Abstract
Purpose
To use classification algorithms to classify swallows as safe, penetration, or aspiration based on measurements obtained from pharyngeal high-resolution manometry (HRM) with impedance.
Study design
Case series evaluating new method of data analysis.
Method
Multilayer perceptron (MLP), an artificial neural network (ANN), was evaluated for its ability to classify swallows as safe, penetration, or aspiration. Data were collected from 25 disordered subjects swallowing 5 or 10 ml boluses. Following extraction of relevant parameters, a subset of the data was used to train the models and the remaining swallows were then independently classified by the ANN.
Results
A classification accuracy of 89.4±2.4% was achieved when including all parameters. Including only manometry-related parameters yielded a classification accuracy of 85.0±6.0% while including only impedance-related parameters yielded a classification accuracy of 76.0±4.9%. Receiver operating characteristic (ROC) analysis yielded areas under the curve (AUC) of 0.8912 for safe, 0.8187 for aspiration, and 0.8014 for penetration.
Conclusions
Classification models show high accuracy in classifying swallows from dysphagic patients as safe or unsafe. HRM-impedance with ANN represents one method which could be used clinically to screen for patients at risk for penetration or aspiration.
Keywords: artificial neural network, classification model, high-resolution manometry, impedance, aspiration, dysphagia
INTRODUCTION
The pharyngeal swallow is a complex physiological event requiring orchestration of muscle contraction to generate the pressure needed to move a bolus from the mouth to the esophagus.1-3 Accurate quantification of these rapidly changing pressures requires high spatial and temporal resolution not previously available with traditional one to three sensor manometers. High-resolution manometry (HRM) represents a promising clinical and research tool capable of capturing the detailed pressure activity during the pharyngeal swallow. HRM uses up to 36 circumferential sensors. Recently, impedance measurement has been added to HRM to increase the information provided by this tool.
Intraluminal impedance detects the speed and direction of bolus movement as it progresses through the digestive tract.4 Combining pressure measurement with impedance measurement provides an assessment of both motility and flow of luminal content.5 Combined manometry with impedance has been used to evaluate esophageal motility disorders.6 Omari et al. used a novel combined manometry and impedance assembly to evaluate the pharyngeal swallow and demonstrated that intraluminal impedance patterns in the pharynx are more complex than those found in the esophagus.5 While impedance may not reliably measure pharyngeal bolus transit time,5 it can provide information on the presence or absence of pharyngeal bolus residue.7-8 Residue leads to a reduction in pharyngeal impedance post-swallow and can be detected as a delay in recovery of pharyngeal impedance post-swallow.
The amount of data points sampled and the high number of variables extracted from HRM-impedance can be intimidating to clinicians seeking to take advantage of a new diagnostic tool. An algorithm for efficient, automated interpretation of these data may be valuable and facilitate increased clinical use. Pattern recognition techniques, including artificial neural networks (ANNs), are powerful mathematical models which can handle large data sets and classify data into groups according to nonlinear statistical analysis.9-11 ANNs have been used to analyze voice and swallow events, differentiating between normal and disordered events, as well as distinguishing among different types of disorders.9-12
Critical to the clinical utility of any swallowing evaluation is the ability to detect aspiration, a potentially life-threatening swallowing abnormality that can lead to aspiration pneumonia. Videofluoroscopy has traditionally been considered the gold standard for evaluating aspiration, but requires radiation exposure and is a fairly poor predictor of aspiration pneumonia.5,13,14 HRM-impedance offers an alternative that does not expose subjects to radiation, can be performed at the bedside, and provides quantitative information on a wide range of parameters relevant to swallowing function.
We aimed to determine if pattern recognition techniques could correctly classify swallows from disordered subjects as safe, penetration, or aspiration. Data were analyzed from dysphagic subjects and feature vectors were extracted that contained relevant parameters such as maximum pressures and timing parameters. Feature vectors form a training set, which is used as the input to train ANNs, including multilayer perceptron (MLP). MLP uses machine learning algorithms to classify data into groups. Parameters were tuned to achieve a higher correct classification rate, and the components of the feature vector were examined to consider their individual contribution to classification. We hypothesized that the highest classification rates would be achieved when including all parameters in the analysis.
MATERIALS AND METHODS
Data collection
Please note that some of the data used in this study were included in a previous paper.7 We employ a novel analysis method on those data and newly collected data.
Equipment and procedure
Data were collected according to the procedure described by Omari et al.7 A 3.2 mm diameter solid state manometric and impedance catheter featuring 25 pressure sensors spaced 1 cm apart and 12 adjoining impedance segments, each 2 cm in length, was employed (Unisensor USA Inc., Portsmouth, NH). Subjects were instrumented after topical application of lignocaine spray and the catheter was positioned with sensors spanning from the velopharynx to the proximal esophagus. Topical anesthesia is required to perform the procedure quickly and effectively. Two sprays of lignocaine anesthetic were applied to the naris through which the catheter was passed. Anesthetic was limited to only the naris and was not applied to the pharynx. Pressure and impedance data were sampled at a rate of 20 Hz (Solar GI Acquisition System, MMS, The Netherlands). Subjects were seated in an upright position. Five and/or ten ml boluses of liquid, semi-solid, or solid material was delivered via syringe (liquid) or spoon (semi-solid, solid). A variety of bolus types were evaluated to test the generalizability of our analysis to a typical range of bolus types which might be given during a standard swallowing assessment.
