Abstract
Introduction
Papaver somniferum is the commercial source of morphine and codeine. The isolation of effective genes involved in the morphine biosynthesis of P. somniferum is very important in the production of specific metabolites achieved using metabolic engi-neering techniques. In this pathway, the key enzyme COR is involved in the conversion of codeinone to codeine and morphinone to morphine.
Methods
the gene encoding of this enzyme was isolated using primers designed on the base of gene sequence available on (NCBI) for P. somniferum. This gene correct size around (960 bp) was first subcloned into pTZ57RIT vector then cloned into expression vectors (pBI121) between BamHI and SacI sites to allow the expression of cor gene driven by the cauliflower mosaic virus 35S pro-moter. The result was confirmed through different molecular methods e.g. PCR and en-zyme digestion by BamHI and SacI. The recombinant plasmid was transformed into the E. coli strain DH5α using a freeze-thaw method. Having selected positive colones on selection medium, plasmid was extracted by miniprep method and recombinant plasmids were selected based on PCR and digestion. The construct was then mobilized in Agrobacterium tumefaciens C58/pGV3850 (KmR RifR). After gene transformation to P. somniferum plants, the agroinfiltration method was also used for transient expression of COR enzyme.
Results
evaluation results showed that morphine and codeine were detectable in the leaves of transgenic plants containing cor transgene and there was significant difference in the final production. After completing this experiment for three times, results showed that in 11 sets from 15 sets of leaves experiment tested, main alkaloids (codeine, morphine, papaverin, noscapine and thebaine) were detectable.
Conclusion
Whereas no signal was detected in non-infiltrated control leaves or in leaves infiltrated with non-recombinant bacteria for morphine and codeine, others such as thebaine and papaverine were detectable.
Keywords: Papaver somniferum, Biosynthesis Pathway, Agroinfiltration, COR Enzyme
Introduction
Alkaloids are a diverse group of low-Mr, nitrogenous compounds found in about 20% of plant species. The benzylisoquinoline alkaloid class, in particular, consists of several important medicinal compounds including morphine, codeine, papaverine, berberine, (+)-tubocurarine and sanguinarine. The limited availability of cloned biosynthetic genes and the inability to genetically transform many alkaloid-producing species has generally restricted the metabolic engineering of plant alkaloid pathways. The enzymatic synthesis of morphine in Opium poppy (Papaver somniferum) has been almost completely elucidated by MH Zenk et al and summarized by Kutchan (1998). Recently, several new gene-encoding enzymes involved in benzylisoquinoline alkaloid biosynthesis have been reported (Facchini 2001) and protocols producing species Opium poppy and California poppy have been established for the genetic transformation of the benzylisoquinoline alkaloid (Belny et al 1997, Park and Facchini 2000a, 2000b, 2000c). These developments have created the opportunity to metabolically engineer benzylisoquinoline alkaloid pathways in plants. Each of the known enzymes of morphine biosynthesis has been detected in both P. somniferum plants and cell suspension culture, yet plant cell cultures have never been shown to accumulate morphine (Kutchan 1998). Isolation of the morphine biosynthesis genes would facilitate the metabolic engineering of Opium poppy to produce plants with specific patterns of alkaloids and could ultimately lead to an understanding of the inability of plant cell culture to accumulate morphine. It is commonly known that morphinan alkaloids assemble in latex vesicles within poppy laticifers. Laticifer ultra structure and differentiation has been analyzed and related to opium alkaloid accumulation (Kutchan et al 1985, 1986, Nessler and Mahlberg 1977, 1978, Roberts et al 1983, Rush et al 1985).
Codeinone reductase is the last step in the two alternative routes between morphine biosynthesis and thebaine. Codeinone reductase catalyzes the NADPH-dependent reduction of codeinone to codeine along the morphine biosynthetic pathway in Opium poppy. For first time, Unterlinner et al (1999) isolated and characterized four cDNAs encoding codeinone reductase isoforms and had functionally expressed them in Escherichia coli. By sequence comparison, codeinone reductase belongs to the aldo/keto reductase family, a group of structurally and functionally related NADPH-dependent oxidoreductases. Larkin et al (2007) demonstrated that Over-expression of COR resulted in an increase in morphinan alkaloid production on a dry-weight basis.
However, transgenic plants have some disadvantages such as the extent of time required to establish a produce line, transformation of recalcitrant cultivars and public concerns about transgene escape in the environment (Miele 1997). In response to such concerns, alternative systems have been suggested. These include, production of transplastomic plants in which the chloroplast genome rather than the nuclear genome is transformed (Daniell et al 2001) and transient gene expression (Fischer et al 1999). Transient expression can be used to verify the expression of constructs and produce small amounts of product for functional analysis before proceeding to the development of transgenic plants. Various approaches, including biolistic delivery of naked DNA, infiltration with recombinant Agrobacteria (agroinfiltration) and infection with modified viral vectors are now being used (Fisher et al 1999). In agroinfiltration, evacuated plant leaves take up large amounts of recombinant DNA and target many cells in a leaf and the T-DNA harboring the gene of interest is actively transferred into the nucleus by the bacterial proteins (Kapilla et al 1997, Fischer et al 1999). Considering other transient expression systems for plants and biosafety regulations that must be considered in virus infected plant techniques as well as regulatory compliance for transgenic plants, agroinfiltration can be used as a suitable technique for the production of biopharmaceuticals important for therapeutic uses especially in low dose.
