Abstract
Introduction
This research investigates the possible potential of Rosa canina (RC) as an immunomodulator in rats and its effects on some biochemical parameters.
Methods
In this experiment, 45 male Wistar rats were obtained and divided into three groups (n = 15). These groups received normal saline (10 mg/kg), RC fruit extract (250 mg/kg) and RC fruit extract (500 mg/kg) as oral gavages every day for a period of four weeks, respective-ly. After obtaining blood samples (at the end of each week), differential white blood cell (WBC) counts, phagocyte activity (number), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphates (ALP) albumin and globulins levels of sam-ples were obtained. The malondialdehyde (MDA) and glutathione (GSH) levels in the se-rum were determined only in day 28 of study. The radical scavenger activity (RSA) of the RC extract was measured spectrophotometrically.
Results
the gamma globulin level, neu-trophil and monocyte counts and phagocyte activity increased significantly in comparison with the normal saline group. ALT, AST and ALP had not significantly differences in compared to control group. RC extract significantly increased thiobarbituric acid reactive substances (TBARS) and also decreased GSH levels in comparing to control group in day 28.
Conclusion
the data suggest that the RC extract has been used in traditional medicine might have immunomodulatory effects.
Keywords: Rosa canina, Immunological Parameters, Biochemical Parameters, Rats
Introduction
Herbal medicine is as ancient as the history of mankind (Atmani et al. 2004). Many of the herbal remedies described by oriental scientists, like Abu Musa Jabir Ben Hayyan and Ibn Wahshiyyah (Saad et al. 2006), are still used today by herbalists (Everest and Ozturk 2005). They are also used in Iranian traditional medicine (Sadigh-Eteghad et al. 2008).
Many plants have been used as immunostimulant and immunomodulator among them Echinacea purpurea, Althaea officinalis, Bryonia cretica, Calendula officinalis, (Sadigh-Eteghad et al. 2011, Agelet et al. 2003).
In the various monographs, fruits (rose-hips, with seeds) of Rosa canina L. (RC), are stated to possess prophylactic and therapeutic activities against the wide range of ailments, including the inflammatory and immune responses (Ercisli 2007, Rein et al. 2004, Wenzig et al. 2008) arthritis, rheumatism, gout, sciatica, fever, colds, infectious diseases, gallstones, biliary complaints (Nojavan et al. 2008, Gurbuz et al. 2003), disorders of the kidney (Kultur 2007) and dropsy (Orhan et al. 2007).
In recent years, it has been established that free radicals and oxidative stress are involved in the pathophysiology of a variety of disorders including immune dysfunction and related diseases. In relation to these findings, an extensive range of antioxidants both exogenous and endogenous, whether synthetic or natural have been presented for the treatment or prophylaxis of disorders attributed to free radical oxidative damages (Souri et al. 2004).
Some chemicals of RC regulate immune and inflammatory responses (Ercisli 2007, Saaby et al. 2010) and possess antioxidant properties too (Deals-Rakotoarison et al. 2002).
RC is a Mediterranean medicinal plant which widely used in Iran northwest. In the present work, we evaluated the possible potential of RC as an immunomodulator in rats and its effects on some biochemical parameters.
Material and methods
Plant material collection and extraction
Samples of RC growing wild in northwest Iran were collected and authenticated at the herbarium of faculty of pharmacy, Tabriz, Iran (Voucher number: 16695). The material was dried in the dark at room temperature before extraction.
Dried fruits (about 60 g) were submitted to extraction with 300 ml methanol (Merck) and distilled water mixture (1:1) in a Soxhlet apparatus for 10 h. After extraction, the solvent was filtered and then evaporated by rotary evaporator in 45ºC and extract yield was recorded and then stored at 4ºC in sealed glass vials until tested and analyzed (Sadigh-Eteghad et al. 2009, Eidi et al. 2004).
Animals and treatments
Male Wistar rats weighing 250–300 g were obtained (Razi Institute, Karaj, Iran) a week before the start of the experimental treatments. Throughout the study, the animals were fed a pelleted commercial chow diet (Pars khurakdam, Shushtar, Iran) and were kept in separate standard cages in a well-ventilated room maintained at 21±2°C with a 12:12 h light:dark cycle (Gharagozlou et al. 2006). After a week of acclimatization, the animals were randomly divided into three groups (I, II and III) of 15 rats each. Group I received normal saline (10 mg/kg) (intact control). Group II, used as a first treated group, was given 250 mg/kg of extract, and group III, used as a second treated group, and was given 500mg/kg of RC extract for 28 days.