Video-loops of the fluoroscopic images of swallows were simultaneously acquired at 25 frames/second. The first swallow that followed bolus administration to the mouth was termed the “first swallow.” If this first swallow did not completely clear the bolus, the subject was asked to swallow again; subsequent swallows were termed “clearing swallows.”
Participants
Twenty-five subjects with a swallowing disorder (15 males and 10 females; mean age: 69.4±15.5 years; range: 30 – 95 years) participated in this study with the approval of the Research Ethics Committee of University Hospitals Leuven, Belgium. Subjects had a variety of dysphagia etiologies, though most were neurologic. Specific etiologies included stroke (6), Arnold-Chiari malformation (1), dementia (3), vocal fold paralysis (1), multiple sclerosis (1), Charcot-Marie-Tooth disease (1), recent cervical surgery (1), spondylodiscitis (1), Parkinson's disease (4), post-tumor resection and radiotherapy for thyroid carcinoma (1), septic cranial embolism (1), revision Nissen fundoplication (1), and unknown (3). Subjects underwent simultaneous manometry and impedance and fluoroscopy.
Data analysis
Videofluoroscopy
Swallows were analyzed for aspiration and penetration using an 8-point scale (1: safe; 2-5: penetration; 6-8: aspiration) described by Rosenbeck et al.15
Data extraction
Impedance data were extracted according to the methods described by Omari et al.7 and Noll et al.16 Average pressure at the pharyngeal impedance nadir (PNadImp) and time lapse between the average peak pressure from NadImp (timing of the pharyngeal impedance nadir) and peak pressure (TNadImp-PeakP) were determined. Pharyngeal flow interval was determined using a method similar to that described by Ghosh et al.17 for measurement of the UES relaxation interval.15 The pharyngeal flow interval is an estimate of the impedance drop interval, which reflects bolus clearance time.
Manometric data were extracted according to the automated analysis method described by Mielens et al.18 Pressure and timing data were extracted using a customized MATLAB program (The MathWorks, Inc., Natick, MA) which locates peak pressures in areas of interest [velopharynx, region of the tongue base/posterior pharyngeal wall, and upper esophageal sphincter (UES)] and then calculates relevant parameters based on those points. Measured parameters in the regions of interest include peak pressure, duration of pressure above baseline, area integral of the pressure curve, and line integral of the pressure curve. The basic workflow is automated, but the user may override program suggestions in cases of misidentification and manually select the correct spatial and temporal location of the areas of interest.
Additional manometric parameters described by Omari et al.7 were also included, including peak UES pressure, UES relaxation interval (UES-RI), UES minimum relaxation pressure (UES-MRP), median UES intrabolus pressure (UES-IBP), and UES deglutitive sphincter resistance (UES-DSR). These measurements were determined according to the method described by Omari et al.7 which was modeled after the method of Ghosh et al.17
Data Processing
All data processing was completed using MATLAB and the Neural Network Toolbox (The MathWorks, Inc.). In total, 163 swallows were analyzed and the derived feature sets were used as a basis for determining models of safe swallow, penetration, and aspiration. Of the 163 swallows, 114 were safe, 17 exhibited penetration, and 32 exhibited aspiration. By attaching the known status of a swallow to its feature vector, machine learning techniques can be applied with the goal of modeling the relationship between the input features and the status of a given swallow. The ANN is first presented with the known data, goes through a training stage, and finally is presented with new data during a test stage. The training and testing data are kept separate to better evaluate the generalizing ability of the classification. Which swallows are used in the training set and testing set changes so all data are used in the test stage at some point. This ensures that the analytic capabilities of the algorithm are not limited to only a select group of swallows.
Data were normalized and each variable in the data set ranged in value from -1 to 1, with a mean of 0 and a standard deviation of 1. Normalizing the data improves algorithm efficiency and accuracy.19 Additionally, principal component analysis was used to reduce dimensionality and improve generalization. The feature set underwent two levels of reduction, during which variables that contributed minimally to classification ability were removed. This was done because such variables can be detrimental to correct classification rates.
The data set was randomly partitioned into a training set (60%), a testing set (20%), and a validation set (20%). A standard Multi-Layer Perceptron (figure 1) was created using sigmoidal activation functions in one hidden layer, and the number of nodes in the hidden layer was varied from 20 to 80 to attain better performance. Ten replicates were performed at each level of hidden nodes to determine the average classification accuracy at that level as well as the reliability of that classification. The Levenberg-Marquardt algorithm was used as a learning algorithm. The goal of this algorithm is to modify the weights associated with the connections between the nodes such that an input vector will produce the specified desired output vector, essentially mapping the input space onto the output classes of safe, penetration, or aspiration.
Figure 1.
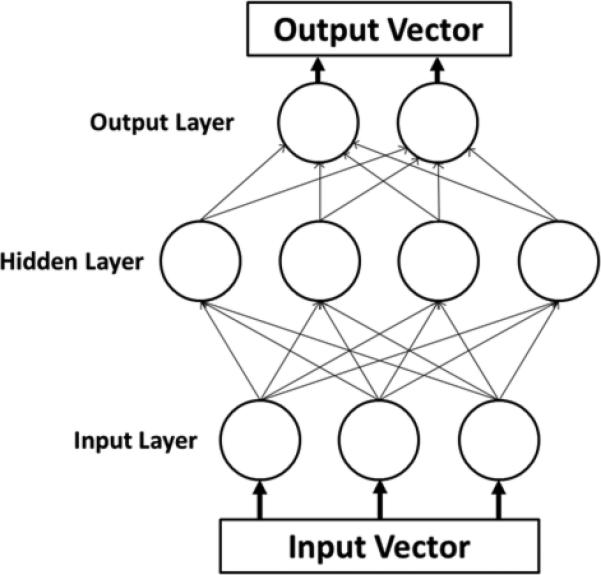
Schematic of a multilayer perceptron neural network. Each parameter of interest in the input vector has a corresponding node in the input layer. The hidden layer contains the nodes, the number of which was varied during the experiment. The output vectors are the possible classifications of data, which were safe, penetration, and aspiration in this study.
