Abstract
We have previously shown that a short course of high-dose tacrolimus induces long-term tolerance to fully mismatched lung allografts procured from healthy MHC-inbred miniature swine. Here, we investigate whether donor brain death affects tolerance induction. Four recipient swine were transplanted with fully mismatched lung grafts from donors that were rendered brain dead and mechanically ventilated for 4 h before procurement (Group 1). These recipients were compared to two control groups (Group 2: 4 h of donor ventilation without brain death [n = 5]; and Group 3: no donor brain death with <1 h of ventilation [n = 6]). All recipients were treated with a 12-day course of tacrolimus. In contrast to both groups of control animals, the swine transplanted with lung allografts from brain dead donors all rejected their grafts by postoperative day 45 and showed persistent responsiveness to donor antigen by MLR. Several additional swine underwent brain death induction and/or mechanical ventilation alone to determine the effects of these procedures on the expression of proinflammatory molecules. Significant increases in serum concentrations of IL-1, TNF-α and IL-10 were seen after brain death. Upregulation of IL-1 and IL-6 gene expression was also observed.
Keywords: Brain death, lung transplantation, swine, tolerance induction
Introduction
Although utilization of organs from living donors continues to increase, the cadaveric donor remains the major source of organs for clinical transplantation. This is particularly true in pulmonary transplantation, where living donor lobar transplantation remains uncommon. In the case of renal transplantation, with the exception of HLA-matched transplants, donor brain death seems to have a more significant effect on survival than the degree of HLA-matching (1). These findings have been attributed to the well-characterized proinflammatory state of brain death, which includes dramatic alterations in hemodynamic parameters, neurohumoral factors, cytokine levels and metabolism, ultimately culminating in the activation of the immune system (reviewed in 2).
Although the systemic and organ-specific effects of brain death have been well characterized in numerous small-animal models, the majority of preclinical models used to study transplantation tolerance employ healthy donor–recipient pairs. Our group has previously reported that a short course of high-dose tacrolimus induces tolerance of fully MHC-disparate lung transplants in miniature swine (3). Here, we report the effects of donor brain death on the induction of tolerance in this well-defined miniature swine model.
Materials and Methods
Animals
For these studies, swine between 5 months and 10 months of age were selected from our herd of partially inbred miniature swine. As previously described, these animals have been bred to homozygosity at the MHC class I and class II loci (4–6). All transplants were performed using fully allogeneic donor–recipient pairs. All animal care and procedures were in compliance with “Principles of Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health.
Experimental design
Three groups of swine were transplanted. In Group 1 (n = 4), recipient swine were transplanted with lung grafts from donors that were rendered brain dead and subsequently ventilated for 4 h before procurement. In Group 2 (n = 5), recipient swine were transplanted with lung grafts from donors that underwent 4 h of ventilation without brain death. In Group 3 (n = 6), recipient swine were transplanted with lung grafts from donors that underwent <1 h of ventilation without brain death (n = 6). All recipients were treated with a 12-day course of tacrolimus.
For all transplanted animals, in vitro assays were performed on each donor–recipient pair to confirm a positive alloresponse before transplantation. On day 0, animals underwent left orthotopic lung transplantation (detailed below). High-dose tacrolimus (Haorui Pharma-Chem Inc., Edison, NJ, USA) was begun on day 0 and infused continuously for 12 days. Levels were monitored daily during this time and the dose adjusted for a target level of 35–50 ng/mL. Animals were followed by daily physical examination and complete blood count. Serial chest radiography and open lung biopsies were performed monthly or sooner if the clinical health of the animal or graft was of concern. Open lung biopsies were performed by a limited thoracotomy. The graft lung was dissected free of adhesions and specimens were taken using sharp dissection or a surgical stapling device. The chest was closed over a drain, which was removed after evacuation of any pneumothorax. Animals were sacrificed if there was evidence of graft loss because of rejection or infection.
Several additional swine underwent brain death induction with 4 h of mechanical ventilation or 4 h of mechanical ventilation alone to document the effects of these procedures on circulating cytokine levels by ELISA and on cytokine and TLR gene expression in the lung by qPCR.
