a platform that confers simultaneous capability for real time detection and therapy of cancer as well as follow-up monitoring (Omidi 2011). Owing to recent advancements in optical imaging using nanomaterials (e.g., gold nanoparticles (AuNPs), quantum dots (QDs) and other photoacoustic imaging contrast agents), it is now possible to construct nanosystems (NSs) with both imaging and therapy potentialities. Nonetheless, we still have to improve the plasma longevity of such NSs to avoid opsonization process and mononuclear phagocyte system (MPS) clearance (Moghimi et al. 2010; Moghimi and Szebeni 2003).
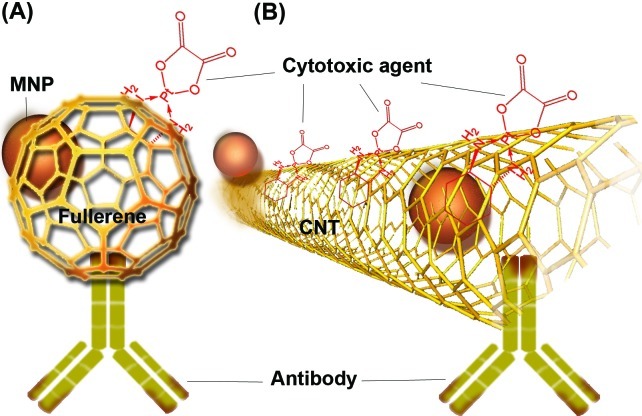
Further, as shown in Fig. 1, carbon nanotubes (CNTs), possessing unique structure, have shown remarkable characteristics as promising nanocarriers (NCs) in real time cancer diagnosis and therapy. The unique surface properties of CNTs make them as suitable NCs for adsorption and/or bioconjugation of various moieties. Such capability enables us to engineer functionalized biocompatible and stealth CNTs with different tagged molecules for different aims as all-in-one NS. They also are able to entrap/adsorb small cytotoxic molecules; whose specific activity within aberrant cells can improve the end point aims of cancer therapy. CNTs possess propensity to absorb the transparent NIR emissions and become heated – a process that makes its useful exploitation for photothermal and photoacoustic therapy (Thakare et al. 2010; Ilbasmis-Tamer et al. 2010). In fact, both fullerenes (C60) and CNTs display great potential for biofunctionalization with different moieties and accordingly can be used as DDS in particular for cancer diagnosis and therapy (Thakare et al. 2010). As shown in Fig. 1, CNTs can be functionalized with targeting device (mAb/scFv), magnetic particles (MNPs) and also cytotoxic agent (doxorubicin, paclitaxel) mainly via molecular adsorption or chemical conjugation methods (e.g., a cleavable ester bond), and the resultant can be used as multimodal NSs (Liu et al. 2008; Ilbasmis-Tamer et al., 2010). It has been reported that the head and neck squamous carcinoma cells (HNSCC) overexpressing EGF treated with CNT-CP-EGF can highly selectively internalize these NSs and effectively kill the cells (Bhirde et al. 2009). The distribution and clearance study of PEGylated CNTs as nanocarrier for delivery of cisplatin (CP) in mice showed that the PEG-CNT-CP were highly dispersed in aqueous media and efficiently inhibited the growth of squamous cell tumors, in which the impact was greater when the NSs were tagged to EGF for better cellular internalization (Bhirde et al. 2010). Further, CNTs are able to absorb NIR radiation (especially between 700 and 1100 nm), in which body tissues are most transparent. This promotes molecular oscillation that can produce efficient heating within the surrounding environment (Levi-Polyachenko et al. 2009; Zhou et al. 2009). Similarly, Kang et al. (2009) have exploited single-walled carbon nanotubes (SWCNTs) for extensive photoacoustic effect and showed activation of a nanosized firecracker-like explosion in suspension by irradiation of a 1064-nm Q-switched millisecond-pulsed laser. As a result, the functionalized SWCNTs with folate moiety eradicated the cancer cells overexpressing folate receptor. These researchers reported that application of 1064-nm millisecond-pulsed laser can kill more than 85% of cancer cells within 20 s, at which they showed profound improvement in cancer therapy (Kang et al. 2009). Besides, SWCNTs were shown to be heated up under a radiofrequency (RF) field – a de novo safe method for selective elimination of malignant cells. Application of 13.56-megahertz RF field had a heating impact on injected functionalized SWCNTs in the hepatic VX2 tumors in rabbits, so that at 48 hrs, all treated tumors displayed complete necrosis (Gannon et al. 2007). This promising photoacoustic property of CNTs used for selective destruction of cancer cells may change the direction of cancer therapy in near future. No longer would it be a science fiction to see tracking down the felon cells by application of “biophotonics”!