The feature set was selectively reduced in an attempt to discover the classification ability of all impedance parameters collectively, all manometry parameters collectively, as well as each parameter individually.
Statistical analysis
Classification accuracy for each category (safe, aspiration, penetration) was determined for all parameters collectively as well as each parameter individually and groups of impedance or manometry parameters. Receiver operating characteristic (ROC) analysis was performed to yield ROC curves and area under the curve (AUC) for each category.
One-way analysis of variance (ANOVA) was also performed for each parameter. If data did not meet the assumptions for parametric testing, ANOVA on ranks was performed. It is important to note that multiple bolus types were included in the analysis; thus, results of statistical testing may reflect differences in individual parameters due to both bolus and swallow characteristics. A significance level of α=0.05 was used for all tests.
RESULTS
Artificial neural network classification
A classification accuracy of 89.4±2.4% was achieved when including all parameters. Including only manometry-related parameters yielded a classification accuracy of 85.0±6.0% while including only impedance-related parameters yielded a classification accuracy of 76.0±4.9% (table 1). ROC analysis yielded curves with AUC of 0.8912 for safe (figure 2a), 0.8187 for aspiration (figure 2b), and 0.8014 for penetration (figure 2c). Summary data for individual parameters are provided in table 2. When analyzing the ability of individual parameters to correctly classify swallows, there was not much variation when considering overall classification accuracy. Percent accuracy ranged from 63.8% to 73.6% (table 3). Individual parameters had relatively low classification rates for penetration and aspiration, with a range of 7.1% to 38.6% (table 3). Maximum velopharynx pressure achieved the highest classification accuracy at 38.6%.
Table 1.
Summary classification accuracies for each category and group of parameters.
| Parameter set | Safe | Penetration | Aspiration | Total |
|---|---|---|---|---|
| All parameters | 94.8 ± 2.9 | 65.9 ± 12.4 | 82.5 ± 5.3 | 89.4 ± 2.4 |
| Manometry | 95.9 ± 2.1 | 51.2 ± 25.3 | 64.4 ± 27.4 | 85.0 ± 6.0 |
| Impedance | 90.7 ± 6.2 | 25.9 ± 13.3 | 50.3 ±22.5 | 76.0 ± 4.9 |
Figure 2.
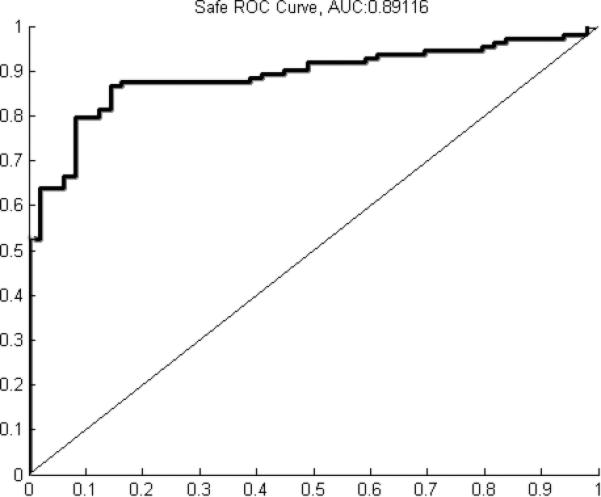
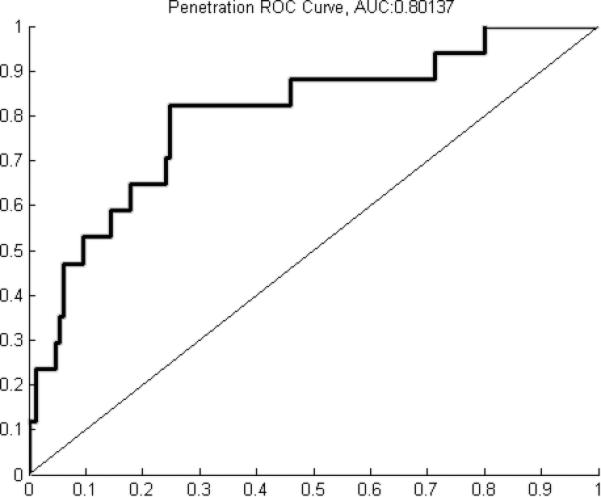
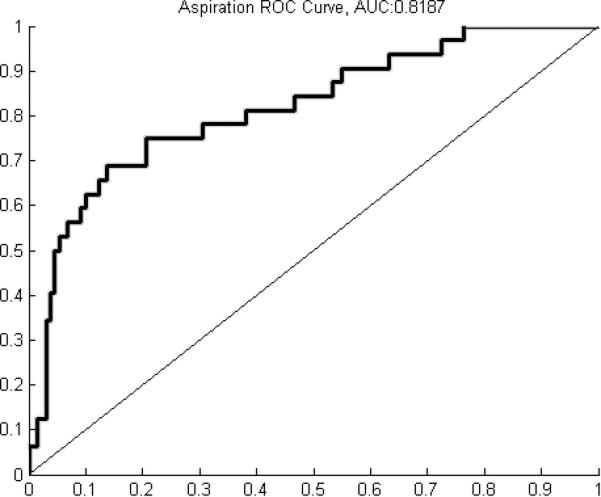
A) Receiver operating characteristic (ROC) curve for classification of safe swallows (area under the curve (AUC) = 0.8912). B) ROC curve for classification of penetration (AUC = 0.8014). C) ROC curve for classification of aspiration (AUC = 0.8187).