Brain death and mechanical ventilation
Donor animals were subjected to brain death with a 4-h period of ventilation (n = 4) or a 4-h period of mechanical ventilation alone (n = 5). Both groups received general anesthesia and underwent continuous hemodynamic monitoring. Brain death was induced by intracranial inflation of a 30 cc Foley catheter over a period of 1 min. All donor animals received hemodynamic support during the brain death period to maintain adequate perfusion before allograft harvest.
Orthotopic lung transplantation
Orthotopic left lung transplantation was performed as previously described (7). In brief, under general anesthesia a left thoracotomy was performed and the recipient hilar structures isolated. Heparin was administered (300 U/kg) and a pneumonectomy was performed. The donor lung was approached by median sternotomy. Heparin was administered (300 U/kg) and the heart and lung cooled with iced saline. The left lung was flushed in situ with 4 L of cold Euro–Collins solution (Fresenius Medical Care AG; Bad Homburg, Germany) containing prostaglandin E1 (500 μg/L). The lung was prepared surgically and immediately transplanted. A thoracostomy tube was placed to evacuate the pleural space and was subsequently removed after recovery from anesthesia. Two indwelling silastic catheters were placed in the external jugular veins. One catheter was used for the administration of tacrolimus and other medications; the second catheter was used to obtain blood for daily clinical monitoring and in vitro studies.
Isolation of PBMCs
Heparinized whole blood was diluted with HBSS (Life Technologies; Grand Island, NY, USA). The mononuclear cells were extracted by gradient centrifugation with lymphocyte separation media (Organon Teknika; Durham, NC, USA). The mononuclear cells were then washed with HBSS and any contaminating red blood cells were lysed with an ammonium chloride-potassium buffer (B&B Research Laboratories; Fiskeville, RI, USA). The mononuclear cells were washed with HBSS after lysis and resuspended in tissue culture media appropriate for the in vitro assays to be performed.
Mixed lymphocyte response assays
Mixed lymphocyte reaction (MLR) responses to self, donor and third-party were determined in a single assay for each animal. MLR media consisted of RPMI 1640 (Life Technologies) supplemented with 6% fetal pig serum (Sigma; St. Louis, MO, USA), 100 U/mL penicillin (GIBCO-Invitrogen Corporation; Carlsbad, CA, USA), 135 μg/mL streptomycin (GIBCO-Invitrogen Corporation), 50 μg/mL gentamicin (GIBCO-Invitrogen Corporation), 10 mM HEPES (Cellgro Mediatech, Inc.; Manassas, VA, USA), 2 mM l-glutamine (Life Technologies), 1 mM sodium pyruvate (BioWhittaker–Cambrex; East Rutherford, NJ, USA), nonessential amino acids (BioWhittaker–Cambrex) and 5 × 10−5 M 2-–mercaptoethanol (Sigma). Cultures containing 4 × 106 responder and 4 × 106 irradiated (2500 cGy) stimulator PBMCs were incubated in 200 μL of media in 96-well flat-bottomed plates (Costar Corning; Lowell, MA, USA) for 5 days at 37°C and 6% CO2. After the 5-day incubation, 1 μCi of [3H]-thymidine was added to each well, followed by an additional 5-h incubation under the same conditions. [3H]-thymidine incorporation was determined in triplicate samples by β-scintillation counting. Absolute counts were compensated for background and then expressed as stimulation indices (SI), calculated as SI = average cpm for a responder–stimulator pair per cpm of the same responder stimulated by an autologous stimulator.
Histology
Biopsies were placed in 10% formalin. Tissue was then imbedded in paraffin and stained with H&E and Verhoeff (elastin) stains. A pathologist evaluated all specimens in a blinded fashion. Acute cellular rejection was scored on the basis of the current ISHLT nosology (grade A0–A4; Ref. 8).
