Abstract
Previous studies have shown that gene therapy with inducible nitric oxide synthase (iNOS) protects against myocardial infarction at 3 days after gene transfer. However, the long-term effects of iNOS gene therapy on myocardial ischemic injury and cardiac function are unknown. To address this issue, we used a recombinant adenovirus 5 (Ad5) vector (Av3) with deletions of the E1, E2a, and E3 regions, which enables long-lasting recombinant gene expression for at least 2 mo due to lack of inflammation. Mice received intramyocardial injections in the left ventricular (LV) anterior wall of Av3/LacZ (LacZ group) or Av3/iNOS (iNOS group); 1 or 2 mo later, they were subjected to myocardial infarction (30-min coronary occlusion followed by 4 h of reperfusion). Cardiac iNOS gene expression was confirmed by immunoblotting and activity assays at 1 and 2 mo after gene transfer. In the iNOS group, infarct size (percentage of risk region) was significantly reduced (P < 0.05) both at 1 mo (24.2 ± 3.4%, n = 6, vs. 48.0 ± 3.6%, n = 8, in the LacZ group) and at 2 mo (23.4 ± 3.1%, n = 8, vs. 36.6 ± 2.4%, n = 7). The infarct-sparing effects of iNOS gene therapy were as powerful as those observed 24 h after ischemic preconditioning (23.1 ± 3.4%, n = 10). iNOS gene transfer had no effect on LV function or dimensions up to 8 wk later (echocardiography). These data demonstrate that iNOS gene therapy mediated by the Av3 vector affords long-term (2 mo) cardioprotection without inflammation or adverse functional consequences, a finding that provides a rationale for further preclinical testing of this therapy.
Keywords: nitric oxide synthase, cardiac function, mouse
Although ischemic preconditioning (PC) is known to be a powerful cardioprotective adaptation, it has not yet found clinical application. The late phase of PC would appear particularly suitable for therapeutic exploitation because it is underlain by the upregulation of cardioprotective genes, which, if expressed chronically, could conceivably induce a long-lasting PC state (3, 4). Among these genes, the inducible isoform of nitric oxide (NO) synthase (iNOS) has been studied most thoroughly. Extensive evidence over the past decade demonstrates that iNOS is an obligatory mediator of the protection afforded by late PC induced by various stimuli, including ischemia, physical exercise, and various pharmacological agents (1–3, 5). Therefore, upregulation of iNOS appears to be a final common pathway whereby diverse stimuli induce a shift of the heart to a defensive (preconditioned) phenotype. We have previously demonstrated that gene transfer of iNOS via recombinant adenovirus 5 (Ad5) effectively limits infarct size 3 days later (18), indicating that upregulation of iNOS via gene therapy is sufficient to replicate the cardioprotective effects of late PC. However, before iNOS gene therapy can be used clinically to protect the ischemic myocardium, it is important to determine whether chronic overexpression of iNOS in the myocardium is cardioprotective.
In this regard, a number of experimental and clinical results have been interpreted to suggest that enhanced production of NO via iNOS is detrimental to the myocardium (13, 20). Increased iNOS activity has been reported in ventricular tissue from patients with dilated cardiomyopathy (6). Increased iNOS activity has also been found in myocarditis, ischemic heart disease, valvular heart disease, and human cardiac allografts, and iNOS levels have been found to correlate with ventricular contractile dysfunction (8, 15, 20, 22). Mungrue et al. (19) reported that chronic cardiac-specific upregulation of iNOS in transgenic mice led to increased production of peroxynitrite, which was associated with cardiac hypertrophy and dilatation. In contrast, Heger et al. (16) found that cardiac-specific over-expression of iNOS at higher levels than those of Mungrue et al. (19) resulted in a relatively normal cardiac phenotype. Therefore, the effects of chronic upregulation of iNOS on the myocardium remain controversial, although many investigators believe that it may have adverse effects on myocardial viability and function.