Blood sampling
Biochemical and immune functions were assessed in the animals at day 0 and at the end of each week using blood samples obtained from the animals’ tail vein. Collected samples emptied into 1.5 ml tubes containing 100 µL of heparin as an anticoagulant. Approximately, 1 ml of blood was drawn from each animal, of which 0.5 ml was immediately microcentrifuged for five min and the resultant supernatant removed and stored at -80 ºC for later monitoring. The remainder was used for differential WBC counts and assessment of phagocytic activity.
Biochemical and hematological assays
Differential WBCs counts indicated the determination of the percentages of each WBC under a light microscope. An air-dried blood smear was stained with Giemsa stain (Sigma) and washed with phosphate buffer (Sigma) and 95% ethanol. Once dried, cell types, including neutrophils, monocytes and lymphocytes, were distinguished by their appearance after staining (one hundred leukocytes were identified). Percentage levels of albumin, alpha, beta and gamma globulins were determined by cellulose acetate electrophoresis and densitometry (Helena system). Plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphates (ALP) levels in all samples were estimated by the methods of Rietman and Frankel (1957) and Kind and King (1954) respectively. The malondialdehyde (MDA) level in the serum was determined using the thiobarbituric acid reactive substance (TBARS) test (Satho 1978). Glutathione (GSH) was measured by the method of Moron et al. (1979), last tow parameters were evaluated only in day 28 of study.
Phagocytic assay
The heparinized blood was immediately used for the phagocytic assay. Briefly, 1 × 10 7 07 cells of Staphylococcus aureus (ATCC 29213) in 0.1 ml of phosphate buffer saline (PBS) were added to 0.1 ml of blood samples in a microplate and incubated for 30 min at 37 ◦C, after thorough mixing. After incubation, the plate was mixed gently and 0.05 ml of this suspension smeared on the glass slide. After air-drying, the smears fixed in ethanol, stained with Giemsa and cells and phagocytosed bacteria were counted (Ispir and Dorucu 2005). Assays were performed in three replicates.
DPPH radical scavenging assay
The radical scavenger activity (RSA) of the hidro-methanolic RC extract and acid ascorbic (as a standard) were measured spectrophotometrically using the 1, 1-diphenyl-2 picrylhydrazyl (DPPH) radical. RSA in RC extract were estimated by the methods of Tayefi-Nasrabadi et al (2011).
Statistical analyses
Data were examined using a commercially available statistical package (SPSS version 17 for Windows), and comparisons were made using the one way ANOVA and regression.
Results
Compare to normal saline group, monocyte levels significantly increased in rats receiving 250 and 500 mg doses of herb extract, while lymphocyte percentages were significantly decries in treatment groups in weeks 2 and 3. Neutrophil levels increased in 250 mg group in days 14 and 21 (Fig. 1).
Fig. 1.
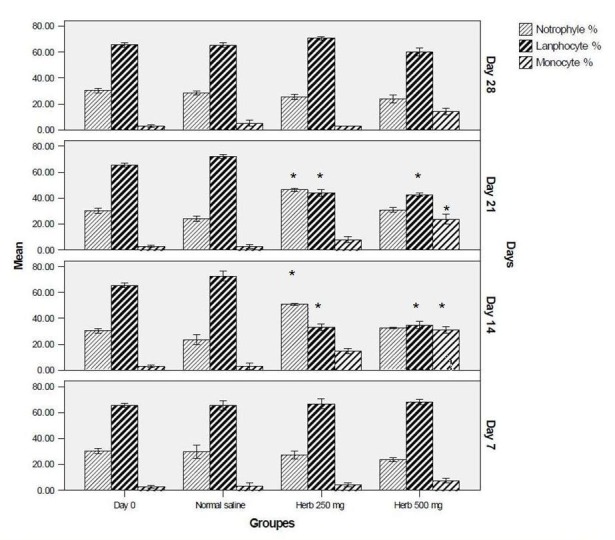
Differential WBCs percentage in various groups and days. Data is expressed as mean, bars represent standard deviation. *p<0.05 significant from normal saline.
