Abstract
Melanocyte proliferation, dendrite formation, and pigmentation are controlled by paracrine factors, particularly following exposure to ultraviolet radiation (UVR). Little is known about autocrine factors for melanocytes. Prostaglandins activate signaling pathways involved in growth, differentiation and apoptosis. Prostaglandin E2 (PGE2) is the most abundant prostaglandin released by keratinocytes following UVR, and stimulates the formation of dendrites in melanocytes. Synthesis of PGE2 is controlled by cPLA2, which releases arachidonic acid from membranes, and COX-2 and prostaglandin E2 synthases (PGES), which convert arachidonic acid to PGH2 and PGH2 to PGE2, respectively. In this report we show that multiple irradiations of human melanocytes with UVR stimulates tyrosinase activity, independent of expression of a functional melanocortin 1 receptor, suggesting the presence of a non-melanocortin autocrine factor. Irradiation of melanocytes activated cPLA2, the rate-limiting step in eicosanoid synthesis, and stimulated PGE2 secretion. PGE2 increased cAMP production, tyrosinase activity and proliferation in melanocytes. PGE2 binds to four distinct G-protein coupled receptors (EP1–4). We show that EP4 receptor signaling stimulates cAMP production in melanocytes. Conversely, stimulation of the EP3 receptor lowered basal cAMP levels. These data suggest that relative levels or activity of these receptors controls effects of PGE2 on cAMP in melanocytes. The data are the first to identify PGE2 as an UVR-inducible autocrine factor for melanocytes that stimulates tyrosinase activity and proliferation, and to show that EP3 and EP4 receptor signaling have opposing effects on cAMP production, a critical signaling pathway that regulates proliferation and melanogenesis in melanocytes.
Keywords: PGE2, melanocyte, UVR, tyrosinase, prostaglandin
Introduction
Melanocytes are long lived cells that reside in the basal epidermal layer and produce melanin, a complex polymer, through a pathway in which tyrosinase activation is rate limiting. Melanin-containing melanosomes are transported along arborizing dendrites in melanocytes, and transferred to keratinocytes, where they form supranuclear caps that protect nuclei from impinging UV rays. Identification of growth factors that stimulate dendricity and the production of melanin are of interest because of their potential chemotherapeutic value in preventing UVR-induced skin cancers, including squamous cell carcinoma and melanoma. Prostaglandin E2 (PGE2) is a lipid factor produced by keratinocytes in response to UVR and inflammatory conditions such as wound healing (1,2). PGE2 regulates a broad range of physiological processes in the skin, including immune function, carcinogenesis, cutaneous barrier function and cell growth and differentiation (3–6). Biosynthesis of PGE2 is controlled by phospholipase A2 (PLA2), cyclooxygenase (COX), and prostaglandin E synthase (PGES) enzymes. Type IV phospholipase A2, also known as cytosolic PLA2 (cPLA2), is the only PLA2 that selectively releases arachidonic acid in preference to other fatty acids, and is a rate-limiting step in prostaglandin production (7,8). cPLA2 is tightly regulated both at the transcriptional and post-transcriptional level and is activated by two primary mechanisms: i) phosphorylation at serine residues, and ii) Ca2+-dependent translocation to the nuclear envelope, which is important for coupling with COX enzymes.
Paracrine factors produced by keratinocytes and fibroblasts play an important role in the regulation of melanocyte function. For example, keratinocytes synthesize and secrete endothelin-1 following UVR (9–12) and keratinocyte-derived endothelin-1 stimulates tyrosinase activity (13), melanocyte dendricity (14) and proliferation (15,16). Fibroblasts produce stem cell factor, which induces melanocyte dendrite formation, and pigmentation (17). Autocrine factors for melanocytes are few, compared with paracrine factors. Alpha-melanocyte stimulating hormone (α-MSH) is produced by melanocytes in response to UVR, and controls melanocyte proliferation, melanin production and dendricity through stimulation of the melanocortin 1 receptor (MC1R, 18–21). Our previous work has shown that PGF2α, an eicosanoid that binds to the FP receptor, is a UV-inducible autocrine factor for melanocytes that stimulates melanocyte dendrite formation and tyrosinase activation, but not proliferation (22).
PGE2 binds to 4 different G-protein coupled receptors termed EP1, EP2, EP3 and EP4. We showed previously that PGE2 stimulates melanocyte dendrite formation through activation of EP1 and EP3, and that melanocytes express the EP3 receptor in vivo (23). The purpose of the present study was to determine if PGE2 is released from melanocytes in response to UVR, and its effects on tyrosinase activation and proliferation, and to begin to identify the prostanoid receptor(s) that mediates PGE2 effects on melanocyte pigmentation. Our data show that UVR stimulates PGE2 synthesis and activates cPLA2 in melanocytes, indicating that PGE2 is an autocrine factor for melanocytes. Treatment of melanocytes with PGE2 stimulated the cAMP/PKA pathway, and increased tyrosinase activity, and modestly increased melanocyte proliferation. Through the use of selective agonists and antagonists of EP receptors, we show that EP4 receptor stimulates, and EP3 receptor inhibits, cAMP production in melanocytes. These data are the first to demonstrate that PGE2 is an UV-inducible autocrine factor for human melanocytes that stimulates tyrosinase activation, and that signaling by EP3 and EP4 receptors modulate the cAMP/PKA signaling pathway, a critical regulatory pathway of melanocyte function.
Materials and Methods
Reagents
Rabbit polyclonal antibodies to β-actin and to the EP4 receptor (H-160) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit polyclonal antibodies against EP2and EP 3 receptor were purchased from Cayman Chemicals (Ann Arbor, MI); rabbit polyclonal antibodies to cPLA2 phosphorylated on Ser 505 and rabbit polyclonal antibodies to cPLA2 were purchased from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibodies were purchased from Sigma Co (St. Louis, MO). Full range rainbow molecular weight markers were purchased from Amersham Life Sciences (Arlington Heights, Il). PGE2, butaprost (EP2 receptor agonist), sulprostone (EP3 receptor agonist) and ELISA Assay kits for analysis of PGE2 were purchased from Cayman Chemicals. This kit has essentially no cross reactivity with PGF1α, PGF2α, PGA2, PGD2, thromboxane or arachidonic acid (all <1.6%), but does cross react with PGE1 (70%) and PGE3 (16.3%). PAH6809 (EP2 receptor antagonist) and L 161982 (EP4 receptor antagonist) were purchased from Tocris Biosciences (Ellisville, MO).
Cells and cell culture
Human melanocyte cultures were derived from individual neonatal foreskins as previously described (18). Melanocytes with loss of function mutations in the MC1 receptor were characterized for the response to the MC1R ligand α-MSH as described (24). For analysis of effects of UVR on PGE2 synthesis and on PGE2 synthetic enzymes, melanocytes were maintained in of MCDB 153, supplemented with 4% fetal bovine serum (FBS), 14 μg/ml bovine pituitary extract (BPE), 5 μg/ml insulin, 10 ng/ml α-tocopherol acetate, 8 nM 12-o-tet-radecanoylphorbol-13-acetate (TPA), and 0.75 ng/ml basic fibroblast growth factor (bFGF). All supplements were purchased from Sigma Co, except FBS, which was purchased from Mediatech (Manassas, VA). For experiments in which effects of exogenous PGE2 and prostanoid receptor agonists/antagonists were examined (tyrosinase activity assays, BrDu uptake assays, and cAMP assays), and for experiments in which EP receptor expression was examined by Western blotting, melanocytes were established in medium as described above, and were changed into medium without TPA and BPE (“−/−” media), 4 days prior to the experiment. Removal of these two factors is important for determining the effects of factors that activate PKC, such as PGE2, since TPA down-regulates protein kinase C, and cAMP activity, since BPE contains high concentrations of melanocortins that stimulate cAMP formation (24–26). This medium does not support proliferation, however, melanocyte survival is maintained by the presence of bFGF and fetal bovine serum (personal observations).
