Abstract
Many epidemics have broken out over the centuries. Hundreds and thousands of humans have died over a disease. Available treatments for infectious diseases have always been limited. Some infections are more deadly than the others, especially viral pathogens. These pathogens have continuously resisted all kinds of medical treatment, due to a need for new treatments to be developed. Drugs are present in nature and are also synthesized in vitro and they help in combating diseases and restoring health. Synthesizing drugs is a hard and time consuming task, which requires a lot of man power and financial aid. However, the natural compounds are just lying around on the earth, may it be land or water. Over a thousand novel compounds isolated from marine organisms are used as antiviral agents. Others are being pharmacologically tested. Today, over forty antiviral compounds are present in the pharmacological market. Some of these compounds are undergoing clinical and preclinical stages. Marine compounds are paving the way for a new trend in modern medicine.
Keywords: Marine Life-Forms, Antiviral Compounds, Pharmacology, HIV
Introduction
In the world, animals, plants and microorganisms live together in an ecological niche. Pathogenicity is a liability, which cannot be completely obliterated; however it can be curable (Mayer and Hamann 2004).
Since the dawn of man, he has been vulnerable to microbial attack, from a mild fever to a fatal disease. Scientists have been working diligently to prepare vaccines or to discover cures for various infections. After experimenting with terrestrial life forms, researchers have obtained many antimicrobial compounds, which have been used for patient care (Marin Lit 1998; Cirne-Santos et al. 2008; Oku et al. 2004; Lee et al. 2006; Mayer and Hamann 2002). However, many new diseases have sprung up over the ages, which are resistant to many antibiotic drugs. The world is in need of more pharmacological agents, especially against viral infections.
For nearly one hundred thousand years, it has been known that marine life-forms can be used against microbial attacks. However, serious investigation started nearly half a century ago (Mayer and Hamann 2002). Researchers have opted for screening marine life forms for finding antiviral activity against viruses, such as HIV, HSV (1 and 2), and HCMV, etc. (Mayer and Hamann 2004; Oku et al. 2004; de Souza et al. 2005; Rodriguez et al. 2005).
The first credited work on studying marine natural products against the microbes was of Bergman, over 60 years ago (Bergman and Feeny 1951). During 1970s-1980s, many research papers on marine natural products were published and reviewed (Munro et al. 1987). Mostly studied phyla were (in order of preference): Porifera, Cnidaria, Chromophycota, Rhodophycota, Mollusca, Chordata, and Echinodermata (Marin Lit 1998).
After a comparative study between the pharmacological benefits from marine life forms and terrestrial ones, it was reported that marine invertebrates were preferred as their cytotoxic activity was quite high, (Garson 1994). Marine life-forms are under clinical trials since the early 1950s, to find anticancer marine metabolites (Fenical 1996; Nakao et al. 2001).
From 1998-2008, a massive amount of marine life forms were studied to extract the antiviral compounds. Many species showed remarkable activities against pathogenic viruses. However, this was observed in vitro. Chemical classification of the marine compounds was done to classify them into the following major marine chemical compounds; Polyketides, Terpenes, Nitrogen-containing compounds and Polysaccharides (Schimtz 1994). Marine compounds show antibacterial, antifungal, anti-malarial, anticoagulant and antiviral activities. They also affect cardiovascular and nervous systems. During 1999, 21 marine compounds underwent clinical trials. The antiviral pharmacology of the natural products of marine water reached its peak during 1999. Antiviral activity was screened against human immunodeficiency virus-1 (HIV-1), herpes simplex virus-2 (HSV-2), junin virus (JV), polio virus (PV), molluscum contagiosum dengue virus, severe acute respiratory syndrome (SARS) virus, measles virus and influenza virus (Comin et al. 1999; Hwang et al. 1999; Mayer and Lehman 2000; Rowley et al. 2002; Rodriguez et al. 2005).
Marine compounds from tunicates and sponges
Antiviral compounds, halichondrin B, homohalichondrin B and isohomohalicondrin B; were derived from the sponge Lissodendoryx sp. These compounds inhibited the proliferation of tumor cells after replication. For this purpose in vivo and in vitro trials have been established (Munro et al. 1987).
Fig. 1.
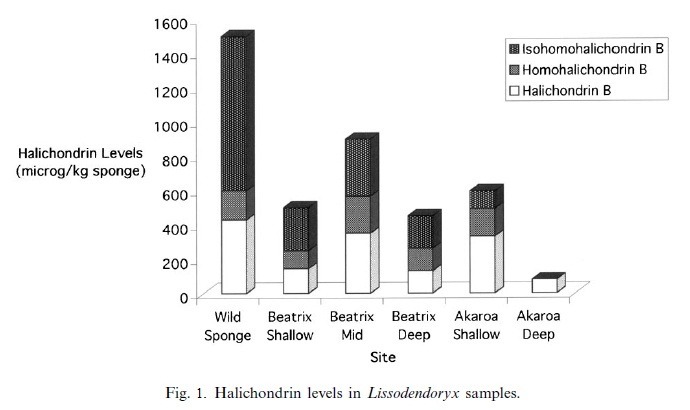
Halichondrin levels in Lissodendoryx samples.
