Abstract
Functional carbon-based nanomaterials (CBNs) have become important due to their unique combinations of chemical and physical properties (i.e., thermal and electrical conductivity, high mechanical strength, and optical properties), extensive research efforts are being made to utilize these materials for various industrial applications, such as high-strength materials and electronics. These advantageous properties of CBNs are also actively investigated in several areas of biomedical engineering. This Perspective highlights different types of carbon-based nanomaterials currently used in biomedical applications.
Graphite is one of the oldest and most widely used natural materials. More traditionally known as the main ingredient of pencil lead, from which the name “graphite” originated, it is now more widely used in several large-scale industrial applications, such as carbon raising in steelmaking, battery electrodes, and industrial-grade lubricants.1 Due to its high demand, the consumption of synthetic graphite has significantly increased in recent years. Extensive scientific investigation into graphite has revealed that its unique combination of physical properties stems from its macromolecular structure, which consists of stacked layers of hexagonal arrays of sp2 carbon.1
With the deeper appreciation and development of nanofabrication techniques and nanomaterials that have progressed within the last two decades, graphite is now being actively used as a starting material to engineer various types of carbon-based nanomaterials (CBNs), including single or multi-walled nanotubes, fullerenes, nanodiamonds, and graphene (Fig. 1).2 These CBNs possess excellent mechanical strength, electrical and thermal conductivity, and optical properties; much of the research efforts have been focused on utilizing these advantageous properties for various applications, such as high-strength composite materials and electronics.1,2
Figure 1.

Various types of carbon-based nanomaterials.
The field of biomedical engineering has also embraced the growing popularity and influence of CBNs in recent years, because many of its applications rely heavily on the performance of biomaterials. Carbon-based nanomaterials have been widely regarded as highly attractive biomaterials due to their multi-functional nature. In addition, incorporating CBNs into existing biomaterials could further augment their functions. Therefore, CBNs have found their way into many areas of biomedical research, including drug delivery systems, tissue scaffold reinforcements, and cellular sensors.3
Carbon nanotubes
Ever since their discovery, carbon nanotubes (CNTs) have become the most widely used CBNs.4,5 Carbon nanotubes are commonly synthesized by arc discharge or chemical vapor deposition of graphite. They have a cylindrical carbon structure, and possess a wide range of electrical and optical properties stemming not only from their extended sp2-carbon, but also from their tunable physical properties (e.g., diameter, length, single-walled vs. multi-walled, surface functionalization, and chirality).5 Due to the diverse array of their useful properties, CNTs have been explored for use in many industrial applications.6 For example, CNTs are well known for their superb mechanical strength: their measured rigidity and flexibility are greater than that of some commercially available high-strength materials (e.g., high tensile steel, carbon fibers, and Kevlar®). Thus, they have been utilized as reinforcing elements for composite materials such as plastics and metal alloys, which have already led to several commercialized products.7 However, the possibility of CNT-incorporated composites as super high-strength load-bearing materials has not been met with satisfactory results, mostly due to their poor interaction with the surrounding matrices, which leads to inefficient load transfer from the matrices to the CNTs.7
Many recent research efforts have been geared toward incorporating CNTs into various materials to utilize their multi-functional nature (i.e., electrical and thermal conductivity, and optical properties) rather than focusing purely on composite mechanical strength. For example, the excellent electrical properties of CNTs coupled with their nanoscale dimensions are of great interest in electronics for the construction of nanoscale electronic circuitry.8,9 In addition, CNTs are known to have low threshold electric fields for field emission, as compared with other common field emitters.10,11 Thus, CNTs are actively explored in high-efficiency electron emission devices such as electron microscopes, flat display panels, and gas-discharge tubes. Carbon nanotubes also display strong luminescence from field emission, which could be used in lighting elements.9
Carbon nanotubes in Biomedical Engineering
There is considerable interest in using CNTs for various biomedical applications. The physical properties of CNTs, such as mechanical strength, electrical conductivity, and optical properties, could be of great value for creating advanced biomaterials.3 Carbon nanotubes can also be chemically modified to present specific moieties (e.g., functional groups, molecules, and polymers) to impart properties suited for biological applications, such as increased solubility and biocompatibility, enhanced material compatibility, and cellular responsiveness.12 Their applications include cell and tissue labeling agents, injectable drug delivery systems, and biomaterial reinforcements.
