Abstract
Beta-catenin-dependent TCF/LEF (T-cell factor/lymphocyte enhancing factor) is known to be mechanosensitive and an important regulator for promoting bone formation. However, the functional connection between TCF/LEF activity and Rho family GTPases is not well understood in osteoblasts. Herein we investigated the molecular mechanisms underlying oscillatory shear stress-induced TCF/LEF activity in MC3T3-E1 osteoblast cells using live cell imaging. We employed fluorescence resonance energy transfer (FRET)-based and green fluorescent protein (GFP)-based biosensors, which allowed us to monitor signal transduction in living cells in real time. Oscillatory (1 Hz) shear stress (10 dynes/cm2) increased TCF/LEF activity and stimulated translocation of β-catenin to the nucleus with the distinct activity patterns of Rac1 and Cdc42. The shear stress-induced TCF/LEF activity was blocked by the inhibition of Rac1 and Cdc42 with their dominant negative mutants or selective drugs, but not by a dominant negative mutant of RhoA. In contrast, constitutively active Rac1 and Cdc42 mutants caused a significant enhancement of TCF/LEF activity. Moreover, activation of Rac1 and Cdc42 increased the basal level of TCF/LEF activity, while their inhibition decreased the basal level. Interestingly, disruption of cytoskeletal structures or inhibition of myosin activity did not significantly affect shear stress-induced TCF/LEF activity. Although Rac1 is reported to be involved in β-catenin in cancer cells, the involvement of Cdc42 in β-catenin signaling in osteoblasts has not been identified. Our findings in this study demonstrate that both Rac1 and Cdc42 GTPases are critical regulators in shear stress-driven β-catenin signaling in osteoblasts.
Keywords: fluorescence resonance energy transfer (FRET), MC3T3-E1, mechanical loading, mechanotransduction, Rho family GTPases, TCF/LEF
1. Introduction
Mechanical loading of bone is known to influence bone growth and remodeling [1,2]. Application of physiological loading to bone accelerates bone formation and fracture healing, whereas removal of loading results in bone loss [3,4]. The process of growth and remodeling of bone involves the coordinated activity of two types of cells present in bone: bone-forming osteoblasts and bone-resorbing osteoclasts. In osteoblasts, β-catenin-dependent Wnt signaling is known to be one of the important regulators to promote bone formation [5] and its activation is regulated by mechanical loading including fluid flow-induced shear stress [6,7] and biaxial stretching [8]. In response to mechanical loading, β-catenin in the cytoplasm is stabilized by the inactivation of a destruction complex such as axin and GSK3β (glycogen synthase kinase 3β) and translocated to the nucleus. The β-catenin in the nucleus associates with TCF/LEF (T-cell factor/lymphocyte enhancing factor) transcription factors, leading to the activation of TCF/LEF and induction of expression of Wnt target genes [9]. Although the regulatory role of mechanical loading in β-catenin signaling activity is well defined, the precise mechanism by which how β-catenin signaling interacts with other mechanoresponsive signaling proteins, such as Rho family GTPases, is not clearly understood.
The Rho family GTPases act as a molecular switch that regulates fundamental processes including morphogenesis, polarity, movement, and cell division [10]. They present an active (GTP-bound) and inactive (GDP-bound) states, which are controlled by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). RhoA, Rac1, and Cdc42 are most well studied members of the GTP-binding proteins and are known to play distinctive roles in regulating actin cytoskeletal dynamics of stress fibers, lamellipodia, and filopodia, respectively [11]. Accumulating evidence indicates that they respond to mechanical loading in various cell types, including osteoblasts [12], smooth muscle cells [13], endothelial cells [14], and mesenchymal progenitor cells [15]. Moreover, the Rho family GTPases are involved in bone resorption by regulating osteoclast functions [16,17,18].
Here we report, for the first time, that Rac1 and Cdc42 are critical components of β-catenin signaling in shear stress-induced osteoblasts. Since the activities of these GTPases can be rapid and transient [13,19], we employed fluorescence resonance energy transfer (FRET) technique and live cell imaging approach to capture their dynamic patterns. We applied oscillatory fluid flow-induced shear stress to MC3T3-E1 cells and imaged the activities of the individual GTPases and TCF/LEF as well as β-catenin translocation at high spatiotemporal resolution. To examine the role of the individual GTPases in mediating shear stress-induced TCF/LEF activity, we used constitutively active and dominant negative GTPase mutants as well as GTPase-specific pharmacological drugs. The roles for cytoskeletal structures and myosin activity in shear-stress-induced TCF/LEF activity were also examined.