Antiviral bioactive compounds from marine Bacteria, Clam and marine Ascidian species
The causative agents of the most fatal diseases are viruses, such as cancer, anti-acquired immune deficiency syndrome (AIDS), herpes simplex (Mayer et al. 1998). Integrase is an integral enzyme of HIV. In 1999, a new compound, “alkaloid lamellarin α 20-sulphate” was discovered in an unidentified ascidian. This compound exhibited inhibition of integrase in vitro (Reddy et al. 1999). A series of antiviral compounds, “cyclic depsipeptides papuamides A, B, C and D” were derived from a family of sponges, Theonella mirabilis and Theonella swinhoei. Compounds A and B inhibited the infection of HIV in T-lymphoblastoid cells (Ford et al. 1999).
Another aromatic alkaloid, “Polycitone A” was isolated from an ascidian, Polycitor sp. It works as a potent inhibitor of reverse transcriptase of HIV and retroviruses as well as an inhibitor of DNA polymerases, (Loya et al. 1999). As it is a general inhibitor it cannot be used as an anti-HIV agent. However, with a few modifications and some new derivatives it can be used as such.
A compound glycosaminoglycan was discovered in a Pseudomonas sp. This compound exhibited antiviral activity against influenza virus A and B, (Ahmad et al. 1999). In some AIDS patients, HIV causes syncitia formation. One infected cell binds with another cell via CD4, resulting in a multinuclear HIV infected cell. Another antiviral compound, Sulphated β-galactan from clam displayed inhibition of HIV binding with CD4, in vitro (Amornrut et al. 1999).
Hydroxysteroids from marine echinoderms
Poly-hydroxysteroids were isolated from the Brittle Star, Astrotoma agassizzi. These compounds caused the reduction of three human pathogenic viruses; HSV-2, JV, and PV-3 in vitro, (Comin et al. 1999). Sulfated polymannuroguluronate is an anti-AIDS drug candidate which targets CD4 in lymphocytes. Scientists have great hopes for this compound to be used against HIV (Miao et al. 2004).
Antiviral activity shown by marine fungus
An antiviral compound sansalvamide was isolated from a fungus, Fusarium sp. Molluscum contagiosum is a poxvirus causing pink bumps (rash) in humans. This compound inhibited the topoisomerase enzyme of the poxvirus (Hwang et al. 1999).
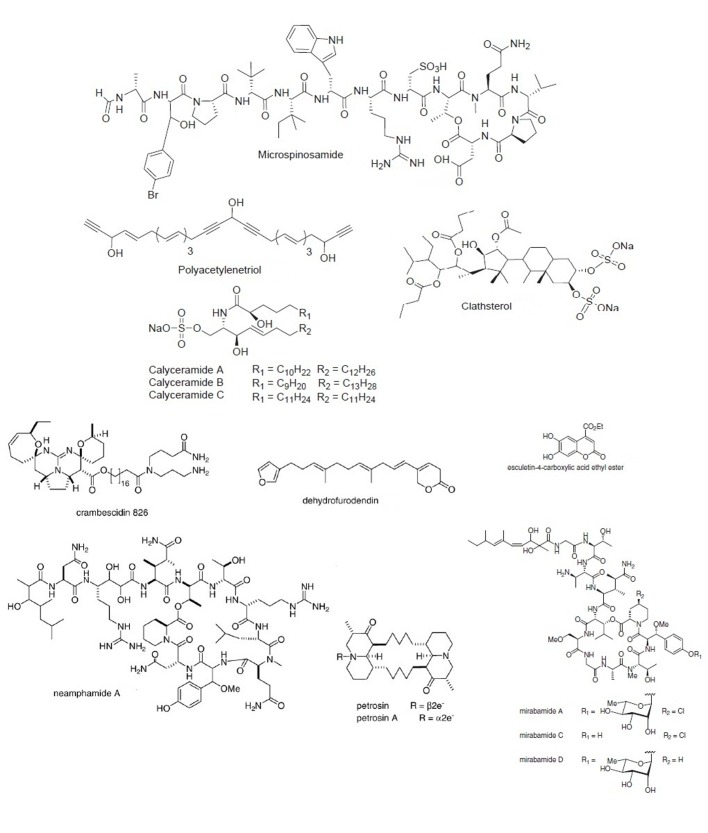
Marine sponges
Rudi et al. (2001) discovered clathsterol from the Red Sea sponge (Clathria sp.). It caused the inhibition of RT-HIV, like the alkaloid compound of 1999 (Loya et al.). Microspinosamide was discovered by Rashid et al. (2001) and it was originated from a sponge, Sidonops microspinosa. It inhibited HIV growth, in vitro. Polyacetylenetriol was derived from a specific sponge (Petriosa sp.). This compound inhibited the DNA and RNA directed DNA-polymerases, by reversible, noncompetitive mechanism, involving hydrophobic interaction (Loya et al. 2002). Calyceramides, derived from the marine sponge Discodermia calyx, showed inhibition of neuraminidase in influenza virus (Nakao et al. 2001).