The goal of the present study was to determine whether long-term (up to 2 mo) overexpression of iNOS confers protection against myocardial infarction in mice. We used a well-established murine model of myocardial infarction (11, 12) and the same recombinant Ad5 vector (Av3) employed in our previous study (18). Because of the deletions of the E1, E2a, and E3 regions, this vector offers the advantage of attenuated inflammatory response compared with first-generation (E1- or E1/E3-deleted) vectors (10). Our results demonstrate that the Av3 vector enables long-lasting iNOS gene expression for at least 2 mo and that this results in significant cardioprotection without adverse functional effects.
MATERIALS AND METHODS
Adenoviral vectors
Recombinant adenoviral vectors deleted in the E1, E2a, and E3 regions and carrying either a nuclear-targeted β-galactosidase reporter gene (Av3/LacZ) or the human iNOS gene (Av3/iNOS) were constructed essentially as previously described (10, 18) by homologous recombination between pAvS6/LacZ or pAvS6/iNOS and the large ClaI fragment constituting the right side of a novel Ad5 mutant that contains deletions in the E2a and E3 regions. Plaque-isolated viral clones were propagated at a high titer in an A549-derived cell line, AE1–2a, which contains the Ad5 E1 and E2a region genes, and then purified over two CsCl gradients and titered by plaque assay (10, 18).
In vivo gene transfer
Male ICR mice (10–12 wk; 37.7 ± 0.4 g) were anesthetized with pentobarbital sodium (50 mg/kg ip) and intubated. After the chest was opened through a midline sternotomy, mice received intramyocardial injections in the anterior left ventricular (LV) wall of Av3/LacZ [2 × 107 pfu (Av3/LacZ group)] or Av3/iNOS [2 × 107 pfu (Av3/iNOS group)]. One or two months later, the mice underwent the infarction protocol described below (Fig. 1). The intramyocardial injection was 10 μl in volume and was performed with a 50-μl syringe using a 30-gauge needle; each mouse received two injections in the soon-to-be-ischemic region of the LV (10, 18).
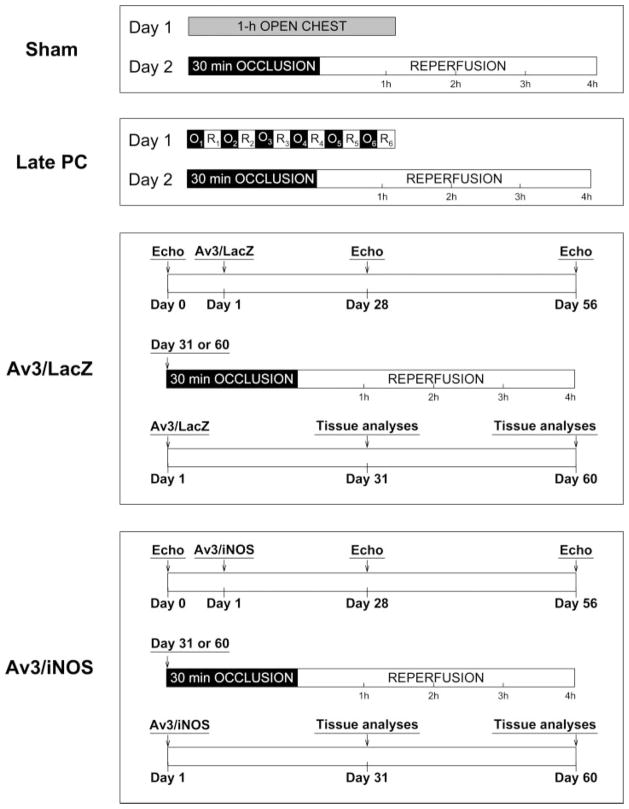
Fig. 1.