In the present study, morphinan and nonmorphinan alkaloid productivity was investigated in leaves of P. somniferum L. We confirm that vacuum infiltration of intact P. somniferum leaves is an efficient system for the transient expression of genes encoding morphinan alkaloids biosynthetic enzymes.
Materials and methods
Plant material
Papaver somniferum seeds were grown in green houses (photoperiod: natural daylight, Temperature regime: 25/18°C day-night). Excised leaves from 6-week seedlings were used for RNA extraction and intact young leaves were used for agroinfiltration.
RNA extraction and cDNA synthesis
Total RNA for cDNA synthesis was isolated using the method of RNA XPLUS Kite (Sinagen). Generation of cDNAs encoding codeinone reductase from P. somniferum was produced by reverse transcription of mRNA isolated from 6-week-old plants. DNA amplification using either Taq or Pfu polymerase was performed under the following conditions: 5 min at 94° C, 30 cycles of 94° C, 60 s; 56° C, 60 s; 72° C, 60 s. At the end of 30 cycles, the reaction mixtures were incubated for an additional 10 min at 72° C. Prior to cooling to 4° C, cor coding regions were amplified by using primers designed to add BamHІ and SacІ restriction sites to the 5′ and 3′ ends, of each primers, respectively. The specific sequences of the oligodeoxynucleotide primers were designed on the complete sequence of cor genes based on the sequences in GenBank (accession number AF108435). Cor gene was amplified from cDNA using primers corresponding to the beginning and end of gene (5′ TTG, GAT, CCG, CCA, CCA, TGG, CAA, CAA, TGT, ATA 3′ and 3′ GAG, AGC, TCT, CAA, ATC, AAT, TCA, AGG, ATT, TCA 5′). Agarose gel electrophoresis was applied for resolving amplified DNA. The bands of approximately correct size around (960 bp) were isolated and subcloned into pTZ57RIT (Cloning Kit # k1214, Fermentase) prior to nucleotide sequence determination.
Construction of binary vector
The recombinant plasmid was transformed into the Escherichia coli strain DH5α by using a freeze-thaw method. Transformed E. coli DH5α was grown on LB agar plates containing ampicillin (100 μg/ml) and positive clones were selected and sequenced. Subsequently, after plasmid extraction, the pTZ57RIT/cor was digested with BamHІ and SacІ to recover the cor fragment. Approximately 200-250 ng of each plasmid DNA were digested with 1μl of BamHІ (10U/μl) and SacІ (20U/μl) in an appropriate buffer at 37ºC for one overnight. The digested products were fractionated on 1% agarose gel. The gel was visualized over UV transilluminator and the desired band was excised with a sterile scalpel then eluted with Genomic DNA Purification Kit (Fermentase). Eluted DNA was used for subcloning into pBI121 between BamHI and SacI sites to allow the expression of cor gene driven by the cauliflower mosaic virus 35S promoter. The recombinant plasmid was transformed into the E. coli strain DH5α using a freeze-thaw method. After the selection of positive colones on selection medium, plasmid was extracted by miniprep method and recombinant plasmids were selected based on PCR and digestion. The construct was then mobilized in Agrobacterium tumefaciens C58/pGV3850 (KmR RifR). A. tumefaciens cultures were grown at 28°C at 180 rpm on a gyratory shaker in liquid Luria-Bertani medium (1% [w/v] tryptone, 0.5% [w/v] yeast extract, and 1% [w/v] NaCl, pH 7.0), containing 50 mg/l kanamycin, to mid-log phase (A600= 0.8).
Transient expression assay in vacuum infiltrated leaves
Preparation of Agrobacterium for the infiltration of plant leaves were performed as previously described by Kapilla et al (1997) with some modifications. Preculture (2 ml) of Agrobacterium was inoculated into 100 ml LB medium supplemented with 50 mg/l of kanamycin and grown overnight to logarithmic phase (OD600 = 0.6) at 28°C. Bacteria were centrifuged and resuspended in half volume of infection medium (Murashige and Skoog (1962): basal medium (pH 5.5) containing 5.0% sucrose, 10 mM MES and 200 mM acetosyringone) and grew at 28°C for 2-3 h to a final OD600 = 0.6. A continuous vacuum in the range of 0.5-1 mbar was applied for 0.5 g intact leaves. For analysis of the effect of infiltration time, papaver leaves were infiltrated for 15, 30 and 45 minutes. The best result was used for the infiltration of papaver leaves. After infiltration, vacuum was broken rapidly; leaves were rinsed in sterile water, kept on a Whatman paper # 40 with adaxial side facing up and put in sealed trays (16/8 h photoperiod, 25°C) for 72h. Leaves were used directly for further TLC and HPLC analysis.