Fig. 1.
Schematic representation of functionalized fullerene (A) and carbon nanotube (B) CNT: carbon nanotube, MNP: magnetic nanoparticle.
Fig. 2 represents schematic illustration of biofunctionalized fullerens and carbon nanotubes used for phothermal erdication of cancer. The CNTs conjugated to a PEGylated mAb/aptamer can readily target the cancer cells, while the magnetic field acts as an external driving force for accumulating magnetic nanoparticle (MNP) tagged CNTs-Ab conjugates in cancer cells (Fig. 2A). Such complex NS can be activated by NIR laser for selective photothermal abolition of cancer (Fig. 2B).
Fig. 2.
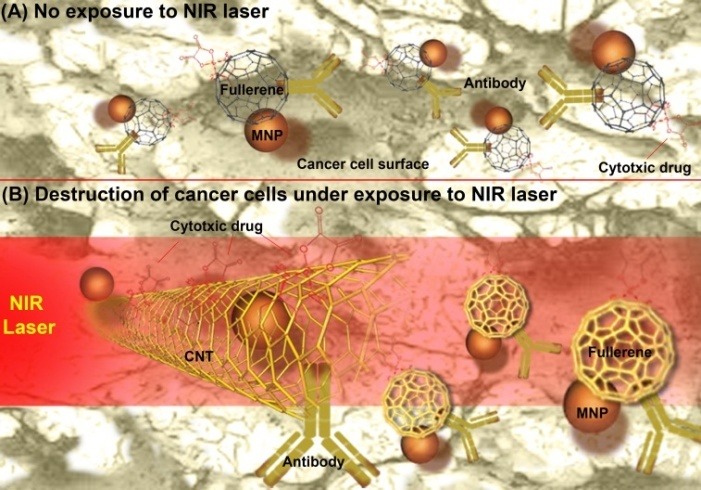
Schematic illustration of biofunctionalized fullerens and carbon nanotubes used for phothermal erdication of cancer. A) Accumulation of CNTs-Ab-Drug-MNP complex NSs in cancer cells. B) Activated of CNTs-Ab-Drug-MNP complex NSs by NIR laser for selective photothermal abolition of cancer. CNT: carbon nanotube. MNP: magnetic nanoparticle. NSs: nanosystems.