ELISA
Solid-phase sandwich ELISAs were performed, according to the manufacturer’s instructions, using swine-specific assay kits (Biosource, Camarillo, CA, USA) for TNF-α, IFN-γ, TGF-β, IL-1, IL-4 and IL-10. Standard curves were created from control concentrations run in duplicate; experimental assays were run in triplicate.
qPCR
Lung biopsy specimens were submerged in RNAlater (Qiagen, Valencia, CA, USA) and snap-frozen in liquid nitrogen. Total RNA was extracted using the RNeasy kit (Qiagen) and reverse transcriptase reactions performed using the Invitrogen Superscript III system. qPCR reactions were run on the MX3000P qPCR system (Stratagene, La Jolla, CA, USA), using the SYBR-Green (Stratagene) reagent. Reaction conditions were optimized and cycles began at 95°C for 3 min, followed by 50 cycles, with a 20-s denaturation step at 95°C, a 30-s annealing step at 55°C and a 30-s extension step at 72°C, during which data were collected. Primer specificity was confirmed with dissociation curve analysis and gel electrophoresis.
qPCR primers were synthesized based on previously published reports of swine-specific assays for IL-1, IL-6, TNF-α, IFN-γ and GAPDH (9,10). Swine-specific primers for TLR-2 and TLR-4 were synthesized based on known homology of mouse and human sequences.
Statistical Analyses
Mann–Whitney U tests and Kaplan–Meier analyses were used to compare graft survival times. Differences in survival time were deemed significant when p < 0.05. Comparisons of cytokine levels before and after brain death were performed using a Student’s t-test. Relative quantification of PCR products was performed using the “delta-delta C(T)” method (11).
Results
Clinical course
We previously reported that five of six animals receiving fully MHC-disparate pulmonary allografts from healthy donors under optimal conditions accepted their grafts long-term (> 1 year) when treated with a short course of high-dose tacrolimus, (Table 1, Group 3; Ref. 3). In contrast, the four experimental animals that received lung grafts from brain dead donors all died of acute cellular rejection by postoperative day (POD) 45 (Table 1, Group 1). The group of animals subjected to 4 h of ventilation without brain death showed an intermediate median survival of 120 days with a high incidence of pneumonia (Table 1, Group 2). Kaplan–Meier survival analyses of uncensored data demonstrated a significant difference between swine transplanted with lungs from healthy donors (Group 3) versus lungs from brain dead donors (Group 1; p = 0.001; Figure 1). The same finding was true for lungs from donors that underwent 4 h of mechanical ventilation alone without brain death (Group 2) versus lungs from brain dead donors (Group 1; p = 0.004).
Table 1.
Summary of outcomes of grafts from brain dead donors (Group 1), donors subjected to mechanical ventilation only (Group 2) and healthy donors (Group 3; previously reported in 3)
| Donor treatment | Recipient/donor animal | Graft survival | Final pathology |
|---|---|---|---|
| Group 1: Brain death + 4 h of ventilation | #17320/#17421 | 311 | ISHLT A4 |
| Group 1: Brain death + 4 h of ventilation | #17447/#17495 | 451 | ISHLT A32 |
| Group 1: Brain death + 4 h of ventilation | #17509/#17551 | 191 | ISHLT A4 |
| Group 1: Brain death + 4 h of ventilation | #17575/#17626 | 421 | ISHLT A4 |
| Group 2: 4 h of ventilation, no brain death | #17555/#17651 | 763 | ISHLT A0 |
| Group 2: 4 h of ventilation, no brain death | #17552/#17658 | 323 | ISHLT A0 |
| Group 2: 4 h of ventilation, no brain death | #17556/#17722 | >5164 | ISHLT A0 |
| Group 2: 4 h of ventilation, no brain death | #17721/#17987 | 1201 | ISHLT A4 |
| Group 2: 4 h of ventilation, no brain death | #18552/#18559 | >1354 | ISHLT A0 |
| Group 3: No brain death, <1-h ventilation | #15515/#15576 | >5154 | ISHLT A2 |
| Group 3: No brain death, <1-h ventilation | #15616/#15606 | 1031 | ISHLT A4 |
| Group 3: No brain death, <1-h ventilation | #15841/#15696 | >4294 | ISHLT A2 |
| Group 3: No brain death, <1-h ventilation | #15843/#15789 | >4811 | ISHLT A0 |
| Group 3: No brain death, <1-h ventilation | #15895/#15786 | >3894 | ISHLT A0 |
| Group 3: No brain death, <1-h ventilation | #16052/#16128 | >4384 | ISHLT A0 |
Graft lost because of ACR.