TLC and HPLC analysis
P. somniferum leaves were extracted with methanol in boiling water and refluxed for three hrs. Extracts were reduced to dryness under vacuum, dissolved in 1.0 ml sodium carbonate/bicarbonate (3:2, w/w), pH 10.0, and extracted three times with ethyl acetate. Pooled ethyl acetate fractions were decreased to dryness and the residue was taken up in 5 ml of methanol. TLC was performed on precoated silica gel HPTLC aluminum plates 60F254 (20 cm × 20 cm, 0.2 mm thickness, 5–6 μm particle size, E-Merck, Germany). Twenty microliters of the standard solutions and samples were spotted as bands of 6 mm width by using the auto sampler fitted with a 100 μl Hamilton syringe. The plates were developed with a mobile phase using toluene-acetone-ethanol-ammonia (40:40:6:2). The developed plates were air dried and scanned. A spectrodensitometer (Scanner 3, CAMAG) possessing ‘win CATS’ planar chromatography manager (version 1.3.0) software was utilized for the densitometry measurements, spectra recording and data processing.
Samples, for HPLC analysis, were filtered (Whatman no. 6 filter paper) directly into an autosampler vial for HPLC injection. alkaloid samples (15 μl) Extracted, were run on an Alltech platinum C18 53 × 7 mm rocket column heated to 40 °C at a flow rate of 1.5 ml/min using an acetonitrile gradient. The gradient began at 100% eluant A and moved to 35% A and 65% B at 18 min (where eluant A was 2% acetonitrile, 0.8% triethylamine, 0.05 M sodium dihydrogen phosphate, adjusted to pH = 3.5 with phosphoric acid; and eluant B was 50% acetonitrile, 0.8% triethylamine, 0.05 M sodium dihydrogen phosphate, adjusted to pH = 3.5 with phosphoric acid). Separated products were analyzed using a Waters 2487 Dual wavelength detector and a Millennium 32 version 3.05.01 data system with UV detection of peaks at 254 nm. Pharmaceutical grade opium alkaloids were used as standards and included morphine sulfate, codeine sulfate, thebaine, papaverine and noscapine (Sigma Chemical Corp.).
Results
RNA extraction, cDNA synthesis and construction of binary vector
Based on the sequences of cor in Gene bank (accession number AF108435), two sets (forward and reverse) of degenerate primers were made and used to amplify the corresponding gene product by PCR using reverse-transcribed mRNA as a template. The PCR mixture was resolved on a 1% agarose gel. Only forward and reverse primers amplified specifically around 960 bp DNA band (Fig. 1).
Fig. 1 .
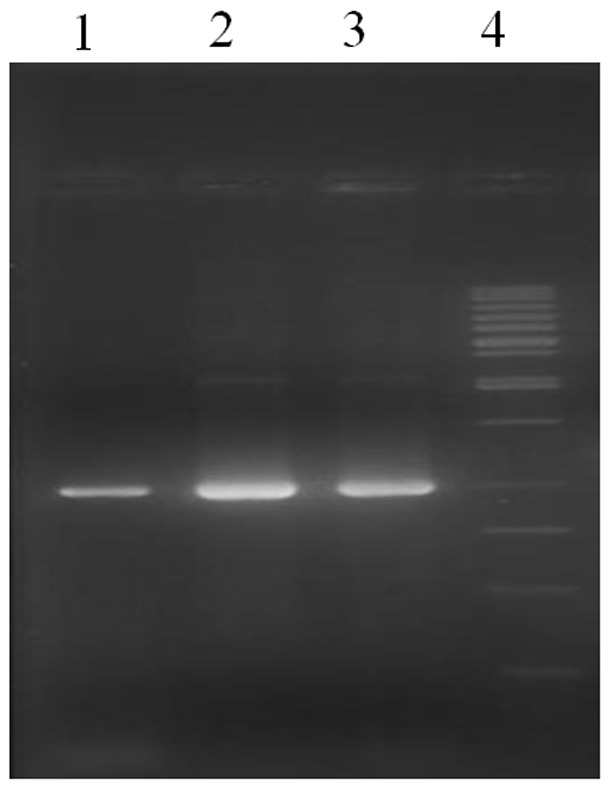
The PCR product of cor gene. Lane 1, 2 and 3: PCR product (960 bp), Lane 4: marker 1 kb fermentase.
The PCR product was then recovered and subsequently cloned into pTZ57RIT. All of the positive clones had the same size inserts around 960 bp. After plasmid extraction, and digestion of pTZ57RIT/cor with BamHІ and SacІ, the cor fragment was recovered and ligated into the pBI121 between BamHI and SacI sites just under the CaMV35S promoter sequence to yield overexpressing plasmid. The ligated vector was named pBI121/cor. The recombinant plasmid was transformed into the Escherichia coli strain DH5α.Recombinant plasmids were selected based on PCR and digestion after the selection of positive colones on selection medium and plasmid extraction, (Figure 2, 3). The construct was then mobilized in Agrobacterium tumefaciens C58/pGV3850 (KmR RifR).
Fig. 2 .