2. Materials and methods
2.1. Rho family GTPase biosensors and mutants
FRET-based GTPase biosensors for monitoring Rac1, Cdc42, and RhoA were used [20,21]. Each biosensor contains two fluorescent proteins, cyan fluorescent protein (CFP) as the FRET donor and yellow fluorescent protein (YFP) as the FRET acceptor. One of the GTPases (Rac1, Cdc42, or RhoA) and a binding domain of an effector protein for the specific GTPases (p21 protein-activated kinase 1 for Rac1 or Cdc42, and protein kinase N for RhoA) are inserted between the CFP and YFP. An activated GTPase promotes the intramolecular binding of the GTPase to a specific binding within the biosensor, which results in the close association of CFP with YFP, and an increase of FRET from CFP to YFP. The YFP/CFP emission ratio was used as a measure of the specific GTPase activity. The biosensors have been well characterized in terms of its specificity [12,13,20,21]. As GTPase mutants, constitutively active Rac1 (Rac1-L61) and Cdc42 (Cdc42-L61), as well as dominant negative Rac1 (Rac1-N17), Cdc42 (Cdc42-N17), and RhoA (RhoA-N19) were used [22,23].
2.2. TCF/LEF reporter and β-catenin probe
A TCF/LEF-green fluorescent protein (GFP) reporter (SA Biosciences, Valencia, CA, USA) was used to monitor the activity of β-catenin signaling since β-catenin activation is known to lead to TCF/LEF transcriptional activation [24]. The TCF/LEF reporter utilizes the inducible transcription factor-responsive GFP and increases the GFP intensity level in the cytoplasm when activated β-catenin forms a complex with TCF/LEF transcription factors. Enhanced GFP (EGFP)-β-catenin fusion proteins were used to monitor translocation of β-catenin [25].
2.3. Cell culture and transfection
MC3T3-El osteoblasts were used for this study (ATCC, Manassas, VA, USA). The cells were cultured in minimum essential alpha medium (αMEM; Invitrogen, Grand Island, NY, USA) containing 10% FBS (Invitrogen) and antibiotics (50 units/ml penicillin and 50 μg/ml streptomycin; Lonza, Basel, Switzerland). Prior to experiments, the cells were maintained at 37°C and 5% CO2 in a humidified incubator. The DNA plasmids were transfected into the cells using a Neon transfection system (Invitrogen). After transfection, the cells were transferred to a type I collagen-coated μ-slide cell culture chamber (Ibidi, Martinsried, Germany) and incubated in αMEM containing 0.5% FBS for 24–36 h before imaging experiments.
2.4. Pharmacological drugs
NSC 23766 (50 μM; Tocris Bioscience, Bristol, UK) was used to prevent Rac1 activation. ML141 (10 μM; Tocris Bioscience) was used to inhibit Cdc42 activity. Rac/Cdc42 activator II (100 ng/ml) was from Cytoskeleton (Denver, CO, USA). XAV939 (1 mM; R&D Systems, Minneapolis, MN, USA) was used to inhibit β-catenin signaling. Cytochalasin D (1 μg/ml; Enzo Life Sciences, Farmingdale, NY, USA) was used to disrupt actin filaments. Nocodazole (1 μM; Sigma-Aldrich, St. Louis, MO, USA) was used to disrupt microtubules. Blebbistatin (50 μM; Toronto Research Chemicals, Toronto, Ontario, Canada) was used to specifically inhibit myosin II activity.
2.5. Shear stress application
To apply oscillatory (1 Hz) flow to the MC3T3-E1 cells, an Osci-Flow controller (Flexcell International, Hillsborough, NC, USA) was used. Shear stress of 10 dyn/cm2 was applied to the cells grown in the μ-slide cell culture chamber (Ibidi) by controlling the flow rate of a peristaltic pump (Cole-Parmer, Vernon Hills, IL, USA). Two pulse dampeners (Cole-Parmer) were connected to both the inlet and outlet of the cell culture chamber to minimize pulsation of the flow. During flow experiments, the flow system was perfused with HEPES-buffered (20 mM) αMEM without serum to maintain the pH at 7.4. The flow experiments were conducted at 37°C.