Table 2.
Summary data for individual parameters.
| Parameter | Safe | Penetration | Aspiration | p-value |
|---|---|---|---|---|
| Impedance parameters | ||||
| PNadImp | 29 ± 27 | 31 ± 29 | 44 ± 36 | 0.015 |
| TPNadImp-PeakP | 0.23 ± 0.15 | 0.21 ± 0.19 | 0.15 ± 0.16 | 0.054 |
| Flow Interval | 0.92 ± 0.73 | 1.44 ± 0.97 | 1.77 ± 0.80 | <0.001 |
| Manometry parameters | ||||
| Peak UES pressure | 207 ± 158 | 206 ± 108 | 221 ± 224 | 0.403 |
| UES RI | 0.87 ± 0.34 | 0.97 ± 0.37 | 0.95 ± 0.49 | 0.482 |
| UES IBP | 16 ± 11 | 29 ± 17 | 24 ± 16 | <0.001 |
| UES MRP | 5 ± 8 | 14 ± 13 | 12 ± 8 | <0.001 |
| UES DSR | 20 ± 20 | 44 ± 48 | 36 ± 40 | 0.001 |
| Peak pharyngeal pressure | 134 ± 75 | 95 ± 72 | 105 ±67 | 0.017 |
| Slope | 12.6 ± 4.8 | 9.0 ±5.5 | 13.1 ±7.3 | 0.008 |
| Maximum VP pressure | 152 ± 60 | 117 ± 106 | 123 ± 81 | <0.001 |
| Maximum TB pressure | 164 ± 148 | 131 ±114 | 133 ±108 | 0.462 |
| Pre-opening UES pressure | 96 ± 77 | 85 ± 56 | 79 ±55 | 0.765 |
| Post-closure UES pressure | 234 ± 157 | 168 ± 117 | 189 ± 205 | 0.004 |
| Rise time VP | 0.29 ± 0.14 | 0.34 ± 0.17 | 0.32 ± 0.13 | 0.338 |
| Rise time TB | 0.31 ± 0.11 | 0.33 ± 0.14 | 0.35 ± 0.15 | 0.322 |
| Rise time pre-opening UES | 0.27 ± 0.11 | 0.32 ±0.15 | 0.25 ± 0.14 | 0.227 |
| Rise time post-closure UES | 0.28 ± 0.15 | 0.39 ±0.23 | 0.24 ± 0.10 | 0.062 |
| Fall time VP | 0.48 ± 0.15 | 0.65 ± 0.36 | 0.53 ±0.19 | 0.159 |
| Fall time TB | 0.34 ± 0.15 | 0.44 ±0.21 | 0.37 ±0.17 | 0.087 |
| Fall time pre-opening UES | 0.19 ± 0.11 | 0.25 ± 0.15 | 0.20 ± 0.14 | 0.366 |
| Fall time post-closure UES | 0.49 ± 0.19 | 0.57 ± 0.23 | 0.49 ±0.21 | 0.381 |
| Duration VP | 0.77 ± 0.13 | 1.01 ± 0.47 | 0.85 ± 0.22 | 0.037 |
| Duration TB | 0.65 ± 0.17 | 0.76 ± 0.26 | 0.72 ± 0.24 | 0.058 |
| Duration UES | 1.71 ± 0.36 | 1.71 ±1.21 | 1.56 ± 0.40 | 0.032 |
| Total swallow duration | 0.71 ± 0.28 | 1.00 ±0.42 | 0.72 ± 0.29 | 0.008 |
| Integral VP | 5935 ± 2462 | 2838 ± 1607 | 4081 ± 2246 | <0.001 |
| Integral TB | 3492 ± 2330 | 2536 ± 1272 | 3408 ±2116 | 0.398 |
| Integral UES | 9135 ± 7374 | 4976 ±2087 | 7431 ±11665 | <0.001 |
| Minimum UES pressure | -8 ± 11 | -3 ±7 | 0 ±7 | <0.001 |
| UES resting pressure | 27 ± 27 | 37 ±27 | 41 ±58 | 0.146 |
UES = upper esophageal sphincter; RI = rest interval; IBP = intrabolus pressure; MRP = mean relaxation pressure; DSR = deglutitive sphincter resistance; PNadImp = pressure at the impedance nadir; TNadImp-PeakP = difference in time between maximum pharyngeal pressure and PNadImp; VP = velopharynx; TB = tongue base.
Table 3.