The phagocyte activity in the test groups was significantly higher than that of the control group during all study days (Fig. 2).
Fig. 2.
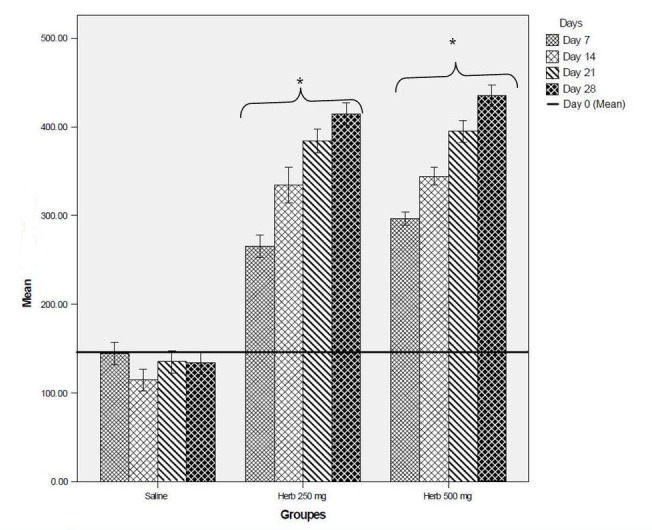
Phagositic activity in various groups and days. Data is expressed as mean, bars represent standard deviation. *p<0.05 significant from normal saline.
There was no significant increase in alpha and beta globulin levels but compare to normal saline group gamma globulin and albumin levels were significantly higher in the 500 mg group during all study days (Fig. 3).
Fig. 3.
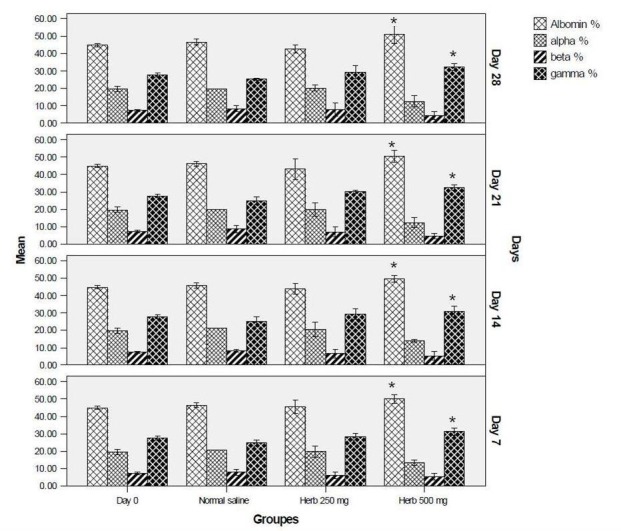
Percentage of albumin and globulins percentage in various groups and days. Data is expressed as mean, bars represent standard deviation. *p<0.05 significant from normal saline.
The Table 1 outlines the ALT, AST and ALP on the follow-up days in each group of rats. Test groups had not significantly differences in ALT, AST and ALP levels, in compared to control group on all days of study.
Table 1. ALP, ALT and AST amounts in different groups.
| ALP (IU/dl) | ALT (IU/dl) | AST (IU/dl) | ||
| Day 0 | 406.81±11.22 | 112.28±15.14 | 110.21±17.41 | |
| Day 7 | Ia | 407.42±08.24 | 114.54±12.71 | 114.29±14.86 |
| IIb | 404.42±12.46 | 117.00±10.95 | 113.05±09.48 | |
| IIIc | 416.86±14.62 | 111.84±13.81 | 108.42±12.70 | |
| Day 14 | I | 409.27±11.51 | 109.42±18.19 | 113.73±11.49 |
| II | 412.72±18.81 | 112.00±16.21 | 118.86±18.20 | |
| III | 421.49±12.14 | 114.12±12.75 | 117.00±10.47 | |
| Day 21 | I | 411.24±10.29 | 114.46±12.41 | 111.54±14.84 |
| II | 414.39±9.27 | 119.84±09.84 | 114.00±12.71 | |
| III | 419.52±14.68 | 117.08±14.15 | 116.47±16.92 | |
| Day 28 | I | 409.71±12.16 | 111.26±14.14 | 109.79±12.45 |
| II | 418.83±15.91 | 121.73±10.48 | 108.14±16.41 | |
| III | 419.48±21.78 | 126.52±18.47 | 114.45±18.24 |
anormal saline group, bherb 250 mg/kg, cherb 500 mg/kg
Regression index between RC and vitamin C in the DPPH assay pointed below (Table 2).