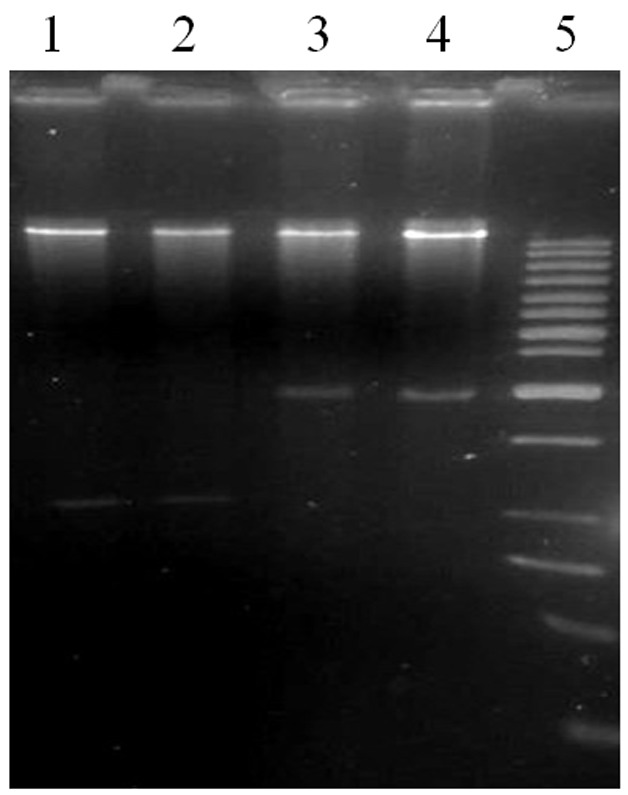
Identification of the insertion of cor gene in pBI121. Lanes 1–2: the recombination plasmid with correctly gene insertion; Line 3–4: the Non-recombinant plasmid, lane 5: marker 1 kb fermentase.
Fig. 3 .
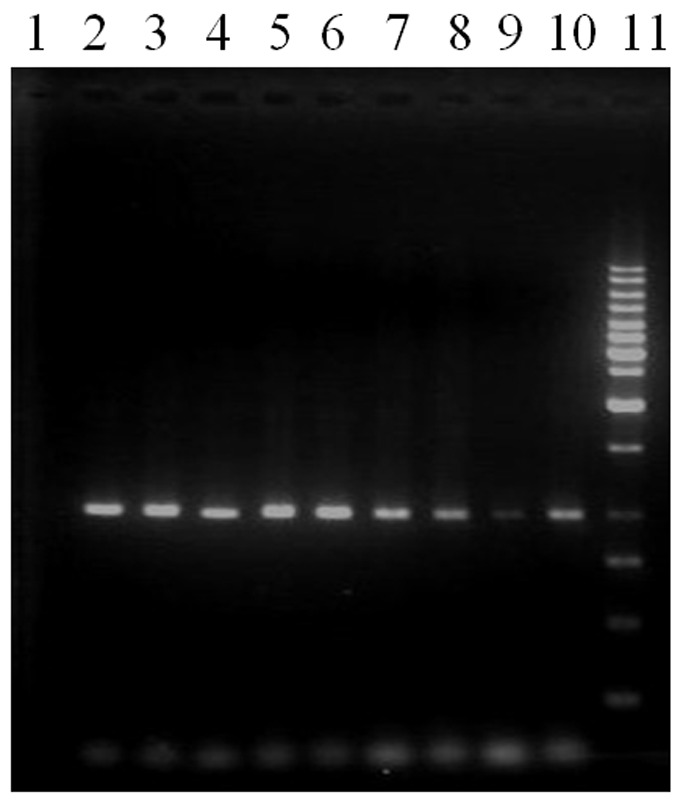
PCR analyses for the presence of cor genes in recombinant plasmid; lane 1: negative control, lanes 2-9, recombinant plasmid, line 10: positive control, 11: marker 1 kb fermentase.
Transient expression assay
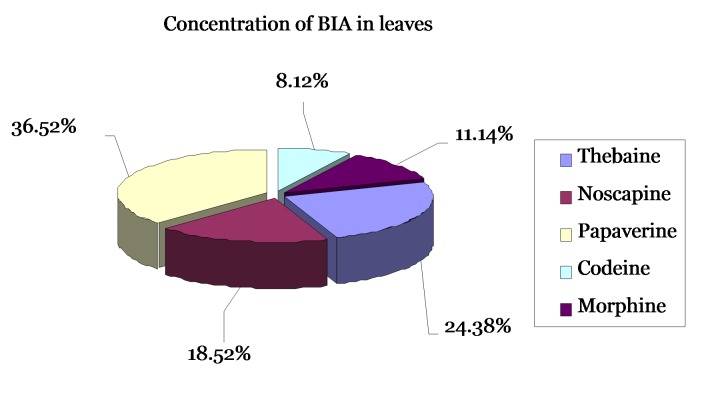
The agroinfiltration method developed by Kapila et al (1997) was used in order to achieve transient overexpression of cor gene in intact P. somniferum leaves. Gene expression was confirmed by means of TLC and HPLC analysis of crude leaf extracts. The role of inoculation time during the agroinfiltration process on papaver leaves showed that this factor plays an important role in the expression level of final products in leaves. We have designed an experiment to study different inoculation times (15, 30 and 45 min) in agroinfiltration on papaver leaves. Analysis of TLC showed that the best expression level could be detected on 30 min. On low times, detection of alkaloids was impossible after three days and all parts of leaves were destroyed in 45 min and were very slimy so that extraction was difficult after three days. After completing this experiment for three times, results showed that in 11 sets from 15 sets of leaves experiment tested, main alkaloids (codeine, morphine, papaverin, noscapine and thebaine) were detectable (Fig. 4, 5). Whereas no signal was detected in non-infiltrated control leaves or in leaves infiltrated with non-recombinant bacteria for morphine and codeine (Fig. 5), others such as thebaine and papaverine were detectable.