Feature reduction analysis demonstrating classification accuracy for individual parameters.
| Parameter | % accuracy for safe | % accuracy for penetration/aspiration | % total accuracy |
|---|---|---|---|
| Impedance parameters | |||
| PNadImp | 87.7 ± 11.1 | 21.6 ± 13.2 | 67.9 ± 5.6 |
| PNadImp-PeakP | 79.1 ± 22.6 | 28.4 ± 13.7 | 63.8 ± 13.0 |
| Flow Interval | 85.5 ± 13.8 | 27.4 ± 18.9 | 68.0 ± 5.8 |
| Manometry parameters | |||
| Peak UES pressure | 90.4 ± 9.4 | 17.2 ± 12.9 | 68.4 ± 4.9 |
| UES RI | 82.9 ± 12.3 | 27.6 ± 13.8 | 66.3 ± 6.2 |
| UES IBP | 84.5 ± 20.6 | 27.0 ± 19.2 | 67.2 ± 12.5 |
| UES MRP | 95.3 ± 4.9 | 23.3 ± 15.7 | 73.6 ± 1.8 |
| UES DSR | 89.4 ± 15.6 | 26.5 ± 22.0 | 70.5 ± 7.6 |
| Peak pharyngeal pressure | 82.1 ± 20.8 | 25.3 ± 15.7 | 65.0 ± 11.5 |
| Slope | 87.0 ± 12.0 | 28.6 ± 17.8 | 69.5 ± 6.0 |
| Maximum VP pressure | 87.0 ± 15.0 | 38.6 ± 22.5 | 72.5 ± 8.0 |
| Maximum TB pressure | 90.2 ± 17.1 | 24.9 ± 11.8 | 70.6 ± 10.9 |
| Pre-opening UES pressure | 85.5 ± 17.6 | 19.6 ± 16.4 | 65.7 ± 8.0 |
| Post-closure UES pressure | 87.4 ± 11.9 | 18.2 ± 16.1 | 66.6 ± 5.8 |
| VP rise time | 89.1 ± 7.6 | 26.5 ± 13.0 | 70.3 ± 3.8 |
| TB Rise time | 84.3 ± 15.9 | 23.1 ± 13.5 | 65.9 ± 9.3 |
| Pre-opening UES rise time | 88.7 ± 6.3 | 23.9 ± 11.7 | 69.2 ± 4.1 |
| Post-closure UES rise time | 86.2 ± 6.6 | 33.1 ± 17.3 | 70.2 ± 5.4 |
| VP Fall time | 89.1 ± 19.7 | 20.0 ± 18.0 | 68.3 ± 9.2 |
| TB Fall time | 94.2 ± 4.7 | 17.2 ± 10.4 | 71.1 ± 2.1 |
| Pre-opening UES fall time | 88.4 ± 10.5 | 25.9 ± 13.9 | 69.6 ± 4.5 |
| Post-closure UES fall time | 89.5 ± 8.6 | 19.6 ± 10.4 | 68.5 ± 4.4 |
| VP duration | 91.7 ± 8.6 | 21.0 ± 11.0 | 70.4 ± 4.7 |
| TB duration | 92.2 ± 6.7 | 18.8 ± 9.5 | 70.1 ± 2.7 |
| UES duration | 96.5 ± 6.0 | 11.63 ± 9.6 | 71.0 ± 2.1 |
| Total swallow duration | 98.2 ± 1.9 | 7.1 ± 3.6 | 70.9 ± 1.4 |
| VP integral | 85.6 ± 7.3 | 36.3 ± 22.2 | 70.8 ± 3.4 |
| TB integral | 84.7 ± 13.8 | 22.0 ± 17.1 | 65.8 ± 5.8 |
| UES integral | 84.5 ± 22.4 | 19.6 ± 22.2 | 65.0 ± 14.3 |
| Minimum UES pressure | 81.8 ± 13.4 | 30.0 ± 16.5 | 66.2 ± 6.8 |
| Resting UES pressure | 90.5 ± 11.0 | 16.7 ± 10.6 | 68.3 ± 5.7 |
UES = upper esophageal sphincter; RI = rest interval; IBP = intrabolus pressure; DSR = deglutitive sphincter resistance; PNadImp = pressure at the impedance nadir; TNadImp-PeakP = difference in time between maximum pharyngeal pressure and PNadImp; VP = velopharynx; TB = tongue base.
One-way ANOVA
Statistical results from ANOVA comparisons are provided in table 2. All impedance parameters were significantly or near significantly different across groups (PNadImp: p=0.015; TPNadImp-PeakP: p=0.054; pharyngeal flow interval: p<0.001). Manometric parameters demonstrating significant differences across groups included UES intrabolus pressure (p<0.001), UES mean relaxation pressure (p<0.001), UES deglutitive sphincter resistance (p=0.001), slope (p=0.008), post-closure UES pressure (p=0.004), velopharyngeal pressure duration (p=0.037), UES duration (p=0.032), total swallow duration (p=0.008), UES pressure integral (p<0.001), peak pharyngeal pressure (p=0.017), maximum velopharyngeal pressure (p<0.001), post-closure UES pressure (p=0.004), velopharyngeal pressure integral (p<0.001), and minimum UES pressure (p<0.001).
DISCUSSION
We applied artificial neural network (ANN) analysis to classify swallows from dysphagic patients as safe, penetration, or aspiration. In our previous work,12 we applied a similar method of analysis to classify swallows from normal and disordered subjects as normal or disordered. This study represents four key advances beyond that initial investigation: both manometry and impedance data were included in the analysis; three classes of data were included, increasing the complexity of the categorization; all swallows were obtained from patients and distinguishing between safe and unsafe swallows in dysphagic subjects demonstrates greater sensitivity than distinguishing between normal and disordered swallows; and a variety of bolus volumes and consistencies were included.