Cell and tissue labeling and imaging
The possibility of using CNTs as labeling and imaging agents has been discussed since their discovery due to their unique optical properties. Carbon nanotubes have optical transitions in the near-infrared (NIR) region, which has been shown to be useful in biological tissue because NIR has greater penetration depth and lower excitation scattering.3 In addition, fluorescence in the NIR region displays much lower autofluorescence than do the ultraviolet or visible ranges. These properties make CNTs potent imaging agents with higher resolution and greater tissue depth for NIR fluorescence microscopy and optical coherence tomography. For example, Cherukuri et al. successfully monitored CNTs in phagocytic cells and those intravenously administered into mice using NIR fluorescence.13
Raman spectroscopy has been used extensively to characterize the structural features of CNTs, as they are highly sensitive to Raman scattering because of their extensive symmetric carbon bonds.14 The characteristic Raman signatures of CNTs have also been utilized as cellular probes. For example, Liu et al. detected CNTs in various tissues after intravenous delivery into mice using Raman spectroscopy.15
Drug delivery systems
Drug delivery has benefited greatly from the advances in nanotechnology by using a variety of nanomaterials (i.e., liposomes, polymersomes, microspheres, and polymer conjugates) as vehicles to deliver therapeutic agents.16 Carbon nanotubes have also been investigated extensively as drug delivery systems, since CNTs have been shown to interact with various biomacromolecules (i.e., proteins and DNA) by physical adsorption.17 In addition, several chemical modification schemes have been developed to conjugate therapeutic molecules or targeting moieties covalently to CNTs.12
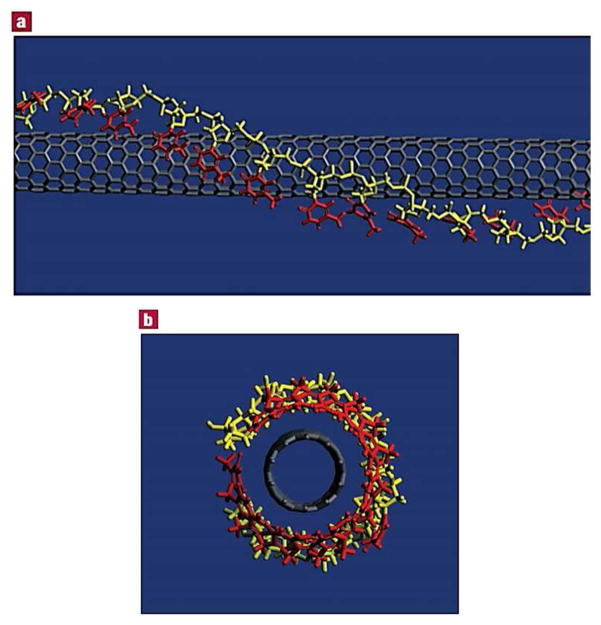
In one interesting study, Zheng et al. provided important insight into the interactions between CNTs and DNA molecules.18 Carbon nanotubes were shown to be effectively dispersed in aqueous media in the presence of single-stranded DNA (ssDNA). Spectroscopic and microscopic analyses provided evidence of strong interactions between DNA molecules and CNTs, resulting in individual dispersion. Molecular dynamics modeling showed that the base of ssDNA interacted with the surface of CNT via π–π stacking, resulting in helical wrapping of ssDNA chains around the CNT (Fig. 2). This research highlights the potential use of CNTs for gene delivery, as well as DNA-specific separation techniques for molecular electronics.
Figure 2.
Binding model of a carbon nanotube (CNT) with a poly(T) DNA sequence. The DNA wraps around the CNT in a right-handed helical structure. The bases (red) orient to stack with the surface of the nanotube, and extend away from the sugar-phosphate backbone (yellow). The DNA wraps to provide a tube within which the CNT can reside, hence converting it into a water-soluble object. Reprinted with permission from ref. 18. Copyright 2003 Nature Publishing Group.