Fig. 2.
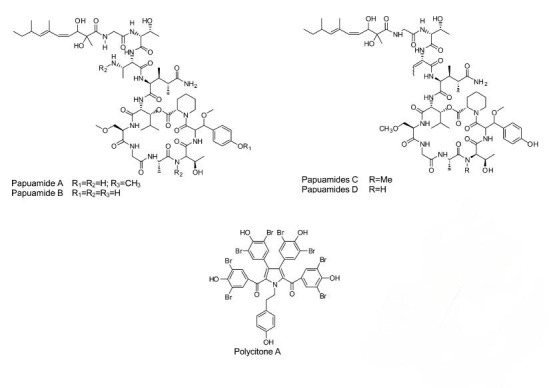
Natural compounds from marine bacteria, ascidians and clams.
For HIV growth inhibition, neamphamide was discovered that was obtained from a marine sponge, Neamphius huxleyi (Oku et al. 2004). Crambescidin was isolated from a Monanchora sponge. It inhibited HIV-1 envelope fusion with normal cells, in vitro (Chang et al. 2003). Chill et al. (2004) discovered dehydrofurodendin from a Madagascan sponge, Lendenfeldia. It inhibits the RT-RNA and DNA directed DNA polymerases like polyacetylenetriol (Loya et al. 2002). Petrosia similis is a marine sponge which produces petrosins, for HIV growth inhibition (Venkateshwar Goud et al. 2003). Esculetin ethyl ester from sponge (Axinella cf. corrugata), this compound inhibits the protease 3CL of the SARS enzyme, (de Lira et al. 2007). It is effective against Corona virus. Mirabamides A, C and D were isolated from a sponge, Siliquariaspongia mirabilis. It inhibits the action of HIV-1 cell fusion (Plaza et al. 2007).
Seagrass and seaweed
Thalassoilins A, B and C were derived from sea grass, Thalassia testudinum. It inhibited HIV growth, HIV enzyme, and integrase, (Rowley et al. 2002). This ability was discovered in an ascidian. Thalassia A was reported to be the most active compound, (Reddy et al. 1999; Rashid et al. 2001). Schizymenia binderi is derived from marine red seaweed and causes the deactivation of HSV-1 and 2 by interfering with the haparan sulphate residues in the viral cells (Matsuhiro et al. 2005). Xylomannan, from red seaweed; Scinaia hatei, showed antiviral activity against HSV-1 and HSV-2 (Mandal et al. 2008).
Fig. 3.
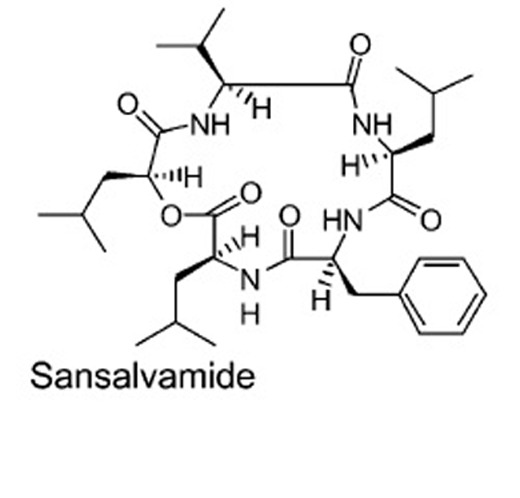
Natural compound obtained from Fusarium sp.
Fig. 4.
Natural products extracted from marine sponges.
Fig. 5.
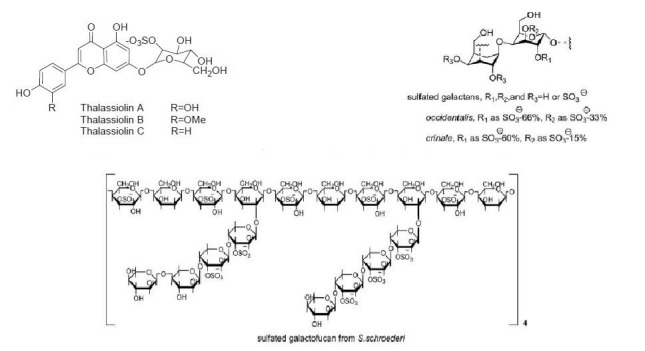
Compounds obtained from Seaweed and Seagrass.