With foci of A4 ACR.
Animal sacrificed for severe pneumonia without evidence of ACR.
Sacrificed with viable graft.
Figure 1. Allograft survival.
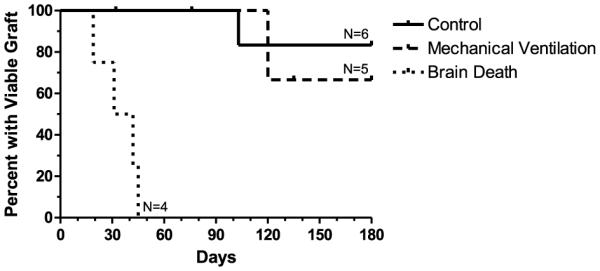
Allografts from brain dead donors (Group 1, n = 4) demonstrate accelerated rejection as compared to historical controls (Group 3; n = 6; p 0.001) and when compared to donor animals that were subjected to mechanical ventilation only (n = 5; p = 0.004).
Pathology
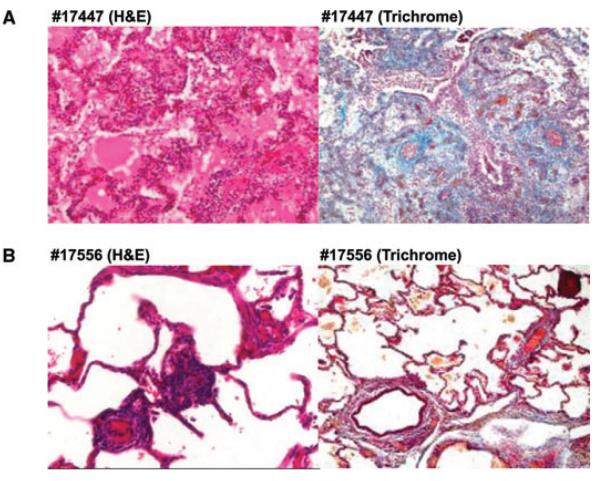
Three animals in Group 1 (#17320, #17509 and #17575), demonstrated International Society for Heart and Lung Transplantation (ISHLT) ACR grade A4 rejection at necropsy and one animal (#17447) demonstrated grade A3 ACR with foci of grade A4 rejection (Figure 2A). In contrast, only one of the animals in Group 2 (#17721) lost its allograft to acute cellular rejection, which occurred on POD 120. Two other recipients in Group 2 (#17555 and #17552) died of pneumonia, without evidence of significant cellular rejection. A fourth animal (#17556) accepted its allograft for over 1 year with no evidence of rejection (Figure 2B). This animal subsequently accepted a donor-matched kidney allograft without immunosuppression (data not shown). The fifth animal in Group 2 (#18552) also accepted its graft long term and was electively sacrificed on POD 135 with a normal functioning healthy allograft.
Figure 2. Graft histology.
(A) Animal #17447 (recipient of lung from a brain dead donor [Group 1]) demonstrates severe acute cellular rejection (ISHLT A4) 45 days after transplantation. The lung was not viable and the animal was sacrificed. (B) Animal #17556 (recipient of lung from an animal subjected to 4 h of mechanical ventilation (Group 2) demonstrates no evidence of rejection (ISHLT A0) at the time of sacrifice (POD 516).