Reinforcing tissue engineering scaffolds
The use of CNTs as composite reinforcements for tissue engineering scaffolds to date has primarily been focused on enhancing their mechanical properties.19 Commonly used scaffold materials, such as hydrogels and fibrous scaffolds, are inherently soft in order to mimic the stiffness of natural tissues and thus often lack structural strength and support. Incorporating CNTs into these materials has been shown to enhance their mechanical properties. For example, Shin et al. demonstrated that incorporating CNTs into photocrosslinkable gelatin hydrogels resulted in significant increases in their tensile strengths (Fig. 3).20 Sen et al. also demonstrated improvement in the tensile strengths of CNT-reinforced polystyrene and polyurethane fibrous membranes.21
Figure 3.
(a) Schematic description of gelatin methacrylate (GelMA) coated onto a carbon nanotube (CNT). (b) High resolution TEM image of CNT-GelMA. (c) Stress-strain curves of CNT-GelMA hydrogels with various concentrations of CNTs. (d) Tensile moduli of CNT-GelMA hydrogels. (e) 3T3 fibroblasts cultured on GelMA hydrogel (A) and CNT-GelMA hydrogel (B).(Scale bar: 100 μm) Reprinted from ref. 20. Copyright 2011 American Chemical Society.
More recently, researchers have turned their attention to utilizing the multi-functional nature of CNTs in engineering tissue scaffolds. Most notably, CNTs have been incorporated to fabricate electrically conductive scaffolds. Most of the biomaterials used for tissue engineering applications are electrically insulating, as they are made from non-conductive polymers.22–24 However, certain applications, such as neural and cardiac tissues, would benefit greatly from conductive scaffolds that can effectively propagate electrical signals across the tissue constructs for proper electrophysiological functions. For example, Kam et al. applied electrical stimulation to neural stem cells (NSCs) grown on CNT-laminin composite films, and demonstrated improved action potentials of NSCs and differentiation into functional neural networks.23 Shin et al. cultured cardiomyocytes on CNT-reinforced gelatin hydrogel and observed their enhanced electrophysiological activities, and ultimately developed functional cardiac tissue.25 These works highlight the ability of CNTs to impart electrical conductivity successfully to otherwise non-conductive biomaterials.
Cytotoxicity of CNTs
Despite many successful applications in biomedical engineering, there is a growing concern for safety with CNTs. Some recent in vitro studies have reported increased cytotoxicity of CNTs due to their cellular uptake, agglomeration, and induced oxidative stress.26–28 These conflicting results regarding the biocompatibility of CNTs largely stem from the variability of CNTs (i.e., size, surface properties, and functionalization) and testing subjects (i.e., in vitro vs. in vivo, types of cells, tissues, and animals tested). In addition, increased cytotoxicity has often been attributed to incomplete removal of metal catalysts used to prepare CNTs.27 Most in vivo studies using CNTs have shown that they did not cause significant toxicity and reported renal clearance from the body, although small portions of CNTs have been shown to accumulate in certain organs, such as lungs, liver, and spleen, and may cause inflammation.26–28 However, the cytotoxicity seems to be more highly variable and more pronounced at the cellular level, based on several in vitro cell culture studies.29,30
With the continued growth of CNTs in various biomedical fields, more systematic biological evaluations of CNTs having various chemical and physical properties are warranted in order to determine their precise pharmacokinetics, cytotoxicity, and optimal dosages. Nevertheless, many studies have shown that toxicity can effectively be minimized by functionalizing the CNTs with biocompatible polymers or surfactants to prevent aggregation.31,32
Graphene