Marine alga
Extracts of marine microalgae exhibited in vitro suppression of the replication of haemorrhagic septicaemia virus (VHSV) and African swine fever virus (ASFV) (Fabregas et al. 1999). Dictyota menstrualis is an alga, from which, dictyota diterpenes was derived for the inhibition of HIV-1 replication in cell lines (Pereira et al. 2004). Rodriguez et al. (2005) isolated three polysaccharides from the marine alga, Callophyllis variegata. It was observed that they displayed antiviral activity against HSV-1 and 2 and dengue type 2. Researchers regarded them as “promising antiviral agents”. Three fractions of sargassam plastiquinones were isolated from marine alga Sargassam micracanthum, for the inhibition of measles and cytomegalovirus, (Iwashima et al. 2005). It was suggested that it can be used as a lead compound in anti-human cytomegalovirus drug. De Souza et al. (2005) isolated griffithsin from a marine red alga, Giffithsia sp. It showed properties of being a microbiocide to prevent the sexual transmission of HIV and AIDS, in vitro. Mori et al. (2005) contributed in the characterization of this HIV inactivating protein compound, Griffithsin, which is a new type lectin. This was a remarkable discovery. D, L-galactan hybrid C2S- 3 from the alga Cryptonemia crenulata inhibits three strains of dengue type 2; it also inhibits the binding and penetrating the virus (Talarico et al. 2007). From the brown alga family, E. cava 6, 6’-Bieckol was extracted which is a phlorglucinol derivative suppressing the syncytia formation caused by HIV-1 infection (Artan et al. 2008). Cirne-Santos et al. (2008) discovered dolabelladienetriol from a family of brown alga, Dictyota pfaffii. This compound had the ability to inhibit HIV-1 replication. It inhibits the RT enzyme of the provirus. Lu et al. (2007) discovered a novel compound sulphated polymannuroguluronate (SPMG) from the brown alga, Laminaria aponica. It has a molecular weight of 8.0 KDa. It deactivated HIV-1 with a very high intensity, in vitro (Meiyu et al. 2003). It is an anti-AIDS drug candidate and is in the phase II of clinical trials in China. SPMG eliminates viral gene product, transactivator of transcription protein (Tat) - induced transduction. Tat stimulated calcium overload and caused apoptosis of cells. SPMG showed neuroprotective effect, i.e. no apoptosis occurred in the neural cells (Hui et al. 2008).
Marine diatoms
Naviculan was derived from a marine diatom Navicula directa, for the inhibition of viral replication of HSV-1 and 2 at early stages (Lee et al. 2006).
Fig. 6.
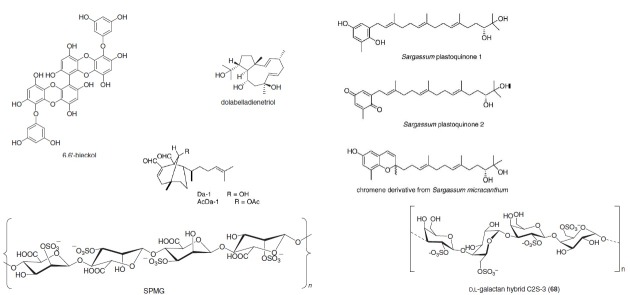
Natural compounds from marine alga exhibiting antiviral activity.
Table 1. Marine compounds having antiviral pharmacological potential since 1999 to 2008.