2.6. Microscopy and image analysis
A Nikon Ti-E inverted microscope equipped with a charge-coupled device (CCD) camera (Evolve 512; Photometrics, Tucson, AZ, USA) and a filter wheel controller (Sutter Instruments, Novato, CA, USA) was used for imaging experiments. The following filter sets (Semrock, Rochester, NY, USA) were used: CFP excitation: 438/24 (center wavelength/bandwidth in nm); CFP emission: 483/32; YFP (FRET) emission: 542/27; GFP excitation: 472/30; GFP emission: 520/35. To minimize photobleaching, a neutral density (ND) 64 filter (~1.5% transmittance) was used during imaging experiments. Time-lapse images were acquired with a 40X (0.75 numerical aperture) objective at an interval of 2 min or 10 min. FRET images for GTPase activity was generated with NIS-Elements software (Nikon) by computing an emission ratio of YFP/CFP for individual cells. The FRET ratio images were scaled according to the color bar. The GFP images for TCF/LEF activity and β-catenin localization were background-subtracted and the fluorescence intensity was averaged over the whole cell area or over the nucleus by using NIS-Elements software (Nikon). The GFP images of β-catenin were scaled according to the color bar as described previously [25].
2.7. Statistical analysis
All statistical data were analyzed using Prism 5 software (GraphPad Software, La Jolla, CA, USA) and presented as the mean ± standard error of the mean (SEM). One-way ANOVA followed by Dunnett’s test was used for multiple comparisons with a control group. Student’s t-test was used to compare the difference between two groups.
3. Results
3.1. Fluid flow-induced shear stress increases TCF/LEF activity
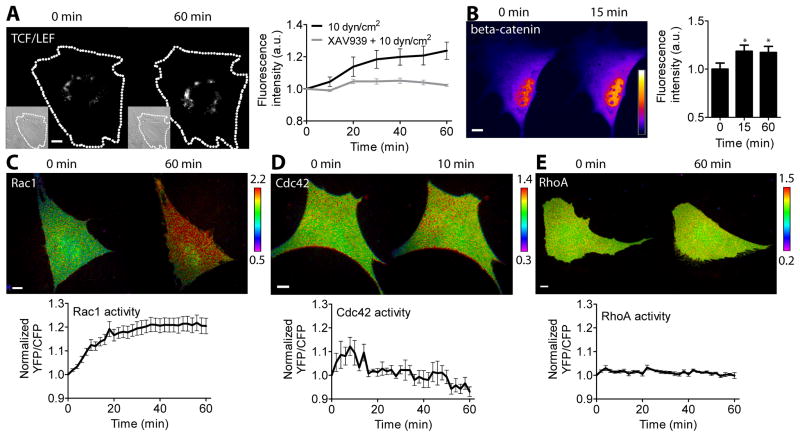
To study the potential role of Rho family GTPases in TCF/LEF activity under flow-induced shear stress, we first evaluated the effect of shear stress in regulating TCF/LEF activity. We transfected MC3T3-E1 cells with a TCF/LEF reporter and conducted real-time monitoring of its activity while applying oscillatory (1 Hz) shear stress of 10 dyn/cm2, which is known to be within the physiological range subjected in bone [26]. In response to shear stress, the TCF/LEF activity, which is evidenced by the changes in GFP intensity of the TCF/LEF reporter within the cell, was increased and maintained a sustained level during shear stress application for 1 hour (Fig. 1A). This result is consistent with previous reports demonstrating the nuclear translocation of β-catenin in response to shear stress [6,7]. We also conducted static control experiments without applying shear stress and observed that static incubation did not increase TCF/LEF activity as expected (data not shown). To test the specificity of the TCF/LEF reporter, we used XAV939, a drug specific to β-catenin signaling by inhibiting tankyrases and stabilizing axin, which in turn stabilizes β-catenin destruction complex and selectively inhibits β-catenin signaling [27]. XAV939 treatment completely blocked shear stress-induced TCF/LEF activity (Fig. 1A). To confirm the effect of shear stress on β-catenin signaling activity, we transfected the cells with an EGFP-β-catenin fusion protein and monitored its activity under shear stress. Within 15 min of shear stress application, the nuclear became brighter, reflecting the increased localization of β-catenin in the nucleus (Fig. 1B). This increased β-catenin nuclear localization was sustained during 1 hour of shear stress application.