Experimental protocol. Four groups of mice were studied. Sham group underwent a thoracotomy and 1 h of open-chest state (without coronary occlusion) on day 1. The 1 h of open-chest state corresponded to the time interval necessary to perform 6 occlusion-reperfusion cycles in the late PC group; 24 h later (day 2), they underwent a 30-min coronary occlusion followed by 4 h of reperfusion. Mice in the late PC group were preconditioned with a sequence of 6 cycles of 4-min occlusion and 4-min reperfusion on day 1; on day 2, they were subjected to a 30-min coronary occlusion followed by 4 h of reperfusion. Mice in the LacZ and iNOS groups underwent serial echocardiographic studies on days 0, 28, and 56; on day 1, they were subjected to intramyocardial injections of Av3/LacZ (LacZ group) or Av3/iNOS (iNOS group), respectively; either on day 31 or on day 60, both groups underwent a 30-min coronary occlusion followed by 4 h of reperfusion for infarct size determination.
Coronary occlusion-reperfusion protocol
The murine model of myocardial ischemia and reperfusion has been described in detail (11, 12). Briefly, mice were anesthetized with pentobarbital sodium (50 mg/kg ip) and ventilated using carefully selected parameters. After administration of antibiotics, the chest was opened through a midline sternotomy, and a nontraumatic balloon occluder was implanted around the midleft anterior descending coronary artery by using an 8-0 nylon suture. To prevent hypotension, blood from a donor mouse was given at serial times during surgery (11, 12). Rectal temperature was carefully monitored and maintained between 36.7°C and 37.3°C throughout the experiment. In all groups, myocardial infarction was produced by a 30-min coronary occlusion followed by 4 h of reperfusion (Fig. 1). The sham group underwent a thoracotomy with 1 h of open-chest state without coronary occlusion-reperfusion. The late PC group was preconditioned with a sequence of six 4-min occlusion/4-min reperfusion cycles 24 h before the 30-min occlusion (Fig. 1). The LacZ and iNOS groups received intramyocardial injections of Av3/LacZ or Av3/iNOS, respectively, as described above, 1 or 2 mo before the 30-min occlusion. In all groups, successful performance of coronary occlusion and reperfusion was verified by visual inspection (i.e., by noting the development of a pale color in the distal myocardium after inflation of the balloon and the return of a bright red color due to hyperemia after deflation) and by observing S-T segment elevation and widening of the QRS on the ECG during ischemia and their resolution after reperfusion. After the coronary occlusion-reperfusion protocol was completed, the chest was closed in layers, and a small catheter was left in the thorax for 10–20 min to evacuate air and fluids. The mice were removed from the ventilator, kept warm with heat lamps, given fluids (1.0–1.5 ml of 5% dextrose in water ip), and allowed 100% oxygen via nasal cone.
Postmortem tissue analysis
At the conclusion of the study, the occluded-reperfused vascular bed and the infarct were identified by postmortem perfusion of the heart with phthalo blue dye and triphenyltetrazolium chloride (11, 12). The corresponding areas were measured by computerized videoplanimetry (Adobe Photoshop version 7.0), and from these measurements infarct size was calculated as a percentage of the region at risk (11, 12).
Echocardiography
Echocardiographic studies were performed using a HDI 5000 SonoCT ultrasound system (Philips Medical Systems, Bothell, WA) equipped with a 15- to 7-MHz linear transducer. Mice were anesthetized with isoflurane (3% induction and 1.5% maintenance). The chest was shaved, and mice were placed in a supine position. A rectal temperature probe was placed, and the body temperature was carefully maintained between 37.0°C and 37.5°C with a heating pad throughout the study. The parasternal short-axis and modified parasternal long-axis views were used to obtain two-dimensional (2-D) and M-mode images. Digital images were analyzed off-line in a blinded fashion using ProSolv (version 2.5) image analysis software (Problem Solving Concepts, Indianapolis, IN), according to the American Society of Echocardiography standards (9, 21).