| Compound/organism | Chemistry | Pharmacological activity | Molecular Mechanism of Action | Country | References |
| Lamellarin a-20- sulfate/Tunicate | Tyrosine-based | In vitro HIV infection | HIV integrase | IND, USA | Reddy et al. 1999 |
| Papuamides A–D/sponge | Depsipeptide | In vitro HIV infection | Undetermined | CAN, USA | Ford et al. 1999 |
| Polycitone A /Tunicate | Tyrosine-based | In vitro anti-RT inhibition | DNA polymerase inhibition | ISRA | Loya et al. 1999 |
| Glycosaminoglycan /Bacterium | Sulfated Polysaccharide | In vitro anti- influenza A & B inhibition | influenza A virus inhibition | JPN | Ahmad et al. 1999 |
| Sulfated ?-galactan /Clam | Sulfated Polysaccharide | In vitro syncitia formation inhibition | Inhibition of HIV binding to CD4 | S. KOR, JPN, USA | Amornrut et al. 1999 |
| Poly-hydroxysteroids /Brittle Star | Sterol | In vitro reduction of HSV-2, JV, PV plaque formation | Sulfate at C-21, C-2 and C-3 critical for inhibition | ARG | Comin et al. 1999 |
| Sansalvamide /Fungus | Depsipeptide | Molluscum contagiosum virus topoisomerase inhibition | DNA binding and relaxation inhibition | USA | Hwang et al. 1999 |
| Clathsterol/sponge | Sulfated sterol | HIV reverse transcriptase inhibition | Undetermined | ISRA, S. AFR | Rudi et al. 2001 |
| Microspinosamide/sponge | Depsipeptide | HIV-growth inhibition | Undetermined | USA | Rashid et al. 2001 |
| Polyacetylenetriol/sponge | Fatty acid | RNA- and DNA-directed DNA polymerase inhibition | Reversible non-competitive inhibition, with hydrophobic interactions | ISRA | Loya et al. 2002 |
| Thalassiolins A–C/sea grass Sulfated flavones | Sulfated flavones | HIV-1 integrase inhibition and HIV growth in vitro | Binding to catalytic domain of HIV-1 integrase | USA | Rowley et al. 2002 |
| Calyceramides A–C/sponge | Fatty acid | Neuraminidase inhibition | Undetermined | JAPN | Nakao et al. 2001 |
| Crambescidin/sponge | Alkaloid | HIV-1 envelope-mediated fusion inhibition in vitro | Undetermined | USA | Chang et al. 2003 |
| Dehydrofurodendin/sponge | Furanoterpene | RT RNAand DNA-directed DNA polymerase inhibition | Undetermined | ISRA, FRA | Chill et al. 2004 |
| Neamphamide A/sponge | Depsipeptide | HIV-growth inhibition | Undetermined | USA | Oku et al. 2004 |
| Dictyota diterpenes/alga | Diterpene | Inhibition of HIV-1 replication in cell line | RNA-dependent DNA-polymerase RT inhibition | BRA | Pereira et al. 2004 |
| Petrosins/sponge | Alkaloid | HIV-growth inhibition | Giant cell formation and RT inhibition | IND | Venkateshwar Goud et al. 2003 |
| Callophylis variegate galactans/alga | Polysaccharide | Herpes simplex and dengue type 2 inhibition | Undetermined | ARG | Rodriguez et al. 2005 |
| Naviculan/diatom | Polysaccharide | Herpes simplex 1 and 2 inhibition | Undetermined | JPN | Lee et al. 2006 |
| Schizymenia binderi sulfated galactan/alga | Polysaccharide | Herpes simplex 1 and 2 inhibition | Interference with HSV-heparan sulfate cellular residues | ARG, CHL | Matsuhiro et al. 2005 |
| Sargassum plastoquinones/sponge | Terpenoid | Measles and cytomegalovirus Inhibition | Lipid peroxidation observed | JPN | Iwashima et al. 2005 |
| Griffithsin/alga | Protein | T- and M-tropic HIV-1 inhibition | Inhibition of CD4- dependent gp120 binding | USA | De Souza et al. 2005 |
| Griffithsin/alga | Protein | HIV inactivation | Binds to viral glycoproteins in a monosacchride manner | USA | Mori et al. 2005 |
| Esculetin ethyl ester/sponge | Polyketide | SARS-Corona virus viral protease 3CL inhibition | Undetermined | BRA, CAN | De Lira et al. 2007 |
| Cryptonemia crenulata galactan/alga | Polysaccharide | Dengue type 2 inhibition | Inhibition of viral binding and cell penetration | ARG, BRA | Talarico et al. 2007 |
| 6,6?-Bieckol/alga | Shikimate | Inhibition of HIV-1 Infection | Viralp24antigen production and reverse transcriptase inhibition | CHN, S. KOR | Artan et al. 2008 |
| Dolabelladienetriol/alga | Terpenoid | Inhibition of HIV-1 Replication | Noncompetitive inhibition of reverse transcriptase | BRA | Cirne-Santos et al. 2008 |
| Mirabamides A, C and D/sponge | Peptide | Inhibition of HIV-1 fusion | InteractionwithHIV-1 envelope glycoproteins | NZL, USA | Plaza et al. 2007 |
| Sulfated SPMG/alga | Polysaccharide | Inhibition of HIV-1 infection | Inhibition of HIV-1 Tat induced angiogenesis | CHN | Lu et al. 2007 |
Conclusion
Since 1940s, scientists have been trying to find cures for fatal viruses like HIV, HSV and HCMV. Marine based antiviral compounds are to be approved for patient use by the US Food and Drug Association. The isolated compounds undergo various stages of preclinical and clinical trials. They pass through different phases before being approved for human use. Then those showing higher activity than the others are subjected to clinical trials, where they are subjected for dry development after QSAR activity.