Graphene is the latest nanomaterial to burst onto the scene. The ground-breaking work by Geim and Novoselov provided a simple method for extracting graphene from graphite via exfoliation and explored its unique electrical properties.33,34 Graphene and CNTs possess similar electrical, optical, and thermal properties, but the two-dimensional atomic sheet structure of graphene enables more diverse electronic characteristics; the existence of quantum Hall effect and massless Dirac fermions help explain the low-energy charge excitation at room temperature and the optical transparency in infrared and visible range of the spectrum.34 In addition, graphene is structurally robust yet highly flexible, which makes it attractive for engineering thin, flexible materials.35,36
Graphene in Biomedical Engineering
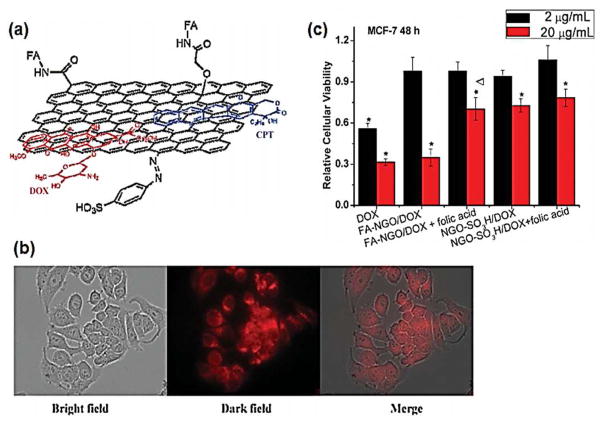
Research into utilizing graphene for biomedical applications has been limited to date, as graphene research itself is still in its infancy. Graphene oxide (GO), produced by oxidation of graphite under acidic conditions, is more commonly used, as it offers several advantages over using pure graphene.37 First, GO is dispersible in aqueous media, which is essential for biological applications. Second, GO presents hydrophilic functional groups that enable chemical functionalization. Third, GO has broader ranges of physical properties than pure graphene due to its structural heterogeneity. Several biomedical applications including injectable cellular labeling agents, drug delivery systems, and scaffold reinforcements have been explored using GO, as has similarly been done with CNTs.38 For example, Zhang et al. functionalized GO with folic acid (FA) as a cancer targeting molecule and loaded doxorubicin and camptothecin, known cancer drugs, onto the large surface area of GO via π–π stacking (Fig. 4).39 The drug-loaded FA-GO showed improved cancer targeting capability and anti-cancer activity as compared with drugs delivered alone or drugs carried with unmodified GO. Sun et al. also demonstrated that poly(ethylene glycol)-conjugated GO with targeting molecules could be used as a cellular sensor by utilizing the intrinsic photoluminescence property of GO at the NIR region.40 In another study, Zhang et al. incorporated GO into poly(vinyl alcohol) hydrogels to improve their mechanical strength.41
Figure 4.
(a) Graphene oxide (GO) was funtionalized with folic acid (FA) as a targeting molecule (FA-NGO), then loaded with anti-cancer drug, doxorubicin (DOX) or camptothecin (CPT). (b) FA-NGO was localized into MCF-7 (human breast cancer) cells, identified with fluorescent labeling with rhodamine. (c) Greater anti-cancer activity of DOX was observed when FA-NGO was used as a drug carrier. Reprinted with permission from ref. 39. Copyright 2010 Wiley-VCH Verlag GmbH & Co.
Other carbon-based nanomaterials
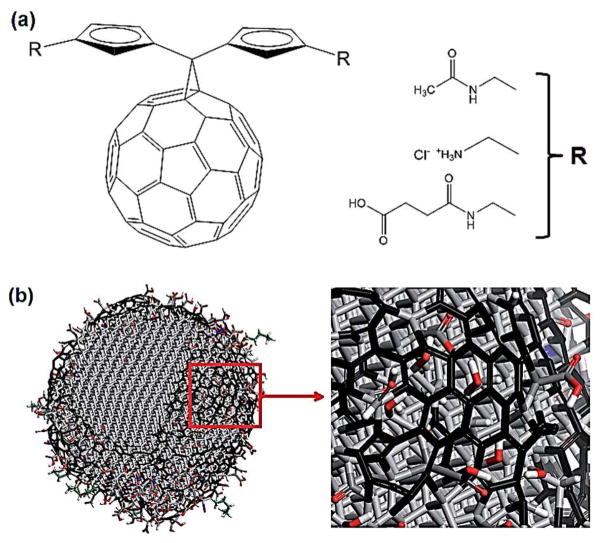
Buckminsterfullerene (C60), also commonly known as the buckyball, is a spherical closed-cage structure (truncated icosahedron) made of sixty sp2 carbon. Its discovery in 1985 and subsequent investigation led to the uncovering of electronic properties, stemming from its highly symmetrical structure, and potential applications, culminating in a Nobel Prize in 1996.42 It can be argued that the scientific pursuit of CBNs and their potential applications began with the discovery of C60. The popularity of C60 has somewhat diminished in recent years with the rise of more scalable and practical CBNs such as CNTs and graphene. However, its uniform size and shape as well as availability for chemical modification led many scientists to develop C60 derivatives for therapeutic purposes.43 Perhaps the most fascinating and highly promising aspect of C60 is its anti-human immunodeficiency virus (HIV) activity. Schinazi et al. first discovered a group of water-soluble C60 derivatives capable of inhibiting HIV protease activity by binding to its active site, due to their unique molecular structure and hydrophobicity (Fig. 5a).44 Various C60 derivatives have since been developed that display anti-HIV activity by targeting other important HIV enzymes, such as reverse transcriptase.43 These results demonstrate that C60 p derivatives may become a potent group of AIDS therapeutics in the future.