Irradiation of melanocytes with UVR
Melanocytes were maintained in complete media and irradiation was carried out in phosphate buffered saline (PBS) using a bank of 6 FS20 sun lamps (Westinghouse) that have more than 75% emission in the UVR range (280–320 nm), with a peak emission of 313 nm, and less than 25% UVA rays (>320 nm). Kodacel filter was used to remove UVC rays. For controls, cells were placed in PBS but not irradiated (‘sham irradiated”).
Western Blotting for EP receptors
Cells were lysed in RIPA buffer (150 mM NaCl, 1%NP-40, 0.5% DOC, 0.1% SDS, 50 mM Tris-HCl) with protease inhibitors (Boehringer Mannheim, Gmbt, Germany) and phosphatase inhibitors (Phosphatase Inhibitor Cocktail Set II, Calbiochem). Total cell lysates were resolved on 10% SDS-PAGE and blotted using standard procedures. Visualization of the immunoreactive proteins was accomplished with an enhanced chemiluminescence reaction (Pierce Chemical, Rockford, IL).
Tyrosinase activity assay
Melanocytes were plated at 104 cells/cm2 in triplicate dishes in complete medium, then switched to −/− media 4 days prior to treatment with PGE2. Melanocytes were treated with PGE2 (1.5 nM, 3 nM or 30 nM) every other day, for 6 days, in the continuous presence of indomethacin (4 μg/ml) to block endogenous PG production. Twenty-four hours after the final treatment with PGE2, 0.7μCi 3H-tyrosine (Perkin Elmer, Boston, MA) was added to each dish, and 24 hours later, tyrosinase activity was assayed according to the modified charcoal absorption method of Pomerantz as previously described (19, 27). Tyrosinase activity was determined using the in situ tyrosine hydroxylase activity, based on measuring the 3H-labeled water that is released to the media as melanocytes metabolize 3H-tyrosine to DOPA during the first reaction catalyzed by tyrosinase in the melanin synthetic pathway. Cell number in each dish was counted using a Coulter Particle Counter (Model Z1 Hialeah, FL).
Proliferation Assay
Proliferation was measured using a BrDu cell proliferation assay kit supplied by Calbiochem (San Diego, CA). Cells were plated at 2 × 104 melanocytes/well (triplicate wells) in a 96 well plate in −/− media in the presence of PGE2, and 8 hours later wells were spiked with BrDu. Twenty-four hours after addition of PGE2, assay for BrDu uptake was performed using manufacturer’s instructions. Cells were pretreated with indomethacin (4 μg/ml for 18 hours prior to the assay) to suppress endogenous prostaglandin production.
Cyclic-AMP assays
Melanocytes (2.6 × 104 cells/cm2) were plated in complete media, then switched to −/− media. Cells were treated with indomethacin (4 μg/ml) overnight to block endogenous prostaglandin production, and indomethacin was maintained in the media for the duration of the experiment. Two hours prior to the addition of PGE2 or prostanoid receptor agonists and antagonists, isobutylmethylxanthine (IBMX, 2 mM) was added to inhibit phosphodiesterase activity. PGE2 or agonists/antagonists were added to the medium and cAMP levels were measured 10 minutes later using the Direct Cyclic AMP EIA kit as per manufacturers instructions (Assay Design, Inc., Ann Arbor, MI). Positive controls consisted of cells treated with 1 μM forskolin, a direct activator of adenylate cyclase, for 10 minutes. The plate was read on a Finstrument™ microplate Reader (MTX Lab Inc, VA) and the data was analyzed with Deltasoft™ 3 software (BioMetallics, Inc., Princeton, NJ). cAMP was normalized to cell number and expressed as pg/ml/cell.
Statistical Evaluation of Data
Data were expressed as mean ± Standard Error (SE) or Standard Deviation (SD) and were analyzed using Fisher’s exact test to determine statistically significant differences in data sets. A p value of <0.05 was considered significant.
Results
UVR stimulates PGE2 secretion in melanocytes
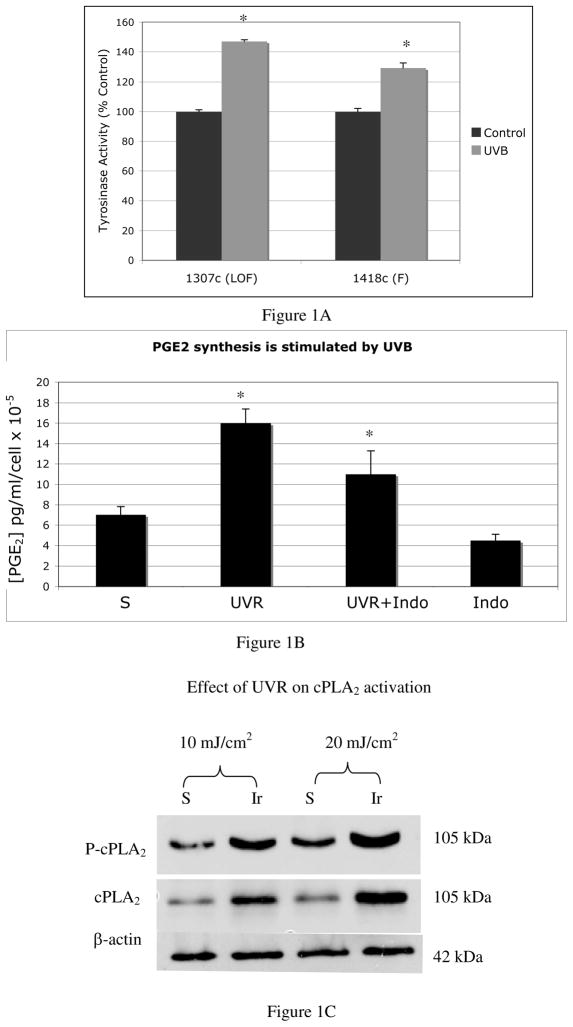
Melanocytes expressing functional or loss of function MC1R were irradiated with UVR (25 mJ/cm2), every other day, for a total of 4 irradiations. Two days after the final irradiation, tyrosinase activity was measured and normalized to cell number (Figure 1A). Results are presented as percent of controls, which consisted of sham-irradiated cells. Melanocytes, regardless of their MC1R status, showed an increase in tyrosinase activity in response to UVR, indicating the presence of a UVR-inducible factor in melanocytes that is not a melanocortin that binds to the MC1R.
Figure 1. Evidence that the UVR-induced autocrine factor is not α-MSH, and that UVR stimulates PGE2 synthesis.