The clinical trials are still going on and it is expected that antiviral compounds will be used for patients soon, as it will be much better than chemotherapies and radiotherapies. It has little side-effects. 97% of the world is marine water, from which only 5% has been explored and these compounds have been extracted. However, there is a lot of area to cover and it beckons us to work on it.
The future trends show the use of “polymer therapeutics”, in which specific target drug designing system is used. Drugs are attached to a water soluble polymer and injected at a specific site of infection/disease. In this process, the pharmacokinetic properties of the drugs are enhanced. It is a huge help in drug designing and cancer chemotherapy (Dumdei et al. 1997).
Ethical Issues
None to be declared.
Conflict of interests
None to be declared.
Acknowledgments
This review was made possible due to the funding of Higher Education Commission (HEC) of Pakistan. The research program project number is; PM-IPFP/HRD/HEC/2010/1815.
References
- Ahmad AS, Matsuda M, Shigeta S and Okutani K . 1999 Revelation of antiviral activities by artificial sulfation of a glycosaminoglycan from a marine Pseudomonas Mar . Biotechnol, 1, 102-106 [DOI] [PubMed] [Google Scholar]
- Amornrut C, Toida T, Imanari T, Woo ER, Park H, Linhardt R, et al. 1999 A new sulfated beta-galactan from clams with anti-HIV activity. Carbohydrate Res, 321, 121-127 [DOI] [PubMed] [Google Scholar]
- Artan M, Li Y, Karadeniz F, Lee SH, Kim MM, Kim SK, et al. 2008 Anti-HIV-1 activity of phloroglucinol derivative, 6, 6′-bieckol, from Ecklonia cava. Bioorg. Med. Chem, 16, 7921-7926 [DOI] [PubMed] [Google Scholar]
- Bergman W and Feeney RJ . 1951 Nucleosides of sponges. J. Org. Chem, 16, 981-987 [Google Scholar]
- Chang L, Whittaker NF and Bewley CA . 2003 Crambescidin 826 and dehydrocrambine A: new polycyclic guanidine alkaloids from the marine sponge Monanchora sp . that inhibit HIV-1 fusion. J. Nat. Prod, 66, 1490-1494 [DOI] [PubMed] [Google Scholar]
- Chill L, Rudi A, Aknin M, Loya S, Hizi A, Kashman Y, et al. 2004 New sesterterpenes from Madagascan Lendenfeldia sponges. Tetrahedron, 60, 10619-10626 [Google Scholar]
- Cirne-Santos CC, Souza TM, Teixeira VL, Fontes CF, Rebello MA, Castello-Branco LR, et al. 2008 The dolabellane diterpene dolabelladienetriol is a typical noncompetitive inhibitor of HIV-1 reverse transcriptase enzyme. Antiviral Res, 77, 64-71 [DOI] [PubMed] [Google Scholar]
- Comin MJ, Maier MS, Roccatagliata AJ, Pujol CA and Damonte EB . 1999 Evaluation of the antiviral activity of natural sulfated polyhydroxysteroids and their synthetic derivatives and analogs. Steroids, 64, 335-340 [DOI] [PubMed] [Google Scholar]
- De Lira SP, Seleghim MH, Williams DE, Marion F, Hamill P, Jean F, et al. 2007 A SARS-coronovirus 3CL protease inhibitor isolated from the marine sponge Axinella cf . corrugata: structure elucidation and synthesis. J. Braz. Chem. Soc, 18, 440-443 [Google Scholar]
- De Souza PH, Leao-Ferreira LR, Moussatche N, Teixeira VL, Cavalcanti DN, Da Costa LJ, et al. 2005. Effects of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis on HIV-1 reverse transcriptase, Planta Med 71, 1019–1024. [DOI] [PubMed]
- Dumdei EJ, Blunt JW, Munro MHG and Pannell LK . 1997 Isolation of calyculins, calyculinamides, and swinholide H from the New Zealand deep-water marine sponge Lamellomorpha strongylata. J. Org. Chem, 62, 2636-2639 [DOI] [PubMed] [Google Scholar]
- Fabregas J, García D, Fernandez-Alonso M, Rocha AI, Gómez-Puertas P, Escribano JM, et al. 1999 In vitro inhibition of the replication of haemorrhagic septicaemia virus (VHSV) and African swine fever virus (ASFV) by extracts from marine microalgae. Antiviral Res, 44, 67-73 [DOI] [PubMed] [Google Scholar]
- Fenical W . 1996 Marine biodiversity and the medicine cabinet. The status of new drugs from marine organisms. Oceanography, 9, 23-27 [Google Scholar]
- Ford PW, Gustafson KR, McKee TC, Shigematsu N, Maurizi LK, Pannell LK, et al. 1999 Papuamides A–D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J. Am. Chem. Soc, 121, 5899-5909 [Google Scholar]
- Garson MJ. 1994. The biosynthesis of sponge secondary metabolites: why it is important. In: van Soest, R.W.M., van Kempen, T.M.G., Braekman, J-C. (Eds.), Sponges in Time and Space. Balkema, Rotterdam, pp. 427–440.