Western immunoblotting analysis
Western immunoblotting analysis was performed as previously described (11, 18, 23). Briefly, 100 μg of protein were separated on an 8% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. Gel transfer efficiency was recorded carefully by making photocopies of membranes dyed with reversible Ponceau staining; gel retention was determined by Coomassie blue staining. Proteins were probed with a specific monoclonal anti-human iNOS antibody (BD Transduction Laboratories, Lexington, KY). Immunoreactive bands were visualized with horseradish peroxidase-conjugated anti-mouse IgG using an ECL detection kit (NEN), quantified by densitometry, and normalized to the Ponceau stain density. Lysates of mouse macrophages pretreated with interferon-γ and lipopolysaccharide (LPS) (Transduction Laboratories) were used as positive control for iNOS immunoblotting. In all samples, the content of iNOS protein was expressed as a percentage of the corresponding protein in the Av3/LacZ group (viral control group).
Measurement of iNOS activity
Calcium-independent NOS activity (iNOS activity) was determined by measuring the conversion of L-[14C]arginine to L-[14C]citrulline as previously described (11, 18). Briefly, isolated proteins were incubated at 37°C for 30 min with reaction buffer containing 50 mM Tris• HCl (pH 7.4), 1 mM NADPH, 5 μM flavin adenine dinucleotide, 5 μM flavin mononucleotide, 10 μM tetrahydrobiopterin, 1 mM EGTA, and purified L-[14C]arginine (348 mCi/mmol, 0.1 μCi per reaction, Amersham) without calcium and calmodulin. In all groups, duplicate assays were performed for each sample, and iNOS activity was expressed as picomoles of L-citrulline per minute per milligram protein.
Immunohistochemistry
Frozen 10-μm sections were fixed in 10% formalin for 2 min at room temperature, washed in PBS, and immunostained respectively with a monoclonal anti-human iNOS antibody (BD Transduction Laboratories) (Fig. 3), a monoclonal anti-CD4+ or anti-CD8+ antibody, and a rabbit polyclonal anti-CD25+ antibody (Santa Cruz Biotechnology) (Fig. 4) by using the ABC kit (Vector Laboratories). Alternate sections were incubated in the absence of the primary antibody (negative control) as previously reported (18).
Fig. 3.
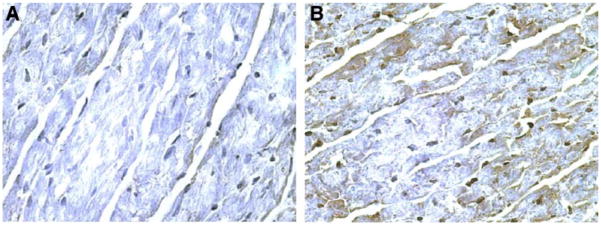
Expression of the iNOS gene 2 mo after gene transfer. Hearts transfected with Av3/LacZ (A, ×400) or Av3/iNOS (B, ×400), respectively, are shown. Robust expression of iNOS (brown stain) was observed in the transfected region of the anterior left venticular (LV) wall 2 mo after gene transfer (n = 3).
Fig. 4.
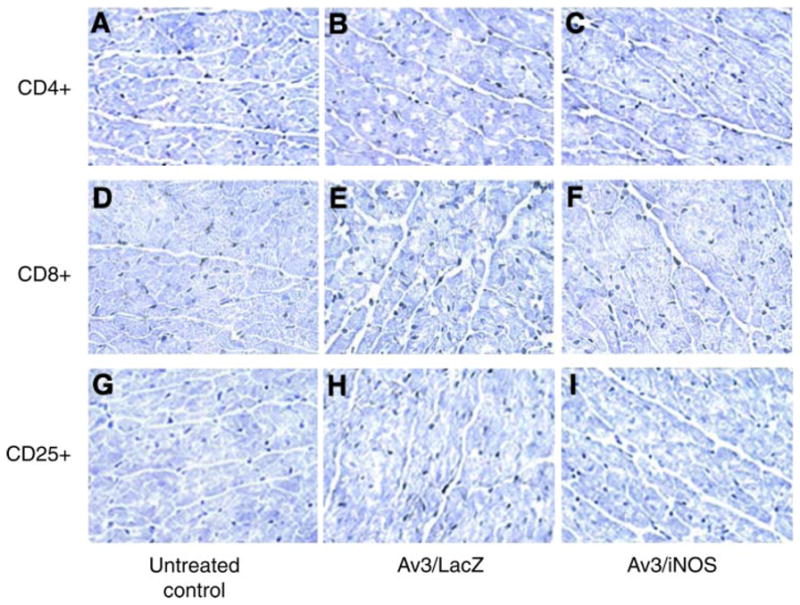
Immunocytochemistry for immune cell markers 2 mo after Av3-mediated gene transfer. In hearts transfected with either Av3/LacZ or Av3/iNOS, few T lymphocytes (CD4+ cells, B and C, ×400; CD8+ cells, E and F, ×400) and interleukin-2 receptor-positive activated T lymphocytes (CD25+ cells, H and I, ×400) were observed in the pericardiomyocyte space, which was not different from the untreated control hearts (A, D, and G, ×400) (n = 3 in each group).