Manometric and impedance parameters performed better collectively than either group did individually, as demonstrated by the differences in classification rates (table 1). Interestingly, including only manometric parameters led to greater classification accuracy than including only impedance parameters. This may be due to HRM analysis being more robust and currently including far more parameters (twenty-eight) than impedance analysis (three). Additionally, generation of pressure gradients is the key to safe swallowing and analysis of pressure may be more reflective of abnormal swallowing than analysis of impedance. The greatest classification accuracy, though, was achieved when combining both pressure and impedance information, demonstrating the importance of a comprehensive assessment. This was particularly evident when classifying swallows exhibiting penetration. Using manometric parameters alone led to a classification accuracy of 51.2% while impedance parameters alone led to a classification accuracy of 25.9%. Neither rate is high enough to provide a basis for clinical decision-making; however, combining manometric and impedance parameters led a classification accuracy of 65.9%. As manometric and impedance parameters are collected simultaneously, we obtain the added synergistic effect without affecting the duration of data collection. HRM-impedance is also still a relatively new research tool; development and inclusion of additional parameters which better distinguish among different disorders and swallowing abnormalities could further improve classification accuracy and thus improve the utility of analysis while not increasing time required for assessment.
All subjects included in the analysis had dysphagia and many had a neurologic etiology of their swallowing disorder. Penetration and aspiration are common in this patient population due to weakness and a poorly coordinated swallow. Identifying which patients are at risk for aspiration can aid clinicians when making feeding recommendations. Notably, there was a wide age range included in this study (30 – 95 years). Despite this range, classification accuracy was rather high (average of 89.4%), demonstrating the power and generalizability of the algorithm. Classification accuracy was higher for aspiration than penetration. This is somewhat expected, as aspiration deviates more from a safe swallow than penetration. The low number of swallows exhibiting penetration included in the analysis (seventeen) may also have contributed to the low classification rate, as introducing fewer examples of one category during the training stage can have an adverse effect during the testing stage. In the future, this may be addressed by including more swallows exhibiting penetration. Higher classification accuracy is preferred for aspiration, as it is a more severe problem and identifying it is critical to prevent such serious sequelae as aspiration pneumonia. Aspiration pneumonia is the leading cause of death in Parkinson's disease.20-21 Additionally, the risk of aspiration pneumonia is increased in other neurologic disorders such as stroke, particularly in those patients with an impaired cough.22-23
A variety of bolus consistencies and volumes were included in our analysis. The majority of penetration and aspiration events occurred on liquid boluses. Of the ten swallows exhibiting penetration, six were with a liquid bolus, three were with a semisolid bolus, and one was with a solid bolus. Of the twenty-nine swallows exhibiting aspiration, twenty-two were with a liquid bolus, two were with a semisolid bolus, and five were with a solid bolus. Additionally, both primary swallows as well as clearing swallows were included in the analysis. These factors increase the variability of the data. As changes in bolus volume and consistency lead to changes in pressure,24-25 one may think including a variety of bolus types would decrease classification accuracy due to confusion distinguishing between changes due to bolus characteristics and changes due to disordered swallowing. However, a main benefit of ANN analysis is the ability to recognize patterns rather than trends in single variables. Bolus characteristics did not appear to negatively affect classification accuracy in this experiment, though a more rigorous study determining if there is an “optimal bolus” for this type of analysis may be warranted. A method of analysis that is generalizable across bolus types is clinically valuable, allowing the analysis to be performed as part of the standard swallowing evaluation rather than in addition to it. This finding also has physiological significance, as the relationships among the variables of interest, rather than absolute values of each parameter in isolation, appear to be the key in distinguishing safe from unsafe swallows. Accordingly, this provides support for a comprehensive multi-parameter analysis such as that provided by ANN analysis of HRM-impedance, rather than isolated measurements of peak pharyngeal pressure or UES pressure, for example.
Feature reduction analysis allowed us to evaluate which individual parameters could classify data with the highest accuracy (table 3). There was not much variation across parameters when considering overall classification or classification of safe swallows. Percent classification accuracy ranged from 79.1% to 98.2% for safe swallows and from 63.8% to 73.6% overall. This may be due to large differences in parameters between safe and unsafe swallows but may also be due to an imbalance in the data set, as many more safe swallows were included in the analysis. More interestingly, no single parameter was able to classify swallows as penetration or aspiration with high accuracy. Percent classification accuracy ranged from 7.1% to 38.6%. It is important to note that parameters with low individual classification rates do not adversely affect overall classification accuracy, as the relationships among parameters are more important and any parameter which would decrease classification ability is weighted such that its effect is minimal. Maximum velopharyngeal pressure (38.6%), velopharyngeal pressure integral (36.3%), and post-closure UES rise time (33.1%) provided the highest classification accuracies. Low classification rates could be due to difficulty distinguishing between the two types of unsafe swallows and could also be due to the relatively low number of unsafe swallows (17 penetration, 32 aspiration) included in the analysis. These classification rates are not high enough to warrant clinical consideration; however, combining all parameters led to significantly higher classification rates of 65.9% for penetration and 82.5% for aspiration. This disparity between individual parameter classification rates and group classification rates demonstrates the importance of multi-parameter assessment. Given the large number of parameters included in this study (thirty-two), human data interpretation would be time intensive at best and likely impossible. Utilizing the machine learning techniques of ANNs allows for analysis to be completed and feedback be provided in seconds. Creating a user friendly interface to take advantage of this for clinical use will be the subject of further method development.