Fig. 1.
Effect of oscillatory fluid flow shear stress on TCF/LEF activity, β-catenin nuclear localization, and Rho family GTPase activity. (A) TCF/LEF activity under shear stress. Dashed lines indicate cell boundaries obtained from the corresponding DIC images (inlets). Fluorescence intensities of the TCF/LEF reporter were normalized to those at 0 min. n = 7 (10 dyn/cm2, black line), and 6 (XAV939 + 10 dyn/cm2, gray line). (B) β-catenin nuclear localization under flow. Color bar represents fluorescence intensity of the EGFP-β-catenin probe. Bar graphs represent relative fluorescence intensities in the nucleus. * p < 0.05. n = 7. (C–E) Cells were transfected with the GTPase biosensors and subjected to shear stress. Color bars represent YFP/CFP emission ratios of the biosensors. The time courses of the emission ratios were normalized to time 0. (C) Rac1 activity. n = 14. (D) Cdc42 activity. n = 6. (E) RhoA activity. n = 8. Scale bars = 10 μm.
3.2. Rac1, Cdc42, and RhoA are differentially regulated by shear stress
Next, we evaluated the role of shear stress in regulating Rho family GTPase activity. We transfected the cells with one of the Rac1, Cdc42, or RhoA biosensors and apply the oscillatory (1 Hz) shear stress at 10 dyn/cm2 for 1 hour. The GTPase activity was determined by the YFP/CFP emission ratio of the GTPase biosensor within the individual cell. In response to shear stress, there was a significant increase in Rac1 and Cdc42 activity (Fig. 1C–D), whereas RhoA activity was not significantly increased (Fig. 1E). Interestingly, Rac1 and Cdc42 displayed distinct dynamics of activation under flow. In response to shear stress application Rac1 activity was gradually increased until it was maximal at ~ 30 min and sustained, but Cdc42 activity was quickly increased within 2 min, maximal at 8 min, and returned to its basal level at 16 min, suggesting that Cdc42 activation might precede Rac1 activation in response to shear stress.
3.3. Inhibition of Rac1 and Cdc42 blocks shear stress-induced TCF/LEF activity
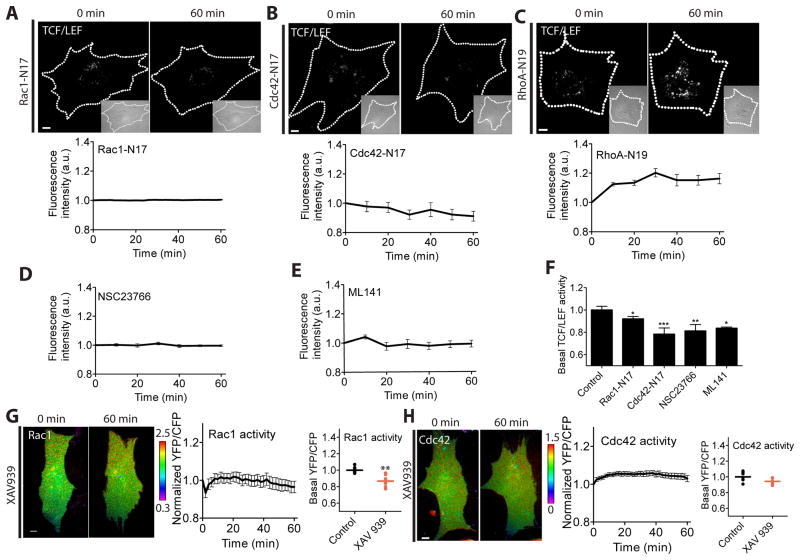
To examine the role of individual GTPases in shear stress-induced TCF/LEF activity, we co-transfected MC3T3-E1 cells with one of the dominant negative GTPase mutants and a TCF/LEF reporter. The dominant negative Rac1 (Rac1-N17) and Cdc42 (Cdc42-N17) prevented the induction of TCF/LEF by shear stress, whereas the dominant negative RhoA (RhoA-N19) did not have a significant effect (Fig. 2A–C). Inhibition of Rac1 or Cdc42 using pharmacological drugs specific to Rac1 (NSC23766) [28] and Cdc42 (ML141) [29] also inhibited shear stress-induced TCF/LEF activity (Fig. 2D–E). Moreover, the basal level of TCF/LEF activity before applying shear stress (at time zero) was significantly decreased by the expression of Rac1-N17 and Cdc42-N17 or inhibitor treatments as compared to the non-treated control cells (Fig. 2F), indicating that endogenous Rac1 and Cdc42 contribute to TCF/LEF responses to shear stress. We also observed that treatment of XAV939 prevented shear stress-induced Rac1 and Cdc42 activity (Fig. 2G–H), consistent with previous reports showing the Wnt/β-catenin signaling-triggered Rac1 and Cdc42 activation [30]. Together, these results suggest that Rac1 and Cdc42 are required for shear stress-induced TCF/LEF activity in MC3T3-E1 cells.