Statistical analysis
Data are reported as means ± SE. Measurements were analyzed by either one-way or two-way repeated-measures ANOVA, as appropriate, followed by unpaired Student’s t-tests with the Bonferroni correction. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using the SAS software system.
RESULTS
iNOS gene expression
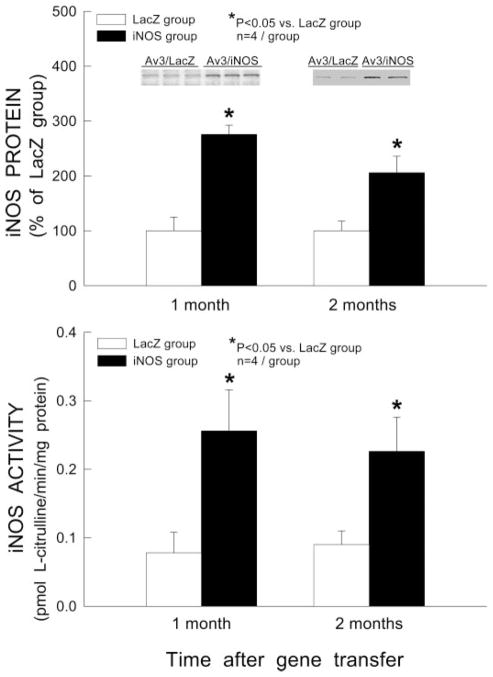
One or two months after intramyocardial injection of Av3/LacZ (LacZ group) or Av3/iNOS (iNOS group), the myocardium surrounding the sites of injection (~20 mg) was harvested for measurement of iNOS protein expression and iNOS activity (each heart received two injections). Western immunoblotting analysis demonstrated that myocardial iNOS expression was significantly increased by iNOS gene transfer at both 1 mo (2.8-fold) and 2 mo (2.1-fold) compared with mice transfected with reporter virus (Av3/LacZ) (Fig. 2, top; P < 0.05). iNOS activity was also significantly increased in mice transfected with Av3/iNOS (3.3-fold at 1 mo and 2.5-fold at 2 mo vs. the corresponding Av3/LacZ group, P < 0.05; Fig. 2, bottom), indicating that the transferred gene expresses functional competent protein for at least 2 mo. Consistent with iNOS immunoblotting and activity measurements, immunohistochemistry demonstrated robust iNOS immunoreactivity in the myocytes of hearts transfected with Av3/iNOS at 2 mo after gene transfer (Fig. 3B). No iNOS immunoreactivity was noted in sections incubated with non-immune serum (not shown). Immunocytochemistry for CD4+ and CD8+ (markers of T lymphocytes) and CD25+ (IL-2 receptor-positive activated T lymphocytes) revealed no evidence of inflammation around the myocytes transduced with Av3/iNOS (Fig. 4).
Fig. 2.
iNOS gene expression 1 and 2 mo after Av3-mediated gene transfer. Top: Western immunoblots and densitometric analyses of iNOS protein signals in the transduced myocardium. Bottom: total calcium-independent NOS (iNOS) activity in the transduced myocardium. Data are means ± SE.