Table 2. RSA in the DPPH assay in RC and vitamin C in various doses.
| % Inhibition DPPH | ||
| Concentration a | RC | Vitamin C |
| 1.0 | 26.82 | 31.86 |
| 2.5 | 39.43 | 42.01 |
| 5.0 | 59.71 | 50.69 |
| 10.0 | 96.54 | 83.37 |
| 20.0 | 100.00 | 85.11 |
a Unit of used extract and vitamin C were (mg/ml) and (µg/ml) respectively.
RSA of RC mg/ml = -14.56 + (1.35 × RSA of Vitamin C μg/ml)
RC extract significantly increased TBARS and also decreased GSH levels in comparing to control group in day 28 (Fig. 4).
Fig. 4.
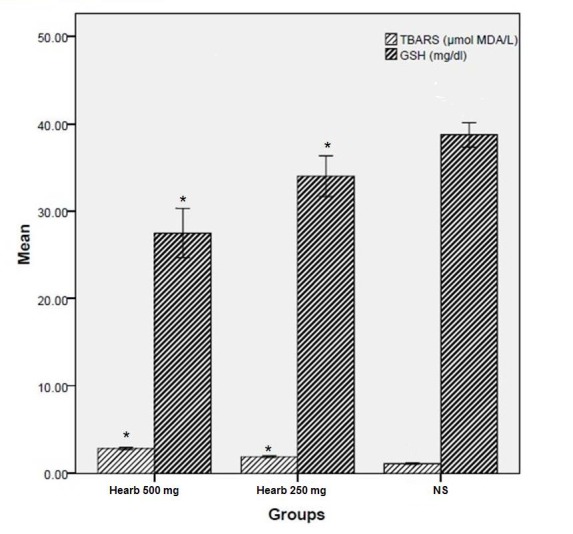
TRABS and GSH levels in serum of experimental and control groups in day 28. *p<0.05 significant from normal saline.
Discussion
RC is a European wild plant and rose hips constitute the used part according to the Xth French Pharmacopoeia. Apart from the anti-inflammatory activity, an anti-oxidant mode of action might contribute to the observed immunological effects of rose hip preparations (Wenzig et al. 2008). The main uses of rose hips are linked to their vitamin C content (Deals-Rakotoarison et al. 2002, Ercisli 2007, Tayefi-Nasrabadi et al. 2011). Vitamin C is a nutrient potentially involved in many aspects of the immune system (Mitchell et al. 2003). The results of Ortuno et al (1999) indicated that the non-specific immune response parameters increased as a consequence of a high vitamin C supply, although it generally acts as an antioxidant in the biologic systems (Mitchell et al. 2003). The anti-oxidant function of the vitamin C could in part, at least, enhance immunity by maintaining the functional and structural integrity of important immune cells (Ortuno et al. 1999)
In our study the vitamin C content of RC extract was found 52 ± 1.9 mg/100g of dray sample. Also linear regression analysis shows a close relation between RC and acid ascorbic in the RSA evaluation (Table 2).
Kumari and Sahoo (2006) studies shows, the humoral and cellular parameters of innate immunity, have maximum efficacy in both healthy and immune-compromised catfishes after vitamin C composition. There are some other antioxidants such as galactolipids, proanthocyanidins and flavonoids in RC that affect on immune-modulation properties of herb (Kharazmi 2008, Daels-Rakotoarison et al. 2002). Medicinal plants used for immunomodulation purpose for decades. Sadigh-Eteghad et al (2011) reported significant increases in monocytes and neutrophils, only after 2 weeks of oral administration of the Echinacea purpurea and levamisole which demonstrated an immediate and sustained increase in the percentage of with blood cells.
The results of this study indicated a significant increase of monocytes and neutrophils in groups which received 250 and 500 mg RC, especially on days 21 and 14 respectively (Fig 1).