Figure 5.
(a) A class of C60 derivatives that display anti-HIV activity.44 (b) Molecular structure of a 5 nm-diameter nanodiamond (ND). The ND is mostly made up of sp3 carbon, but the outer layer is functionalized with sp2 carbon and other functional groups. Reprinted with permission from ref. 45. Copyright 2012 Nature Publishing Group.
Nanodiamond (ND) has also generated interest in the field of biomedical engineering in recent years (Fig. 5b).45 Nanodiamonds are synthesized by high energy treatment of graphite, most commonly via detonation, and are smaller than 10 nm. They have similar physical properties as bulk diamond, such as fluorescence and photoluminescence, as well as biocompatibility. Unlike other CBNs, NDs are made up mostly of tetrahedral clusters of sp3-carbon. The surface of nanodiamonds, however, is functionalized with various functional groups or sp2-carbon for colloidal stability, which enables chemical modification for targeted drug and gene delivery and tissue labeling. For example, Lien et al. recently used fluorescent and magnetic NDs for cell labeling.46 Zhang et al. also demonstrated that polyethyleneimine (PEI)-conjugated NDs were highly effective as gene carriers, without the cytotoxicity associate with PEI alone.47
Conclusions and Future Outlook
Extensive research efforts over the last two decades have elevated the status of CBNs as one of the most widely used classes of nanomaterials. Owing to their unique combinations of mechanical, optical, and electrical properties, CBNs have been explored in various industrial applications. Several areas of biomedical engineering have also benefited greatly from CBNs in recent years, since incorporating CBNs is effective not only as injectable nanoscale devices, but also as components to enhance the function of existing biomaterials significantly. Despite safety concerns over CBNs, many studies have reported the successful use of CBNs in biological applications. In addition, several chemical modification strategies have been developed to circumvent toxicity issues and to increase the biocompatibility and functionality of CBNs. Nevertheless, it should be noted that more systematic toxicology studies are needed to determine the toxicity and pharmacokinetics of CBNs.
This article has introduced several successful applications of CBNs in drug delivery, tissue imaging, and scaffold reinforcement. With the popularity of CBNs as highly versatile and useful nanomaterials, we expect to see continued use of CBNs in many facets of biomedical engineering. In particular, there is great promise in applying the biocompatible and multi-functional nature of CBNs to the areas that interconnect mechatronics and biology, such as microelectromechanical systems (‘MEMS’) for biological sensors and actuators.48 Furthermore, more recent studies suggest that CBNs may also be used to regulate cellular behavior.49,50 Although research efforts have largely been focused on utilizing CNTs, other types of CBNs—especially graphene, which have gained wide recognition in recent years—are expected to be investigated extensively in the near future.
Acknowledgments
The authors would like to acknowledge funding from the National Science Foundation CAREER Award (DMR 0847287), the Office of Naval Research Young Investigator Award, the National Institutes of Health (HL092836, DE019024, EB012597, AR057837, DE021468, HL099073, EB008392), and the Presidential Early Career Award for Scientists and Engineers (PECASE). N.A. acknowledges the support from the National Health and Medical Research Council of Australia.
Footnotes
Conflict of Interest
The authors claim no conflict of interest.