Fig. 2.
Rac1 and Cdc42 are required for shear stress-induced TCF/LEF activity. (A–C) TCF/LEF activity in cells expressing Rac1-N17 (n = 16), Cdc42-N17 (n = 14), or RhoA-N19 (n = 8). Cells were co-transfected with a TCF/LEF reporter and one of Rac1-N17, Cdc42-N17, or RhoA-N19. (D–E) TCF/LEF activity in cells pretreated with Rac1 inhibitor (NSC23766, n = 7) or Cdc42 inhibitor (ML141, n = 12). (F) Basal levels of TCF/LEF activity. Data were normalized and compared to a control group. * p < 0.05, ** p < 0.01, *** p < 0.001. (G–H) Cells were transfected with Rac1 or Cdc42 biosensor and treated with XAV939. During imaging, the cells were subjected to shear stress. (G) Rac1 activity and its basal levels. n = 7. ** p < 0.01 compared to control. (H) Cdc42 activity and its basal levels. n = 7. Scale bars = 10 μm.
3.4. Activated Rac1 and Cdc42 enhances shear stress-induced TCF/LEF activity
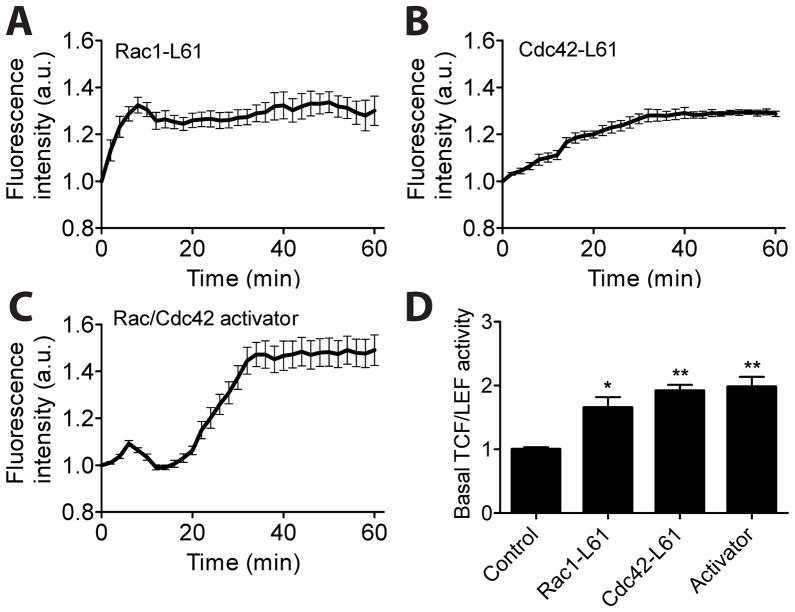
To further explore the involvement of Rac1 and Cdc42 in TCF/LEF activity by flow shear stress, we co-transfected MC3T3-E1 cells with one of constitutively active mutants and a TCF/LEF reporter. The data revealed that both constitutively active Rac1 (Rac1-L61) and Cdc42 (Cdc42-L61) significantly enhanced TCF/LEF activity in response to shear stress (compare Fig. 3A-B and Fig. 1A). To test further the roles of Rac1 and Cdc42 in TCF/LEF activity, we pre-treated MC3T3-E1 cells with Rac/Cdc42 activator II, and monitored TCF/LEF activity under shear stress. The Rac/Cdc42 activator II-treated cells exhibited a marked increase in TCF/LEF activity by shear stress (~50% at 60 min as compared to ~30% in Rac1-L61 or Cdc42-L61 transfected cells), possibly due to the additional activation of other Rac GTPase members such as Rac2 and Rac3 by the drug (Fig. 3C). Activation of Rac1 and Cdc42 also significantly increased the basal activity of TCF/LEF by ~2 fold (Fig. 3D). These results together with those of Rac1 or Cdc42 inhibition (see Fig. 2) strongly suggest that both Rac1 and Cdc42 are critical mediators of shear stress-induced TCF/LEF activity in MC3T3-E1 cells.