According to Ortuno et al (1999) high Acid ascorbic diets may increase the activity of non-specific immune responses in gilthead seabream, enhance serum complement activity as well as leucocytes function via respiratory burst and phagocytic activities. By attention to high levels of acid ascorbic in the RC extract, it seems to be affect in phagocytic activity.
In the present study, the phagocyte activity of RC in the test groups was significantly higher than that of the control group during all study days (Fig 2).
Another healthy function of fruits is their essential fatty acid composition that animals cannot synthesize, and must obtain through diet. These chemicals regulate numerous body functions, including blood pressure, blood viscosity, immune and inflammatory responses. The most abundant fatty acids were linoleic, α-linolenic, oleic and palmitic acids, (Ercisli 2007) that can be affect in immune reaction and globulin regulation in the body. The results showed that RC 500 mg also significantly increased the gamma globins levels during all study days (Table 3). Experiments demonstrated that most antibodies are located in the gamma globulin fraction of serum proteins (Kindt et al. 2007), therefore, increasing this fraction, has positive effects on the immune system.
Hepatic function has been monitored by evaluating the serum levels of ALP, ALT and AST. These enzymes activities are known to be cytosolic marker enzymes that reflect hepatocellular necrosis (Atyabi 2005). According to results RC extract did not affect on serum ALP, AST and ALT levels. Such results have been reported in some other medicinal plants (Wang et al. 2009, Saaby et al. 2010).
According to Winther et al (2008) RC also significantly improved the antioxidant capacity and the vitamin C content of serum. The Daels-Rakotoarison et al (2002) results showed that the extract can inhibit reactive oxygen species tested in acellular and cellular systems. Antioxidative effects of Rosa canina are due not only to vitamin C but also to polyphenolics. Usage of this extract, led to a reduction of superoxide anion liberation in the serum.
In the last day of our study, the RC treatment groups' shows significantly increased in the MDA level, and also decreased GSH level in compare to normal saline group. Therefore, it can show the supplementation of RC contributed to maintaining the serum antioxidants at an optimum level in rats. Such results have been reported in the Tayefi-Nasrabadi et al (2010) study too.
In conclusion, the data suggest that the RC extract used in traditional medicine might have immunomodulatory effects. Further experimental and clinical studies are required to elucidate the chemical constituents of the extracts and the mechanism(s) that are responsible for the pharmacological activities.
Ethical Issues
All the procedures were carried out under the ethical guidelines of Tabriz University of Medical sciences.
Conflict of interests
Authors declare no conflict of interests.
Acknowledgments
The authors are thankful to research office of Tabriz Medical University for spiritual auspices.
References
- Agelet A and Valles J . 2003 Studies on Pharmaceutical Ethnobotany in the Region of Pallars (Pyrenees, Catalonia, Iberian Peninsula) . Part II . New or Very Rare Uses of Previously Known Medicinal Plants. Journal of Ethnopharmacology, 84(2-3), 211-27 [DOI] [PubMed] [Google Scholar]
- Atmani F, Slimani Y, Mimouni M, Aziz M, Hacht B and Ziyyat A . 2004 Effect of Aqueous Extract from Herniaria hirsute L . on Experimentally Nephrolithiasic Rats. Journal of Ethnopharmacology, 95(1), 87-93 [DOI] [PubMed] [Google Scholar]
- Atyabi N . 2005 Veterinary Clinical Pathology Laboratory Methods. University of Tehran Press: Tehran, 338-349
- Ben-Baruch B . 2006 Inflammation-Associated Immune Suppression in Cancer: The Roles Played by Cytokines, Chemokines and Additional Mediators. Seminars in Cancer Biology, 16(1), 38-52 [DOI] [PubMed] [Google Scholar]
- Buege JA and Aust SD . 1978 Microsomal Lipid Peroxidation. Methods in Enzymology, 52, 302-10 [DOI] [PubMed] [Google Scholar]
- Daels-Rakotoarison DA, Gressier B, Trotin F, Brunet C, Luyckx M, Dine T, et al. 2002 Effects of Rosa canina Fruit Extract on Neutrophil Respiratory Burst. Phytotherapy Research, 16(2), 157-61 [DOI] [PubMed] [Google Scholar]
- Eidi M, Eidi A and Zamanizadeh H . 2005 Effect of Salvia officinalis L . Leaves on Serum Glucose and Insulin in Healthy and Streptozotocin-Induced Diabetic Rats. Journal of Ethnopharmacology, 100(3), 310-13 [DOI] [PubMed] [Google Scholar]
- Ercisli S . 2007 Chemical Composition of Fruits in Some Rose (Rosa spp .) Species. Food Chemistry, 104(4), 1379-84 [Google Scholar]
- Everest A and Ozturk E. 2005. Focusing on the Ethnobotanical Uses of Plants in Mersin and Adana Provinces (Turkey). Journal of Ethnobiology and Ethnomedicine, 1(6) doi:10.1186/1746-4269-1-6. [DOI] [PMC free article] [PubMed]
- Gharagozlou MJ, Araghchian M, Shahtaheri SM and Radmehr B. 2006. Laboratory Animals, Biology, Anatomy, Application and Pathology. Bizhan publication, p.111–27. ISBN: 9649567011.