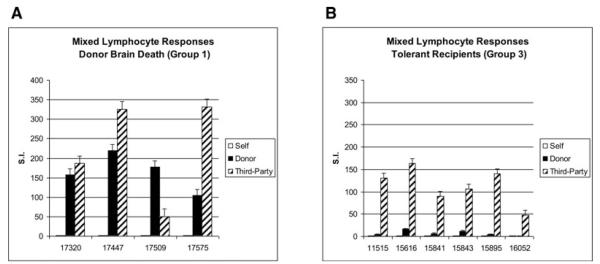
Mixed lymphocyte responses
Figure 3A shows the MLR responses of each of the recipients of the brain dead lung allografts (Group 1) at the time of sacrifice. Consistent with graft rejection, these animals show strong responses to both donor and third-party stimulators. In contrast, MLRs performed at a similar time point in the historical tolerant controls (Group 3) show donor-specific hyporesponsiveness (Figure 3B; data not previously shown).
Figure 3. Mixed lymphocyte responses.
(A) The MLR responses (mean±SD) of each of the recipients of the brain dead lungs (Group 1) at the time of sacrifice is shown (n = 4). As expected, these animals show strong responses to both donor and third-party stimulators. (B) In contrast, MLRs performed at a similar time point in the historical tolerant controls (Group 3) show donor-specific hyporesponsiveness (n = 6).
Cytokine and TLR assays: ELISA
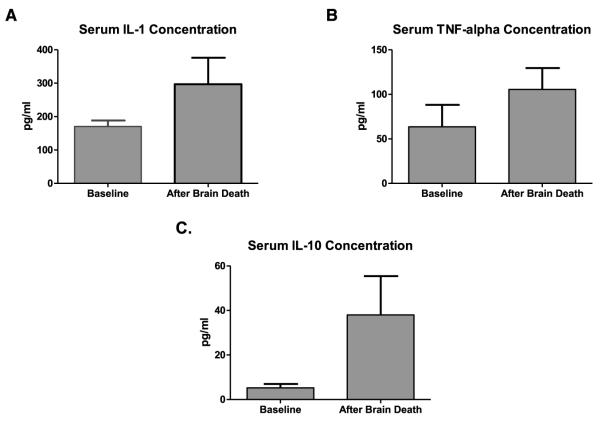
ELISA was performed on donor serum of four additional swine to evaluate the effects of brain death and mechanical ventilation on a panel of relevant cytokines. Serum was collected before the induction of brain death and compared to serum collected 4 h later. There were reproducible increases in serum concentrations of IL-1, TNF-α and IL-10 after the induction of brain death (Figure 4). Four hours of mechanical ventilation on two additional swine under general anesthesia had no significant effect on serum cytokine levels (data not shown).
Figure 4. Serum cytokines.
Significant increases in serum concentrations of (A) IL-1, (B) TNF-α and (C) IL-10 after the induction of brain death are shown (171 ± 17.9 pg/mL vs. 297 ± 79.2 pg/mL [p = 0.046]; 63.5 ± 24.6 pg/mL vs. 105 ± 24.0 pg/mL [p ± 0.050]; 5.25 ± 1.71 pg/mL vs. 38.0 ± 17.4 pg/mL [p = 0.031]; respectively; n = 4 in all cases).
Cytokine and TLR assays: quantitative PCR (qPCR)
PCR assessments of lung tissue obtained before and 4 h after the induction of brain death (n = 4) showed marked elevations in the expression of the proinflammatory genes for IL-1 and IL-6 (10.85 ± 1.3 and 16.37 ± 4.0-fold increases ±SD, respectively). Lesser increases were seen in TNF and IFN-gamma gene expression (2.94 ± 2.9 and 2.39 ± 2.8 fold increases ±SD, respectively). The expression of TLR-2 and TLR-4 was also elevated (1.95 ± 3.5 and 4.63 ± 2.2 fold increases ±SD, respectively).
Discussion
The Massachusetts General Hospital miniature swine herd is a preclinical large-animal model that has been used in the study of transplantation for over 30 years. After the discovery that a short course of high-dose cyclosporine induces tolerance to MHC class-I disparate renal allografts (12), a similar approach was also successfully applied to fully allogeneic kidney transplants using tacrolimus (13). Our group has recently reported that 12 days of high-dose tacrolimus was sufficient to induce tolerance to fully MHC-disparate pulmonary allografts (3). Five animals (83%) treated in this manner accepted their allografts long-term (> 1 year) and demonstrated donor-specific hyporesponsiveness by various assays of immune alloreactivity.