References
- 1.Pierson HO. Handbook of Carbon, Graphite, Diamond, and Fullerenes: Properties, Processing, and Applications. Noyes Publications; Park Ridge, New Jersey: 1993. [Google Scholar]
- 2.Krüger A. Carbon Materials and Nanotechnology. Wiley-VCH; Weinheim: 2010. [Google Scholar]
- 3.Harrison BS, Atala A. Carbon Nanotube Applications for Tissue Engineering. Biomaterials. 2007;28:344–353. doi: 10.1016/j.biomaterials.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 4.Iijima S, Ichihashi T. Single-Shell Carbon Nanotubes of 1-nm Diameter. Nat Nanotechnol. 1993;363:603–605. [Google Scholar]
- 5.Saito R, Dresselhaus G, Dresselhaus MS. Physical Properties of Carbon Nanotubes. Imperial College Press; London: 1998. [Google Scholar]
- 6.Baughman RH, Zakhidov AA, de Heer WA. Carbon Nanotubes–The Route Toward Applications. Science. 2002;297:787–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Watanabe K, Hernandez ER. Mechanical Properties, Thermal Stability and Heat Transport in Carbon Nanotubes. In: Jorio A, Dresselhaus G, Dresselhaus MS, editors. Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications. Springer; Berlin: 2008. [Google Scholar]
- 8.Choi WB, Bae E, Kang D, Chae S, Cheong B-h, Ko J-h, Lee E, Park W. Aligned Carbon Nanotubes for Nanoelectronics. Nanotechnology. 2007;15:S512–S516. [Google Scholar]
- 9.Endo M, Strano MS, Ajayan PM. Potential Applications of Carbon Nanotubes. In: Jorio A, Dresselhaus G, Dresselhaus MS, editors. Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications. Springer; Berlin: 2008. pp. 13–61. [Google Scholar]
- 10.Bonard JM, Salvetat JP, Stöckli T, Forró L, Châtelain A. Field Emission from Carbon Nanotubes: Perspectives for Applications and Clues to the Emission Mechanism. Appl Phys A. 1999;69:245–254. [Google Scholar]
- 11.Ajayan PM, Zhou OZ. Applications of Carbon Nanotubes. In: Dresselhaus MS, Dresselhaus G, Avouris P, editors. Carbon Nanotubes: Synthesis, Structure, Properties, and Applications. Springer-Verlag; New York: 2001. pp. 391–425. [Google Scholar]
- 12.Bianco A, Kostarelos K, Partidos CD, Prato M. Biomedical Applications of Functionalised Carbon Nanotubes. Chem Comm. 2005:571–577. doi: 10.1039/b410943k. [DOI] [PubMed] [Google Scholar]
- 13.Cherukuri P, Bachilo SM, Litovsky SH, Weisman RB. Near-Infrared Fluorescence Microscopy of Single-Walled Carbon Nanotubes in Phagocytic Cells. J Am Chem Soc. 2004;126:15638–15639. doi: 10.1021/ja0466311. [DOI] [PubMed] [Google Scholar]
- 14.Dresselhaus MS, Dresselhaus G, Jorio A, Souza Filho AG, Saito R. Raman Spectroscopy on Isolated Single Wall Carbon Nanotubes. Carbon. 2002;40:2043–2061. [Google Scholar]
- 15.Liu Z, Davis C, Cai W, He L, Chen X, Dai H. Circulation and Long-Term Fate of Functionalized, Biocompatible Single-Walled Carbon Nanotubes in Mice Probed by Raman Spectroscopy. Proc Nat Acad Sci US A. 2008;105:1410–1415. doi: 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable Polymeric Nanoparticles as Drug Delivery Devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Liu G, Jan MR. Ultrasensitive Electrical Biosensing of Proteins and DNA:3 Carbon-Nanotube Derived Amplification of the Recognition and Transduction Events. J Am Chem Soc. 2004;126:3010–3011. doi: 10.1021/ja031723w. [DOI] [PubMed] [Google Scholar]