Effects of iNOS gene transfer on cardiac function
Cardiac function was assessed by 2-D echocardiography at baseline and at 4 and 8 wk after gene transfer. As shown in Table 1, there were no appreciable changes in LV end-diastolic or end-systolic diameter, in LV fractional shortening, or in LV ejection fraction at either time point after iNOS gene transfer.
Table 1.
Echocardiographic parameters in response to long-term iNOS gene therapy
| n | Av3/LacZ
|
Av3/iNOS
|
||||
|---|---|---|---|---|---|---|
| Baseline | 4 Wk | 8 Wk | Baseline | 4 Wk | 8 Wk | |
| 7 | 5 | 5 | 8 | 8 | 8 | |
| LVEDD, mm | 4.31±0.08 | 4.27±0.08 | 4.37±0.11 | 4.19±0.03 | 4.14±0.07 | 4.10±0.09 |
| LVESD, mm | 2.81±0.09 | 2.73±0.14 | 2.81±0.13 | 2.59±0.03 | 2.52±0.09 | 2.55±0.13 |
| FS, % | 34.0±1.5 | 36.0±3.5 | 35.7±2.3 | 38.5±0.9 | 39.3±1.3 | 38.2±2.3 |
| EF, % | 57.5±1.3 | 58.5±4.7 | 58.4±3.2 | 61.8±1.2 | 62.9±1.7 | 60.9±2.9 |
| HR, beats/min | 502±18 | 513±32 | 466±17 | 562±19 | 534±15 | 504±22 |
Data are means ± SE; n, number of mice. Av3, recombinant adenovirus 5 vector deleted in the E1, E2a, and E3 regions; iNOS, inducible nitric oxide synthase; LVEDD, left ventricular (LV) end-diastolic diameter; LVESD, LV end-systolic diameter; FS, fractional shortening; EF, ejection fraction; HR, heart rate.
Infarct size
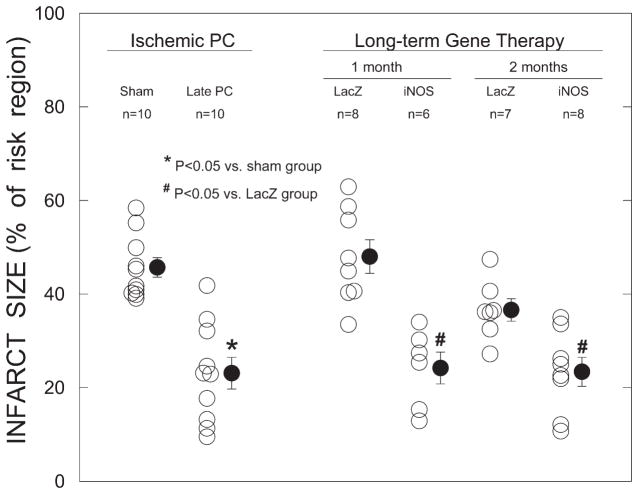
To characterize the efficacy of iNOS gene therapy, the infarct-sparing effects of Av3/iNOS gene transfer were compared with those of the late phase of ischemic PC. When mice were preconditioned with six cycles of 4-min coronary occlusion/4-min reperfusion 24 h before the 30-min coronary occlusion, infarct size was reduced by an average of 49.5% versus sham preconditioned mice, indicating a late PC effect (Fig. 5). In mice given Av3/LacZ, infarct size did not differ from that measured in mice undergoing sham PC, at either 1 mo (48.0 ± 3.6% of the risk region, n = 8) or 2 mo (36.6 ± 2.4% of the risk region, n = 7) (Fig. 5). However, in mice transduced with Av3/iNOS, infarct size was smaller than in the control virus group (Av3/LacZ group) at both time points (24.2 ± 3.4% of the risk region at 1 mo, n = 6, and 23.4 ± 3.1% of the risk region at 2 mo, n = 8) (Fig. 5), demonstrating that the expression of iNOS was associated with long-term cardioprotection. The average infarct size observed in the Av3/iNOS group was similar to that observed in the late PC group (Fig. 5).