Fig. 4. Determination of tetrahydrobenzylisoquinoline, alkaloids in leaves. (a) Present the average alkaloid content of opium poppy plants transient expression leaves. HPLC analysis of latex from Papaver somniferum wild type (WT) and transgenic plants transformed with S4S4:anti-bbe.
Fig. 5 .
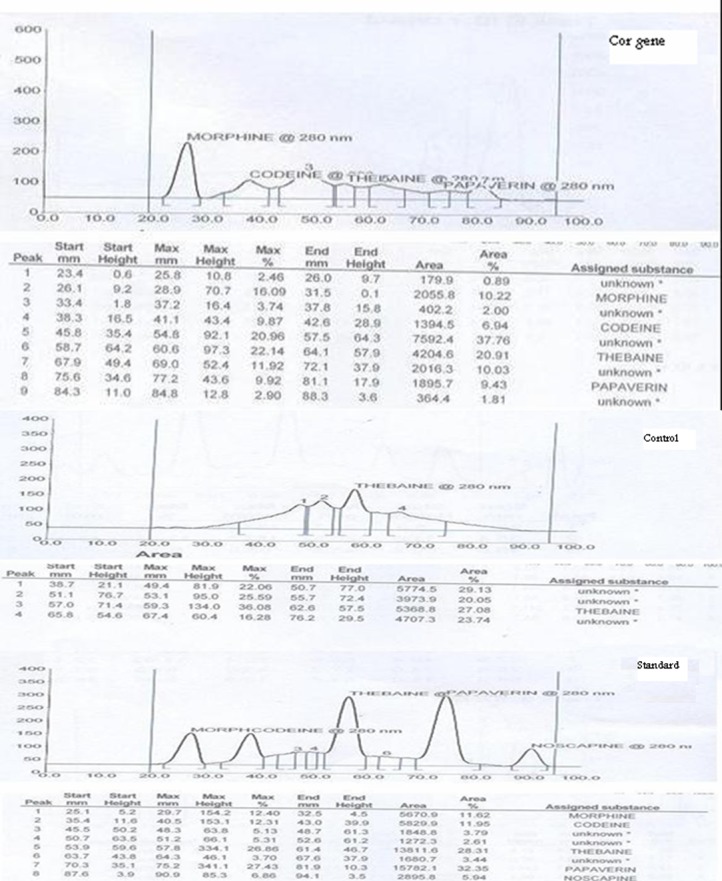
TLC analysis of transient expression of Papaver somniferum leaves. Cor gene, Control and Standard samples.
a.
b.
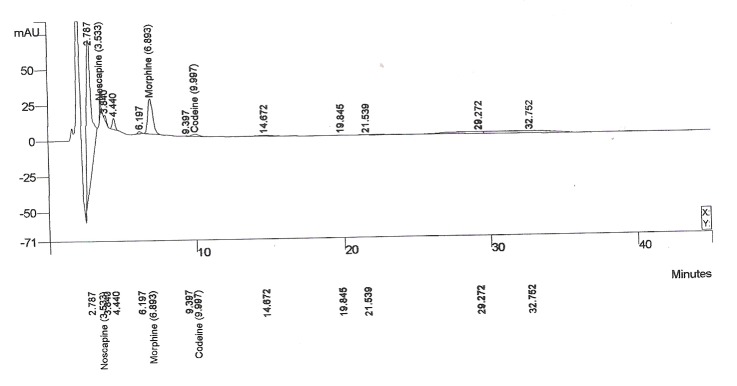
These results show that transient expression can induce the production of these pharmaceutically important analgesic and narcotic drugs precursors. The alkaloids in leaves and stem sections were calculated as in alkaloid/100 mg fresh weight.
Discussion
Papaver somniferum is now the commercial source of the narcotic analgesics morphine and codeine. Along with these two morphinans, P. somniferum produces approximately eighty alkaloids belonging to various tetrahydrobenzylisoquinoline-derived classes. Morphine, along with the chemotherapeutic agents' vincristine, vinblastine and camptothecin, is one of the most important alkaloids commercially isolated from medicinal plants. Isolation of the genes of morphine biosynthesis would facilitate the metabolic engineering of O. poppy to produce plants with specific patterns of alkaloids and could ultimately lead to an understanding of the inability of plant cell cultures to accumulate morphine. In this paper, we take both of these goals with the isolation and characterization of cDNAs and genes that encode codeinone reductase in P. somniferum. To our knowledge, this is the first report of transient expression systems in P. somniferum plants. After total RNA extraction, full-length cDNAs were generated by RT-PCR using the specific degenerated primers and RNA isolated from P. somniferum leaves and after cDNA synthesis, cor gene was amplified by specific primers and into pTZ57RIT vector then cloned into expression vectors (pBI121). Agrobacterium tumefaciens was transformed by recombinant pBI121cor plasmid.