One-way ANOVA revealed several interesting trends. Most notably, there was not a consistent correlation between p-values resulting from ANOVA and classification accuracy. This may be due to relatively large standard deviations causing overlap which precluded differentiation based on standard statistical testing. Also, one-way ANOVA cannot determine the value of relationships among parameters. The nonlinear capabilities of ANN analysis allow for efficient evaluation and weighting of these relationships. Individual parameter analyses are more susceptible to identifying differences due to bolus type rather than swallow type. As the majority of unsafe swallows were on a liquid bolus (thirty-eight of forty-nine), differences detected in individual parameters across groups cannot be completely attributed to swallow status (i.e., safe, penetration, or aspiration). Each of the three impedance parameters (PNadImp, TPNadImp-PeakP, pharyngeal flow interval) was significantly different across categories. As impedance can detect bolus clearance and poor bolus clearance is more likely to result in penetration or aspiration, significant differences across the three categories can be expected. While a greater proportion of impedance parameters were significant (two of three) compared to manometric parameters (thirteen of twenty-eight), classification accuracy was higher when considering only manometric parameters. This demonstrates the importance of including a wide range of parameters in the ANN. Of the manometric parameters demonstrating significant differences, many were related to the UES. Manometric parameters describing the muscular activity in this region are strong predictors of pharyngeal function, as an inability to pass the bolus through the UES due to poorly controlled muscular activity can result in penetration and aspiration of materials retained in the pharynx.26-27
Our efforts to improve performance by modifying the architecture of the ANN, such as increasing the number of hidden nodes, had a minimal effect on classification accuracy (table 4). This is likely a consequence of implementing measures to prevent overfitting in large networks. Increasing the number of data points available by analyzing a larger subject pool could potentially prevent this overfitting and allow these larger networks to run longer, potentially improving accuracy and generalization to new data.
Table 4.
Total classification accuracies (%) at each number of hidden nodes evaluated. Values are presented as mean ± standard deviation.
| Nodes | HRM-impedance | HRM | Impedance |
|---|---|---|---|
| 20 | 82.0 ± 4.5 | 79.0 ± 8.2 | 73.4 ± 2.7 |
| 40 | 87.6 ± 2.8 | 81.1 ± 8.2 | 72.4 ± 3.6 |
| 60 | 85.0 ± 3.2 | 81.2 ± 6.2 | 73.8 ± 5.9 |
| 80 | 89.4 ± 2.4 | 85.0 ± 3.4 | 76.0 ± 4.9 |
CONCLUSION
The pattern recognition techniques employed here appear to be clinically useful in identifying patients at risk for aspiration. We recognize that the ultimate goal of a swallowing evaluation is to define the underlying physiologic abnormality that impairs successful swallow function; however, an immediate screening report on whether a subject's swallow is safe or unsafe is clinically valuable. The high accuracy in this preliminary study provides evidence that HRM-impedance has potential as an alternative clinical assessment tool, especially when coupled with ANN techniques.
ACKNOWLEDGEMENTS
This research was supported by NIH grant numbers R21 DC011130A and T32 DC009401 from the National Institute on Deafness and other Communicative Disorders, NHMRC grant number 1009344, and a grant from the Thrasher Research Fund, Salt Lake City, Utah.
Footnotes
This study was accepted for presentation as an oral and poster presentation at the 20th Annual Dysphagia Research Society Meeting. March 7-10, 2012. Toronto, Canada.
Conflicts of interest: None.
REFERENCES
- 1.Kim SM, McCulloch TM, Rim K. Pharyngeal pressure analysis by the finite element method during liquid bolus swallow. Ann Otol Rhinol Laryngol. 2000 Jun;109(6):585–9. doi: 10.1177/000348940010900610. [DOI] [PubMed] [Google Scholar]
- 2.McConnel FM. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope. 1988 Jan;98(1):71–8. doi: 10.1288/00005537-198801000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Cook IJ. Normal and disordered swallowing: new insights. Bailliere's Clinical Gastroenterology. 1991 Jun;5(2):245–67. doi: 10.1016/0950-3528(91)90029-z. [DOI] [PubMed] [Google Scholar]
- 4.Fass J, Silny J, Braun J, Heindrichs U, Dreuw B, Schumpelick V, Rau G. Measuring esophageal motility with a new intraluminal impedance device. First clinical results in reflux patients. Scand J Gastroenterol. 1994;29:693–702. doi: 10.3109/00365529409092496. [DOI] [PubMed] [Google Scholar]
- 5.Omari TI, Rommel N, Szczesniak MM, Fuentealba S, Dinning PG, Davidson GP, Cook IJ. Assessment of intraluminal impedance for the detection of pharyngeal bolus flow during swallowing in healthy adults. Am J Physiol Gastrointest Liver Physiol. 2006;290:G183–8. doi: 10.1152/ajpgi.00011.2005. [DOI] [PubMed] [Google Scholar]