Fig. 5.
Myocardial infarct size expressed as a percentage of the region at risk. Open circles, individual mice; solid circles, means ± SE.
DISCUSSION
Although previous studies have shown that iNOS gene therapy protects the heart against ischemia-reperfusion injury in the short term (at 3 days) (18), the long-term effects are unknown. The present study demonstrates that transfer of the iNOS gene via the Av3 vector, a vector with deletions of the E1, E2a, and E3 regions, results in expression of the transgenic protein for at least 2 mo, with no immune response and no adverse effect on cardiac function. The upregulation of iNOS expression is associated with a reduction in infarct size for at least 2 mo after gene transfer. These results suggest that iNOS gene therapy may be a potentially useful therapeutic approach to ischemic heart disease.
Replication-deficient Ad5 vectors offer a number of advantages for gene transfer, including low pathogenicity, the capacity to transfect nonproliferating cells, the ability to accommodate relatively large segments of DNA, the fact that they can be prepared at higher titers than other vital vectors, and increased stability. The major problem with the first-generation Ad5 vectors (deleted only in the E1 and/or E3 regions) has been the short duration of gene expression and the strong inflammatory response that occurs after gene transfer in vivo. Studies of the immunopathogenesis of Ad5 vectors have revealed that the adenoviral gene products trigger an immune response in which CD4+, CD8+, and CD25+ cells are important components (14, 17). For this reason, we utilized an Ad5 vector with deletions of E1, E2a, and E3 regions (Av3). In agreement with previous studies (7, 10), we found that at 2 mo after gene transfer, the levels of CD4+, CD8+, and CD25+ cells in the area of gene transfer (as assessed by immunocytochemistry) were not increased compared with the untreated control hearts. These data indicate that the Av3 vector does not elicit an inflammatory response, which may be the major reason for its ability to direct prolonged transgene expression for at least 2 mo. In addition, serial echocardiographic measurements of cardiac function showed no evidence of an adverse effect of iNOS gene transfer on LV dimensions or contractility up to 8 wk. These results, coupled with the fact that the cardioprotection persisted for 2 mo, suggest that the Av3 vector may have potential clinical utility. Whereas additional studies are needed with longer follow-up, the present findings provide a rationale for pursuing preclinical testing of iNOS gene therapy against myocardial infarction.
Acknowledgments
The expert technical assistance of Xiaoping Zhu is gratefully acknowledged.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grants R01-HL-55757, HL-70897, HL-76794, and HL-78825.
References
- 1.Arnaud C, Godin-Ribuot D, Bottari S, Peinnequin A, Joyeux M, Demenge P, Ribuot C. iNOS is a mediator of the heat stress-induced preconditioning against myocardial infarction in vivo in the rat. Cardiovasc Res. 2003;58:118–125. doi: 10.1016/s0008-6363(02)00812-x. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R, Dawn B, Tang XL, Qiu Y, Ping P, Xuan YT, Jones WK, Takano H, Guo Y, Zhang J. The nitric oxide hypothesis of late preconditioning. Basic Res Cardiol. 1998;93:325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Belder AJ, Radomski MW, Why HJ, Richardson PJ, Bucknall CA, Salas E, Martin JF, Moncada S. Nitric oxide synthase activities in human myocardium. Lancet. 1993;341:84–85. doi: 10.1016/0140-6736(93)92559-c. [DOI] [PubMed] [Google Scholar]