- 18.Zheng M, Jagota A, Semke ED, Diner BA, Mclean RS, Lustig SR, Richardson RE, Tassi NG. DNA-Assisted Dispersion and Separation of Carbon Nanotubes. Nat Mater. 2003;2:338–342. doi: 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- 19.Sahithi K, Swetha M, Ramasamy K, Srinivasan N, Selvamurugan N. Polymeric Composites Containing Carbon Nanotubes for Bone Tissue Engineering. Int J Biol Macromol. 2010;46:281–283. doi: 10.1016/j.ijbiomac.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Shin SR, Bae H, Cha JM, Mun JY, Chen YC, Tekin H, Shin H, Farshchi S, Dokmeci MR, Tang S, Khademhosseini A. Carbon Nanotube Reinforced Hybrid Microgels as Scaffold Materials for Cell Encapsulation. ACS Nano. 2011;6:362–372. doi: 10.1021/nn203711s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen R, Zhao B, Perea D, Itkis ME, Hu H, Love J, Bekyarova E, Haddon RC. Preparation of Single-Walled Carbon Nanotube Reinforced Polystyrene and Polyurethane Nanofibers and Membranes by Electrospinning. Nano Lett. 2004;4:459–464. [Google Scholar]
- 22.Lau C, Cooney MJ, Atanassov P. Conductive Macroporous Composite Chitosan–Carbon Nanotube Scaffolds. Langmuir. 2008;24:7004–7010. doi: 10.1021/la8005597. [DOI] [PubMed] [Google Scholar]
- 23.Kam NWS, Jan E, Kotov NA. Electrical Stimulation of Neural Stem Cells Mediated by Humanized Carbon Nanotube Composite Made with Extracellular Matrix Protein. Nano Lett. 2008;9:273–278. doi: 10.1021/nl802859a. [DOI] [PubMed] [Google Scholar]
- 24.Worsley MA, Kucheyev SO, Kuntz JD, Hamza AV, Satcher JJH, Baumann TF. Stiff and Electrically Conductive Composites of Carbon Nanotube Aerogels and Polymers. J Mater Chem. 2009;19:3370–3372. [Google Scholar]
- 25.Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim Sb, Nikkhah M, Khabiry M, Azize M, Kong J, et al. Carbon-Nanotube-Embedded Hydrogel Sheets for Engineering Cardiac Constructs and Bioactuators. ACS Nano. 2013;7 doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang ST, Luo J, Zhou Q, Wang H. Pharmacokinetics, Metabolism and Toxicity of Carbon Nanotubes for Biomedical Purposes. Theranostics. 2012;2:271–282. doi: 10.7150/thno.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam C-w, James JT, McCluskey R, Arepalli S, Hunter RL. A Review of Carbon Nanotube Toxicity and Assessment of Potential Occupational and Environmental Health Risks. Crit Rev Toxicol. 2006;36:189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- 28.Firme CP, III, Bandaru PR. Toxicity Issues in the Application of Carbon Nanotubes to Biological Systems. Nanomed Nanotechnol Biol Med. 2010;6:245–256. doi: 10.1016/j.nano.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Sato Y, Yokoyama A, Shibata K-i, Akimoto Y, Ogino S-i, Nodasaka Y, Kohgo T, Tamura K, Akasaka T, Uo M, et al. Influence of Length on Cytotoxicity of Multi-Walled Carbon Nanotubes Against Human Acute Monocytic Leukemia Cell Line THP-1 In Vitro and Subcutaneous Tissue of Rats In Vivo. Mol BioSyst. 2005;1:176–182. doi: 10.1039/b502429c. [DOI] [PubMed] [Google Scholar]
- 30.Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S, Stark WJ, Bruinink A. The Degree and Kind of Agglomeration Affect Carbon Nanotube Cytotoxicity. Toxicol. Lett. 2007;168:121–131. doi: 10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Duch MC, Budinger GRS, Liang YT, Soberanes S, Urich D, Chiarella SE, Campochiaro LA, Gonzalez A, Chandel NS, Hersam MC, et al. Minimizing Oxidation and Stable Nanoscale Dispersion Improves the Biocompatibility of Graphene in the Lung. Nano Lett. 2011;11:5201–5207. doi: 10.1021/nl202515a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang K, Wan J, Zhang S, Zhang Y, Lee ST, Liu Z. In Vivo Pharmacokinetics, Long-Term Biodistribution, and Toxicology of PEGylated Graphene in Mice. ACS Nano. 2011;5:516–522. doi: 10.1021/nn1024303. [DOI] [PubMed] [Google Scholar]