The initial biochemical analysis of codeinone reductase showed evidence for only two isoforms in the poppy plant and one isoform in poppy cell suspension culture was observed (Lenz and Zenk 1995). Then, Unterlinner et al (1999) suggested the presence of at least six genes that could encode codeinone reductase in Opium poppy. They isolated and characterized four cDNAs encoding codeinone reductase isoforms and functionally expressed them in Escherichia coli. They suggested that at least six alleles appear to be present in the poppy genome. In this study, we isolated and cloned codeinone reductase sequence based on their four sequences for cor1-cor4.
Until this time, many enzymes included in morphine biosynthesis were characterized and in some studies were overexpressed or blocked by RNAi or RNA antisense. The site of morphine biosynthesis in P. somniferum is a long-standing question. Grothe et al (2001), showed that salAT was expressed in each major plant part analyzed—root, stem, leaf, and capsule. This is compatible with the detection of transcript of another morphine biosynthesis-specific gene, cor1, in each plant organ analyzed (Huang and Kutchan 2000). Moreover, salutaridinol 7-O-acetyltransferase and codeinone reductase enzyme activity have each been detected in the cytosolic fraction of isolated latex (Huang and Kutchan 2000, Unterlinner et al 1999). The gene cyp80b1 takes part in (S)-reticuline biosynthesis taking place before a bifurcation in the biosynthetic pathway which results in more than 80 isoquinoline alkaloids. Cyp80b1 is, hence, frequent to several biosynthetic pathways such as morphine, sanguinarine, and noscapine. cyp80b1 Transcript was also detected in all analyzed plant organs (Huang and Kutchan 2000). morphinan alkaloids Accumulation is thought to be related with the appearance of laticifer cells in the developing plant and in differentiating plant cell culture (Rush et al 1985, Kutchan et al 1985). A reticulated laticifer system relevant to the vascular tissue exists through the aerial parts of the poppy plant. The localization of three genes of morphine biosynthesis, cyp80b1, salAT, and cor1, is thus far consistent in assuming that this biosynthesis is, partly at least, associated with laticifer cells (Grothe et al 2001).
The analysis of RNA and enzyme activity of various stages of developing Opium poppy seedlings and roots, stem, leaf and capsule of mature poppy plants indicated that transcript from these alleles is present throughout the plant at all developmental stages, and that the highest total enzyme activity is localized to the capsule after petal fall. These results would propose that morphine biosynthesis occurs in all major plant organs initiating within the first 7 days after seed germination. This biosynthesis continues throughout the lifecycle of this annual plant with the highest biosynthetic activity taking place in the capsule after petal fall, consistent with the amount of biosynthetic enzyme present (Unterlinner et al 1999). With the identification of many of the alkaloid biosynthesis enzymes in this plant, biochemical data proposed involvement of multiple cell types in alkaloid biosynthesis in poppy (Weid et al 2004). However, morphinan alkaloids (i.e. morphine, codeine and thebaine) have been detected in only some cases, and mostly in quite low levels (Staba et al 1982).
In 1989, Williams and Ellis tried the age and tissue distribution of some major alkaloids in the cultivar “Marianne”. They noticed a time- and tissue-specific accumulation of morphine, codeine, noscapine, and narcotoline in aerial and root tissues. In another experiment, Frick et al (2005) showed that detection of some important alkaloids possible in leaves and stems of two P. somniferum cultivars (C048-6- 14-64 and Marianne) two days after petals dropped. They showed the alkaloids in the leaves display the same pattern as in latex and stems; 75-80% of the alkaloids in leaves were morphine, codeine, and thebaine. In another experiment, researchers showed that gene expression in O. poppy is restricted to some tissues and developmental stages. In situ hybridization analysis presented that TYDC genes expression is developmentally regulated. TYDC transcripts are associated with vascular tissue in mature roots and stems, but they are also expressed in cortical tissues at earlier stages of development. TYDC genes Expression is restricted to metaphloem and to protoxylem in the vascular bundles of mature aerial organs. Localization of TYDC transcripts in the phloem is compatible with the expected developmental stem of laticifers, which are specialized internal secretory cells accompanying vascular tissues in all organs of selected species and that contain the alkaloid-rich latex in aerial organs. A coordinated regulation of specific alkaloid biosynthetic genes is proposed by the differential expression of TYDC genes and the organ-dependent accumulation of different alkaloids that are eventually controlled by specific developmental programs (Facchini and De Luca 1995).
Another objective of this study was to evaluate the capacity of P. somniferum leaves for the overexpression of codeinone reductase by agroinfiltration. As the efficiency of agroinfiltration is highly dependent on the ability of bacterial penetration inside the leaf tissue (Fischer et al 2004), it seems that by increasing infiltration time, there is more chance for passing the epidermis barrier and infecting the neighboring cells and transferring T-DNA containing the desired gene into the nucleus. While standardizing for maximum expression level at different inoculation time was 15, 30 and 45 min, we found that after 30 min infiltration time, highest expression was achieved. Therefore, 30 minutes for infiltration experiments was used for further study. In previous studies, in other plant leaves (tobacco, potato and lettuce), results showed that in 35 minutes, the highest expression of human growth hormone was achieved (Hashemi et al 2005). As prolonged exposure to vacuum could be rapidly decreased, the temperature of bacterial suspension may have additionally reduced the expression (Wroblewski et al 2005). Therefore, we did not use longer inoculation time. Low increase in amount of final product in leaves may be caused by the synthesis of low yield, but it could be managed by increasing the amount of leaves. For example, researchers at Medicago Inc. have described how the agroinfiltration of alfalfa leaves can be scaled up to 7500 leaves per week producing micrograms of recombinant protein (Fischer et al 2004).