- 7.Engelhardt JF, Ye X, Doranz B, Wilson JM. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreiro CR, Chagas AC, Carvalho MH, Dantas AP, Scavone C, Souza LC, Buffolo E, da Luz PL. Expression of inducible nitric oxide synthase is increased in patients with heart failure due to ischemic disease. Braz J Med Biol Res. 2004;37:1313–1320. doi: 10.1590/s0100-879x2004000900005. [DOI] [PubMed] [Google Scholar]
- 9.Gardin JM, Siri FM, Kitsis RN, Edwards JG, Leinwand LA. Echocardiographic assessment of left ventricular mass and systolic function in mice. Circ Res. 1995;76:907–914. doi: 10.1161/01.res.76.5.907. [DOI] [PubMed] [Google Scholar]
- 10.Gorziglia MI, Kadan MJ, Yei S, Lim J, Lee GM, Luthra R, Trapnell BC. Elimination of both E1 and E2 from adenovirus vectors further improves prospects for in vivo human gene therapy. J Virol. 1996;70:4173–4178. doi: 10.1128/jvi.70.6.4173-4178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 1998;275:H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habib FM, Springall DR, Davies GJ, Oakley CM, Yacoub MH, Polak JM. Tumour necrosis factor and inducible nitric oxide synthase in dilated cardiomyopathy. Lancet. 1996;347:1151–1155. doi: 10.1016/s0140-6736(96)90610-8. [DOI] [PubMed] [Google Scholar]
- 14.Harvey BG, Worgall S, Ely S, Leopold PL, Crystal RG. Cellular immune responses of healthy individuals to intradermal administration of an E1–E3-adenovirus gene transfer vector. Hum Gene Ther. 1999;10:2823–2837. doi: 10.1089/10430349950016555. [DOI] [PubMed] [Google Scholar]
- 15.Haywood GA, Tsao PS, von der Leyen HE, Mann MJ, Keeling PJ, Trindade PT, Lewis NP, Byrne CD, Rickenbacher PR, Bishopric NH, Cooke JP, McKenna WJ, Fowler MB. Expression of inducible nitric oxide synthase in human heart failure. Circulation. 1996;93:1087–1094. doi: 10.1161/01.cir.93.6.1087. [DOI] [PubMed] [Google Scholar]
- 16.Heger J, Godecke A, Flogel U, Merx MW, Molojavyi A, Kuhn-Velten WN, Schrader J. Cardiac-specific overexpression of inducible nitric oxide synthase does not result in severe cardiac dysfunction. Circ Res. 2002;90:93–99. doi: 10.1161/hh0102.102757. [DOI] [PubMed] [Google Scholar]
- 17.Kajiwara K, Byrnes AP, Charlton HM, Wood MJ, Wood KJ. Immune responses to adenoviral vectors during gene transfer in the brain. Hum Gene Ther. 1997;8:253–265. doi: 10.1089/hum.1997.8.3-253. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Guo Y, Xuan YT, Lowenstein CJ, Stevenson SC, Prabhu SD, Wu WJ, Zhu Y, Bolli R. Gene therapy with inducible nitric oxide synthase protects against myocardial infarction via a cyclooxygenase-2-dependent mechanism. Circ Res. 2003;92:741–748. doi: 10.1161/01.RES.0000065441.72685.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mungrue IN, Gros R, You X, Pirani A, Azad A, Csont T, Schulz R, Butany J, Stewart DJ, Husain M. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 2002;109:735–743. doi: 10.1172/JCI13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoh M, Nakamura M, Tamura G, Makita S, Segawa I, Tashiro A, Satodate R, Hiramori K. Inducible nitric oxide synthase and tumor necrosis factor-alpha in myocardium in human dilated cardiomyopathy. J Am Coll Cardiol. 1997;29:716–724. doi: 10.1016/s0735-1097(96)00567-0. [DOI] [PubMed] [Google Scholar]
- 21.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 22.Wildhirt SM, Weis M, Schulze C, Conrad N, Pehlivanli S, Rieder G, Enders G, von Scheidt W, Reichart B. Expression of endomyocardial nitric oxide synthase and coronary endothelial function in human cardiac allografts. Circulation. 2001;104:I336–I343. doi: 10.1161/hc37t1.094598. [DOI] [PubMed] [Google Scholar]
- 23.Xuan YT, Guo Y, Zhu Y, Han H, Langenbach R, Dawn B, Bolli R. Mechanism of cyclooxygenase-2 upregulation in late preconditioning. J Mol Cell Cardiol. 2003;35:525–537. doi: 10.1016/s0022-2828(03)00076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]