A). Tyrosinase activity (represented as percent of controls) is increased in loss-of-function (LOF:1307c) MC1R mutants by UVR (*p<0.005), indicating the presence of an autocrine factor that is not α-MSH stimulates tyrosinase activity in response to UVR. Results are the average of four separate experiments performed on melanocytes cultured from MC1R mutant melanocytes (1307c) and melanocytes with confirmed functional MC1R (1408c) +/− standard error of the mean (SEM).
B) Melanocytes showed a 2-fold increase in levels of PGE2 in response to UVR, which was inhibited by indomethacin, indicating that COX enzymes are activated by UVR in melanocytes. Differences in PGE2 were statistically significant between irradiated and sham irradiated cells, and between irradiated cells and cells irradiated in the presence of indomethacin (*p=0.12). Each bar represents the average amount of PGE2 from 3 independent experiments, +/− SEM, in which each condition was performed in triplicate wells, and in which each experiment was performed using melanocytes cultured from a separate Caucasian donor (n=3).
C) UVR induced the phosphorylation of cPLA2 and increased levels of cPLA2 protein in melanocytes. Results are representative of 2 experiments. For each experiment, melanocytes cultured from a separate Caucasian donor were used (n=2).
We next determined whether UVR stimulates the synthesis of PGE2 in melanocytes. Cells were irradiated with UVR (50 mJ/cm2, five times over the course of 10 days) and 2 days after the final dose of UVR, culture supernatants were collected and PGE2 levels were determined (Figure 1B). In some dishes, cells were incubated with indomethacin (4 μg/ml) for the duration of the experiment to suppress UVR-dependent activation of COX enzymes. Controls consisted of sham-irradiated cells. Sham-irradiated melanocytes express low but detectable amounts of PGE2 (7 pg/ml/cell × 10−5) that were reduced by the addition of indomethacin, indicating low but detectable constitutive activity of COX enzymes. Following irradiation, PGE2 levels were significantly higher than sham-irradiated cells (16 pg/ml/cell × 10−5 vs 7 pg/ml/cell × 10−5, p=0.12). UVR-dependent up-regulation of PGE2 synthesis was decreased significantly (p=0.12) by indomethacin, but was not completely eliminated, suggesting that UVR-inducible enzymes other than COX-2 regulate PGE2 synthesis in melanocytes. The rate limiting step in eicosanoid synthesis is catalyzed by cPLA2, which is activated by UVR in human keratinocytes (28). To determine if UVR activates cPLA2 in melanocytes, cells were irradiated (10 mJ/cm2 or 20 mJ/cm2) and 24 hours later, lysates were blotted for phosphorylated (active) cPLA2 and total cPLA2 (Figure 1C). Irradiation stimulated phosphorylation and expression of cPLA2, similar to effects described in human keratinocytes.
PGE2 stimulates tyrosinase activity, proliferation, and cAMP production, in melanocytes
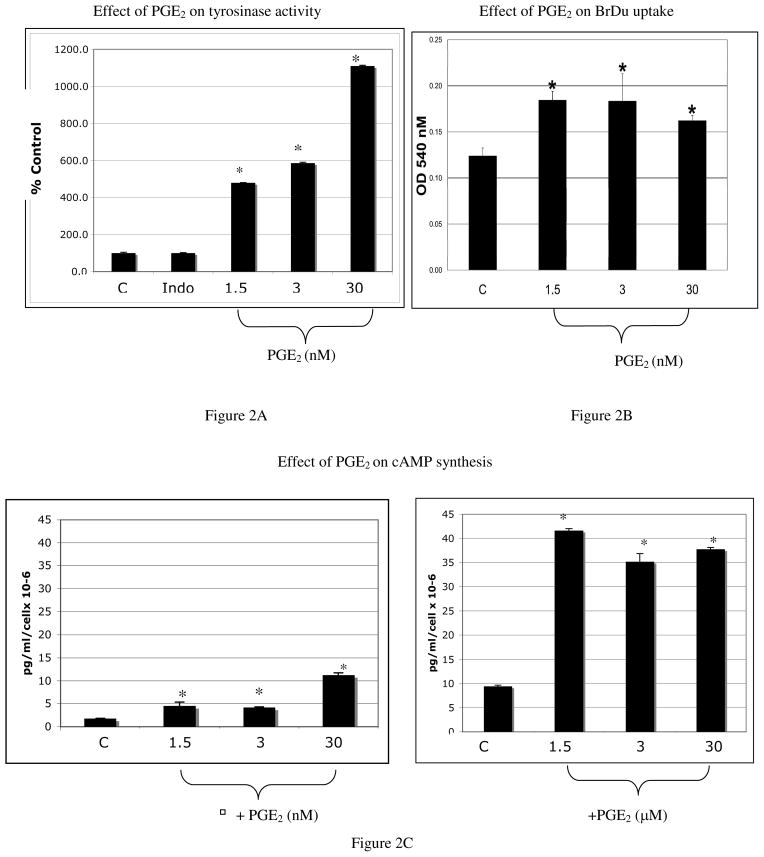
Melanocytes were treated with PGE2 or vehicle every other day for 6 days, and tyrosinase activity was quantified (Figure 2A). Indomethacin (4 μg/ml) was present during the duration of the experiment to block endogenous prostaglandin production. A dose dependent increase in tyrosinase activity in response to PGE2 was observed. PGE2 at doses of 1.5 nM and 3 nM induced a 4.5 and 5.8 fold increase in tyrosinase activity compared with cells treated with vehicle (p<0.05). At the highest dose of PGE2 (30 nM) a 10-fold increase in tyrosinase activity, over vehicle-treated cells, was observed (p<0.05). To determine the effect of PGE2 on melanocyte proliferation, cells were pre-treated with indomethacin (4 μg/ml for 18 hours), and plated in triplicate in 96 well plates with PGE2 or vehicle. Cells were spiked with BrDu 8 hours after plating, and 24 hours after addition of PGE2, ELISA assay for BrDu uptake was performed (Figure 2B). PGE2 stimulated a modest (1.2 fold) yet statistically significant (p<0.001) increase in BrDu uptake, at doses as low as 1.5 nM.
Figure 2. PGE2 stimulates tyrosinase activation, proliferation, and cAMP production.
A) A dose-dependent increase in tyrosinase activity was observed with PGE2 treatment. Results represent the average of three separate experiments in which triplicate dishes were analyzed, +/− standard error of the mean (SEM). For each experiment, melanocytes cultured from a separate Caucasian donor were used (n=3).
B) Melanocyte proliferation was stimulated by PGE2 (*p<0.001) at each concentration tested. Each bar represents the average of 3 separate experiments +/− standard deviation (SD). For each experiment, melanocytes cultured from a separate Caucasian donor were used (n=3).
C) At doses as low as 1.5 nM, PGE2 stimulated a statistically significant (*p<0.05) increase in cAMP compared with vehicle treated controls (4.4 pg/ml/cell × 10−6 compared with 1.8 pg/ml/cell × 10−6 respectively); a dose of 30 nM PGE2 resulted in a cAMP response of 11 pg/ml/cell × 10−6. Micromolar doses PGE2 stimulated a maximum increase in cAMP of 42 pg/ml/cell × 10−6, compared with vehicle treated cells. Each bar represents the averaged cAMP levels of 3 separate experiments +/−SD, in which each experiment was performed in duplicate wells. For each experiment, melanocytes cultured from a separate Caucasian donor were used (n=3).