Fig. 3.
Elevated activation of Rac1 or Cdc42 enhances shear stress-induced TCF/LEF activity. (A–B) TCF/LEF activity in cells expressing Rac1-L61 (n = 9) or Cdc42-L61 (n = 7). Cells were co-transfected with a TCF/LEF reporter and one of Rac1-L61 or Cdc42-L61. (C) TCF/LEF activity in cells pretreated with Rac/Cdc42 activator (n = 9). (D) Basal levels of TCF/LEF activity. Data were normalized and compared to a control group. * p < 0.05, ** p < 0.01.
3.5. Inhibition of cytoskeletal structures does not effectively alter shear stress-induced TCF/LEF activity
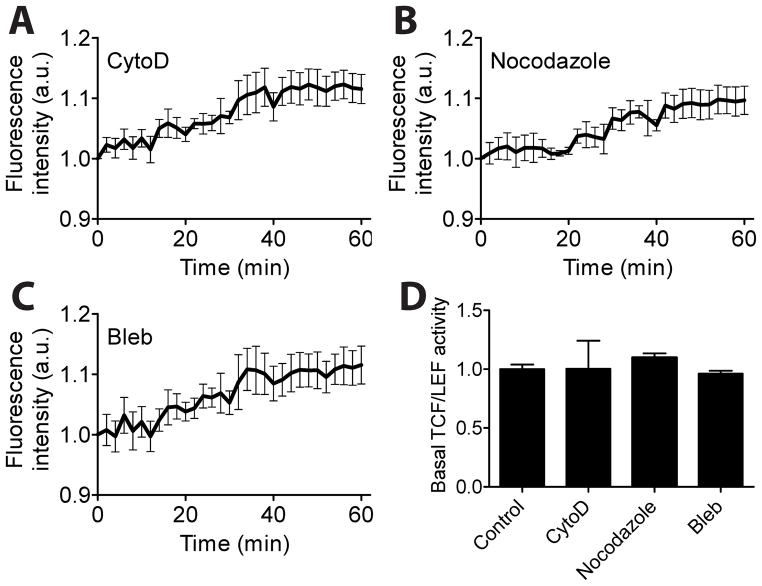
Rac1 and Cdc42 are known to regulate cytoskeletal structures, specifically lamellipodia, and filopodia, respectively [11]. To examine the role of cytoskeletal structures in shear stress-induced TCF/LEF activity, we transfected MC3T3-E1 cells with a TCF/LEF reporter and treated with one of the drugs that specifically disrupt or inhibit actin filaments (cytochalasin D), microtubules (nocodazole), or myosin II activity (blebbistatin). Both disruption of cytoskeleton and inhibition of myosin activity did not effectively block shear stress-induced TCF/LEF activity (compare Fig. 4A–C and Fig. 1A). The treatment with these drugs also did not significantly alter the basal TCF/LEF activation level (Fig. 4D). These results suggest that cytoskeletal structures and myosin activity may not be the major contributor to shear stress-induced TCF/LEF activity in MC3T3-E1 cells.
Fig. 4.
Cytoskeletal integrity and its contractile activity do not effectively control shear stress-induced TCF/LEF activity. (A–C) TCF/LEF activity in cells treated with cytochalasin D (CytoD, n = 6), nocodazole (n = 5), or blebbistatin (Bleb, n = 6). (D) Basal levels of TCF/LEF activities in the drug-treated cells.
4. Discussion
By using live cell imaging with FRET-based GTPase biosensors and a GFP-based TCF/LEF reporter and β-catenin probe, we have shown that fluid flow shear stress induces dynamic changes in Rho family GTPases as well as TCF/LEF with the distinct activity patterns in MC3T3-E1 cells. Furthermore, our study using Rho family GTPase mutants and pharmacological drugs revealed that Rac1 and Cdc42 GTPases mediate shear stress-induced TCF/LEF activity. Our findings thus suggest that Rac1 and Cdc42 activity may play roles in regulating TCF/LEF-mediated transcription process and consequently contribute to load-induced bone repair and remodeling.