- Hui B, Li J and Geng MY . 2008 Sulfated polymannuroguluronate, a novel anti-acquired immune deficiency syndrome drug candidate, decreased vulnerability of PC12 cells to human immunodeficiency virus tat protein through attenuating calcium overload. J. Neurosci. Res, 86, 1169-1177 [DOI] [PubMed] [Google Scholar]
- Hwang Y, Rowley D, Rhodes D, Gertsch J, Fenical W, Bushman F, et al. 1999 Mechanism of inhibition of a poxvirus topoisomerase by the marine natural product sansalvamide A. Mol. Pharmacol, 55, 1049-1053 [DOI] [PubMed] [Google Scholar]
- Iwashima M, Mori J, Ting X, Matsunaga T, Hayashi K, Shinoda D, et al. 2005 Antioxidant and antiviral activities of plastoquinones from the brown alga Sargassum micracanthum, and a new chromene derivative converted from the plastoquinones, . Biol. Pharm. Bull, 28, 374-377 [DOI] [PubMed] [Google Scholar]
- Lee JB, Hayashi K, Hirata M, Kuroda E, Suzuki E, Kubo Y, et al. 2006 Antiviral sulfated polysaccharide from Navicula directa, a diatom collected from deep-sea water in Toyama Bay. Biol. Pharm. Bull, 29, 2135-2139 [DOI] [PubMed] [Google Scholar]
- Loya S, Rudi A, Kashman Y and Hizi A . 1999 Polycitone A, a novel and potent general inhibitor of retroviral reverse transcriptases and cellular DNA polymerases. Biochem. J, 344, 85-92 [PMC free article] [PubMed] [Google Scholar]
- Loya S, Rudi A, Kashman Y and Hizi A . 2002 Mode of inhibition of HIV-1 reverse transcriptase by polyacetylenetriol, a novel inhibitor of RNA- and DNA-directed DNA polymerases. Biochem. J, 362, 685-692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CX, Li J, Sun YX, Qi X, Wang QJ, Xin XL, et al. 2007 Sulfated polymannuroguluronate, a novel anti-AIDS drug candidate, inhibits HIV-1 Tatinduced angiogenesis in Kaposi's sarcoma cells. Biochem. Pharmacol, 74, 1330-1339 [DOI] [PubMed] [Google Scholar]
- Mandal P, Pujol CA, Carlucci MJ, Chattopadhyay K, Damonte EB and Ray B . 2008 Anti-herpetic activity of a sulfated xylomannan from Scinaia hatei. Phytochemistry, 69, 2193-2199 [DOI] [PubMed] [Google Scholar]
- Marin Lit. 1998. A marine literature database maintained by the Marine Chemistry Group, University of Canterbury, Christchurch, New Zealand.
- Matsuhiro B, Conte AF, Damonte EB, Kolender AA, Matulewicz MC, Mejias EG, et al. 2005. Structural analysis and antiviral activity of a sulfated galactan from the red seaweed Schizymenia binderi (Gigartinales, Rhodophyta), Carbohydr. Res. 340 2392–2402. [DOI] [PubMed]
- Mayer AMS . 1998 Therapeutic implications of microglia activation by lipopolysaccharide and reactive oxygen species generation in septic shock and central nervous system pathologies: a review. Medicina (Buenos Aires), 58, 377-385 [PubMed] [Google Scholar]
- Mayer AMS and Hamann MT . 2002 Marine pharmacology in 1999: compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities; affecting the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C, Comp. Pharmacol. Toxicol, 132, 315-339 [DOI] [PubMed] [Google Scholar]
- Mayer AMS and Hamann MT . 2004 Marine pharmacology in 2000: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar. Biotechnol(NY), 6, 37-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AMS and Lehmann VKB . 2000 Marine pharmacology in 1998: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities; with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Pharmacologist, 42, 62-69 [DOI] [PubMed] [Google Scholar]
- Meiyu G, Fuchuan L, Xianliang X, Jing L, Zuowei Y and Huashi G . 2003 The potential molecular targets of marine sulfated polymannuroguluronate interfering with HIV-1 entry . Interaction between SPMG and HIV-1 rgp120 and CD4 molecule. Antivir. Res, 59, 127-135 [DOI] [PubMed] [Google Scholar]
- Miao B, Geng M, Li J, Li F, Chen H, Guan H, et al. 2004 Sulfated polymannuroguluronate, a novel anti-acquired immune deficiency syndrome (AIDS) drug candidate, targeting CD4 in lymphocytes. Biochem. Pharmacol, 68, 641-649 [DOI] [PubMed] [Google Scholar]
- Mori T, O'Keefe BR, Sowder RC, Bringans S, Gardella R, Berg S, et al. 2005 Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem, 280, 9345-9353 [DOI] [PubMed] [Google Scholar]
- Munro MHG, Luibrand RT and Blunt JW. 1987. The search for antiviral and anticancer compounds from marine organisms. In: Scheuer PJ. (Ed.), Bioorganic Marine Chemistry, vol. 1. Verlag Chemie, Berlin, pp. 93– 176.