To determine the effects of PGE2 on cAMP production, melanocytes were pre-treated with indomethacin for 18 hours (4 μg/ml), and 2 hours prior to the assay, IBMX was added to block phosphodiesterase activity. PGE2 was added and cAMP was measured 10 minutes later. PGE2 at nanomolar concentrations stimulated a 2.6 fold and 6.5-fold increase in cAMP at a dose of 1.5 nM and 30 nM respectively, compared with vehicle treated controls (Figure 2C). At higher doses of PGE2 (1.5–30 μM) cAMP levels reached a maximum of 42 pg/ml/cell × 10−6. While PGE2 stimulated a cAMP response in melanocytes, the increase in cAMP induced by PGE2 was much less than that observed following treatment with 1 μM forskolin for 10 minutes (296.6 pg/ml/cell × 10−6, data not shown).
EP4 receptor signaling stimulates cAMP release
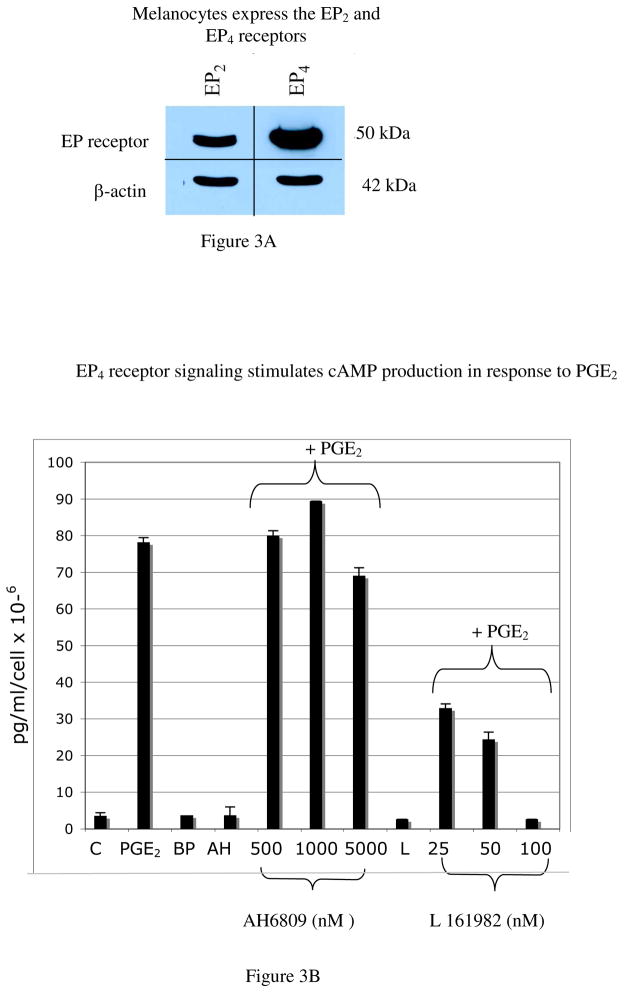
The cAMP/PKA pathway is the main regulator of melanocyte function, through its effects on Micropthalmia transcription factor (Mitf), which stimulates the synthesis of tyrosinase and tyrosinase related protein-1 (TRP-1), and through activation of the MAP kinase pathway, which stimulates proliferation and maintains survival (29–33). The EP2 and EP4 receptors couple to Gs to stimulate cAMP production (34). To determine if melanocytes express the EP2 and EP4 receptors, melanocytes maintained in −/− media for 4 days were blotted with antibodies against the EP2 and EP4 receptors (Figure 3A). Melanocytes expressed both receptors. We next sought to determine the prostanoid receptor that mediates effects of PGE2 on cAMP production. To determine if EP2 or EP4 receptor signaling stimulates cAMP production, melanocytes were treated with PGE2 alone, or with the EP2-receptor specific agonist butaprost (BP;100 nM). In some experiments, cells were treated with PGE2 (1.5 μM) in the presence of the EP2 or EP4 receptor antagonists AH6809 (AH; 500 nM, 1 μM, 5 μM) and L 161982 (L; 25 nM, 50 nM, 100 nM) respectively. The lack of a commercially available EP4 receptor agonist prevented us from directly assessing the effect of EP4 stimulation on cAMP. Controls consisted of cells treated with AH6809 alone (5 μM) or L 161982 alone (100 nM). Ten minutes after treatment with prostanoids or agonists/antagonists, cAMP levels were measured (Figure 3B). As expected, PGE2 stimulated cAMP production compared with vehicle treated controls (78 pg/ml/cell × 10−6 vs. 3.5 pg/ml/cell × 10−6 respectively). Butaprost failed to stimulate cAMP production, and pre-treatment of cells with the EP2 receptor antagonist AH6809 prior to the addition of PGE2, failed to block PGE2-dependent increases in cAMP. Higher doses of butaprost (up to 500 nM) also failed to elicit cAMP production (data not shown). In contrast, pre-treatment of melanocytes with the EP4 receptor antagonist L 161982 resulted in a dose dependent blockade of PGE2-dependent cAMP production in melanocytes, with complete loss of PGE2-dependent cAMP response at a dose of inhibitor of 100 nM. These data show that EP4 receptor signaling stimulates cAMP production in melanocytes.
Figure 3. EP3 and EP4 receptor signaling have opposing effects on cAMP production in melanocytes.
A) Melanocytes maintained in −/− media for 4 days were lysed and resolved on 10% SDS-PAGE and blotted with antibodies against EP2 and EP4. Melanocytes express both EP2 and EP4, although steady state levels of EP4 are higher. Results are representative of blots on melanocyte cultures from three separate Caucasian donors (n=3).
B) Treatment of melanocytes with PGE2 (1.5 μM) stimulates cAMP production (78 pg/ml/cell × 10−6), where as butaprost (“BP”, 100 nM), an EP2 receptor agonist, had no effect on cAMP. Pre-treatment of melanocytes with the EP2 receptor antagonist AH6809 (“AH”) failed to block PGE2-dependent cAMP production, further confirming that EP2 receptor signaling does not mediate effects of PGE2 on cAMP. Pre-treatment of melanocytes with the EP4 receptor antagonist L 161982 (“L”), blocked PGE2-dependent cAMP production in a dose-dependent manner. Treatment with AH6809 (5 μM) or L 161982 (100 nM) alone had no effect on cAMP levels. Results are representative of 2 separate experiments performed on melanocytes from a single Caucasian donor (n=2), done in duplicate wells, +/−SD.