- 33.Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA. Electric Field Effect in Atomically Thin Carbon Films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 34.Geim AK, Novoselov KS. The Rise of Graphene. Nat Mater. 2007;6:183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 35.Kim KS, Zhao Y, Jang H, Lee SY, Kim JM, Kim KS, Ahn JH, Kim P, Choi JY, Hong BH. Large-Scale Pattern Growth of Graphene Films for Stretchable Transparent Electrodes. Nature. 2009;457:706–710. doi: 10.1038/nature07719. [DOI] [PubMed] [Google Scholar]
- 36.Eda G, Fanchini G, Chhowalla M. Large-Area Ultrathin Films of Reduced Graphene Oxide as a Transparent and Flexible Electronic Material. Nat Nanotechnol. 2008;3:270–274. doi: 10.1038/nnano.2008.83. [DOI] [PubMed] [Google Scholar]
- 37.Dreyer DR, Park S, Bielawski CW, Ruoff RS. The Chemistry of Graphene Oxide. Chem Soc Rev. 2010;39:228–240. doi: 10.1039/b917103g. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Li Z, Wang J, Li J, Lin Y. Graphene and Graphene Oxide: Biofunctionalization and Applications in Biotechnology. Trends Biotechnol. 2011;29:205–212. doi: 10.1016/j.tibtech.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Xia J, Zhao Q, Liu L, Zhang Z. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small. 2010;6:537–544. doi: 10.1002/smll.200901680. [DOI] [PubMed] [Google Scholar]
- 40.Sun X, Liu Z, Welsher K, Robinson J, Goodwin A, Zaric S, Dai H. Nano-Graphene Oxide for Cellular Imaging and Drug Delivery. Nano Res. 2008;1:203–212. doi: 10.1007/s12274-008-8021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Wang Z, Xu C, Li Y, Gao J, Wang W, Liu Y. High Strength Graphene Oxide/Polyvinyl Alcohol Composite Hydrogels. J Mater Chem. 2011;21:10399–10406. [Google Scholar]
- 42.Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162–163. [Google Scholar]
- 43.Jensen AW, Wilson SR, Schuster DI. Biological Applications of Fullerenes. Bioorgan Med Chem. 1996;4:767–779. doi: 10.1016/0968-0896(96)00081-8. [DOI] [PubMed] [Google Scholar]
- 44.Schinazi RF, Sijbesma R, Srdanov G, Hill CL, Wudl F. Synthesis and Virucidal Activity of a Water-Soluble, Configurationally Stable, Derivatized C60 Fullerene. Antimicrob Agents Ch. 1993;37:1707–1710. doi: 10.1128/aac.37.8.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The Properties and Applications of Nanodiamonds. Nat Nanotechnol. 2012;7:11–23. doi: 10.1038/nnano.2011.209. [DOI] [PubMed] [Google Scholar]
- 46.Lien ZY, Hsu TC, Liu KK, Liao WS, Hwang KC, Chao JI. Cancer Cell Labeling and Tracking Using Fluorescent and Magnetic Nanodiamond. Biomaterials. 2012;33:6172–6185. doi: 10.1016/j.biomaterials.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Zhang XQ, Chen M, Lam R, Xu X, Osawa E, Ho D. Polymer-Functionalized Nanodiamond Platforms as Vehicles for Gene Delivery. ACS Nano. 2009;3:2609–2616. doi: 10.1021/nn900865g. [DOI] [PubMed] [Google Scholar]
- 48.Li C, Thostenson ET, Chou TW. Sensors and Actuators Based on Carbon Nanotubes and their Composites: A Review. Compos Sci Technol. 2008;68:1227–1249. [Google Scholar]
- 49.Shi X, Chang H, Chen S, Lai C, Khademhosseini A, Wu H. Regulating Cellular Behavior on Few-Layer Reduced Graphene Oxide Films with Well-Controlled Reduction States. Adv Funct Mater. 2012;22:751–759. [Google Scholar]
- 50.Li X, Liu H, Niu X, Yu B, Fan Y, Feng Q, Cui F-z, Watari F. The Use of Carbon Nanotubes to Induce Osteogenic Differentiation of Human Adipose-Derived MSCs In Vitro and Ectopic Bone Formation In Vivo. Biomaterials. 2012;33:4818–4827. doi: 10.1016/j.biomaterials.2012.03.045. [DOI] [PubMed] [Google Scholar]