Mechanotransduction in bone has been implicated in maintaining the dynamic balance between bone formation and bone resorption [1,2]. The mechanotransduction process is mediated by various molecules including integrins, kinases, GTPases, intracellular ions, prostaglandin and nitric oxide signaling as well as β-catenin-dependent Wnt signaling [2,31,32]. We have previously shown that Src kinase as well as Rac1 and RhoA GTPases can be activated by mechanical loading [12,13,33]. Despite these studies, much remains to be elucidated on how individual GTPases are activated and interact with β-catenin-mediated TCF/LEF activity. Since Rho family GTPases are known to influence bone cell functions [12,17,18,34], the distinct activity pattern of individual Rho family GTPases in response to shear stress, demonstrated by our FRET data, will be of significance in regulation of the GTPase-mediated mechanotransduction in bone cells. We also showed that shear stress increased the activity of TCF/LEF in MC3T3-E1 cells. This result is consistent with previous findings that shear stress promotes nuclear translocation of β-catenin [6,7]. The biological significance of the changes in TCF/LEF activation and β-catenin translocation by fluid flow shear stress is supported by their roles in regulating bone formation [35].
Here we identified Rac1 and Cdc42 as potential mediators for TCF/LEF activity in MC3T3-E1 cells in response to fluid flow shear stress. Recent findings suggest that, although demonstrated in different types of cells without load application, Rac1 activity is required for β-catenin signaling in a bone marrow-derived stromal cell line [30] and a colon cancer cell line [36]. Rac1 and Cdc42 are also known to regulate bone growth and remodeling. Recent in vivo data show that Rac1 proteins are required for the osteoblastic maturation and normal bone development [34]. Cdc42 is also essential for osteoclast survival and ostoclastic bone resorption by modulating RANKL/M-CSF (receptor activator of nuclear factor kappa-B ligand/macrophage colony-stimulating factor) signaling [17]. Thus, it is becoming increasingly clear that Rho family GTPases may play a significant role in regulating bone physiology.
In summary, our findings of the distinct activation patterns of Rac1 and Cdc42 suggest their critical role in the responses of osteoblasts to fluid flow shear stress. By mediating TCF/LEF activity, Rac1 and Cdc42 GTPases interact with β-catenin-dependent regulatory pathways important for proliferation and differentiation of osteoblasts. The work herein defines Rac1 and Cdc42 as critical nodes within the signaling axis of mechanotransduction that regulates β-catenin under mechanical stimulation.
Highlights.
Shear stress increased TCF/LEF activity and stimulated β-catenin nuclear localization.
Rac1, Cdc42, and RhoA displayed distinct dynamic activity patterns under flow.
Rac1 and Cdc42, but not RhoA, regulate shear stress-driven TCF/LEF activation.
Cytoskeleton did not significantly affect shear stress-induced TCF/LEF activation.
Acknowledgments
We thank Dr. M. Matsuda (Kyoto University, Japan) and Dr. J. Miyazaki (Osaka University, Japan) for the gift of the GTPase biosensors, Dr. E. Schuman (California Institute of Technology, Pasadena, CA, USA) for the EGFP-β-catenin; Dr. Y. Wang (University of Illinois, Urbana-Champaign, IL, USA) for the RhoA mutant, and Dr. A. Hall (Memorial Sloan-Kettering Cancer Center, New York, NY, USA) for the Rac1 and Cdc42 mutants. This work was supported by Indiana University (S.N.), and National Institutes of Health Grant AR052144 (H.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yokota H, Leong DJ, Sun HB. Mechanical loading: bone remodeling and cartilage maintenance. Curr Osteoporos Rep. 2011;9:237–242. doi: 10.1007/s11914-011-0067-y. [DOI] [PubMed] [Google Scholar]
- 2.Huang C, Ogawa R. Mechanotransduction in bone repair and regeneration. FASEB J. 2010;24:3625–3632. doi: 10.1096/fj.10-157370. [DOI] [PubMed] [Google Scholar]
- 3.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66:397–402. [PubMed] [Google Scholar]
- 4.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. Journal of Bone and Mineral Research. 2004;19:1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 5.Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, Nusse R, Helms JA. Wnt proteins promote bone regeneration. Sci Transl Med. 2010;2:29ra30. doi: 10.1126/scitranslmed.3000231. [DOI] [PubMed] [Google Scholar]