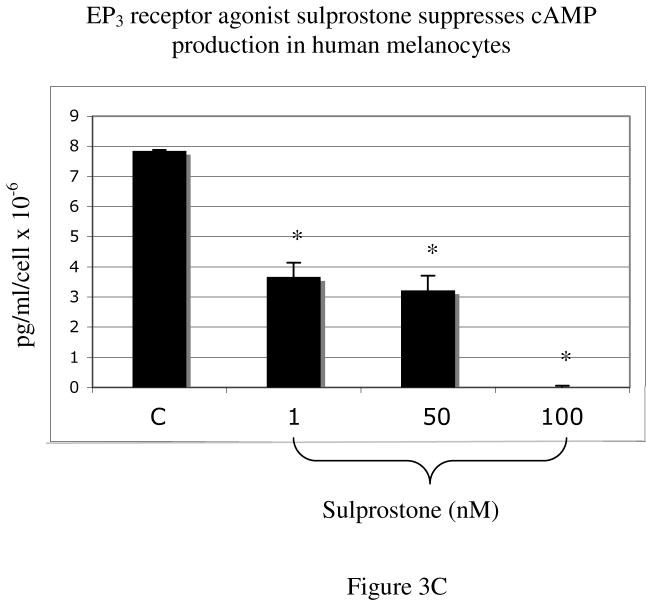
C) Treatment of melanocytes with sulprostone, an EP3 receptor agonist, lowered baseline cAMP production in a dose dependent manner At a dose of 1 nM, sulprostone lowered cAMP from 7.8 pg/ml/cell × 10−6 to 3.6 pg/ml/cell × 10−6; at a dose of 100 nM, sulprostone completely eliminated basal cAMP production. Results are representative of 2 separate experiments performed on melanocytes from a single Caucasian donor (n=2), done in duplicate wells, +/−SD.
Human tissues express eight isoforms of the EP3 receptor that differ in their carboxyl terminal domain, and while most couple to Gi to inhibit cAMP production, some EP3 isoforms couple to Gs (35–39). Because melanocytes express EP3 receptor (23, 40), we also tested the effect of sulprostone (1 nM, 50 nM, 100 nM), a selective EP3 receptor agonist, on cAMP production in melanocytes (Figure 3C). Sulprostone decreased cAMP in melanocytes in a dose dependent manner, completely eliminating detectable cAMP at a dose of 100 nM. These data show that the EP3 and EP4 receptor signaling have opposing effects on cAMP production in melanocytes in response to PGE2.
Discussion
The effects of repetitive UVR on skin are complex, and a recent report indicates that repetitive doses of UVR are protective of subsequent UV-induced DNA damage in vivo (41). Another effect of repetitive UVR is increased pigmentation (tanning response) due to induction of paracrine factors by keratinocytes (42). PGE2 is a lipid factor that is released by keratinocytes following UVR and in inflammatory conditions, such as wound healing and has multiple functions in the skin including proliferation, differentiation and immune function (43–48). PGE2 binds to 4 distinct EP receptors with differing affinities for PGE2, which stimulate multiple signaling pathways including cAMP/PKA (EP2 and EP4), Ca+2 mobilization (EP1) and the inositol-triphosphate-diacyl glycerol pathway (EP3; 49). In the present study we show that normal melanocytes with non-functional MC1R respond to repetitive low dose UVR with increased tyrosinase activity, suggesting the production of a potent, UVR-inducible autocrine factor, distinct from α-MSH or related melanocortins. Melanocytes produce POMC-derived bioactive peptides that function independent of the MC1R, such as β-endorphin, which binds to the mu-opiate receptor in melanocytes. Therefore, we cannot exclude the possibility that some of the effects of UVR on tyrosinase activity in our MC1R mutants are due to the production of melanocortins that bind to receptors other than the MC1R (50–53). Our previous studies have shown that PGF2α is produced by melanocytes in response to UV irradiation, and stimulates tyrosinase activity, thus it is likely that some of the effects of tyrosinase activity in MC1 receptor mutants are due to autocrine production of PGF2α. However, in this report we show that PGE2 production is also stimulated by UVR, and PGE2 can be added to the short list of UV inducible autocrine factors for melanocytes. Levels of PGE2 and PGF2α produced by melanocytes under unstimulated conditions are low and are similar (22 and present report). It is difficult to directly compare levels of PGE2 and PGF2α produced by melanocytes in response to UVR because our previous studies examining effects of UVR on PGF2α production were performed using a solar simulator, which predominantly emits wavelengths in the UVA range. However, both are clearly inducible by UVR, and it is likely that PGE2 and PGF2α both contribute to melanocyte dendricity and tyrosinase activation. While PGE2 and PGF2α stimulate melanocyte dendrite formation and tyrosinase activation, PGE2, in contrast with PGF2α, stimulates melanocyte proliferation, suggesting that PGE2 may contribute to UVR-dependent melanocyte proliferation. While the amount of PGE2 produced by melanocytes is quite small compared with keratinocytes, because prostaglandins are rapidly oxidized, localized synthesis of PGE2 at the melanocyte cell membrane may result in efficient receptor binding and signaling, however, it is likely that the primary effect of PGE2 on melanocytes occurs through paracrine production of PGE2 by keratinocytes. However, it is not known whether the regulation of prostaglandin synthesis in response to UVR differs between melanocytes and keratinocytes. Potential differences in activation of phospholipases, COX-2 enzyme, or PGE synthases in melanocytes and keratinocytes in response to UVR could result in selective production of prostaglandins by melanocytes at certain doses of UVR. The rate-limiting step in eicosanoid production is the release of arachidonic acid from lipid membranes by cPLA2 (7, 54). UVR stimulates cPLA2 activation and synthesis in keratinocytes (28), and our data indicate that UVR stimulates the activation of cPLA2 in melanocytes. Two other phospholipases release arachidonic acid from cell membranes; the Ca+2 independent phospholipase A2 (iPLA2), which has recently been shown to be expressed by melanocytes (55), and secretory phospholipase A2 (sPLA2), which we have shown is expressed by keratinocytes, but not melanocytes (56). While cPLA2 is the rate-limiting step in arachidonic acid release, and is inducible by UVR in both melanocytes and keratinocytes, it would be of interest to examine the role of iPLA2 in UV-dependent prostaglandin synthesis in melanocytes. cPLA2 is activated by the MAP kinase p38, which is involved in the stress responses of keratinocytes and melanocytes (57–61). It will be of interest to determine whether stress-related MAP kinases (p38 and JNK) play a role in UVR-dependent cPLA2 activation in melanocytes.