It seems that, with the optimization of this protocol, the likely production of most important pharmaceutical substances would be possible. The agroinfiltration system allows scaling up and is comparable to transgenic plants in terms of quantity and quality. Therefore, considering some limitations for production in a commercial scale, it could be possible to have an optimized condition; agroinfiltration could be equal from economical point of view or even inexpensive and a suitable replacement for releasing of transgenic plants. Furthermore, transient expression is a very fast technique to analyze interested genes. Transient expression takes only a few days, whereas stable transformed plants are generated after several months. Sparkes et al (2006) showed that transient transformation of tobacco leaf epidermal cells is a relatively fast technique to assess the expression of genes of interest. While stable lines are generated after approximately 2 to 4 months, Transient expression takes from 2 to 4 days. In other crops, such as Catharanthus roseus, the transient expression technique was used to overexpress tryptophan decarboxylase (tdc) and strictosidine synthase (str1) genes, which encode two key enzymes of the terpenoid indole alkaloid (TIA) biosynthesis pathway. Immunoblot analysis of crude leaf extracts showed that recombinant TDC and STR1 were accumulated to detectable levels when targeted to their native subcellular compartments (i.e., the cytosol and vacuole, respectively) or to the chloroplast (Stefano et al 2004).
In spite of other reports that the production of morphine and codeine is only possible in laticfiers, now it seems that with transient expression of COR enzyme in leaves, there is a possibility to produce these pharmaceutically important analgesics in leaves. Transient expression can be used to verify the expression of constructs and produce small amounts of products for functional analysis before proceeding to the development of transgenic plants.
Conclusion
We showed that agroinfiltration could be an efficient tool for the transient expression of genes encoding enzymes of the Benzylisoquinoline alkaloids (BIA) pathway in intact P. somniferum leaves. The transient expression assay described here is reliable and inexpensive and can be a suitable test-system for our further understanding of the biosynthetic BIA pathway to develop metabolic engineering strategies for the production of valuable pharmaceutical precursors.
Ethical Issues
Not applicable in this study.
Conflict of interests
Authors declared no conflicts of interests.
Acknowledgments
We would like to thank Mrs. Esmat Jourabchi, Mr. Ali Sharafi and Hooman Mirzaei for providing plant materials and primer synthesis.
References
- Belny M, Herouart D, Thomasset B, David H, Jacquin-Dubreuil A, et al. 1997 Transformation of Papaver somniferum cell suspension cultures with sam-1 from A. thaliana results in cell lines of different S-adenosyl-lmethionine synthase activity. Physiol Plant, 99(2), 233-240 [Google Scholar]
- Daniell H, Stephen J, Stratfield SJ and Wycoff K . 2001 Medical molecular farming production of antibodies biopharmaceuticals and edible vaccine in plants. Trends plant Sci, 6(5), 219-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini PJ . 2001 Alkaloid biosynthesis in plants: biochemistry, cell biology, molecular regulation, and metabolic engineering applications. Annu Rev Plant Physiol Plant Mol Biol, 51(1), 29-66 [DOI] [PubMed] [Google Scholar]
- Facchini PJ and De Luca . 1995 Phloem-specific expression of tyrosine / dopa decarboxylase and isoquinoline alkaloid biosynthesis in opium poppy. Plant Cell, 7(11), 1811-1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Stoger E, Schillberg S, Christou P and Twyman R . 2004 Plant based production of Biopharmaceuticals. Current opinion in plant Biology, 7(2), 152-158 [DOI] [PubMed] [Google Scholar]
- Fischer R, Vaquero C, Sack M, Drossard J, Emans N and Commandeur U . 1999 Toward Molecular Farming in the future: transient protein expression in plants. Biotechnol Appl Biochem, 30(2), 113-116 [PubMed] [Google Scholar]
- Frick S, Frick R, Kramell J, Schmidt AJF and Kutchan TM . 2005 Comparative qualitative and quantitative determination of alkaloids in narcotic and condiment Papaver somniferum cultivars . J . Nat. Prod, 68(5), 666-673 [DOI] [PubMed] [Google Scholar]
- Grothe T, Lenz R, and Kutchan TM . 2001 Molecular characterization of the salutaridinol 7-O-acetyltransferase involved in morphine biosynthesis in opium poppy Papaver somniferum. J. Biol. Chem, 276(33), 30717-30723 [DOI] [PubMed] [Google Scholar]
- Hashemi HS, Jourabchi E, and Kutchanand Khodabandeh M . 2005 Transient expression of human growth hormone in potato (Solanum tuberosum), tobacco (Nicotiana tobacum) and lettuce (Lactuca sativa) leaves by agro infiltration. Iran.J.Biotech, 3(2), 109-113 [Google Scholar]
- Huang FC and Kutchan TM . 2000 Distribution of morphinanand benzo[c]phenanthridine alkaloid gene transcript accumulation in Papaver somniferum. Phytochemistry, 53(5), 53(5) 555-564 [DOI] [PubMed] [Google Scholar]
- Kapila J, and Kutchanand KhodabandehDe Rycke R, Van Montagu and Angenon G . 1997 An Agrobacterium mediated transient gene expression system for intact leaves. Plant Sci, 122(1), 101-108 [Google Scholar]
- Kutchan TM. 1998. Molecular genetics of plant alkaloid biosynthesis, In: The Alkaloids, Vol. 50. Cordel, G (Ed.), San Diego, Academic Press, pp 257-316.