Similar to other animal models, however, these experiments used healthy donor and recipient pairs. Although this approach may lend itself to preclinical investigations relevant to living-donor transplantation, conclusions may not be applicable to the cadaveric donor transplant, where the overwhelming majority of donors have suffered a major neurologic insult.
The reproducible effects of increased intracranial pressure were first described by Cushing (14). Numerous subsequent reports in different experimental models have characterized the systemic effects of brain death, as well as the nonspecific inflammatory response that ensues. In experimental models of explosive brain death, rapid inflation of an intracranial Foley catheter leads to cerebral ischemia because of brainstem herniation. A transient period of autonomic dysregulation ensues, characterized by hypertension and tachycardia. Subsequently, hemodynamic parameters normalize, but eventually vasomotor tone and cardiac function diminish, and cardiovascular collapse occurs.
The immunologic consequences of brain death, relevant to clinical transplantation, can be viewed in terms of systemic and organ-specific effects. Changes in IL-1, IL-6, TNF-α, ICAM-1, VCAM-1 and numerous other inflammatory products have been documented (2, 15–18). Despite subtle differences among different experimental models, it is readily apparent that brain death results in significant changes in the expression of cytokines, cellular adhesion molecules and inflammatory cell products.
In this study, we have evaluated whether the immune activation that follows brain death impacts the induction of tolerance in our large-animal model of lung transplant tolerance. We demonstrate here that explosive brain death before organ procurement is sufficient to inhibit the induction of tolerance. All four animals treated transplanted with lungs from brain dead donors acutely rejected within 45 days and donor-specific immune responses persisted. This is in stark contrast to historic controls, as well as the concurrent series of animals presented here that were subjected to the 4-h period of ventilation without brain death. These data are consistent with a recent report showing that brain death abrogates tolerance in a rat kidney model and extend this finding to a fully mismatched large-animal construct (19).
Our in vitro data from animals undergoing brain death demonstrate that brain death leads to increased circulating levels of IL-1, TNF-α and IL-10. In lung tissue, IL-1 and IL-6 expression is consistently upregulated. These findings do not necessarily suggest a mechanism of tolerance inhibition, but rather confirm that donor brain death leads to an altered immune profile in donor serum and the lung allograft itself. It is interesting to note that although the detrimental effects of IFN-γ on allografts can be blocked by tolerance induction (20), this particular cytokine does not seem to be a key mediator of brain death related rejection. Also interesting was the finding that some recipients of lungs from brain dead donors demonstrated a persistent response to donor cells by MLR assay, which was not the case in our previously reported tolerant series. This result demonstrates that innate immune activation enhances alloimmunity and that a relatively brief period of physiologic dysregulation and inflammation can have a profound impact on the nature of the immune response (rejection vs. tolerance).
In summary, we have demonstrated that donor brain death abrogates tolerance induction in fully allogeneic lung transplantation in miniature swine. Subsequent research will attempt to define the mechanism behind this effect. More-over, we hope to use this model to develop protocols that will permit tolerance induction despite donor brain death, as successful tolerance protocols in clinical thoracic transplantation must take into account the significant impact of donor brain death.
Acknowledgments
Supported by NIH R01HL67110 (J.S.A.), NIH R01HL45211 (J.C.M.) and fellowships from the Thoracic Surgery Foundation for Research and Education (K.M.K.) and the Yoshida Scholarship Foundation (A.A.).
The authors would like to thank Scott Arn for his support in managing the inbred miniature swine herd and for his role as Senior Technical Specialist.