PGE2 at nanomolar concentrations stimulated tyrosinase activation in melanocytes. Therefore, conditions in which PGE2 is released, including exposure to UVR and inflammatory mediators, are expected to increase skin pigmentation in a PGE2-dependent manner. While several signaling pathways modulate tyrosinase activity, the cAMP/protein kinase A pathway is the best characterized. PGE2 stimulated cAMP in melanocytes, suggesting a likely mechanism for PGE2-dependent tyrosinase activation. We previously examined the effect of PGE2 on cAMP response in melanocytes and found a small, yet statistically insignificant increase in cAMP in response to PGE2 (40). The lack of a significant change in cAMP in the previous report is most likely due to the presence of BPE in the media, which has been shown to suppress cAMP responses to growth factors in melanocytes (18, 19). The amount of cAMP produced in response to PGE2 varied from culture to culture; differences in cAMP levels could reflect different levels or activity of prostanoid receptors in individual cultures, or different activities of adenylate cyclase. The EP2 and EP4 couple to Gs to stimulate the synthesis of cAMP (34). In addition to Gs, the EP4 receptor is coupled to the PI3 kinase/AKT pathway (62). Melanocytes express EP2 and EP4 receptors by Western blotting, suggesting they are potential candidates for PGE2-dependent tyrosinase activation through cAMP production. We also examined the effect of EP3 receptor stimulation on cAMP production, in melanocytes. The selective EP2 agonist butaprost had no effect on cAMP production even at high doses, and pre-treatment of melanocytes with AH6809, a selective inhibitor of the EP2 receptor, also failed to block PGE2-dependent cAMP release, indicating that EP2 does not mediate effects of PGE2 on cAMP in melanocytes. Because the EP2 receptor is coupled to Gs in virtually all cell types examined, the lack of a response of melanocytes to EP2 receptor agonists, suggests that the receptor identified by Western blotting is non-functional. In contrast, the EP4 receptor functionally couples to cAMP in melanocytes because pre-treatment of melanocytes with L 161982, a selective inhibitor of EP4 receptor, completely blocked PGE2-dependent cAMP production in a dose-dependent manner, even at high concentrations of PGE2. Unfortunately, because of the lack of a commercially available EP4 receptor agonist, we are unable to test the effect of stimulation of EP4 receptor on cAMP production. Sulprostone, an EP3 receptor agonist, lowered cAMP levels in melanocytes, showing that EP3 and EP4 receptor signaling has opposing effects on cAMP in melanocytes; the net effect of activating these two receptors by PGE2 is increased cAMP production.
Our previous data shows that PGE2 stimulates melanocyte dendrite formation through EP3 receptors(23). Data presented here show that PGE 2 stimulates cAMP release, tyrosinase activity, and melanocyte proliferation. EP3 and EP4 receptors are high affinity receptors activated by nanomolar doses of PGE2. Our data suggest that the relative levels of expression and/or signaling of EP3 and EP4 receptors may control tyrosinase activation in melanocytes in response to PGE2 (Figure 4). EP3 receptor activation is predicted suppress pigmentation (decreased cAMP) and stimulate dendrite formation (activation of PKCζ), whereas EP4 receptor activation is predicted to stimulate pigmentation (increased cAMP), in response to PGE2. However, because EP receptors stimulate multiple pathways, such as PI3-kinase, protein kinase C and MAP kinase (63), which regulate tyrosinase activity (64, 65), the contribution of EP receptor(s) signaling on tyrosinase activity is likely to be complex, and is currently being studied. Regulation of EP receptors by UVR has been demonstrated in murine epidermal keratinocytes in vivo and in vitro (66), and the FP receptor for PGF2α is regulated by UVR in melanocytes in vitro and in human skin in vivo. Therefore, regulation of EP3 and EP4 receptors by UVR is a potential mechanism by which effects of PGE2 on melanocyte pigmentation may be modulated. These data suggest the intriguing possibility that EP3 and EP4 receptors could be therapeutic targets in situations in which PGE2 is released, including post-inflammatory hyper-pigmentation.
Figure 4. Effects of PGE2 on melanocyte pigmentation are controlled by EP3 and EP4 receptors.
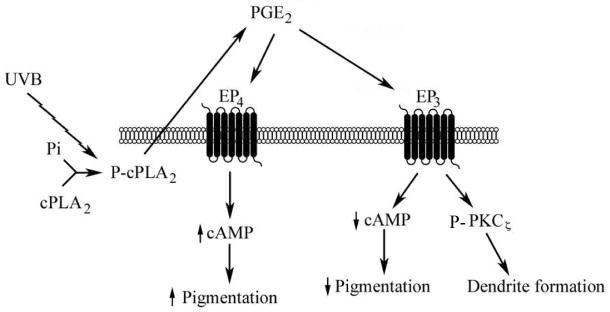
UVR stimulates the activation of cPLA2, the rate-limiting step in eicosanoid synthesis. Autocrine production of PGE2, stimulated by UVR, activates the high affinity receptors EP3 and EP4. EP3 receptor stimulation activates PKCζ, resulting in dendrite formation, and lowers cAMP levels; EP4 receptor signaling increases cAMP release. The relative levels and or activity of EP3 and EP4 receptors is predicted to control the effect of PGE2 on melanocyte tyrosinase activation and melanin synthesis, in part through modulation of cAMP levels.
Supplementary Material
Acknowledgments
This work was supported by RO1AR45427-04 (Glynis Scott) and by 2R01ES009110 (Zalfa Abdel Malek).
References
- 1.Pentland AP, Mahoney M, Jacobs SC, Holtzman MJ. Enhanced prostaglandin synthesis after ultraviolet injury is mediated by endogenous histamine stimulation. A mechanism for irradiation erythema. J Clin Invest. 1990;86:566–574. doi: 10.1172/JCI114746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pentland AP, Mahoney MG. Keratinocyte prostaglandin synthesis is enhanced by IL-1. The Journal of investigative dermatology. 1990;94:43–46. doi: 10.1111/1523-1747.ep12873337. [DOI] [PubMed] [Google Scholar]
- 3.Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000;30:3–21. [PubMed] [Google Scholar]
- 4.Pentland AP, Needleman P. Modulation of keratinocyte proliferation in vitro by endogenous prostaglandin synthesis. J Clin Invest. 1986;77:246–251. doi: 10.1172/JCI112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziboh VA, Cho Y, Mani I, Xi S. Biological significance of essential fatty acids/prostanoids/lipoxygenase-derived monohydroxy fatty acids in the skin. Arch Pharm Res. 2002;25:747–758. doi: 10.1007/BF02976988. [DOI] [PubMed] [Google Scholar]
- 6.Holtzman MJ, Zhang V, Hussain H, Roswit WT, Wilson JD. Prostaglandin H synthase and lipoxygenase gene families in the epithelial cell barrier. Annals of the New York Academy of Sciences. 1994;744:58–77. doi: 10.1111/j.1749-6632.1994.tb52724.x. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A2 family. Progress in lipid research. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Murakami M. Hot topics in phospholipase A2 field. Biological & pharmaceutical bulletin. 2004;27:1179–1182. doi: 10.1248/bpb.27.1179. [DOI] [PubMed] [Google Scholar]
- 9.Gordon PR, Mansur CP, Gilchrest BA. Regulation of human melanocyte growth, dendricity, and melanization by keratinocyte derived factors. J Invest Dermatol. 1989;92:565–572. doi: 10.1111/1523-1747.ep12709595. [DOI] [PubMed] [Google Scholar]