- Gurbuz I, Ustun O, Yesilada E, Sezik E and Kutsal O . 2003 Anti-Ulcerogenic Activity of Some Plants Used as Folk Remedy in Turkey. Journal of Ethnopharmacology, 88(1), 93-7 [DOI] [PubMed] [Google Scholar]
- Ispir U and Dorucu M . 2005 A Study on the Effects of Levamisole on the Immune System of Rainbow Trout (Oncorhynchus mykiss, Walbaum). Turk Journal of Veterinary and Animal Science, 29, 1169-76 [Google Scholar]
- Kharazmi A . 2008 Laboratory and Preclinical Studies on the Anti-Inflammatory and Anti-Oxidant Properties of Rosehip Powder – Identification and Characterization of the Active Component GOPO®. Osteoarthritis and Cartilage, 16, 5-7 [Google Scholar]
- Kind PRN and King EJ . 1954 Estimation of Plasma Phosphatase by Determination of Hydrolysed Phenol with Anti-Pyrine. Journal of Clinical Pathology, 7(4), 322-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt TJ, Osborne BA, Goldsby RA and Kuby J. 2007. Kuby Immunology. 6th ed. W.H. Freeman; p. 76. ISBN: 1429202114.
- Kultur S . 2007 Medicinal Plants Used in Kırklareli Province (Turkey). Journal of Ethnopharmacology, 111(2), 341-64 [DOI] [PubMed] [Google Scholar]
- Kumari J and Sahoo PK . 2006 Non-Specific Immune Response of Healthy and Immunocompromised Asian Catfish (Clarias batrachus) to Several Immunostimulants. Aquaculture, 255(1-4), 133-41 [DOI] [PubMed] [Google Scholar]
- Mitchell BL, Ulrich CM and McTiernan A . 2003 Supplementation with Vitamins or Minerals and Immune Function: Can the Elderly Benefit? Nutrition Research, 23(8), 1117-39 [Google Scholar]
- Moron MS, Defierre JW and Mannervik B . 1979 Levels of Glutathione, Glutathione Reductase, Glutathione S-transferase Activities in Rat Lung and Liver. Biochemistry and Biophysics Acta, 582(1), 67-78 [DOI] [PubMed] [Google Scholar]
- Nojavan S, Kha1i1ian F, Momen-Kiaie F, Rahimi A, Arabanian A and Chalavi S . 2008 Extraction and Quantitative Determination of Ascorbic Acid during Different Maturity Stages of Rosa canina L . fruit. Journal of Food Composition and Analysis, 21(4), 300-5 [Google Scholar]
- Orhan DD, Hartevioglu A, Kupeli E and Yesilada E . 2007 In vivo Anti-Inflammatory and Antinociceptive Activity of the Crude Extract and Fractions from Rosa canina L . Fruits. Journal of Ethnopharmacology, 112(2), 394-400 [DOI] [PubMed] [Google Scholar]
- Ortuno J, Esteban MA and Meseguer J . 1999 Effect of High Dietary Intake of Vitamin C on Non-Specific Immune Response of Gilthead Seabream (Sparus aurata L.). Fish & Shellfish Immunology, 9(5), 429-43 [DOI] [PubMed] [Google Scholar]
- Rein E, Kharazmi A and Winther K . 2004 A Herbal Remedy, Hyben Vital (stand. powder of a Subspecies of Rosa canina Fruits), Reduces Pain and Improves General Wellbeing in Patients with Osteoarthritis—a Double-Blind, Placebo-Controlled, Randomised Trial. Phytomedicine, 11(5), 383-91 [DOI] [PubMed] [Google Scholar]
- Reitman S and Frankel S . 1957 A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. American Journal of Clinical Pathology, 28(1), 56-63 [DOI] [PubMed] [Google Scholar]