Abbreviations
- ACR
acute cellular rejection
- CML
cell-mediated lympholysis
- ISHLT
International Society for Heart and Lung Transplantation
- MLR
mixed lymphocyte reaction
- POD
postoperative day
- qPCR
quantitative polymerase chain reaction
Footnotes
Disclosures The authors have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333:333–336. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 2.Mertes PM. Physiology of Brain Death. In: Tilney NL, Strom TB, Paul LC, editors. Transplantation biology: Cellular and molecular aspects. Lippincott-Raven; Philadelphis, PA: 1996. pp. 275–289. [Google Scholar]
- 3.Shoji T, Sahara H, Muniappan A, et al. An MHC class II disparity raises the threshold for tolerance induction in pulmonary allografts in miniature swine. Transplant Proc. 2006;38:3268–3270. doi: 10.1016/j.transproceed.2006.10.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachs DH, Leight G, Cone J, Schwarz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Leight GS, Sachs DH, Rosenberg SA. Transplantation in miniature swine. II. In vitro parameters of histocompatibility in MSLA homozygous minipigs. Transplantation. 1977;23:271–276. [PubMed] [Google Scholar]
- 6.Sachs DH. MHC homozygous miniature swine. In: Swindle MM, Moody DC, Phillips LD, editors. Swine as models in biomedical research. Iowa State University Press; Ames, IA: 1992. pp. 3–15. [Google Scholar]
- 7.Allan JS, Wain JC, Schwarze ML, et al. Modeling chronic lung allograft rejection in miniature swine. Transplantation. 2002;73:447–453. doi: 10.1097/00007890-200202150-00020. [DOI] [PubMed] [Google Scholar]
- 8.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Duvigneau JC, Hartl RT, Groiss S, Gemeiner M. Quantitative simultaneous multiplex real-time PCR for the detection of porcine cytokines. J Immunol Methods. 2005;306:16–27. doi: 10.1016/j.jim.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Watters JM, Tieu BH, Todd SR, et al. Fluid resuscitation increases inflammatory gene transcription after traumatic injury. J Trauma. 2006;61:300–308. doi: 10.1097/01.ta.0000224211.36154.44. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Rosengard BR, Ojikutu CA, Guzzetta PC, et al. Induction of specific tolerance to class I disparate renal allografts in miniature swine with cyclosporine. Transplantation. 1992;54:490–497. doi: 10.1097/00007890-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Utsugi R, Barth RN, Lee RS, et al. Induction of transplantation tolerance with a short course of tacrolimus (FK506): I. Rapid and stable tolerance to two-haplotype fully MHC-mismatched kidney allografts in miniature swine. Transplantation. 2001;71:1368–1379. doi: 10.1097/00007890-200105270-00003. [DOI] [PubMed] [Google Scholar]
- 14.Cushing H. Some experimental and clinical observations concerning states of increased intracranial tension. Am J Med Sci. 1902;124:375–400. [Google Scholar]
- 15.Mertes PM. Physiology of Brain Death. In: Tilney NL, Strom TB, Paul LC, editors. Transplantation biology: Cellular and molecular aspects. Lippincott-Raven; Philadelphis, PA: 1996. p. 289. [Google Scholar]
- 16.Pratschke J, Wilhelm MJ, Kusaka M, et al. Brain death and its influence on donor organ quality and outcome after transplantation. Transplantation. 1999;67:343–348. doi: 10.1097/00007890-199902150-00001. [DOI] [PubMed] [Google Scholar]
- 17.Kaminska D, Tyran B, Mazanowska O, et al. Cytokine gene expression in kidney allograft biopsies after donor brain death and ischemia-reperfusion injury using in situ reverse-transcription polymerase chain reaction analysis. Transplantation. 2007;84:1118–1124. doi: 10.1097/01.tp.0000287190.86654.74. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Fructuoso A, Naranjo GP, Calvo RN, et al. Effect of the brain-death process on acute rejection in renal transplantation. Transplant Proc. 2007;39:2214–2216. doi: 10.1016/j.transproceed.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 19.Francuski M, Reutzel-Selke A, Weiss S, et al. Donor brain death significantly interferes with tolerance induction protocols. Transpl Int. 2009;22:482–93. doi: 10.1111/j.1432-2277.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoerbelt R, Benjamin LC, Shoji T, et al. The effects of tolerance on allograft damage caused by the innate immune system. Transplantation. 2008;85:314–322. doi: 10.1097/TP.0b013e3181629b05. [DOI] [PMC free article] [PubMed] [Google Scholar]