- 6.Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am J Gastroenterol. 2004:1011–9. doi: 10.1111/j.1572-0241.2004.30035.x. [DOI] [PubMed] [Google Scholar]
- 7.Omari TI, Dejaeger E, Van Beckevoort D, Goeleven A, Davidson GP, Dent J, Tack J, Rommel N. A method to objectively assess swallow function in adults with suspected aspiration. Gastroenterology. 2011;140:1454–63. doi: 10.1053/j.gastro.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 8.Omari TI, Papathanasopoulos A, Dejaeger E, et al. Reproducibility and agreement of pharyngeal automated impedance manometry with videofluoroscopy. Clin Gastroenterol Hepatol. 2011 Oct;9(10):862–7. doi: 10.1016/j.cgh.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Cross SS, Harrison RF, Kennedy RL. Introduction to neural networks. Lancet. 1995 Oct 21;346(8982):1075–9. doi: 10.1016/s0140-6736(95)91746-2. [DOI] [PubMed] [Google Scholar]
- 10.Baxt WG. Application of artificial neural networks to clinical medicine. Lancet. 1995 Oct 28;346(8983):1135–8. doi: 10.1016/s0140-6736(95)91804-3. [DOI] [PubMed] [Google Scholar]
- 11.Santos R, Haack HG, Maddalena D, Hansen RD, Kellow JE. Evaluation of artificial neural networks in the classification of primary oesophageal dysmotility. Scandanavian J Gastroenterol. 2006 Mar;41(3):257–63. doi: 10.1080/00365520500234030. [DOI] [PubMed] [Google Scholar]
- 12.Mielens JD, Hoffman MR, Ciucci MR, McCulloch TM, Jiang JJ. Application of classification models to pharyngeal high-resolution manometry. J Speech Lang Hear Res. 2012 Jun;55(3):892–902. doi: 10.1044/1092-4388(2011/11-0088). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croghan JE, Burke EM, Caplan S, Denman S. Pilot study of 12-month outcomes of nursing home patients with aspiration on videofluoroscopy. Dysphagia. 1994;9:141–6. doi: 10.1007/BF00341256. [DOI] [PubMed] [Google Scholar]
- 14.Aviv JE, Sacco RL, Mohr JP, Thompson JL, Levin B, Sunshine S, Thomson J, Close LG. Laryngopharyngeal sensory testing with modified barium swallow as predictors of aspiration pneumonia after stroke. Laryngoscope. 1997;107:1254–60. doi: 10.1097/00005537-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbeck JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 16.Noll L, Rommel N, Davidson GP, Omari TI. Pharyngeal flow interval: a novel impedance-based parameter correlating with aspiration. Neurogastroenterol Motil. 2011;23:551–e206. doi: 10.1111/j.1365-2982.2010.01634.x. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Kahrilas PJ. Deglutitive upper esophageal sphincter relaxation: a study of 75 volunteer subjects using solid-state high-resolution manometry. Am J Physiol Gastrointest Liver Physiol. 2006;291(3):G525–31. doi: 10.1152/ajpgi.00081.2006. [DOI] [PubMed] [Google Scholar]
- 18.Mielens JD, Hoffman MR, Ciucci MR, Jiang JJ, McCulloch TM. Automated analysis of pharyngeal pressure data obtained with high-resolution manometry. Dysphagia. 2011 Mar;26(1):3–12. doi: 10.1007/s00455-010-9320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saarinen S, Bramley R, Cybenko G. Ill-conditioning in neural network training problems. SIAM J Sci Comp. 1993;14:693–714. [Google Scholar]
- 20.Beyer MK, Herlofson K, Arsland D, Larsen JP. Causes of death in a community-based study of Parkinson's disease. Acta Neurol Scand. 2001;103:7–11. doi: 10.1034/j.1600-0404.2001.00191.x. [DOI] [PubMed] [Google Scholar]
- 21.D'Amelio M, Ragonese P, Morgante L, et al. Long-term survival of Parkinson's disease: a population-based study. J Neurol. 2006;253:33–7. doi: 10.1007/s00415-005-0916-7. [DOI] [PubMed] [Google Scholar]
- 22.Ebihara S, Saito H, Kanda A, Nakajoh M, Takahashi H, Arai H, Sasaki H. Impaired efficacy of cough in patients with Parkinson disease. Chest. 2003;124:1009–1015. doi: 10.1378/chest.124.3.1009. [DOI] [PubMed] [Google Scholar]
- 23.Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56:502–6. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman MR, Ciucci MR, Mielens JD, Jiang JJ, McCulloch TM. Pharyngeal swallow adaptations to bolus volume measured with high-resolution manometry. Laryngoscope. 2010;120:2367–2373. doi: 10.1002/lary.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler SG, Stuart A, Castell D, Russell GB, Kock K, Kemp S. Effects of age, gender, bolus condition, viscosity, and volume on pharyngeal and upper esophageal sphincter pressure and temporal measurements during swallowing. J Speech Lang Hear Res. 2009 Feb;52(1):240–53. doi: 10.1044/1092-4388(2008/07-0092). [DOI] [PubMed] [Google Scholar]
- 26.Higo R, Tayama N, Watanabe T. Manometric abnormality in dysphagic patients after medullary cerebrovascular accidents. ORL J Otorhinolaryngol Relat Spec. 2002;64:368–72. doi: 10.1159/000066075. [DOI] [PubMed] [Google Scholar]
- 27.Bian RX, Choi IS, Kim JH, Han JY, Lee SG. Impaired opening of the upper esophageal sphincter in patients with medullay infarctions. Dysphagia. 2009;24:238–45. doi: 10.1007/s00455-008-9179-7. [DOI] [PubMed] [Google Scholar]