- Saaby L, Katharina-Jager A, Moesby L, Wind-Hansen E and Brogger-Christensen S. 2010. Isolation of Immunomodulatory Triterpene Acids from a Standardized Rose Hip Powder (Rosa canina L.), Phytotherapy Research,25(2), 195-201, doi: 10.1002/ptr.3241. [DOI] [PubMed]
- Saad B, Azaize H, Abu-Hijleh G and Said O . 2006 Safety of Traditional Arab Herbal Medicine. eCAM, 3(4), 433-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadigh-Eteghad S, Khayat-Nuri H, Abadi N, Ghavami S, Golabi M and Shanebandi D . 2011 Synergetic Effects of Oral Administration of Levamisole and Echinacea purpurea on Immune Response in Wistar Rat. Research in Veterinary Science, 91(1), 82-5 [DOI] [PubMed] [Google Scholar]
- Sadigh-Eteghad S, Mirzaei H, Farzam-Pour S and Kahnamui S . 2009 Inhibitory Effects of Endemic Thymus vulgaris and Mentha piperita Essential Oils on Escherichia coli O157:H7. Research Journal of Biological Science, 4(3), 340-4 [Google Scholar]
- Sadigh-Eteghad S, Ghavami S, Mortazavi J and Mirzayi H . 2008 Anesthetic Effects of Valerian officinalis, Melissa officinalis, Papaver somniferum, Papaver bracteatum Herbs on Goldfish (Carassius auratus). Iranian Scientific Fisheries Journal, 17, 91-8 [Google Scholar]
- Satho K . 1978 Serum Lipid Peroxidation in Cerebrovascular Disorders Determined by a New Colorimetric Method. Clinical Chemistry Acta, 90(1), 37-43 [DOI] [PubMed] [Google Scholar]
- Souri E, Amin G, Dehmobed-Sharifabadi A, Nazifi A and Farsam H . 2004 Antioxidative Activity of Sixty Plants from Iran. Iranian Journal of Pharmaceutical Research, 3(1), 55-9 [Google Scholar]
- Tayefi‐Nasrabadi H,Sadigh‐Eteghad S and Aghdam Z . 2010Preventive Effects of Rosa Canina L. Against Oxalate Mediated Free Radical Toxicity in a Rat Urolithiasis Model. Proceedings of 2010 International Conference on Chemical Engineering and Applications, 430-433
- Tayefi‐Nasrabadi H. Sadigh‐Eteghad S and Aghdam Z. 2011. The Effects of the Hydroalcohol Extract of Rosa canina L. Fruit on Experimentally Nephrolithiasic Wistar Rats. Phytotherapy Research, doi: 10.1002/ptr.3519. [DOI] [PubMed]
- Wang J, Li J, Zou Y, Cheng W, Lu C, Zhang L, et al. 2009 Preventive Effects of Total Flavonoids of Litsea coreana Leave on Hepatic Steatosis in Rats Fed with High Fat Diet. Journal of Ethnopharmacology, 121(1), 54-60 [DOI] [PubMed] [Google Scholar]
- Wenzig EM, Widowitz U, Kunert O, Chrubasik S, Bucar F, Knauder E, et al. 2008 Phytochemical Composition and In vitro Pharmacological Activity of Two Rose Hip (Rosa canina L .) Preparations. Phytomedicine, 15(10), 826-35 [DOI] [PubMed] [Google Scholar]
- Winther K, Falk-Ronne J, Kharazmi A, Hansen AV and Hansen EW . 2008 Dose Litovet, a Herbal Remedy Made from Rosa canina, Act as an Anti-Inflamantory Agent in Horses Exposed to Strenuous Exercise- a Randomized, Placebo-Controlled, Parallel, Double-Blinded Study on the Immune System of Horses, Their Working Capacity and Behavior. Osteoarthritis and Cartilage, 16, 44-5 [Google Scholar]