- 6.Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid shear stress induces beta-catenin signaling in osteoblasts. Calcified Tissue International. 2004;75:396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- 7.Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of beta-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: Implications for the study of mechanosensation in bone. Bone. 2010;47:872–881. doi: 10.1016/j.bone.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. Journal of Biological Chemistry. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novak A, Dedhar S. Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci. 1999;56:523–537. doi: 10.1007/s000180050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 11.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 12.Hamamura K, Swarnkar G, Tanjung N, Cho E, Li J, Na S, Yokota H. RhoA-mediated signaling in mechanotransduction of osteoblasts. Connect Tissue Res. 2012;53:398–406. doi: 10.3109/03008207.2012.671398. [DOI] [PubMed] [Google Scholar]
- 13.Poh YC, Na S, Chowdhury F, Ouyang M, Wang Y, Wang N. Rapid activation of Rac GTPase in living cells by force is independent of Src. PLoS One. 2009;4:e7886. doi: 10.1371/journal.pone.0007886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res. 2006;98:176–185. doi: 10.1161/01.RES.0000200162.94463.d7. [DOI] [PubMed] [Google Scholar]
- 15.Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics. Journal of Cell Science. 2009;122:546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chellaiah MA, Biswas RS, Rittling SR, Denhardt DT, Hruska KA. Rho-dependent Rho kinase activation increases CD44 surface expression and bone resorption in osteoclasts. J Biol Chem. 2003;278:29086–29097. doi: 10.1074/jbc.M211074200. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Teitelbaum SL, Zou W, Zheng Y, Johnson JF, Chappel J, Ross FP, Zhao H. Cdc42 regulates bone modeling and remodeling in mice by modulating RANKL/M-CSF signaling and osteoclast polarization. J Clin Invest. 2010;120:1981–1993. doi: 10.1172/JCI39650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razzouk S, Lieberherr M, Cournot G. Rac-GTPase, osteoclast cytoskeleton and bone resorption. Eur J Cell Biol. 1999;78:249–255. doi: 10.1016/S0171-9335(99)80058-2. [DOI] [PubMed] [Google Scholar]
- 19.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N, Nagashima K, Matsuda M. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162:223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol. 2002;22:6582–6591. doi: 10.1128/MCB.22.18.6582-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Chen BP, Azuma N, Hu YL, Wu SZ, Sumpio BE, Shyy JY, Chien S. Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J Clin Invest. 1999;103:1141–1150. doi: 10.1172/JCI5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clevers H, van de Wetering M. TCF/LEF factor earn their wings. Trends Genet. 1997;13:485–489. doi: 10.1016/s0168-9525(97)01305-x. [DOI] [PubMed] [Google Scholar]
- 25.Murase S, Mosser E, Schuman EM. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- 26.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 27.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 28.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surviladze Z, Waller A, Strouse JJ, Bologa C, Ursu O, Salas V, Parkinson JF, Phillips GK, Romero E, Wandinger-Ness A, Sklar LA, Schroeder C, Simpson D, Noth J, Wang J, Golden J, Aube J. A Potent and Selective Inhibitor of Cdc42 GTPase, Probe Reports from the NIH Molecular Libraries Program. Bethesda MD: 2010. [PubMed] [Google Scholar]
- 30.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz MA, Shattil SJ. Signaling networks linking integrins and rho family GTPases. Trends Biochem Sci. 2000;25:388–391. doi: 10.1016/s0968-0004(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 33.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci U S A. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane SW, De Vita S, Alexander KA, Karaman R, Milsom MD, Dorrance AM, Purdon A, Louis L, Bouxsein ML, Williams DA. Rac signaling in osteoblastic cells is required for normal bone development but is dispensable for hematopoietic development. Blood. 2012;119:736–744. doi: 10.1182/blood-2011-07-368753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 36.Esufali S, Bapat B. Cross-talk between Rac1 GTPase and dysregulated Wnt signaling pathway leads to cellular redistribution of beta-catenin and TCF/LEF-mediated transcriptional activation. Oncogene. 2004;23:8260–8271. doi: 10.1038/sj.onc.1208007. [DOI] [PubMed] [Google Scholar]