- 10.Imokawa G, Yada Y, Kimura M, Morisaki N. Granulocyte/macrophage colony-stimulating factor is an intrinsic keratinocyte-derived growth factor for human melanocytes in UVA-induced melanosis. The Biochemical journal. 1996;313 (Pt 2):625–631. doi: 10.1042/bj3130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuboi R, Sato C, Oshita Y, et al. Ultraviolet B irradiation increases endothelin-1 and endothelin receptor expression in cultured human keratinocytes. FEBS Lett. 1995;371:188–190. doi: 10.1016/0014-5793(95)00912-s. [DOI] [PubMed] [Google Scholar]
- 12.Yohn JJ, Morelli JG, Walchak SJ, Rundell KB, Norris DA, Zamora MR. Cultured human keratinocytes synthesize and secrete endothelin-1. J Invest Dermatol. 1993;100:23–26. doi: 10.1111/1523-1747.ep12349932. [DOI] [PubMed] [Google Scholar]
- 13.Imokawa G, Kobayashi T, Miyagishi M, Higashi K, Yada Y. The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment Cell Res. 1997;10:218–228. doi: 10.1111/j.1600-0749.1997.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 14.Hara M, Yaar M, Gilchrest BA. Endothelin-1 of keratinocyte origin is a mediator of melanocyte dendricity. J Invest Dermatol. 1995;105:744–748. doi: 10.1111/1523-1747.ep12325522. [DOI] [PubMed] [Google Scholar]
- 15.Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992;267:24675–24680. [PubMed] [Google Scholar]
- 16.Tada A, Suzuki I, Im S, Davis MB, Cornelius J, Babcock G, Nordlund JJ, Abdel-Malek ZA. Endothelin-1 is a paracrine growth factor that modulates melanogenesis of human melanocytes and participates in their responses to ultraviolet radiation. Cell Growth and Differentiation. 1998;9:575–584. [PubMed] [Google Scholar]
- 17.Grabbe J, Welker P, Dippel E, Czarnetzki BM. Stem cell factor, a novel cutaneous growth factor for mast cells and melanocytes. Archives of dermatological research. 1994;287:78–84. doi: 10.1007/BF00370723. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Malek Z, Swope VB, Suzuki I, et al. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1789–1793. doi: 10.1073/pnas.92.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki I, Cone RD, Im S, Nordlund J, Abdel-Malek ZA. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology. 1996;137:1627–1633. doi: 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- 20.Hunt G, Todd C, Cresswell JE, Thody AJ. A-Melanocyte stimulating hormone and its analogue Nle4 DPhe7 α-MSH affect morphology, tyrosinase activity, and melanogenesis of cultured human melanocytes. J Cell Sci. 1994;107:205–211. doi: 10.1242/jcs.107.1.205. [DOI] [PubMed] [Google Scholar]
- 21.Chakraborty AK, Funasaka Y, Slominiski A, Ermak G, Hwang J, Pawelek J, Ichihashi M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochem Biophysic Acta. 1996;1313:130–138. doi: 10.1016/0167-4889(96)00063-8. [DOI] [PubMed] [Google Scholar]
- 22.Scott G, Jacobs S, Leopardi S, et al. Effects of PGF(2alpha) on human melanocytes and regulation of the FP receptor by ultraviolet radiation. Experimental cell research. 2005;304:407–416. doi: 10.1016/j.yexcr.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Scott G, Fricke A, Fender A, McClelland L, Jacobs S. Prostaglandin E2 regulates melanocyte dendrite formation through activation of PKCzeta. Experimental cell research. 2007;313:3840–3850. doi: 10.1016/j.yexcr.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott MC, Wakamatsu K, Ito S, Kobayashi N, Groden J, Kavanagh R, Takakuwa T, Virador V, Hearing VI, Abdel-Malek ZA. Human melanocortin 1 receptor variants, receptor function and melanocyte response to UV radiation. J Cell Science. 2002;115:2349–2355. doi: 10.1242/jcs.115.11.2349. [DOI] [PubMed] [Google Scholar]
- 25.Oka M, Ogita K, Ando H, et al. Deletion of specific protein kinase C subspecies in human melanoma cells. J Cell Physiol. 1996;167:406–412. doi: 10.1002/(SICI)1097-4652(199606)167:3<406::AID-JCP4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 26.Oka M, Ogita K, Ando H, Kikkawa U, Ichihashi M. Differential down-regulation of protein kinase C subspecies in normal human melanocytes: possible involvement of the zeta subspecies in growth regulation. The Journal of investigative dermatology. 1995;105:567–571. doi: 10.1111/1523-1747.ep12323485. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki I, Tada A, Ollmann MM, et al. Agouti signaling protein inhibits melanogenesis and the response of human melanocytes to alpha-melanotropin. The Journal of investigative dermatology. 1997;108:838–842. doi: 10.1111/1523-1747.ep12292572. [DOI] [PubMed] [Google Scholar]
- 28.Gresham A, Masferrer J, Chen X, Leal-Khouri S, Pentland AP. Increased synthesis of high-molecular-weight cPLA2 mediates early UV-induced PGE2 in human skin. Am J Physiol. 1996;270:C1037–1050. doi: 10.1152/ajpcell.1996.270.4.C1037. [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Malek Z, Suzuki I, Tada A, Im S, Akcali C. The melanocortin-1 receptor and human pigmentation. Annals of the New York Academy of Sciences. 1999;885:117–133. doi: 10.1111/j.1749-6632.1999.tb08669.x. [DOI] [PubMed] [Google Scholar]
- 30.Bertolotto C, Abbe P, Hemesath TJ, et al. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. The Journal of cell biology. 1998;142:827–835. doi: 10.1083/jcb.142.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertolotto C, Bille K, Ortonne JP, Ballotti R. Regulation of tyrosinase gene expression by cAMP in B16 melanoma cells involves two CATGTG motifs surrounding the TATA box: implication of the microphthalmia gene product. The Journal of cell biology. 1996;134:747–755. doi: 10.1083/jcb.134.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herlyn M, Mancianti ML, Jambrosic J, Bolen JB, Koprowski H. Regulatory factors that determine growth and phenotype of normal human melanocytes. Experimental cell research. 1988;179:322–331. doi: 10.1016/0014-4827(88)90271-6. [DOI] [PubMed] [Google Scholar]
- 33.Englaro W, Rezzonico R, Durand-Clement M, Lallemand D, Ortonne JP, Ballotti R. Mitogen-activated protein kinase pathway and AP-1 are activated during cAMP-induced melanogenesis in B-16 melanoma cells. The Journal of biological chemistry. 1995;270:24315–24320. doi: 10.1074/jbc.270.41.24315. [DOI] [PubMed] [Google Scholar]
- 34.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 35.Kotani M, Tanaka I, Ogawa Y, et al. Structural organization of the human prostaglandin EP3 receptor subtype gene (PTGER3) Genomics. 1997;40:425–434. doi: 10.1006/geno.1996.4585. [DOI] [PubMed] [Google Scholar]
- 36.Adam M, Boie Y, Rushmore TH, et al. Cloning and expression of three isoforms of the human EP3 prostanoid receptor. FEBS letters. 1994;338:170–174. doi: 10.1016/0014-5793(94)80358-7. [DOI] [PubMed] [Google Scholar]
- 37.Fabre JE, Nguyen M, Athirakul K, et al. Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation. J Clin Invest. 2001;107:603–610. doi: 10.1172/JCI10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hatae N, Sugimoto Y, Ichikawa A. Prostaglandin receptors: advances in the study of EP3 receptor signaling. J Biochem (Tokyo) 2002;131:781–784. doi: 10.1093/oxfordjournals.jbchem.a003165. [DOI] [PubMed] [Google Scholar]