To overcome limitations attributed with toxicity and lower NIR adsorption by CNTs, Kim et al (2009) made golden CNTs (i.e., GNTs), which was conjugated with Ab specific to the lymphatic endothelial hyaluronan receptor-1 (LYVE-1) and exploited for Photoacoustic (PA)/Photothermal (PT) mapping of lymphatic endothelial cells (LECs), lining on the internal surface of lymphatic vessels. For preparation of GNTs, as shown in Fig. 18 (panels A-C), these researchers used shortened SWCNTs (1.5–2 nm diameter) and coated them by a thin gold layer (4–8 nm thick). These highly water-soluble uniform rod-shaped NSs (~100 nm length and 11 nm diameter) showed photonic properties similar to that of gold nanorods with a longitudinal resonance peak in the NIR region near 850 nm. GNTs were coated with rabbit antimouse antibodies to LYVE-1 of LECs and examined in nude mice with the results showing GNTs potential to be used as photoacoustic and photothermal contrast agents with enhanced NIR contrast (10 2 -fold) using very low laser fluence levels (Kim et al., 2009). Later on, Galanzha et al (2009) established functionalized AuNPs and MNPs as tool for ultra-sensitive molecular detection of the CD44+ circulating tumor cells in a mouse model of human breast cancer (Galanzha et al. 2009). Such approach appears to be the next step for real time specific cancer monitoring and therapy – a process that may be termed as “CNT nanobombs”. This promising photoacoustic property of CNTs used for selective destruction of cancer cells may change the direction of cancer therapy in near future. No longer would it be a science fiction to see tracking the felon cancerous cells down by application of biophotonics-based CNT nanobombs!
Ethical issues
No ethical issues to be declared.
Conflict of interests
No conflict of interest to be declared.
Acknowledgments
Author expresses his sincere gratitude to Prof. George Coukos (Ovarian Cancer Research Center, School of Medicine, University of Pennsylvania, Philadelphia, USA) for his visionary advice upon new directionality of cancer theranostics.
References
- Bhirde AA, Patel S, Sousa AA, Patel V, Molinolo AA, Ji Y, et al. 2010 Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice. Nanomedicine (Lond), 5(10), 1535-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhirde AA, Patel V, Gavard J, Zhang G, Sousa AA, Masedunskas A, et al. 2009 Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano, 3(2), 307-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanzha EI, Kim JW and Zharov VP . 2009 Nanotechnology-based molecular photoacoustic and photothermal flow cytometry platform for in-vivo detection and killing of circulating cancer stem cells. J Biophotonics, 2(12), 725-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, et al. 2007 Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer, 110(12), 2654-65 [DOI] [PubMed] [Google Scholar]
- Ilbasmis-Tamer S, Yilmaz S, Banoglu E and Degim IT . 2010 Carbon nanotubes to deliver drug molecules. J Biomed Nanotechnol, 6(1), 20-7 [DOI] [PubMed] [Google Scholar]
- Kang B, Yu D, Dai Y, Chang S, Chen D and Ding Y . 2009 Cancer-cell targeting and photoacoustic therapy using carbon nanotubes as "bomb" agents. Small, 5(11), 1292-301 [DOI] [PubMed] [Google Scholar]
- Kim JW, Galanzha EI, Shashkov EV, Moon HM and Zharov VP . 2009 Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nat Nanotechnol, 4(10), 688-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Polyachenko NH, Merkel EJ, Jones BT, Carroll DL and Stewart JHT . 2009 Rapid photothermal intracellular drug delivery using multiwalled carbon nanotubes. Mol Pharm, 6(4), 1092-9 [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, et al. 2008 Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res, 68(16), 6652-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimi SM, Andersen AJ, Hashemi SH, Lettiero B, Ahmadvand D, Hunter AC, et al. 2010 Complement activation cascade triggered by PEG-PL engineered nanomedicines and carbon nanotubes: the challenges ahead. J Control Release, 146(2), 175-81 [DOI] [PubMed] [Google Scholar]
- Moghimi SM and Szebeni J . 2003 Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res, 42(6), 463-78 [DOI] [PubMed] [Google Scholar]
- Omidi Y . 2011 Smart Multifunctional Theranostics: Simultaneous Diagnosis and Therapy of Cancer. BioiImpacts, 1(3), 145-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakare VS, Das M, Jain AK, Patil S and Jain S . 2010 Carbon nanotubes in cancer theragnosis. Nanomedicine (Lond), 5(8), 1277-301 [DOI] [PubMed] [Google Scholar]
- Zhou F, Xing D, Ou Z, Wu B, Resasco DE and Chen WR . 2009 Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J Biomed Opt, 14(2), 021009 [DOI] [PMC free article] [PubMed] [Google Scholar]