- Kutchan TM, Ayabe S, Coscia CJ, Phillipson JD, Roberts MF and Zenk MH. 1985. Cytodifferentiation and Papaver alkaloid accumulation. In: Chemistry and Biology of Isoquinoline Alkaloids. Berlin, Springer-Verlag, pp 281-294.
- Kutchan TM, Rush MD and Coscia CJ . 1986 Subcellular localization of alkaloids and dopamine in different vacuolar compartments of Papaver bracteatum. Plant Physiol, 81(1), 161-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin PJ, Miller JAC, Allen RS, Chitty JA, Gerlach WL, Frick S, et al. 2007 Increasing morphinan alkaloid production by over-expressing codeinone reductase in transgenic Papaver somniferum. Plant Biotechnol J, 5(1), 26-37 [DOI] [PubMed] [Google Scholar]
- Lenz R and Zenk MH . 1995 Purification and properties of codeinone reductase (NADPH) from Papaver somniferum cell cultures and differentiated plants. Eur. J. Biochem, 233, 132-139 [DOI] [PubMed] [Google Scholar]
- Miele L . 1997 Plants as bioreactor for biopharmaceuticals, regulation consideration. Trends Biotechnol, 15(2), 45-50 [DOI] [PubMed] [Google Scholar]
- Murashige T and Skood F . 1962 A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473-497 [Google Scholar]
- Nessler CL and Mahlberg PG . 1977 Ontogeny and cytochemistry of alkaloidal vesicles in laticifers of Papaver somniferum L . (Papaveraceae) . Am. J. Bot., 64(5), 541-551 [Google Scholar]
- Nessler CL and Mahlberg PG . 1978 Laticifer ultrastructure and differentiation in seedlings of Papaver bracteatum L . Population Arya II (Papaveraceae) Am. J. Bot., 65(9), 978-983 [Google Scholar]
- Park SU and Facchini PJ . 2000. aHigh-efficiency somatic embryogenesis and plant regeneration in California poppy, Eschscholzia californica Cham. Plant Cell Rep, 19(4), 421-426 [DOI] [PubMed] [Google Scholar]
- Park SU and Facchini PJ . 2000. b Agrobacterium-mediated transformation of opium poppy, Papaver somniferum L ., via shoot organogenesis. J Plant Physiol, 157, 207-214 [Google Scholar]
- Park SU and Facchini PJ . 2000. c Agrobacterim rhizogenesmediated transformation of opium poppy, Papaver somniferum L ., and California poppy, Eschscholzia californica Cham ., root cultures. J Exp Bot, 51(347), 1005-1016 [DOI] [PubMed] [Google Scholar]
- Roberts MF, Carthy DMc, Kutchan TM and Coscia CJ . 1983 Localization of enzymes and alkaloidal metabolites in Papaver latex. Arch Biochem Biophys, 222(2), 599-609 [DOI] [PubMed] [Google Scholar]
- Rush MD, Kutchan TM and Coscia CJ . 1985 Correlation of the appearance of morphinan alkaloids and laticifer cells in germinating Papaver bracteatum seedlings. Plant Cell Report, 4(5), 237-240 [DOI] [PubMed] [Google Scholar]
- Sparkes IA, Runions1 J, Kearns1 A and Hawes C . 2006 Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nature Protocols, 1(4), 2019-2025 [DOI] [PubMed] [Google Scholar]
- Staba EJ, Zito S and Amin M . 1982 Alkaloid production from Papaver tissue cultures . J. Natural Products, 45(3), 256-262 [Google Scholar]
- Stefano D, Verena H, Rainer F and Schillberg S . 2004 Transient Gene Expression of Recombinant Terpenoid Indole Alkaloid Enzymes in Catharanthus roseus Leaves. Plant Molecular Biology Reporter, 22(1), 15-22 [Google Scholar]
- Unterlinner B, Lenz R and Kutchan TM . 1999 Molecular cloning and functional expression of codeinone reductase: the penultimate enzyme in morphine biosynthesis in the opium poppy Papaver somniferum. Plant J, 18(5), 465-475 [DOI] [PubMed] [Google Scholar]
- Weid M, Ziegler J and Kutchan TM . 2004 The roles of latex and the vascular bundle in morphine biosynthesis in the opium poppy, Papaver somniferum, Proc. Natl. Acad. Sci. USA, 101(38), 13957-13962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RD and Ellis BE . 1989 Age and tissue distribution of alkaloids in Papaver somniferum. Phytochemistry, 28(8), 2085-2088 [Google Scholar]
- Wroblewski T, Tomczak A and Michelmore R . 2005 Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnology Journal, 3(2), 259-273 [DOI] [PubMed] [Google Scholar]