- Nakao Y, Takada K, Matsunaga S and Fusetani N . 2001 Calyceramides A– C: neuraminidase inhibitory sulfated ceramides from the marine sponge Discodermia calyx. Tetrahedron, 57, 3013-3017 [Google Scholar]
- Oku N, Gustafson KR, Cartner LK, Wilson JA, Shigematsu N, Hess S, et al. 2004 Neamphamide A, a new HIV-inhibitory depsipeptide from the Papua New Guinea marine sponge Neamphius huxleyi. J. Nat. Prod, 67, 1407-1411 [DOI] [PubMed] [Google Scholar]
- Pereira HS, Leao-Ferreira LR, Moussatche N, Teixeira VL, Cavalcanti DN, Costa LJ, et al. 2004 Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (HIV-1). Antivir. Res, 64, 69-76 [DOI] [PubMed] [Google Scholar]
- Plaza A, Gustchina E, Baker HL, Kelly M and Bewley CA . 2007 Mirabamides A–D, depsipeptides from the sponge Siliquariaspongia mirabilis that inhibit HIV-1 fusion. J. Nat. Prod, 70, 1753-1760 [DOI] [PubMed] [Google Scholar]
- Rashid MA, Gustafson KR, Cartner LK, Shigematsu N, Pannell LK and Boyd MR . 2001 Microspinosamide, a new HIV-inhibitory cyclic depsipeptide from the marine sponge Sidonops microspinosa. J. Nat. Prod, 64, 117-121 [DOI] [PubMed] [Google Scholar]
- Reddy MV, Rao MR, Rhodes D, Hansen MS, Rubins K, Bushman FD, et al. 1999 Lamellarin alpha 20-sulfate, an inhibitor of HIV-1 integrase active against HIV-1 virus in cell culture. J. Med. Chem, 42, 1901-1907 [DOI] [PubMed] [Google Scholar]
- Rodriguez MC, Merino ER, Pujol CA, Damonte EB, Cerezo AS and Matulewicz MC . 2005 Galactans from cystocarpic plants of the red seaweed Callophyllis variegata (Kallymeniaceae, Gigartinales). Carbohydr. Res, 340, 2742-2751 [DOI] [PubMed] [Google Scholar]
- Rowley DC, Hansen MS, Rhodes D, Sotriffer CA, Ni H, McCammon JA, et al. 2002 Thalassiolins A– C: new marine-derived inhibitors of HIV cDNA integrase. Bioorg. Med. Chem, 10, 3619-3625 [DOI] [PubMed] [Google Scholar]
- Rudi A, Yosief T, Loya S, Hizi A, Schleyer M and Kashman Y . 2001 Clathsterol, a novel anti-HIV-1 RT sulfated sterol from the sponge Clathria species. J. Nat. Prod, 64, 1451-1453 [DOI] [PubMed] [Google Scholar]
- Schmitz FJ. 1994. Cytotoxic compounds from sponges and related microfauna. In: van Soest, R.W.M., van Kempen, T.M.G., Braekman, J-C. (Eds.), Sponges in Time and Space. Balkema, Rotterdam, pp. 485–496.
- Talarico LB, Duarte ME, Zibetti RG, Noseda MD and Damonte EB . 2007 An algal derived DL-galactan hybrid is an efficient preventing agent for in vitro dengue virus infection. Planta Med, 73, 1464-1468 [DOI] [PubMed] [Google Scholar]
- De LiraVenkateshwar Goud T, De LiraVenkateshwar GoudSrinivasa Reddy N, De LiraVenkateshwar GoudSrinivasa ReddyRaghavendra Swamy N, Siva Ram and Venkateswarlu Y . 2003 Anti-HIV active petrosins from the marine sponge Petrosia similis. Biol. Pharm. Bull, 26, 1498-1501 [DOI] [PubMed] [Google Scholar]