- 39.Negishi M, Hasegawa H, Ichikawa A. Prostaglandin E receptor EP3gamma isoform, with mostly full constitutive Gi activity and agonist-dependent Gs activity. FEBS letters. 1996;386:165–168. doi: 10.1016/0014-5793(96)00354-7. [DOI] [PubMed] [Google Scholar]
- 40.Scott G, Leopardi S, Printup S, Malhi N, Seiberg M, Lapoint R. Proteinase-activated receptor-2 stimulates prostaglandin production in keratinocytes: analysis of prostaglandin receptors on human melanocytes and effects of PGE2 and PGF2alpha on melanocyte dendricity. The Journal of investigative dermatology. 2004;122:1214–1224. doi: 10.1111/j.0022-202X.2004.22516.x. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi Y, Coelho SG, Zmudzka BZ, et al. Cyclobutane pyrimidine dimer formation and p53 production in human skin after repeated UV irradiation. Exp Dermatol. 2008;17:916–924. doi: 10.1111/j.1600-0625.2008.00722.x. [DOI] [PubMed] [Google Scholar]
- 42.Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment cell research/sponsored by the European Society for Pigment Cell Research and the International Pigment. Cell Society. 2004;17:96–110. doi: 10.1111/j.1600-0749.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 43.Konger RL, Brouxhon S, Partillo S, VanBuskirk J, Pentland AP. The EP3 receptor stimulates ceramide and diacylglycerol release and inhibits growth of primary keratinocytes. Exp Dermatol. 2005;14:914–922. doi: 10.1111/j.1600-0625.2005.00381.x. [DOI] [PubMed] [Google Scholar]
- 44.Konger RL, Malaviya R, Pentland AP. Growth regulation of primary human keratinocytes by prostaglandin E receptor EP2 and EP3 subtypes. Biochimica et biophysica acta. 1998;1401:221–234. doi: 10.1016/s0167-4889(97)00114-6. [DOI] [PubMed] [Google Scholar]
- 45.Konger RL, Scott GA, Landt Y, Ladenson JH, Pentland AP. Loss of the EP2 prostaglandin E2 receptor in immortalized human keratinocytes results in increased invasiveness and decreased paxillin expression. The American journal of pathology. 2002;161:2065–2078. doi: 10.1016/S0002-9440(10)64485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greaves MW, Camp RD. Prostaglandins, leukotrienes, phospholipase, platelet activating factor, and cytokines: an integrated approach to inflammation of human skin. Archives of dermatological research. 1988;280 (Suppl):S33–41. [PubMed] [Google Scholar]
- 47.Kabashima K, Miyachi Y. Prostanoids in the cutaneous immune response. J Dermatol Sci. 2004;34:177–184. doi: 10.1016/j.jdermsci.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Fischer SM. Prostaglandins and cancer. Front Biosci. 1997;2:d482–500. doi: 10.2741/a207. [DOI] [PubMed] [Google Scholar]
- 49.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. The international journal of biochemistry & cell biology. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Wood JM, Gibbons NC, Schallreuter KU. Melanocortins in human melanocytes. Cell Mol Biol (Noisy-le-grand) 2006;52:75–78. [PubMed] [Google Scholar]
- 51.Rousseau K, Kauser S, Pritchard LE, et al. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J. 2007;21:1844–1856. doi: 10.1096/fj.06-7398com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tobin DJ, Kauser S. Beta-endorphin: the forgotten hair follicle melanotropin. J Investig Dermatol Symp Proc. 2005;10:212–216. doi: 10.1111/j.1087-0024.2005.10108.x. [DOI] [PubMed] [Google Scholar]
- 53.Kauser S, Schallreuter KU, Thody AJ, Gummer C, Tobin DJ. Regulation of human epidermal melanocyte biology by beta-endorphin. The Journal of investigative dermatology. 2003;120:1073–1080. doi: 10.1046/j.1523-1747.2003.12242.x. [DOI] [PubMed] [Google Scholar]
- 54.Clark JD, Schievella AR, Nalefski EA, Lin LL. Cytosolic phospholipase A2. J Lipid Mediat Cell Signal. 1995;12:83–117. doi: 10.1016/0929-7855(95)00012-f. [DOI] [PubMed] [Google Scholar]
- 55.Scuderi MR, Anfuso CD, Lupo G, Motta C, Romeo L, Guerra L, Cappellani A, Ragusa N, Cantarella G, Alberghina M. Expression of Ca(2+)-independent and Ca(2+)-dependent phospholipases A(2) and cyclooxygenases in human melanocytes and malignant melanoma cell lines. Biochim Biophys Acta. 2008;1781 (10):635–642. doi: 10.1016/j.bbalip.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Scott G, Jacobs S, Pentland AP. sPLA2-X stimulates cutaneous melanocyte dendricity and pigmentation through a lysophosphatidylcholine dependent mechanism. The Journal of investigative dermatology. 2006;126(4):855–861. doi: 10.1038/sj.jid.5700180. [DOI] [PubMed] [Google Scholar]
- 57.Zhang JP, Liang WY, Luo ZH, Yang ZC, Chan HC, Huang YS. Involvement of p38 MAP kinase in burn-induced degradation of membrane phospholipids and upregulation of cPLA2 in cardiac myocytes. Shock (Augusta, Ga. 2007;28:86–93. doi: 10.1097/SHK.0b013e31802f9d9a. [DOI] [PubMed] [Google Scholar]
- 58.Schmidlin F, Loeffler S, Bertrand C, Landry Y, Gies JP. PLA2 phosphorylation and cyclooxygenase-2 induction, through p38 MAP kinase pathway, is involved in the IL-1beta-induced bradykinin B2 receptor gene transcription. Naunyn-Schmiedeberg’s archives of pharmacology. 2000;361:247–254. doi: 10.1007/s002109900191. [DOI] [PubMed] [Google Scholar]
- 59.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 60.Chen W, Bowden GT. Activation of p38 MAP kinase and ERK are required for ultraviolet-B induced c-fos gene expression in human keratinocytes. Oncogene. 1999;18:7469–7476. doi: 10.1038/sj.onc.1203210. [DOI] [PubMed] [Google Scholar]
- 61.Tada A, Pereira E, Beitner-Johnson D, Abdel-Malek Z. Mitogen and ultraviolet-induced signaling pathways in normal human melanocytes. J lnvest Dermatol. 2002;118:316–322. doi: 10.1046/j.0022-202x.2001.01694.x. [DOI] [PubMed] [Google Scholar]
- 62.George RJ, Sturmoski MA, Anant S, Houchen CW. EP4 mediates PGE2 dependent cell survival through the PI3 kinase/AKT pathway. Prostaglandins & other lipid mediators. 2007;83:112–120. doi: 10.1016/j.prostaglandins.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Negishi M, Sugimoto Y, Ichikawa A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochimica et biophysica acta. 1995;1259:109–119. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- 64.Kim DS, Park SH, Park KC. Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. The international journal of biochemistry & cell biology. 2004;36:1482–1491. doi: 10.1016/j.biocel.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 65.Park HY, Russakovsky V, Ohno S, Gilchrest BA. The beta isoform of protein kinase C stimulates human melanogenesis by activating tyrosinase in pigment cells. The Journal of biological chemistry. 1993;268:11742–11749. [PubMed] [Google Scholar]
- 66.Tober KL, Thomas-Ahner JM, Kusewitt DF, Oberyszyn TM. Effects of UVB on E prostanoid receptor expression in murine skin. The Journal of investigative dermatology. 2007;127:214–221. doi: 10.1038/sj.jid.5700502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.